Submitted:
26 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials & Methods
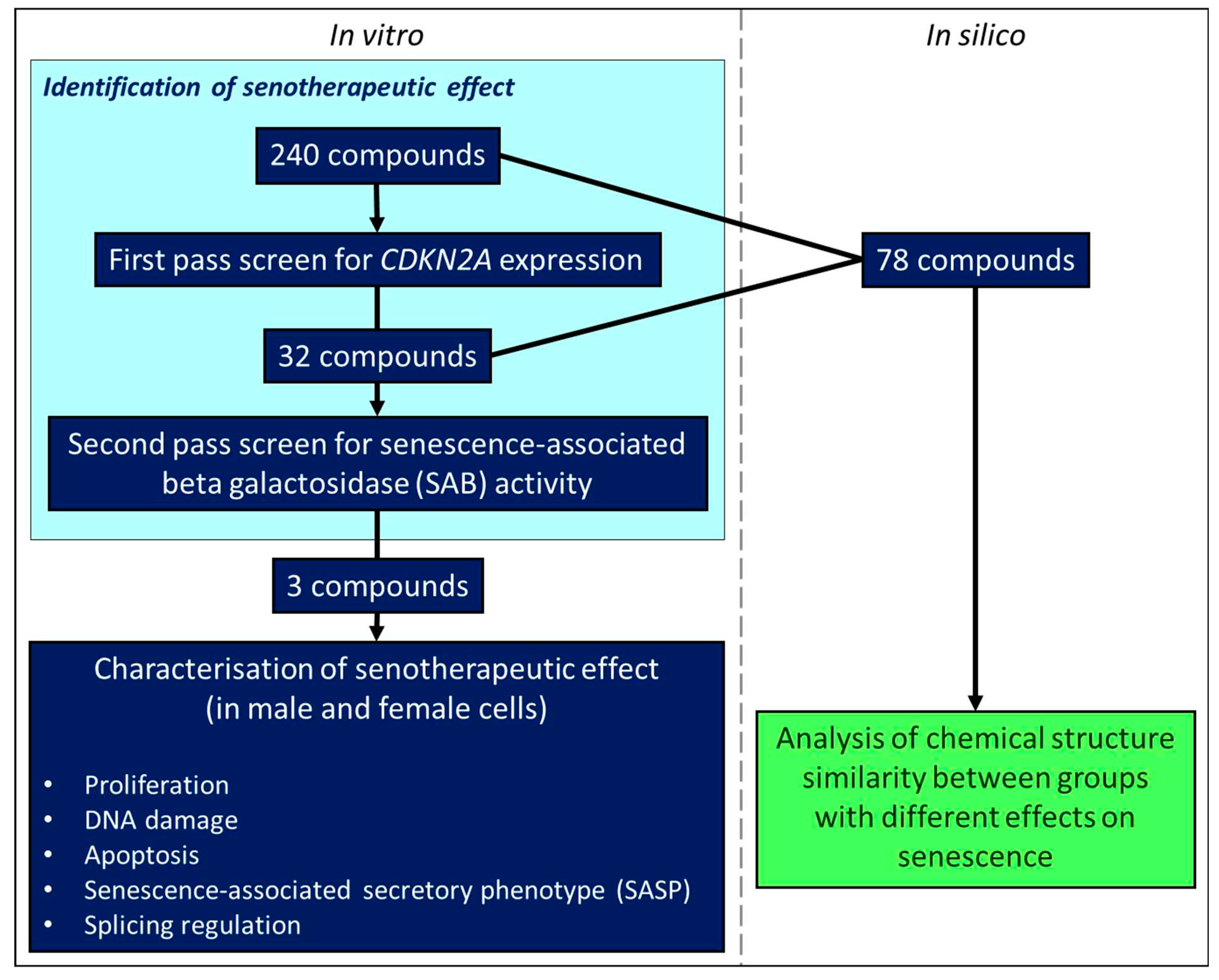
Drug Panel, Screen Design and Preparation
Cells used in this study
Primary screen
Tissue culture and drug treatment conditions
Quantification of CDKN2A expression
Secondary screen
In depth characterisation of female synthetic hormone compounds
Tissue culture and dosing regime
Quantification of senescent cell load using SAB staining
Quantification of cellular proliferation and DNA damage repair using immunocytochemical staining for Ki67 and γH2AX
Quantification of apoptosis using TUNEL assay
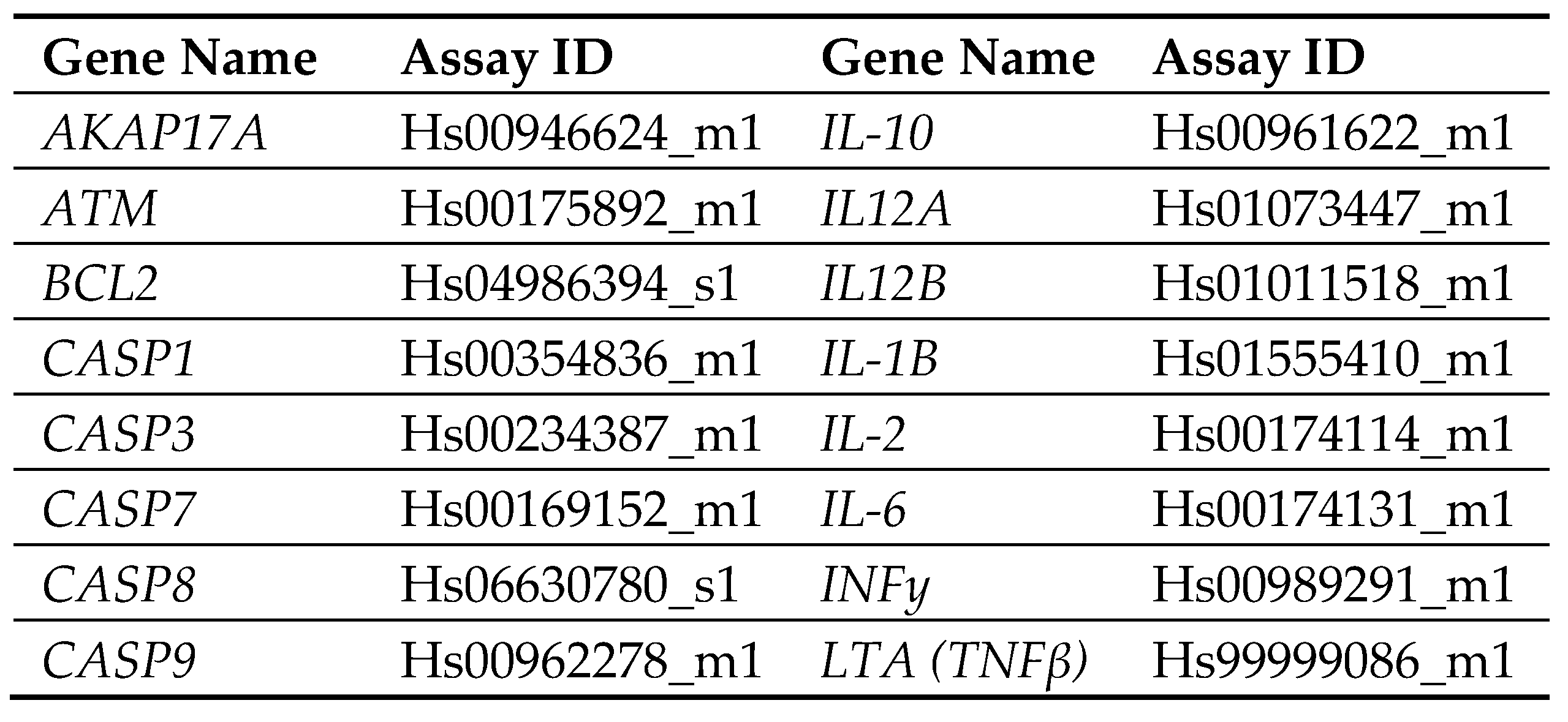
