Submitted:
03 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
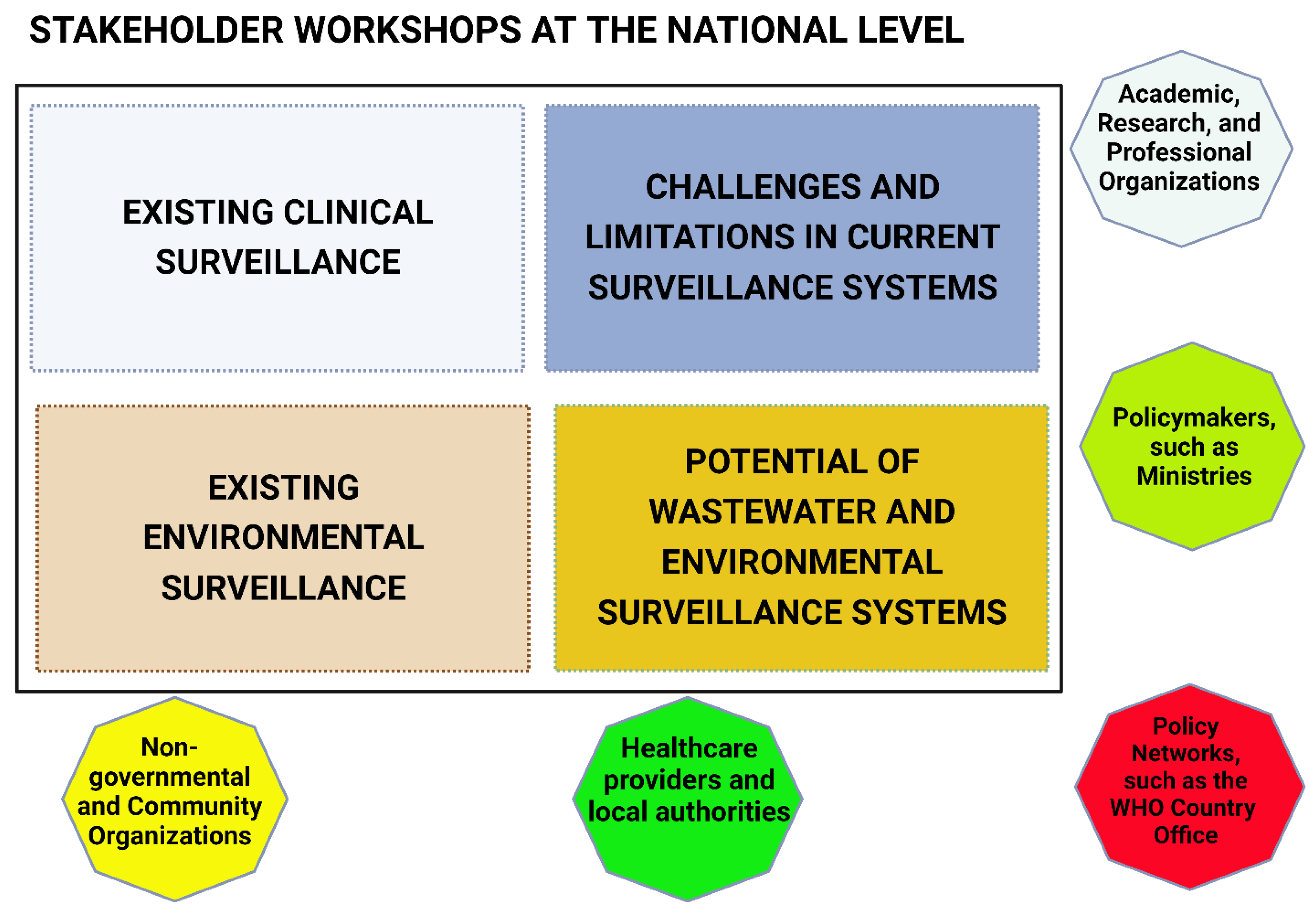
2. Methodology
3. Results
3.1. Priority Pathogens
3.2. Existing Clinical and Epidemiological Surveillance
3.3. Existing Environmental Surveillance
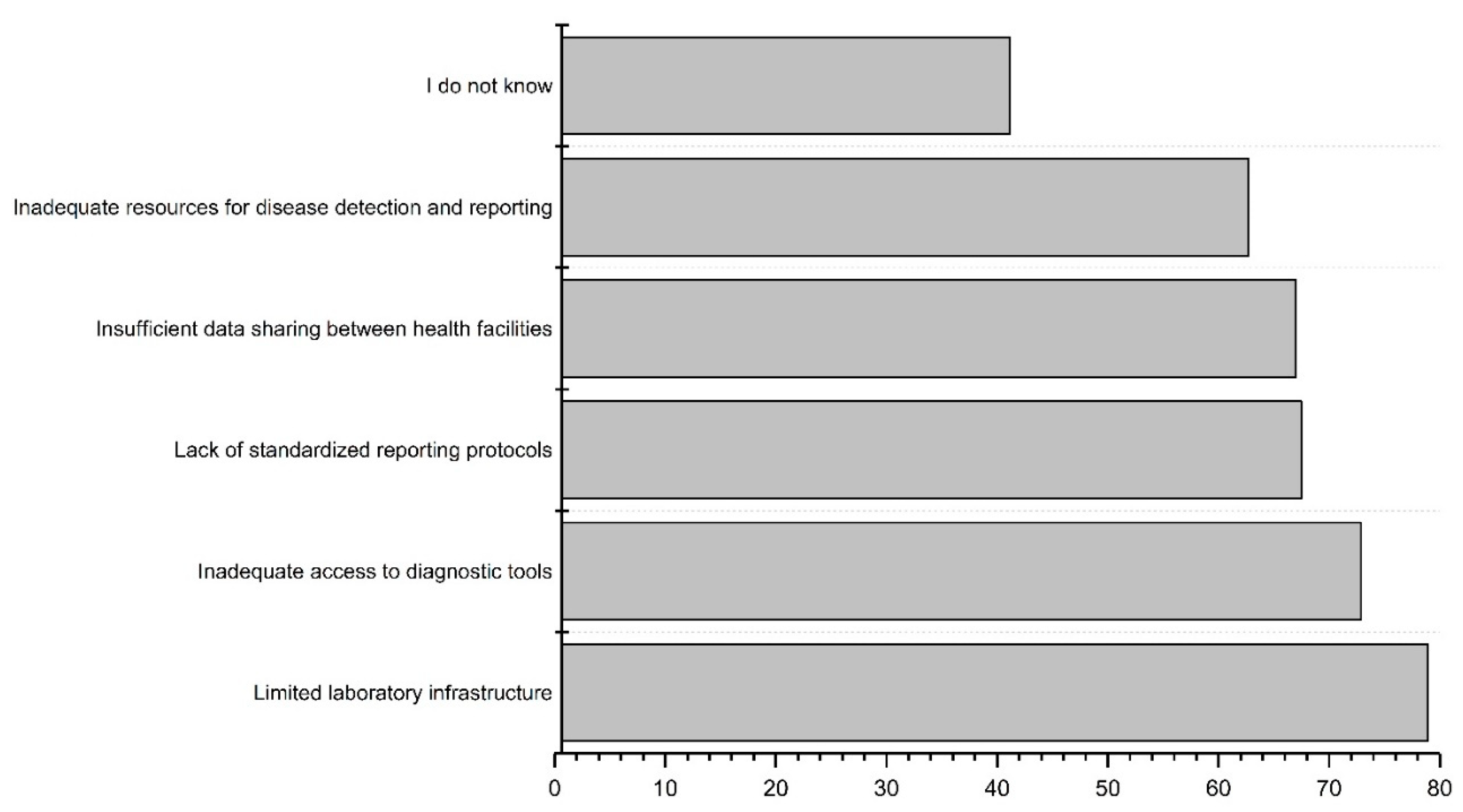
3.4. Challenges and Limitations in the Current Surveillance Systems
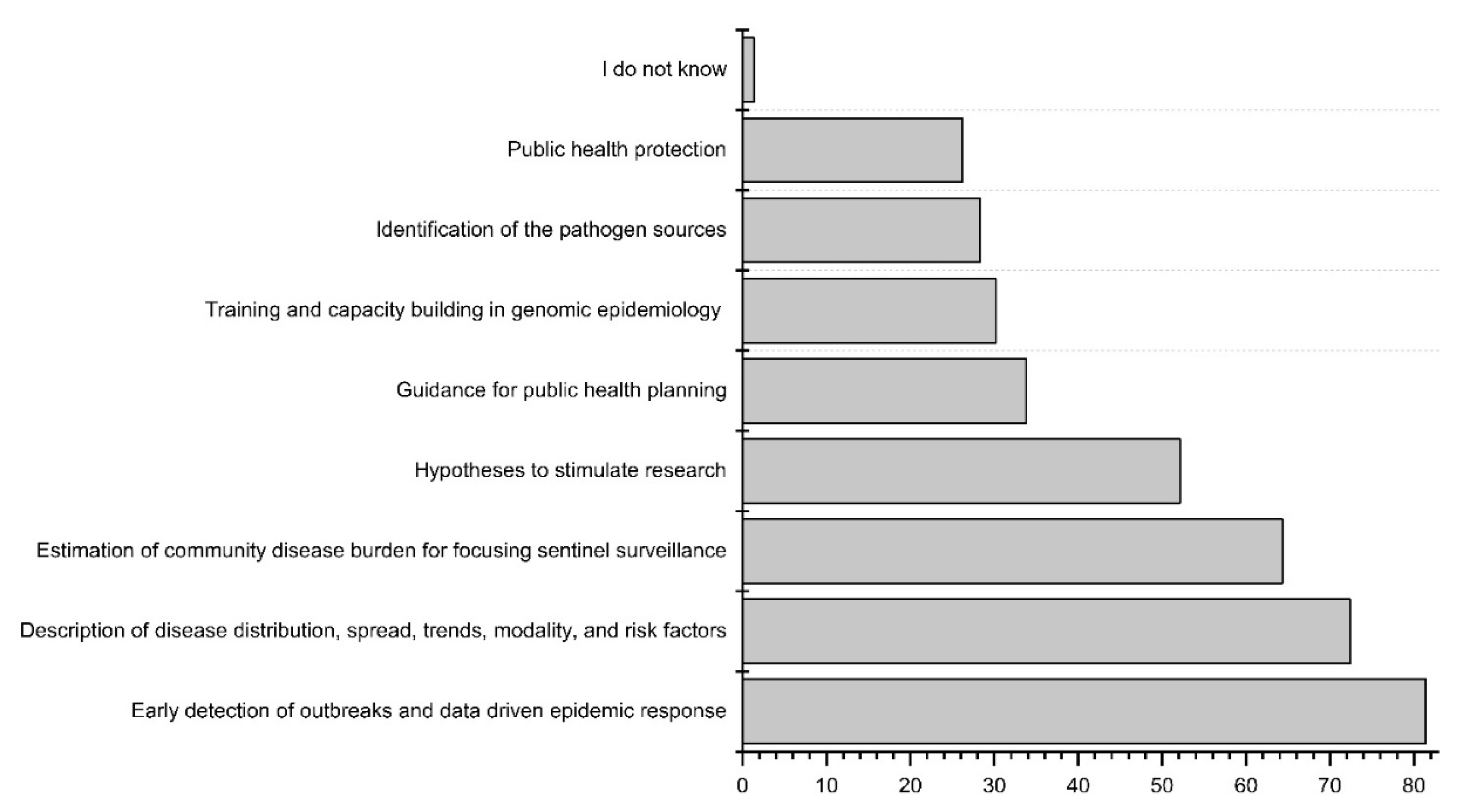