Quantitative RT-qPCR assessment of gene expression
Bioinformatic assessment of structure-function relationships
Methodological validation
Structure-function analysis of in vitro screen results
Results
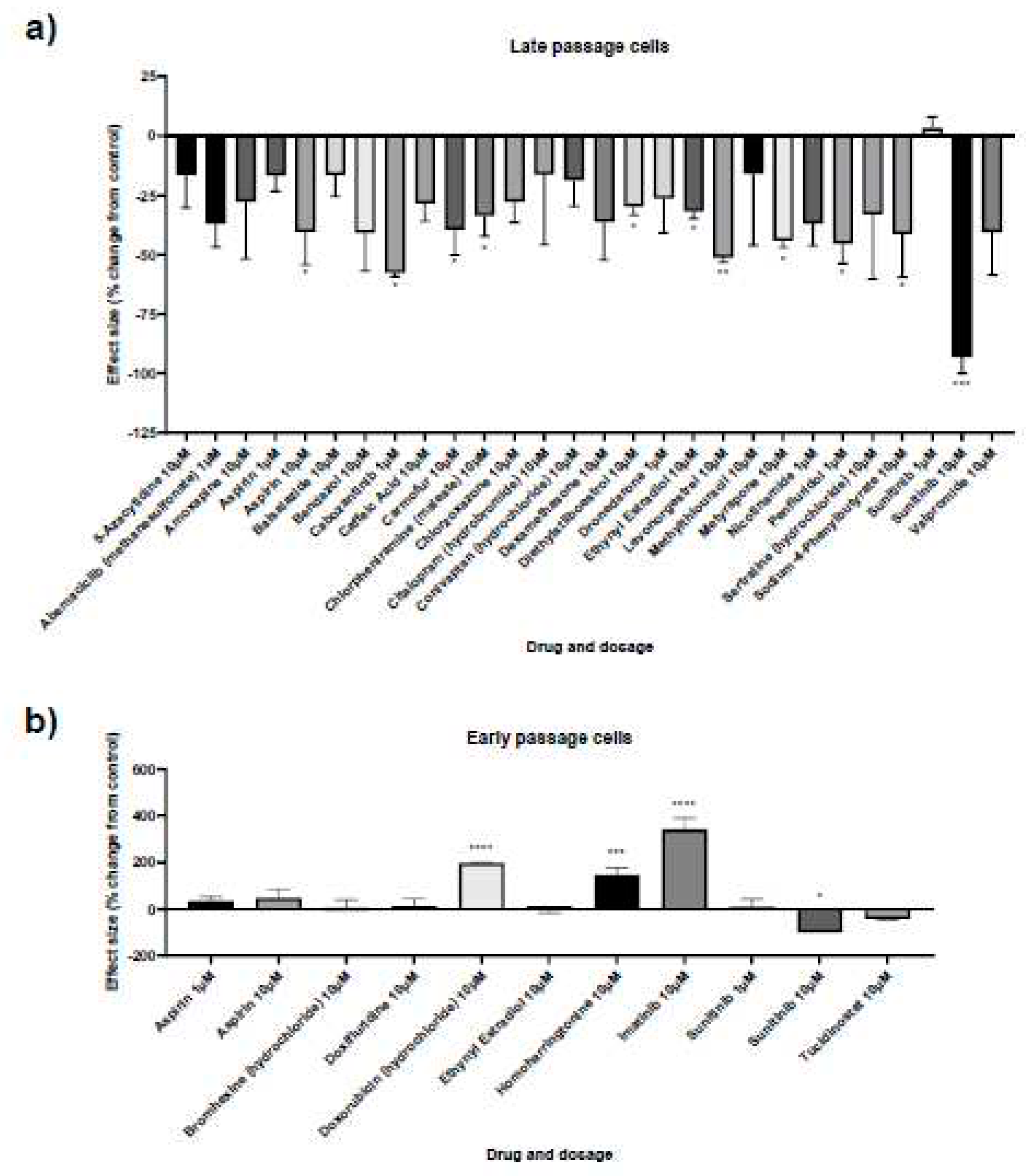
Primary and secondary screens
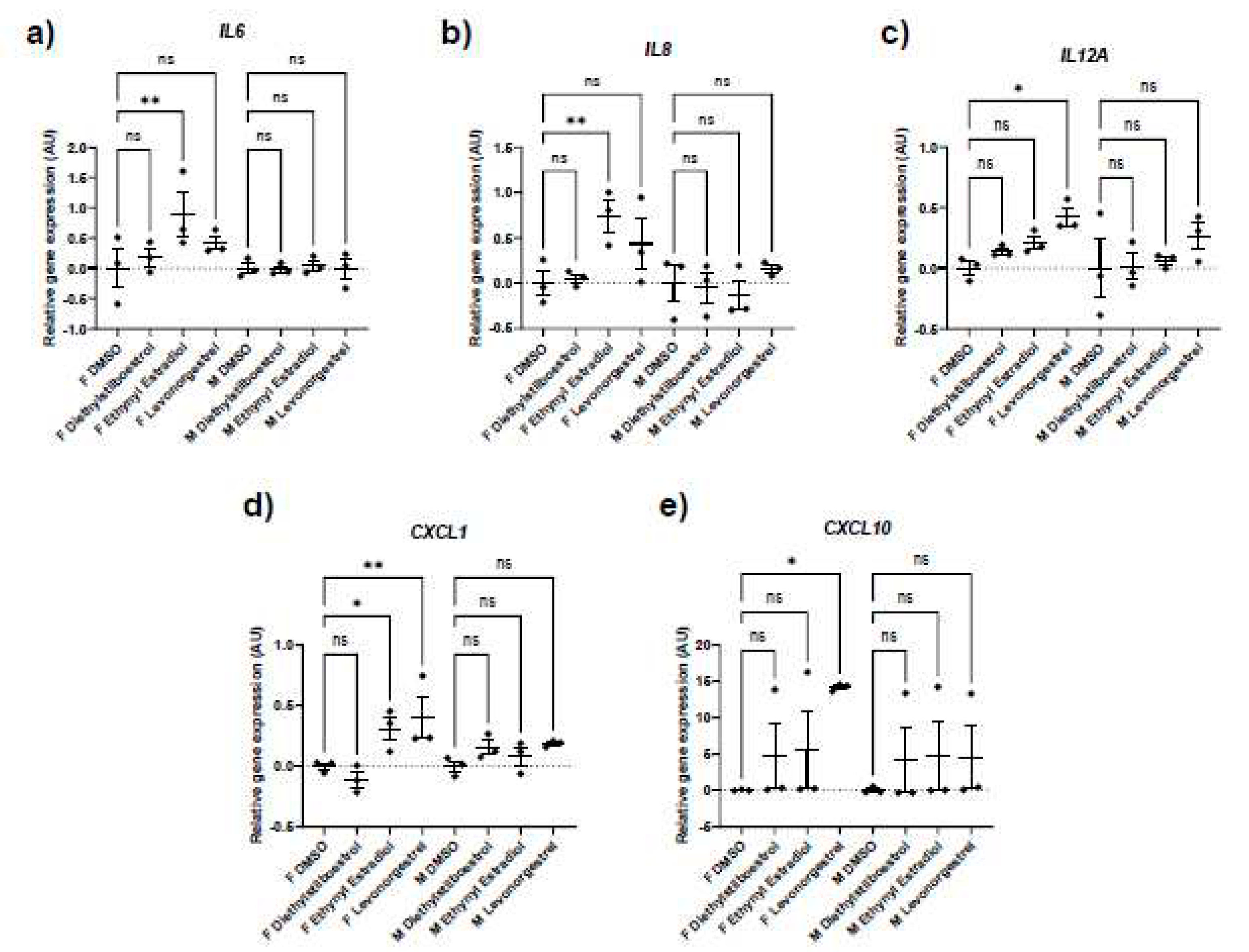
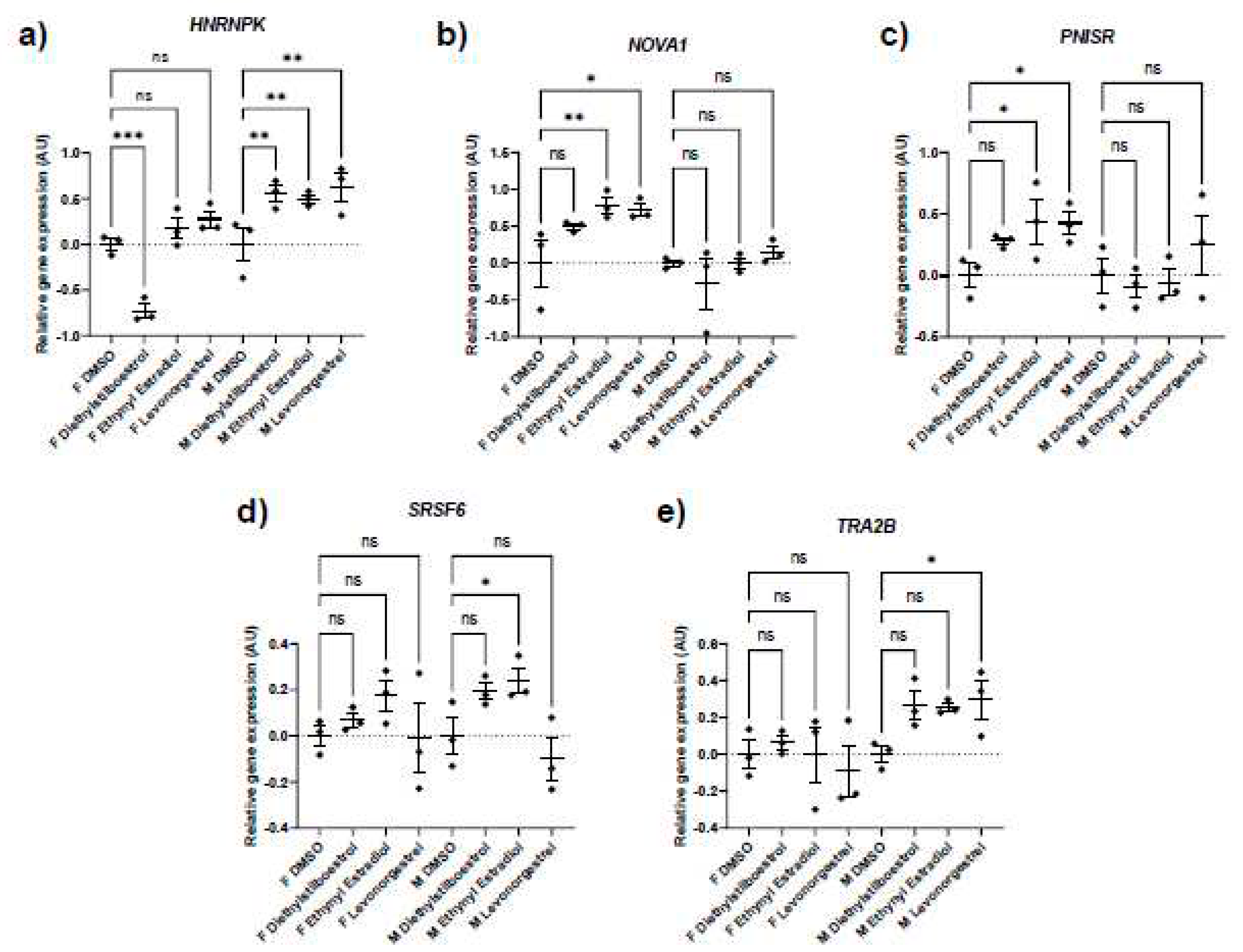
Potential donor characteristic-specific differences in cellular senescence kinetics in response to treatment with female synthetic sex hormones.
A common substructure was identified for compounds that decreased CDKN2A
Discussion
Ethical and conflict of interest statements
Ethical statement:
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- M. Y. Terzi, M. Izmirli, and B. Gogebakan, “The cell fate: senescence or quiescence,”. Mol. Biol. Rep. 2016, 43, 1213–1220. [CrossRef]
- J. Tigges et al., “The hallmarks of fibroblast ageing,”. Mech. Ageing Dev. 2014, 138, 26–44. [CrossRef] [PubMed]
- D. Muñoz-Espín et al., “Programmed cell senescence during mammalian embryonic development,”. Cell 2013, 155, 1104. [CrossRef] [PubMed]
- M. Storer et al., “Senescence Is a Developmental Mechanism that Contributes to Embryonic Growth and Patterning,”. Cell 2013, 155, 1119–1130. [CrossRef] [PubMed]
- L. Hayflick and P. S. Moorhead, “The serial cultivation of human diploid cell strains,”. Exp. Cell Res. 1961, 25, 585–621. [CrossRef] [PubMed]
- Toussaint et al., From the Hayflick mosaic to the mosaics of ageing. Role of stress-induced premature senescence in human ageing,”. Int. J. Biochem. Cell Biol. 2002, 34, 1415–1429. [CrossRef]
- M. Serrano, A. W. Lin, M. E. McCurrach, and D. Beach, “Oncogenic ras Provokes Premature Cell Senescence Associated with Accumulation of p53 and p16 INK4a,”. Cell 1997, 88, 593–602. [CrossRef] [PubMed]
- L. J. Niedernhofer and P. D. Robbins, “Senotherapeutics for healthy ageing,”. Nat. Publ. Gr. 2018. Available online: www.nature.com/nrd (accessed on 1 January 2020).