3.5. Potential of Wastewater and Environmental Surveillance Systems
4. Discussion
4.1. Current Clinical Surveillance
4.2. Wastewater and Environmental Surveillance
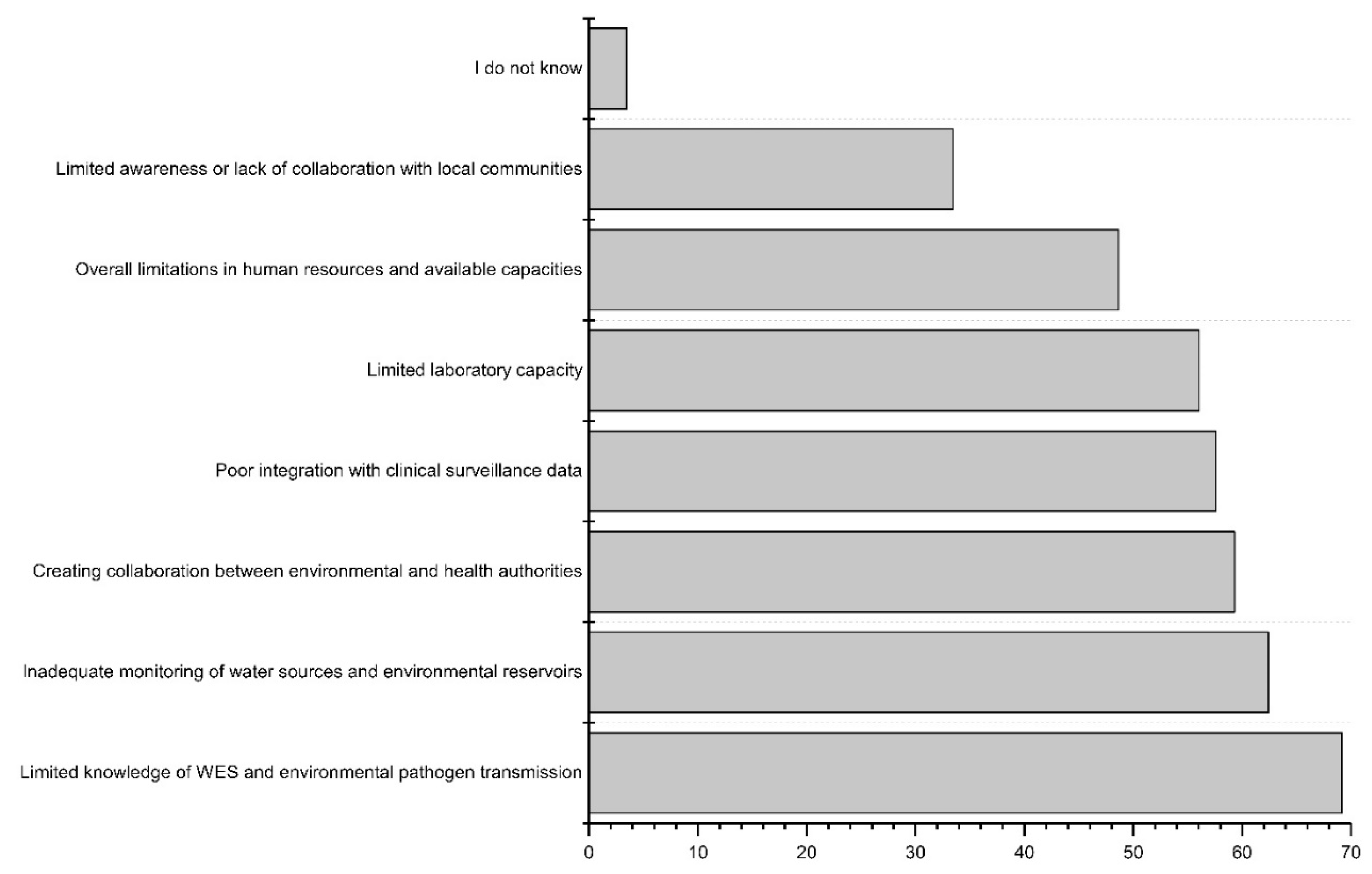
4.3. Challenges and Future Directions in WES
4.4. One Health Perspective for Waterborne Pathogens
4.5. Limitation of Current Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Drinking Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 7 November 2023).