- B. P. Lee and L. W. Harries, “Senotherapeutic drugs: A new avenue for skincare?,”. Plast. Reconstr. Surg. 2021, 148, 21S–26S. [CrossRef] [PubMed]
- J. L. Kirkland, T. Tchkonia, Y. Zhu, L. J. Niedernhofer, and P. D. Robbins, “The Clinical Potential of Senolytic Drugs,”. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [CrossRef]
- D. J. Baker et al., “Clearance of p16 Ink4a-positive senescent cells delays ageing-associated disorders,”. Nature 2011, 479, 232–236. [CrossRef]
- D. J. Baker et al., “Naturally occurring p16Ink4a-positive cells shorten healthy lifespan,”. Nature 2016, 530, 184–189. [CrossRef]
- Y. Gu et al., “The tyrosine kinase inhibitor Dasatinib reduces cardiac steatosis and fibrosis in obese, type 2 diabetic mice,”. Cardiovasc. Diabetol. 2023, 22, 22. [CrossRef]
- Krzystyniak et al., “Combination of dasatinib and quercetin improves cognitive abilities in aged male Wistar rats, alleviates inflammation and changes hippocampal synaptic plasticity and histone H3 methylation profile,”. Aging (Albany. NY). 2022, 14, 572–595. [CrossRef]
- M. Dungan et al., “Deletion of SA β-Gal+ cells using senolytics improves muscle regeneration in old mice,”. Aging Cell 2022, 21. [CrossRef]
- L. J. Hickson et al., “Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease,”. EBioMedicine 2019, 47, 446–456. [CrossRef]
- J. N. Justice et al., “Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study,”. EBioMedicine 2019, 40, 554–563. [CrossRef]
- E. Latorre, R. Torregrossa, M. E. Wood, M. Whiteman, and L. W. Harries, “Mitochondria-targeted hydrogen sulfide attenuates endothelial senescence by selective induction of splicing factors HNRNPD and SRSF2,”. Aging (Albany. NY). 2018, 10, 1666–1681. [CrossRef]
- M. Hay, D. W. Thomas, J. L. Craighead, C. Economides, and J. Rosenthal, “Clinical development success rates for investigational drugs,”. Nat. Biotechnol. 2014, 32, 40–51. [CrossRef]
- E. Latorre, E. L. Ostler, R. G. A. Faragher, and L. W. Harries, “FOXO1 and ETV6 genes may represent novel regulators of splicing factor expression in cellular senescence,”. FASEB J. 2019, 33, 1086–1097. [CrossRef]
- E. Latorre et al., “Small molecule modulation of splicing factor expression is associated with rescue from cellular senescence,”. BMC Cell Biol. 2017, 18. [CrossRef]
- V. Barra, R. F. Chiavetta, S. Titoli, I. M. Provenzano, P. S. Carollo, and A. Di Leonardo, “Specific Irreversible Cell-Cycle Arrest and Depletion of Cancer Cells Obtained by Combining Curcumin and the Flavonoids Quercetin and Fisetin,”. Genes (Basel). 2022, 13, 1125. [CrossRef]
- F. Xie, P. Xiao, D. Chen, L. Xu, and B. Zhang, “miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs,”. Plant Mol. Biol. 2012, 80, 75–84. [CrossRef] [PubMed]
- M. W. Pfaffl, “A new mathematical model for relative quantification in real-time RT–PCR,”. Nucleic Acids Res. 2001, 29, 2002–2007. [CrossRef]
- C. A. Schneider, W. S. Rasband, and K. W. Eliceiri, “NIH Image to ImageJ: 25 years of image analysis,”. Nat. Methods 2012, 9, 671–675. [CrossRef] [PubMed]
- Y. K. Kim, J. Yeo, B. Kim, M. Ha, and V. N. Kim, “Short Structured RNAs with Low GC Content Are Selectively Lost during Extraction from a Small Number of Cells,”. Mol. Cell 2012, 46, 893–895. [CrossRef] [PubMed]
- Y. Cao, A. Charisi, L. C. Cheng, T. Jiang, and T. Girke, “ChemmineR: a compound mining framework for R,”. Bioinformatics 2008, 24, 1733–1734. [CrossRef]
- T. W. H. Backman, Y. Cao, and T. Girke, “ChemMine tools: An online service for analyzing and clustering small molecules,”. Nucleic Acids Res. 2011, 39, 486–491. [CrossRef]
- Y. Wang, T. W. H. Backman, K. Horan, and T. Girke, “FmcsR: Mismatch tolerant maximum common substructure searching in R,”. Bioinformatics 2013, 29, 2792–2794. [CrossRef]
- RStudio Team, “RStudio: Integrated Development Environment for R.” RStudio, PBC, Boston, Massachusetts, 2020. Available online: http://www.rstudio (accessed on 20 October 2020).
- C. Holly et al., “Changes in splicing factor expression are associated with advancing age in man,”. Mech. Ageing Dev. 2013, 134, 356–366. [CrossRef]
- R. Strong et al., “Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice,”. Aging Cell 2008, 7, 641–650. [CrossRef]
- H. Jahn, M. Schick, F. Kiefer, M. Kellner, A. Yassouridis, and K. Wiedemann, “Metyrapone as Additive Treatment in Major Depression: A Double-blind and Placebo-Controlled Trial,”. Arch. Gen. Psychiatry 2004, 61, 1235–1244. [CrossRef]
- M. Holczer, M. Márton, A. Kurucz, G. Bánhegyi, and O. Kapuy, “A Comprehensive Systems Biological Study of Autophagy-Apoptosis Crosstalk during Endoplasmic Reticulum Stress,”. Biomed Res. Int. 2015, 2015. [CrossRef]
- H. L. Kang, S. Benzer, and K. T. Min, “Life extension in Drosophila by feeding a drug,”. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 838–843. [CrossRef] [PubMed]
- K. S. Olascoaga-Del Angel, H. Gutierrez, M. Königsberg, J. Pérez-Villanueva, and N. E. López-Diazguerrero, “Exploring the fuzzy border between senolytics and senomorphics with chemoinformatics and systems pharmacology,”. Biogerontology 2022, 23, 453–471. [CrossRef] [PubMed]
- FSRH, “FSRH Clinical Guideline: Combined Hormonal Contraception,”. 2020. Available online: https://www.fsrh (accessed on 21 October 2020).