- WHO Improving Access to Water, Sanitation and Hygiene Can Save 1.4 Million Lives per Year, Says New WHO Report 2023.
- WHO Global Action Plan on Antimicrobial Resistance; World Health Organization, Geneva , Switzerland, 2015.
- Pieri, A.; Aschbacher, R.; Fasani, G.; Mariella, J.; Brusetti, L.; Pagani, E.; Sartelli, M.; Pagani, L. Country Income Is Only One of the Tiles: The Global Journey of Antimicrobial Resistance among Humans, Animals, and Environment. Antibiotics 2020, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- WHO Key Facts Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 10 January 2024).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Wordometer Coronavirus Cases. Available online: https://www.worldometers.info/coronavirus/#countries.
- WHO-ECDC WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2022 – 2020 Data. Copenhagen: WHO Regional Office for Europe.; 2022.
- WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. World Health Organization; World Health Organization, Geneva, Switzerland, 2022.
- Tiwari, A.; Kurittu, P.; Al-Mustapha, A.I.; Heljanko, V.; Johansson, V.; Thakali, O.; Mishra, S.K.; Lehto, K.-M.; Lipponen, A.; Oikarinen, S.; et al. Wastewater Surveillance of Antibiotic-Resistant Bacterial Pathogens: A Systematic Review. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Inzaule, S.C.; Tessema, S.K.; Kebede, Y.; Ogwell Ouma, A.E.; Nkengasong, J.N. Genomic-Informed Pathogen Surveillance in Africa: Opportunities and Challenges. Lancet Infect. Dis. 2021, 21, e281–e289. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, G.; Lillis, L.; Meschke, J.S. Review of Methods Suitable for Environmental Surveillance of Salmonella Typhi and Paratyphi. Clin. Infect. Dis. 2020, 71, S79–S83. [Google Scholar] [CrossRef]
- Murray, J.; Cohen, A.L. Infectious Disease Surveillance. In International Encyclopedia of Public Health; Elsevier, 2017; pp. 222–229.
- Tiwari, A.; Adhikari, S.; Kaya, D.; Islam, M.A.; Malla, B.; Sherchan, S.P.; Al-Mustapha, A.I.; Kumar, M.; Aggarwal, S.; Bhattacharya, P.; et al. Monkeypox Outbreak: Wastewater and Environmental Surveillance Perspective. Sci. Total Environ. 2022, 856, 159166. [Google Scholar] [CrossRef] [PubMed]
- Kilaru, P.; Hill, D.; Anderson, K.; Collins, M.B.; Green, H.; Kmush, B.L.; Larsen, D.A. Wastewater Surveillance for Infectious Disease: A Systematic Review. Am. J. Epidemiol. 2023, 192, 305–322. [Google Scholar] [CrossRef] [PubMed]
- von Kalckreuth, V.; Konings, F.; Aaby, P.; Adu-Sarkodie, Y.; Ali, M.; Aseffa, A.; Baker, S.; Breiman, R.F.; Bjerregaard-Andersen, M.; Clemens, J.D.; et al. The Typhoid Fever Surveillance in Africa Program (TSAP): Clinical, Diagnostic, and Epidemiological Methodologies. Clin. Infect. Dis. 2016, 62, S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Marks, F.; von Kalckreuth, V.; Aaby, P.; Adu-Sarkodie, Y.; El Tayeb, M.A.; Ali, M.; Aseffa, A.; Baker, S.; Biggs, H.M.; Bjerregaard-Andersen, M.; et al. Incidence of Invasive Salmonella Disease in Sub-Saharan Africa: A Multicentre Population-Based Surveillance Study. Lancet Glob. Heal. 2017, 5, e310–e323. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Phan, N.; Tandukar, S.; Ashoori, R.; Thakali, O.; Mousazadesh, M.; Dehghani, M.H.; Sherchan, S.P. Persistence and Occurrence of SARS-CoV-2 in Water and Wastewater Environments: A Review of the Current Literature. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Bisseux, M.; Colombet, J.; Mirand, A.; Roque-Afonso, A.-M.; Abravanel, F.; Izopet, J.; Archimbaud, C.; Peigue-Lafeuille, H.; Debroas, D.; Bailly, J.-L.; et al. Monitoring Human Enteric Viruses in Wastewater and Relevance to Infections Encountered in the Clinical Setting: A One-Year Experiment in Central France, 2014 to 2015. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.; Kasprzyk-Hordern, B. Future Perspectives of Wastewater-Based Epidemiology: Monitoring Infectious Disease Spread and Resistance to the Community Level. Environ. Int. 2020, 139, 105689. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Zhang, K.; Du, W.; Ali, W.; Feng, X.; Zhang, H. The Potential of Wastewater-Based Epidemiology as Surveillance and Early Warning of Infectious Disease Outbreaks. Curr. Opin. Environ. Sci. Heal. 2020, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lundy, L.; Fatta-Kassinos, D.; Slobodnik, J.; Karaolia, P.; Cirka, L.; Kreuzinger, N.; Castiglioni, S.; Bijlsma, L.; Dulio, V.; Deviller, G.; et al. Making Waves: Collaboration in the Time of SARS-CoV-2 - Rapid Development of an International Co-Operation and Wastewater Surveillance Database to Support Public Health Decision-Making. Water Res. 2021, 199, 117167. [Google Scholar] [CrossRef] [PubMed]
- Bibby, K.; Bivins, A.; Wu, Z.; North, D. Making Waves: Plausible Lead Time for Wastewater Based Epidemiology as an Early Warning System for COVID-19. Water Res. 2021, 202, 117438. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.; Radniecki, T.S.; Kaya, D.; Alegre, D.; Geniza, M.; Girard, A.-M.; Carter, K.; Dasenko, M.; Sanders, J.L.; Cieslak, P.R.; et al. Detection of SARS-CoV-2 B.1.351 (Beta) Variant through Wastewater Surveillance before Case Detection in a Community, Oregon, USA. Emerg. Infect. Dis. 2022, 28, 1–16. [Google Scholar] [CrossRef] [PubMed]
- EU Regulation 2020/741 Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL Concerning Urban Wastewater Treatment (Recast). Off. J. Eur. Union 2022, 0345, 1–68.
- Keshaviah, A.; Diamond, M.B.; Wade, M.J.; Scarpino, S. V; Ahmed, W.; Amman, F.; Aruna, O.; Badilla-Aguilar, A.; Bar-Or, I.; Bergthaler, A.; et al. Wastewater Monitoring Can Anchor Global Disease Surveillance Systems. Lancet Glob. Heal. 2023, 11, e976–e981. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Krolicka, A.; Tran, T.T.; Räisänen, K.; Ásmundsdóttir, Á.M.; Wikmark, O.-G.; Lood, R.; Pitkänen, T. Antibiotic Resistance Monitoring in Wastewater in the Nordic Countries: A Systematic Review. Environ. Res. 2024, 246, 118052. [Google Scholar] [CrossRef] [PubMed]
- Bowes, D.A.; Darling, A.; Driver, E.M.; Kaya, D.; Maal-Bared, R.; Lee, L.M.; Goodman, K.; Adhikari, S.; Aggarwal, S.; Bivins, A.; et al. Structured Ethical Review for Wastewater-Based Testing in Support of Public Health. Environ. Sci. Technol. 2023, 57, 12969–12980. [Google Scholar] [CrossRef] [PubMed]
- Mansfeldt, C.; Maal-Bared, R.; Kaya, D.; Bowes, D.A.; Keenum, I.; Aggarwal, S.; Tiwari, A.; Hutchison, J.M. Unveiling the Targeted Opportunities and Universal Challenges of Wastewater-Based Surveillance for Public Health. ACS ES&T Water 2023, 3, 1987–1989. [Google Scholar] [CrossRef]
- Meckawy, R.; Stuckler, D.; Mehta, A.; Al-Ahdal, T.; Doebbeling, B.N. Effectiveness of Early Warning Systems in the Detection of Infectious Diseases Outbreaks: A Systematic Review. BMC Public Health 2022, 22, 2216. [Google Scholar] [CrossRef] [PubMed]
- Jakariya, M.; Ahmed, F.; Islam, M.A.; Al Marzan, A.; Hasan, M.N.; Hossain, M.; Ahmed, T.; Hossain, A.; Reza, H.M.; Hossen, F.; et al. Wastewater-Based Epidemiological Surveillance to Monitor the Prevalence of SARS-CoV-2 in Developing Countries with Onsite Sanitation Facilities. Environ. Pollut. 2022, 311, 119679. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.C.; Thompson, J.R.; Filho, C.R.M.; Street, R.; Li, X.; Castiglioni, S.; Thomas, K. V. A World of Wastewater-Based Epidemiology. Nat. Water 2023, 1, 408–415. [Google Scholar] [CrossRef]
- Thakali, O.; Raya, S.; Malla, B.; Tandukar, S.; Tiwari, A.; Sherchan, S.P.; Sherchand, J.B.; Haramoto, E. Pilot Study on Wastewater Surveillance of Dengue Virus RNA: Lessons, Challenges, and Implications for Future Research. Environ. Challenges 2022, 100614. [Google Scholar] [CrossRef]
- Islam, M.A.; Rahman, M.A.; Jakariya, M.; Bahadur, N.M.; Hossen, F.; Mukharjee, S.K.; Hossain, M.S.; Tasneem, A.; Haque, M.A.; Sera, F.; et al. A 30-Day Follow-up Study on the Prevalence of SARS-COV-2 Genetic Markers in Wastewater from the Residence of COVID-19 Patient and Comparison with Clinical Positivity. Sci. Total Environ. 2023, 858, 159350. [Google Scholar] [CrossRef]
- Tiwari, A.; Adhikari, S.; Zhang, S.; Solomon, T.B.; Lipponen, A.; Islam, M.A.; Thakali, O.; Sangkham, S.; Shaheen, M.N.F.; Jiang, G.; et al. Tracing COVID-19 Trails in Wastewater: A Systematic Review of SARS-CoV-2 Surveillance with Viral Variants. Water 2023, 15, 1018. [Google Scholar] [CrossRef]
- Santiso-Bellón, C.; Randazzo, W.; Pérez-Cataluña, A.; Vila-Vicent, S.; Gozalbo-Rovira, R.; Muñoz, C.; Buesa, J.; Sanchez, G.; Rodríguez Díaz, J. Epidemiological Surveillance of Norovirus and Rotavirus in Sewage (2016–2017) in Valencia (Spain). Microorganisms 2020, 8, 458. [Google Scholar] [CrossRef]
- Shrestha, S.; Malla, B.; Haramoto, E. Estimation of Norovirus Infections in Japan: An Application of Wastewater-Based Epidemiology for Enteric Disease Assessment. Sci. Total Environ. 2024, 912, 169334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, J.; Li, X.; Tiwari, A.; Gao, S.; Zhou, X.; Sun, X.; O’Brien, J.W.; Coin, L.; Hai, F.; et al. Wastewater-Based Epidemiology of Campylobacter Spp.: A Systematic Review and Meta-Analysis of Influent, Effluent, and Removal of Wastewater Treatment Plants. Sci. Total Environ. 2023, 903, 166410. [Google Scholar] [CrossRef]
- Adhikari, S.; Halden, R.U. Opportunities and Limits of Wastewater-Based Epidemiology for Tracking Global Health and Attainment of UN Sustainable Development Goals. Environ. Int. 2022, 163, 107217. [Google Scholar] [CrossRef] [PubMed]
- THL Strengthening Environmental Surveillance to Advance Public Health Action (ODIN). Available online: https://thl.fi/tutkimus-ja-kehittaminen/tutkimukset-ja-hankkeet/strengthening-environmental-surveillance-to-advance-public-health-action-odin- (accessed on 23 January 2023).
- 2003.
- Hovi, T.; Stenvik, M.; Partanen, H.; Kangas, A. Poliovirus Surveillance by Examining Sewage Specimens. Quantitative Recovery of Virus after Introduction into Sewerage at Remote Upstream Location. Epidemiol. Infect. 2001, 127, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lanrewaju, A.A.; Enitan-Folami, A.M.; Sabiu, S.; Edokpayi, J.N.; Swalaha, F.M. Global Public Health Implications of Human Exposure to Viral Contaminated Water. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- WHO Global Polio Surveillance Action Plan 2022–2024 2022.
- WHO Poliomyelitis Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/poliomyelitis#:~:text=Cases due to wild poliovirus,at risk of contracting polio (accessed on 23 January 2023).
- Kim, C.L.; Cruz Espinoza, L.M.; Vannice, K.S.; Tadesse, B.T.; Owusu-Dabo, E.; Rakotozandrindrainy, R.; Jani, I. V; Teferi, M.; Bassiahi Soura, A.; Lunguya, O.; et al. The Burden of Typhoid Fever in Sub-Saharan Africa: A Perspective. Res. Rep. Trop. Med. 2022, Volume 13, 1–9. [Google Scholar] [CrossRef]
- Crump, J.A.; Sjölund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef] [PubMed]
- Dougan, G.; Baker, S. Salmonella Enterica Serovar Typhi and the Pathogenesis of Typhoid Fever. Annu. Rev. Microbiol. 2014, 68, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Madullu, M.T.; Thomas, D.S.K.; Nyanza, E.C.; Seni, J.; Ngallaba, S.E.; Kiluvia, S.; Asori, M.; Kangmennaang, J. Spatial Distribution of Suspected and Confirmed Cholera Cases in Mwanza City, Northern Tanzania. PLOS Glob. Public Heal. 2023, 3, e0001261. [Google Scholar] [CrossRef] [PubMed]
- ECDC Cholera Worldwide Overview. Available online: https://www.ecdc.europa.eu/en/all-topics-z/cholera/surveillance-and-disease-data/cholera-monthly (accessed on 12 January 2024).
- Ali, M.; Nelson, A.R.; Lopez, A.L.; Sack, D.A. Updated Global Burden of Cholera in Endemic Countries. PLoS Negl. Trop. Dis. 2015, 9, e0003832. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, A.; Hardy, C.; Kamwaga, S.; Sebunya, K.; Massa, K.; Mulungu, J.; Martinsen, A.; Nyasani, E.; Hulland, E.; Russell, S.; et al. Evaluation of an Emergency Bulk Chlorination Project Targeting Drinking Water Vendors in Cholera-Affected Wards of Dar Es Salaam and Morogoro, Tanzania. Am. J. Trop. Med. Hyg. 2019, 100, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- WHO Disease Outbreak News Cholera – Global Situation. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON437 (accessed on 12 January 2024).
- WHO Typhoid. Available online: https://www.who.int/news-room/fact-sheets/detail/typhoid (accessed on 10 January 2024).