- C. J. Pike, “Sex and the development of Alzheimer’s disease,”. J. Neurosci. Res. 2017, 95, 671–680. [CrossRef] [PubMed]
- B. Tramunt et al., “Sex differences in metabolic regulation and diabetes susceptibility,”. Diabetologia 2020, 63, 453–461. [CrossRef] [PubMed]
- V. Regitz-Zagrosek and G. Kararigas, “Mechanistic pathways of sex differences in cardiovascular disease,”. Physiol. Rev. 2017, 97, 1–37. [CrossRef] [PubMed]
- Ruggierii, S. Anticoli, A. D’ambrosio, L. Giordani, and M. Mora, “The influence of sex and gender on immunity, infection and vaccination,”. Ann. Ist. Super. Sanita 2016, 52, 198–204. [CrossRef]
- Borrás, J. Sastre, D. García-Sala, A. Lloret, F. V. Pallardó, and J. Viña, “Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males,”. Free Radic. Biol. Med. 2003, 34, 546–552. [CrossRef] [PubMed]
- S. Groß, U.-D. Mmel, M. Klintschar, and F. Bartel, “Germline Genetics of the p53 Pathway Affect Longevity in a Gender Specific Manner,”. Curr. Aging Sci. 2014, 7. [CrossRef]
- Y. Han, S. A. Wennersten, J. M. Wright, R. W. Ludwig, E. Lau, and M. P. Y. Lam, “Proteogenomics reveals sex-biased aging genes and coordinated splicing in cardiac aging,”. Am. J. Physiol. Hear. Circ. Physiol. 2022, 323, H538–H558. [CrossRef]
- S. Hägg and J. Jylhävä, “Sex differences in biological aging with a focus on human studies,”. Elife 2021, 10, 1–27. [CrossRef]
- J. B. Rubin et al., “Sex differences in cancer mechanisms,”. Biol. Sex Differ. 2020, 11, 1. [CrossRef]
- M. Ng and L. N. Hazrati, “Evidence of sex differences in cellular senescence,”. Neurobiol. Aging, vol. 2022, 120, 88–104. [CrossRef]
- M. J. Yousefzadeh et al., “Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice,”. Aging Cell 2020, 19, e13094. Available online: https://pubmed.ncbi.nlm.nih.gov/31981461/ (accessed on 25 October 2020).
- M. Waskar et al., “Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy,”. Aging (Albany. NY). 2009, 1, 903–936. [CrossRef]
- M. Rall-Scharpf, T. W. P. Friedl, S. Biechonski, M. Denkinger, M. Milyavsky, and L. Wiesmüller, “Sex-specific differences in DNA double-strand break repair of cycling human lymphocytes during aging,”. Aging. 2021, 13, 21066–21089. [CrossRef] [PubMed]
- R. Trzeciak et al., “Age, sex, and race influence single-strand break repair capacity in a human population NIH Public Access,”. Radic Biol Med 2008, 45, 1631–1641. [CrossRef] [PubMed]
- T. L. Rochelle, D. K. Y. Yeung, M. H. Bond, and L. M. W. Li, “Predictors of the gender gap in life expectancy across 54 nations,”. Psychol. Heal. Med. 2015, 20, 129–138. [CrossRef]
- Y. Sasaki, Y. Ikeda, T. Miyauchi, Y. Uchikado, Y. Akasaki, and M. Ohishi, “Estrogen-sirt1 axis plays a pivotal role in protecting arteries against menopause-induced senescence and atherosclerosis,”. J. Atheroscler. Thromb. 2020, 27, 47–59. [CrossRef] [PubMed]
- M. Vernier and V. Giguère, “Aging, senescence and mitochondria: The PGC-1/ERR axis,”. J. Mol. Endocrinol. 2021, 66, R1–R14. [CrossRef] [PubMed]
- C. H. Diep, N. J. Charles, C. B. Gilks, S. E. Kalloger, P. A. Argenta, and C. A. Lange, “Progesterone receptors induce FOXO1-dependent senescence in ovarian cancer cells,”. Cell Cycle 2013, 12, 1433–1449. [CrossRef]
- D. E. Harrison et al., “17-a-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex,”. Aging Cell 2021, 00, e13328. [CrossRef]
- H. N. Wilkinson and M. J. Hardman, “The Role of Estrogen in Cutaneous Ageing and Repair,”. Maturitas 2017, 103, 60–64. [CrossRef] [PubMed]
- S. J. Russell and C. R. Kahn, “Endocrine regulation of ageing,”. Nat. Rev. Mol. Cell Biol. 2007, 8, 681–691. [CrossRef] [PubMed]
- W. van den Beld, J. M. Kaufman, M. C. Zillikens, S. W. J. Lamberts, J. M. Egan, and A. J. van der Lely, “The physiology of endocrine systems with ageing,”. Lancet Diabetes Endocrinol. 2018, 6, 647–658. [CrossRef]
- X. Dai et al., “Estradiol-induced senescence of hypothalamic astrocytes contributes to aging-related reproductive function declines in female mice,”. Aging. 2020, 12, 6089–6108. [CrossRef]
- M. Garratt, K. A. Lagerborg, Y. M. Tsai, A. Galecki, M. Jain, and R. A. Miller, “Male lifespan extension with 17-α estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice,”. Aging Cell 2018, 17, e12786. [CrossRef]
- C. H. Song, N. Kim, R. H. Nam, S. I. Choi, H. N. Lee, and Y. J. Surh, “17β-Estradiol supplementation changes gut microbiota diversity in intact and colorectal cancer-induced ICR male mice,”. Sci. Rep. 2020, 10, 12283. [CrossRef]
- Y. Fang et al., “Sexual dimorphic metabolic and cognitive responses of C57BL/6 mice to Fisetin or Dasatinib and quercetin cocktail oral treatment,”. GeroScience 2023. [CrossRef]
- L. Saemann, P. Naujoks, L. Hartrumpf, S. Pohl, A. Simm, and G. Szabó, “Sex-Specific Protection of Endothelial Function after Vascular Ischemia/Reperfusion Injury by the Senomorphic Agent Ruxolitinib,”. Int. J. Mol. Sci. 2023, 24, 11727. [CrossRef] [PubMed]
- National Institute on Aging, “Intervention Testing Program: Supported Interventions.,”. 2023. Available online: https://www.nia.nih.gov/research/dab/in- terventions-testing-program-itp/supported-interventions (accessed on 25 January 2024).