- Antillón, M.; Warren, J.L.; Crawford, F.W.; Weinberger, D.M.; Kürüm, E.; Pak, G.D.; Marks, F.; Pitzer, V.E. The Burden of Typhoid Fever in Low- and Middle-Income Countries: A Meta-Regression Approach. PLoS Negl. Trop. Dis. 2017, 11, e0005376. [Google Scholar] [CrossRef] [PubMed]
- Msemo, O.A.; Mbwana, J.; Mahende, C.; Malabeja, A.; Gesase, S.; Crump, J.A.; Dekker, D.; Lusingu, J.P.A. Epidemiology and Antimicrobial Susceptibility of Salmonella Enterica Bloodstream Isolates Among Febrile Children in a Rural District in Northeastern Tanzania: A Cross-Sectional Study. Clin. Infect. Dis. 2019, 68, S177–S182. [Google Scholar] [CrossRef] [PubMed]
- Luhata Lungayo, C.; Burke, R.M.; Cikomola, A.; Mukamba, E.; Burnett, E.; Tate, J.E.; Samuel Otomba, J.; Albert, M.K.; Nimpa, M.M.; Dommergues, M.A.; et al. Epidemiology and Pre-Vaccine Burden of Rotavirus Diarrhea in Democratic Republic of Congo (DRC): Results of Sentinel Surveillance, 2009–2019. Vaccine 2022, 40, 5933–5941. [Google Scholar] [CrossRef] [PubMed]
- WHO Multi-Country Outbreak of Cholera. External Situation Report #4, Published 6 July 2023 2023.
- Zohra, T.; Ikram, A.; Salman, M.; Amir, A.; Saeed, A.; Ashraf, Z.; Ahad, A. Wastewater Based Environmental Surveillance of Toxigenic Vibrio Cholerae in Pakistan. PLoS One 2021, 16, e0257414. [Google Scholar] [CrossRef]
- Chigwechokha, P.; Nyirenda, R.L.; Dalitsani, D.; Namaumbo, R.L.; Kazembe, Y.; Smith, T.; Holm, R.H. Vibrio Cholerae and Salmonella Typhi Culture-Based Wastewater or Non-Sewered Sanitation Surveillance in a Resource-Limited Region. J. Expo. Sci. Environ. Epidemiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bwire, G.; Debes, A.K.; Orach, C.G.; Kagirita, A.; Ram, M.; Komakech, H.; Voeglein, J.B.; Buyinza, A.W.; Obala, T.; Brooks, W.A.; et al. Environmental Surveillance of Vibrio Cholerae O1/O139 in the Five African Great Lakes and Other Major Surface Water Sources in Uganda. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Bendhaou, F. Le Burkina Faso Déclare Une Épidémie de Choléra - Après La Confirmation d’un Cas de Contamination Dans Un Marché, Dans l’Est Du Pays.
- Platform Cholera Cholera Outbreaks in Central and West Africa : 2022 Regional Update - Week 1 - 26.
- UNICEF Malawi Appeal Humanitarian Action for Children. Available online: https://www.unicef.org/appeals/malawi (accessed on 23 January 2023).
- WHO Zambia Races to Curb Fast-Spreading Cholera Outbreak. Available online: https://www.afro.who.int/countries/zambia/news/zambia-races-curb-fast-spreading-cholera-outbreak#:~:text=The government declared a new,the epicentre of the outbreak (accessed on 23 January 2024).
- WHO Guidelines for Drinking-Water Quality: First Addendum to the Fourth Edition. Geneva: World Health Organization; 2017; Vol. 109; ISBN 9789241550017.
- WHO Water Safety Plan Manual: Step-by-Step Risk Management for Drinking-Water Suppliers. 2023.
- UNICEF Diarrhoea Remains a Leading Killer of Young Children, despite the Availability of a Simple Treatment Solution. Available online: https://data.unicef.org/topic/child-health/diarrhoeal-disease/.
- Hugho, E.A.; Kumburu, H.H.; Amani, N.B.; Mseche, B.; Maro, A.; Ngowi, L.E.; Kyara, Y.; Kinabo, G.; Thomas, K.M.; Houpt, E.R.; et al. Enteric Pathogens Detected in Children under Five Years Old Admitted with Diarrhea in Moshi, Kilimanjaro, Tanzania. Pathogens 2023, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Lipponen, A.; Hokajärvi, A.-M.; Luomala, O.; Sarekoski, A.; Rytkönen, A.; Österlund, P.; Al-Hello, H.; Juutinen, A.; Miettinen, I.T.; et al. Detection and Quantification of SARS-CoV-2 RNA in Wastewater Influent in Relation to Reported COVID-19 Incidence in Finland. Water Res. 2022, 215, 118220. [Google Scholar] [CrossRef] [PubMed]
- Sherchan, S.P.; Shahin, S.; Ward, L.M.; Tandukar, S.; Aw, T.G.; Schmitz, B.; Ahmed, W.; Kitajima, M. First Detection of SARS-CoV-2 RNA in Wastewater in North America: A Study in Louisiana, USA. Sci. Total Environ. 2020, 743, 140621. [Google Scholar] [CrossRef] [PubMed]
- Haramoto, E.; Malla, B.; Thakali, O.; Kitajima, M. First Environmental Surveillance for the Presence of SARS-CoV-2 RNA in Wastewater and River Water in Japan. Sci. Total Environ. 2020, 737, 140405. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Joshi, M.; Jiang, G.; Yamada, R.; Honda, R.; Srivastava, V.; Mahlknecht, J.; Barcelo, D.; Chidambram, S.; Khursheed, A.; et al. Response of Wastewater-Based Epidemiology Predictor for the Second Wave of COVID-19 in Ahmedabad, India: A Long-Term Data Perspective. Environ. Pollut. 2023, 337, 122471. [Google Scholar] [CrossRef] [PubMed]
- Hokajärvi, A.M.; Rytkönen, A.; Tiwari, A.; Kauppinen, A.; Oikarinen, S.; Lehto, K.M.; Kankaanpää, A.; Gunnar, T.; Al-Hello, H.; Blomqvist, S.; et al. The Detection and Stability of the SARS-CoV-2 RNA Biomarkers in Wastewater Influent in Helsinki, Finland. Sci. Total Environ. 2021, 770, 145274. [Google Scholar] [CrossRef]
- Boehm, A.B.; Hughes, B.; Duong, D.; Chan-Herur, V.; Buchman, A.; Wolfe, M.K.; White, B.J. Wastewater Concentrations of Human Influenza, Metapneumovirus, Parainfluenza, Respiratory Syncytial Virus, Rhinovirus, and Seasonal Coronavirus Nucleic-Acids during the COVID-19 Pandemic: A Surveillance Study. The Lancet Microbe 2023, 4, e340–e348. [Google Scholar] [CrossRef]
- Lehto, K.-M.; Hyder, R.; Länsivaara, A.; Luomala, O.; Lipponen, A.; Hokajärvi, A.-M.; Heikinheimo, A.; Pitkänen, T.; Oikarinen, S.; Group, W.S. Wastewater-Based Surveillance Is an Efficient Monitoring Tool for Tracking Influenza A Virus in the Community. medRxiv 2023, 2008–2023. [Google Scholar]
- Ahmed, W.; Smith, W.J.M.; Tiwari, A.; Bivins, A.; Simpson, S.L. Unveiling Indicator, Enteric, and Respiratory Viruses in Aircraft Lavatory Wastewater Using Adsorption-Extraction and Nanotrap® Microbiome A Particles Workflows. Sci. Total Environ. 2023, 2, 165007. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Bivins, A.; Stephens, M.; Metcalfe, S.; Smith, W.J.M.; Sirikanchana, K.; Kitajima, M.; Simpson, S.L. Occurrence of Multiple Respiratory Viruses in Wastewater in Queensland, Australia: Potential for Community Disease Surveillance. Sci. Total Environ. 2023, 864, 161023. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.K.; Paulos, A.H.; Zulli, A.; Duong, D.; Shelden, B.; White, B.J.; Boehm, A.B. Wastewater Detection of Emerging Arbovirus Infections: Case Study of Dengue in the United States. Environ. Sci. Technol. Lett. 2024, 11, 9–15. [Google Scholar] [CrossRef]
- Gentry, Z.; Zhao, L.; Faust, R.A.; David, R.E.; Norton, J.; Xagoraraki, I. Wastewater Surveillance beyond COVID-19: A Ranking System for Communicable Disease Testing in the Tri-County Detroit Area, Michigan, USA. Front. Public Heal. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Mawson, A.R.; Croft, A.M. Rubella Virus Infection, the Congenital Rubella Syndrome, and the Link to Autism. Int. J. Environ. Res. Public Health 2019, 16, 3543. [Google Scholar] [CrossRef] [PubMed]
- WHO Tuberculose (TB). 2019.
- Mtetwa, H.N.; Amoah, I.D.; Kumari, S.; Bux, F.; Reddy, P. Wastewater-Based Surveillance of Antibiotic Resistance Genes Associated with Tuberculosis Treatment Regimen in KwaZulu Natal, South Africa. Antibiotics 2021, 10, 1362. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global Trends in Antimicrobial Resistance in Animals in Low- and Middle-Income Countries. Science 2019, 365. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Bortolaia, V.; Tate, H.; Tyson, G.H.; Aarestrup, F.M.; McDermott, P.F. Using Genomics to Track Global Antimicrobial Resistance. Front. Public Heal. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Pärnänen, K.; Narciso-da-Rocha, C.; Kneis, D.; Berendonk, T.U.; Cacace, D.; Do, T.T.; Elpers, C.; Fatta-Kassinos, D.; Henriques, I.; Jaeger, T.; et al. Antibiotic Resistance in European Wastewater Treatment Plants Mirrors the Pattern of Clinical Antibiotic Resistance Prevalence. Sci. Adv. 2019, 5, eaau9124. [Google Scholar] [CrossRef] [PubMed]
- Markkanen, M.A.; Haukka, K.; Pärnänen, K.M.M.; Dougnon, V.T.; Bonkoungou, I.J.O.; Garba, Z.; Tinto, H.; Sarekoski, A.; Karkman, A.; Kantele, A.; et al. Metagenomic Analysis of the Abundance and Composition of Antibiotic Resistance Genes in Hospital Wastewater in Benin, Burkina Faso, and Finland. mSphere 2023, 8. [Google Scholar] [CrossRef] [PubMed]
- Diallo, C.O.; Schiøler, K.L.; Samuelsen, H.; Drabo, K.M. Information System as Part of Epidemic Management in Burkina Faso: From Plan to Reality (Field Findings). BMC Public Health 2022, 22, 1726. [Google Scholar] [CrossRef] [PubMed]
- PUBLIC HEALTH ACT THE PUBLIC HEALTH ACT, ACT NO. 1 OF 2009 2009.
- Government Notice The Personal Data Protection Act, 2022 2023.
- Faleye, T.O.C.; Bowes, D.A.; Driver, E.M.; Adhikari, S.; Adams, D.; Varsani, A.; Halden, R.U.; Scotch, M. Wastewater-Based Epidemiology and Long-Read Sequencing to Identify Enterovirus Circulation in Three Municipalities in Maricopa County, Arizona, Southwest United States between June and October 2020. Viruses 2021, 13, 1803. [Google Scholar] [CrossRef] [PubMed]
- Dehghan Banadaki, M.; Torabi, S.; Rockward, A.; Strike, W.D.; Noble, A.; Keck, J.W.; Berry, S.M. Simple SARS-CoV-2 Concentration Methods for Wastewater Surveillance in Low Resource Settings. Sci. Total Environ. 2024, 912, 168782. [Google Scholar] [CrossRef] [PubMed]
- Pruden, A.; Vikesland, P.J.; Davis, B.C.; de Roda Husman, A.M. Seizing the Moment: Now Is the Time for Integrated Global Surveillance of Antimicrobial Resistance in Wastewater Environments. Curr. Opin. Microbiol. 2021, 64, 91–99. [Google Scholar] [CrossRef]
- Karkman, A.; Berglund, F.; Flach, C.-F.; Kristiansson, E.; Larsson, D.G.J. Predicting Clinical Resistance Prevalence Using Sewage Metagenomic Data. Commun. Biol. 2020, 3, 711. [Google Scholar] [CrossRef] [PubMed]
- George, C.M.; Perin, J.; Parvin, T.; Bhuyian, M.S.I.; Thomas, E.D.; Monira, S.; Zohura, F.; Hasan, M.T.; Alam, M.; Tofail, F. Diarrhea Prevalence and Child Growth Faltering Are Associated with Subsequent Adverse Child Developmental Outcomes in Bangladesh (CHoBI7 Program). Am. J. Trop. Med. Hyg. 2022, 106, 233–238. [Google Scholar] [CrossRef] [PubMed]
- de Wit, E.; Falzarano, D.; Onyango, C.; Rosenke, K.; Marzi, A.; Ochieng, M.; Juma, B.; Fischer, R.J.; Prescott, J.B.; Safronetz, D.; et al. The Merits of Malaria Diagnostics during an Ebola Virus Disease Outbreak. Emerg. Infect. Dis. 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- WHO Chikungunya Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/chikungunya.
- Shrestha, S.; Yoshinaga, E.; Chapagain, S.K.; Mohan, G.; Gasparatos, A.; Fukushi, K. Wastewater-Based Epidemiology for Cost-Effective Mass Surveillance of COVID-19 in Low- and Middle-Income Countries: Challenges and Opportunities. Water 2021, 13, 2897. [Google Scholar] [CrossRef]
- Joshi, M.; Kumar, M.; Srivastava, V.; Kumar, D.; Rathore, D.S.; Pandit, R.; Graham, D.W.; Joshi, C.G. Genetic Sequencing Detected the SARS-CoV-2 Delta Variant in Wastewater a Month Prior to the First COVID-19 Case in Ahmedabad (India). Environ. Pollut. 2022, 310, 119757. [Google Scholar] [CrossRef] [PubMed]
- Uprety, S.; Sherchan, S.P.; Narayanan, P.; Dangol, B.; Maggos, M.; Celmer, A.; Shisler, J.; Amarasiri, M.; Sano, D.; Nguyen, T.H. Microbial Assessment of Water, Sanitation, and Hygiene (WaSH) in Temporary and Permanent Settlements Two Years after Nepal 2015 Earthquake. Sci. Total Environ. 2023, 877, 162867. [Google Scholar] [CrossRef] [PubMed]
- UN Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 7 January 2024).
- Ahmed, W.; Bivins, A.; Korajkic, A.; Metcalfe, S.; Smith, W.J.M.; Simpson, S.L. Comparative Analysis of Adsorption-Extraction (AE) and Nanotrap® Magnetic Virus Particles (NMVP) Workflows for the Recovery of Endogenous Enveloped and Non-Enveloped Viruses in Wastewater. Sci. Total Environ. 2023, 859, 160072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sharma, E.; Tiwari, A.; Chen, Y.; Sherchan, S.P.; Gao, S.; Zhou, X.; Shi, J.; Jiang, G. The Reduction of SARS-CoV-2 RNA Concentration in the Presence of Sewer Biofilms. Water 2023, 15, 2132. [Google Scholar] [CrossRef]
- Armas, F.; Chandra, F.; Lee, W.L.; Gu, X.; Chen, H.; Xiao, A.; Leifels, M.; Wuertz, S.; Alm, E.J.; Thompson, J. Contextualizing Wastewater-Based Surveillance in the COVID-19 Vaccination Era. Environ. Int. 2023, 171, 107718. [Google Scholar] [CrossRef] [PubMed]
- Dobrowsky, P.H.; De Kwaadsteniet, M.; Cloete, T.E.; Khan, W. Distribution of Indigenous Bacterial Pathogens and Potential Pathogens Associated with Roof-Harvested Rainwater. Appl. Environ. Microbiol. 2014, 80, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, T. Review of Campylobacter Spp. in Drinking and Environmental Waters. J. Microbiol. Methods 2013, 95, 39–47. [Google Scholar] [CrossRef]
- Zahedi, A.; Monis, P.; Deere, D.; Ryan, U. Wastewater-Based Epidemiology—Surveillance and Early Detection of Waterborne Pathogens with a Focus on SARS-CoV-2, Cryptosporidium and Giardia. Parasitol. Res. 2021, 120, 4167–4188. [Google Scholar] [CrossRef] [PubMed]
- Al-Mustapha, A.I.; Raufu, I.A.; Ogundijo, O.A.; Odetokun, I.A.; Tiwari, A.; Brouwer, M.S.M.; Adetunji, V.; Heikinheimo, A. Antibiotic Resistance Genes, Mobile Elements, Virulence Genes, and Phages in Cultivated ESBL-Producing Escherichia Coli of Poultry Origin in Kwara State, North Central Nigeria. Int. J. Food Microbiol. 2023, 389, 110086. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Pal, S.; Sunder, J.; Sujatha, T.; De, A.K.; Mondal, T.; Singh, A.D.; Joardar, S.N.; Batabyal, K.; Dutta, T.K.; et al. Exploring Broilers and Native Fowls of Andaman and Nicobar Islands as a Source of β-Lactamase-Producing Enterobacteriaceae Even with Limited Anthropogenic Activities and Docking-Based Identification of Catalytic Domains in Novel β-Lactamase Variants. Front. Vet. Sci. 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhang, L. Wastewater Pathogen Surveillance Based on One Health Approach. The Lancet Microbe 2023, 4, e297. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; Geneva, Switzerland, 2022.
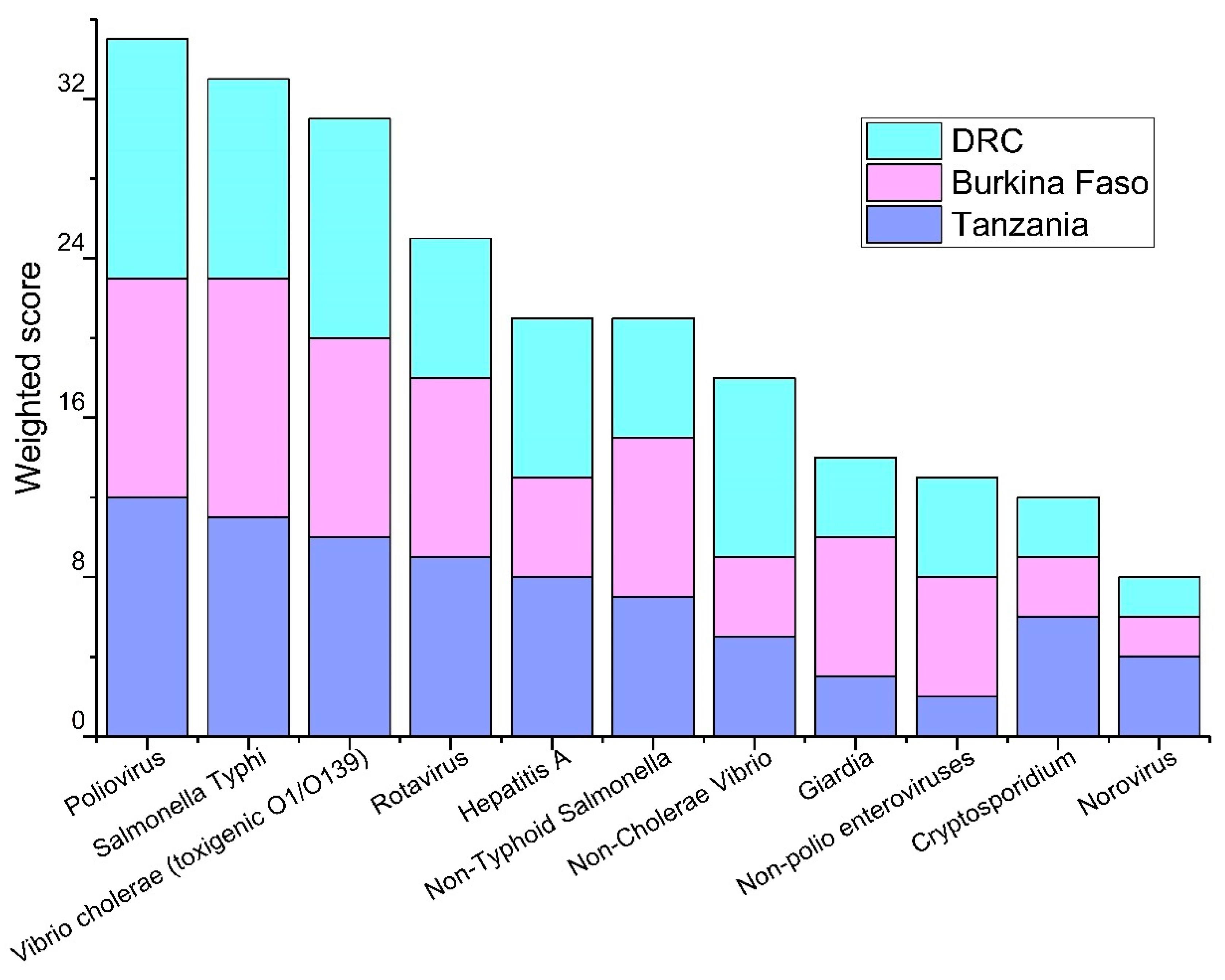
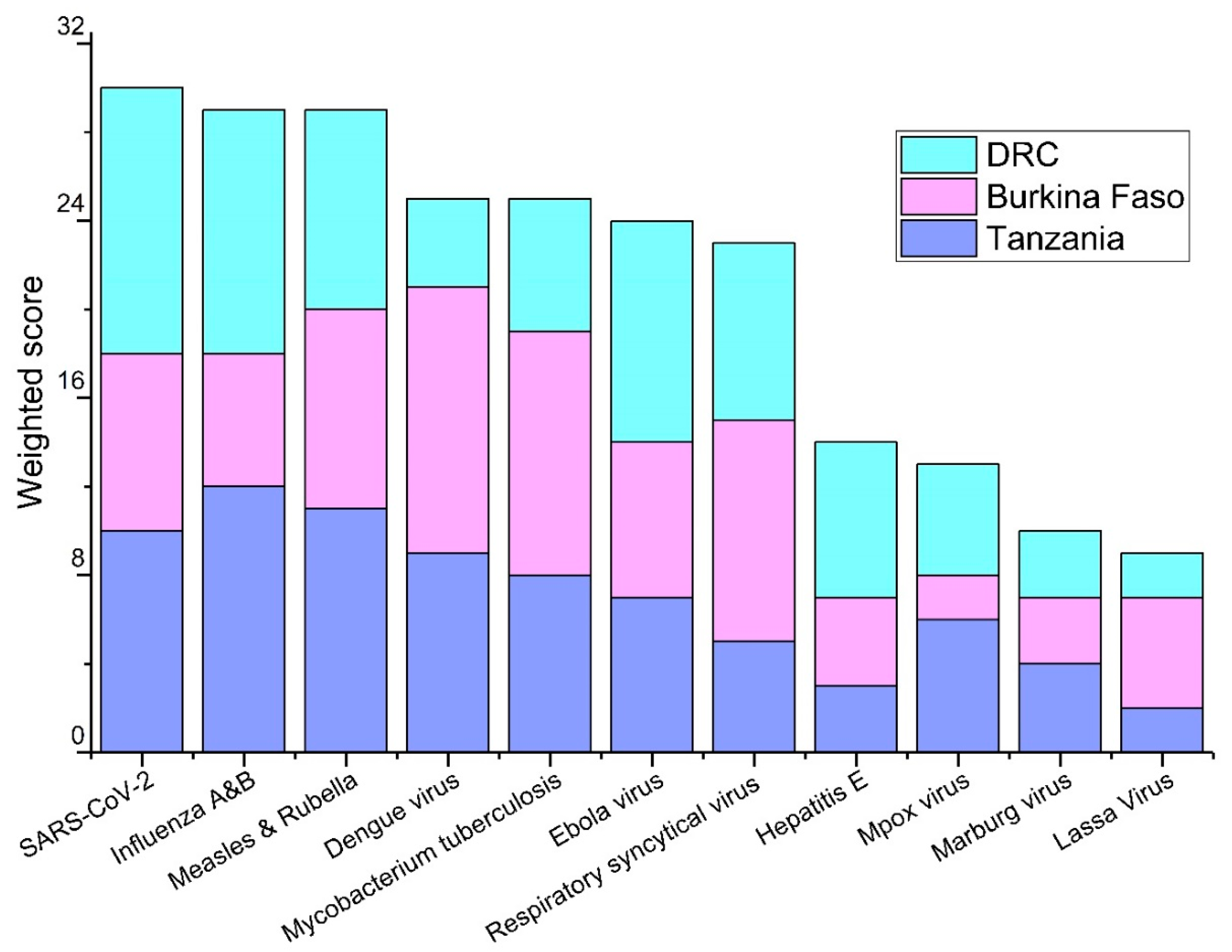
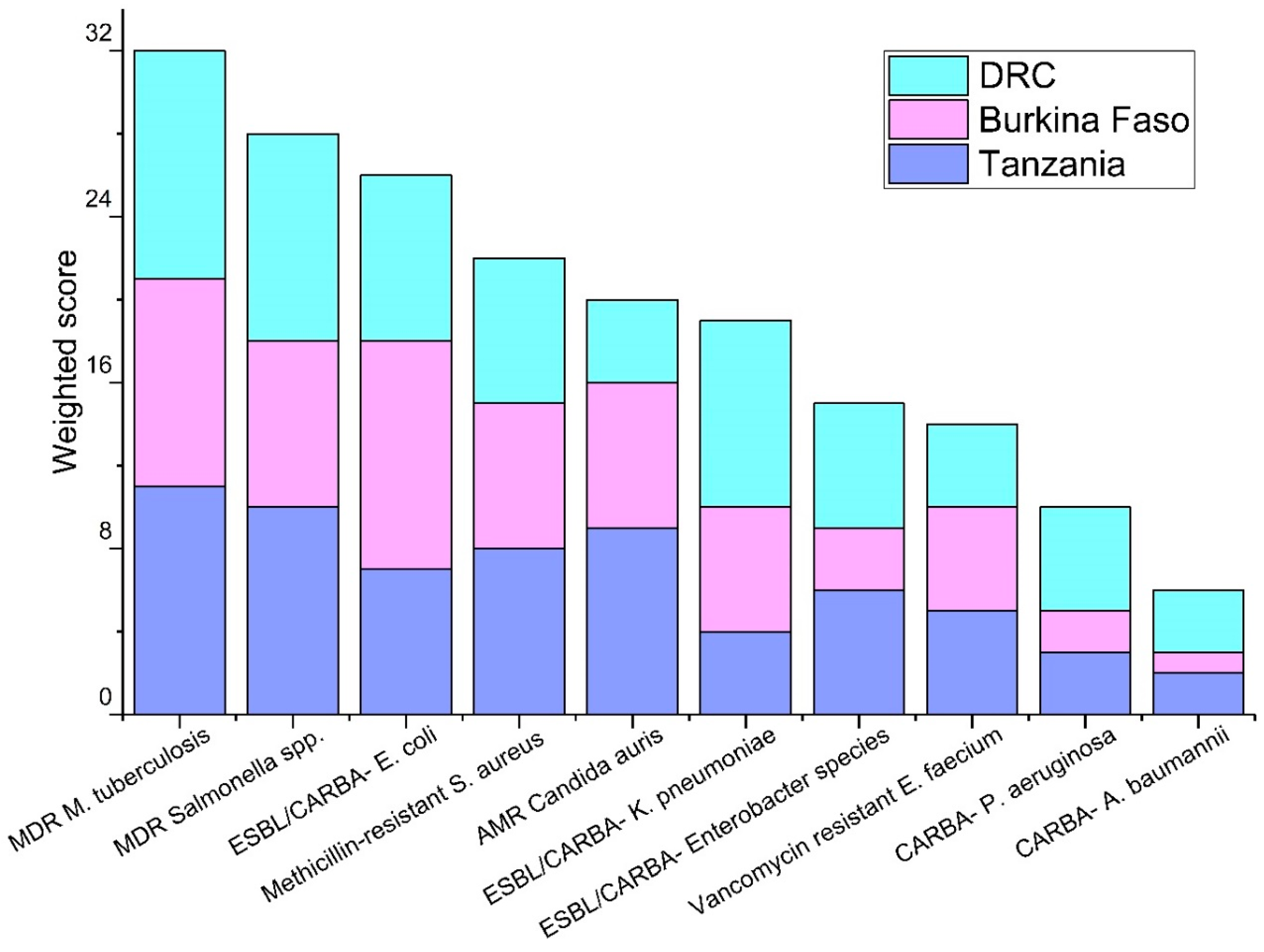
| Tanzania | Burkina Faso | DRC |
| Academicians and researchers | Academicians, Researchers | Academicians and researchers |
| Policymakers and representatives from ministry of Health, and National Public Health Laboratory | Policymakers and representatives from five- ministries of the One health program:
|
Policymakers and representatives from ministries of Health and Environment |
| Healthcare providers and authorities (local) | Head of the Nanoro District Head of the CMA Saint Camille de Nanoro |
- |
| Representatives from national and international non-governmental organizations and community organizations such as healthcare providers and regional water and sewerage authorities (local) | WaterAid, Directorate-General for Water Resources, the National Office for Water and Sanitation (ONEA), Director General of Wastewater and Excreta Disposal, and Water and environment quality analysis laboratory | Water Analysis Infrastructure (REGIDESO), and the National Institute of Biomedical Research |
| Characteristic |
Tanzania Number (%) Total number of respondents, N=20 |
Burkina Faso Number (%) Total number of respondents, N=24 |
DRC Number (%) Total number of respondents, N=16* |
| Clinical surveillance/epidemiologist | 6 (30 %) | 4 (17 %) | 1 (6 %) |
| Environmental surveillance/water supply | 6 (30 %) | 3 (13 %) | 3 (19 %) |
| Healthcare professional | 2 (10 %) | 9 (38 %) | 3 (19 %) |
| International organization | 1 (5 %) | 2 (8 %) | - |
| Non-governmental organization (NGO)/civil society organization (CSO) | 1 (5 %) | 1 (4 %) | - |
| Researcher | 3 (15 %) | 4 (17 %) | 5 (31 %) |
| Other | 1 (5 %) | 1 (4 %) | 3 (19 %) |
| *For the DRC, one respondent did not define the field of expertise | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