- N. Jiang and J. F. Nelson, “Sex Differences in Mouse Longevity and Responses to Geroprotective Drugs: Implications for Human Intervention,”. Public Policy Aging Rep. 2023, 33, 120–124. [CrossRef] [PubMed]
- P. Soldin and D. R. Mattison, “Sex Differences in Pharmacokinetics and Pharmacodynamics,”. Clin. Pharmacokinet. 2009, 48, 143. [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Understanding the Biology of Sex and Gender, T. M. Wizemann, and M.-L. Pardue, “Exploring the Biological Contributions to Human Health: Does Sex Matter?: Sex Begins in the Womb,” Washington (DC): National Academies Press (US). 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222286/.
- M. Oettel and A. K. Mukhopadhyay, “Progesterone: the forgotten hormone in men?,”. Aging Male 2004, 7, 236–257. [CrossRef] [PubMed]
- S. Hiller-Sturmhöfel and A. Bartke, “The endocrine system: an overview,”. Alcohol Heal. Res World 1998, 22, 153–164, [Online] Available: https://wwwncbinlmnihgov/pubmed/15706790.
- N. Fuentes and P. Silveyra, “Estrogen receptor signaling mechanisms,”. Adv. Protein Chem. Struct. Biol. 2019, 116, 135. [CrossRef]
- P. Ranganathan, N. Nadig, and S. Nambiar, “Non-canonical Estrogen Signaling in Endocrine Resistance,”. Front. Endocrinol. 2019, 10, 708. [CrossRef]
- P. Thomas, “Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics,”. Cells 2022, 11. [CrossRef]
- C. Slack, N. Alic, A. Foley, M. Cabecinha, M. P. Hoddinott, and L. Partridge Correspondence, “The Ras-Erk-ETS-Signaling Pathway Is a Drug Target for Longevity,”. Cell 2015, 162, 72–83. [CrossRef]

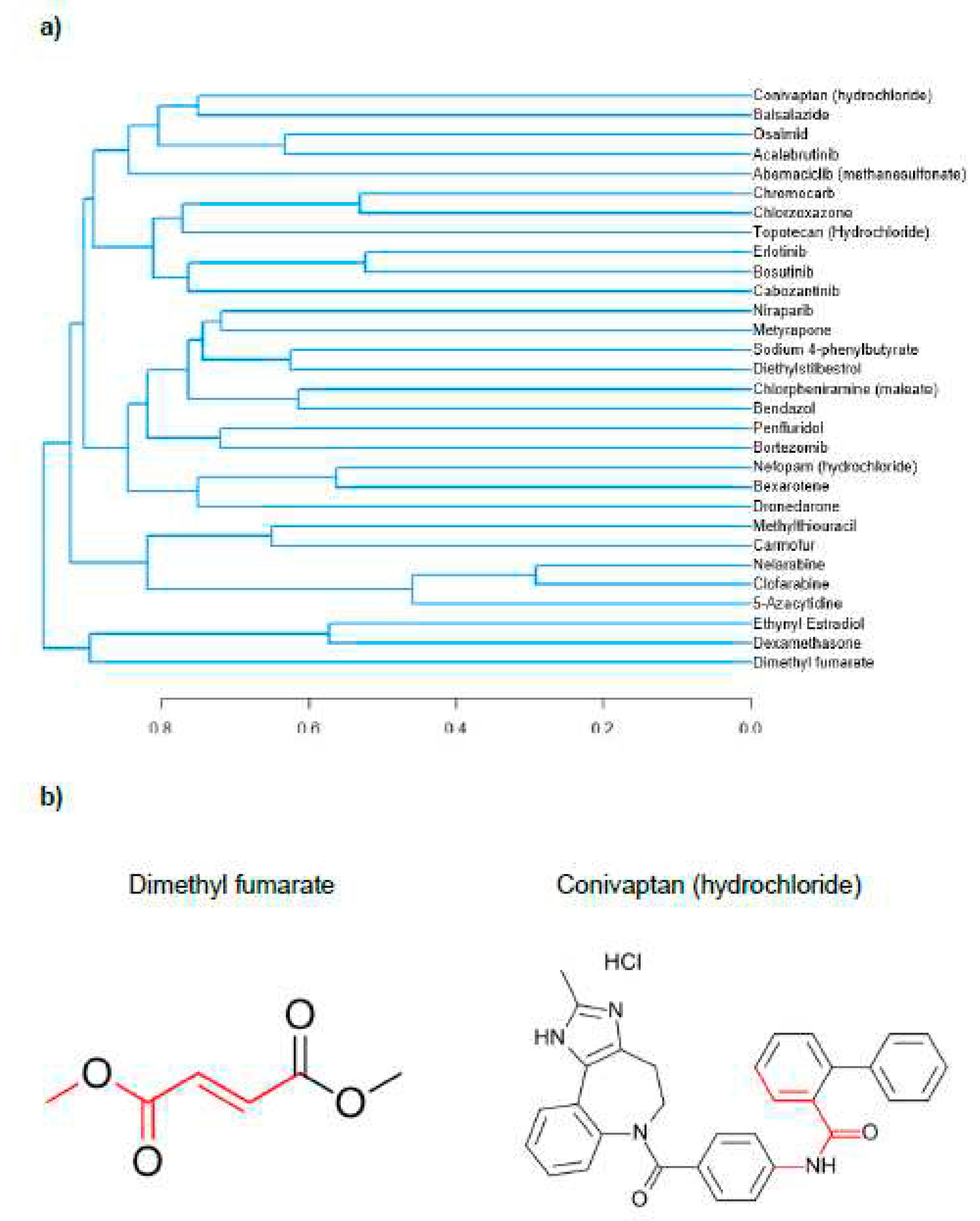
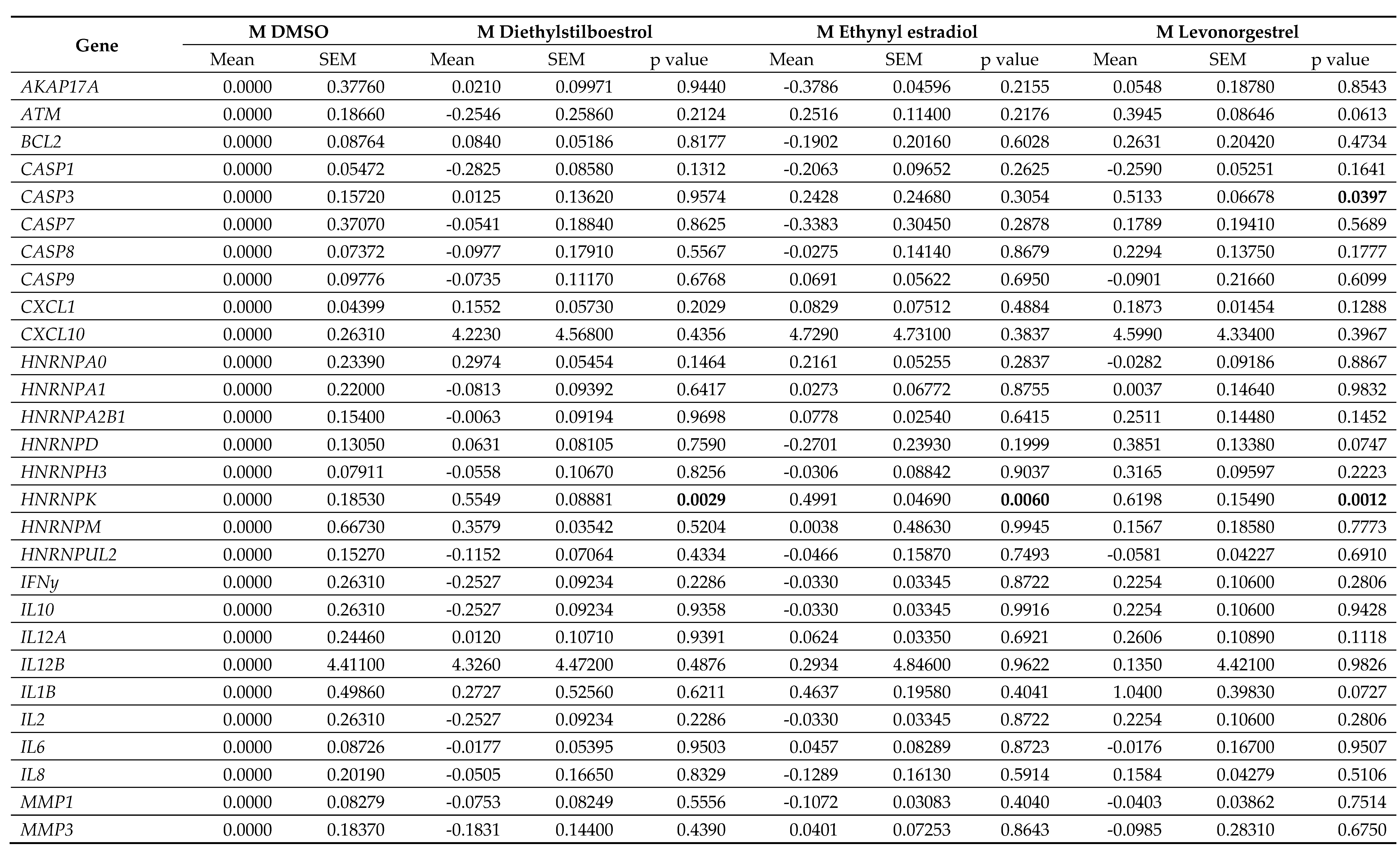
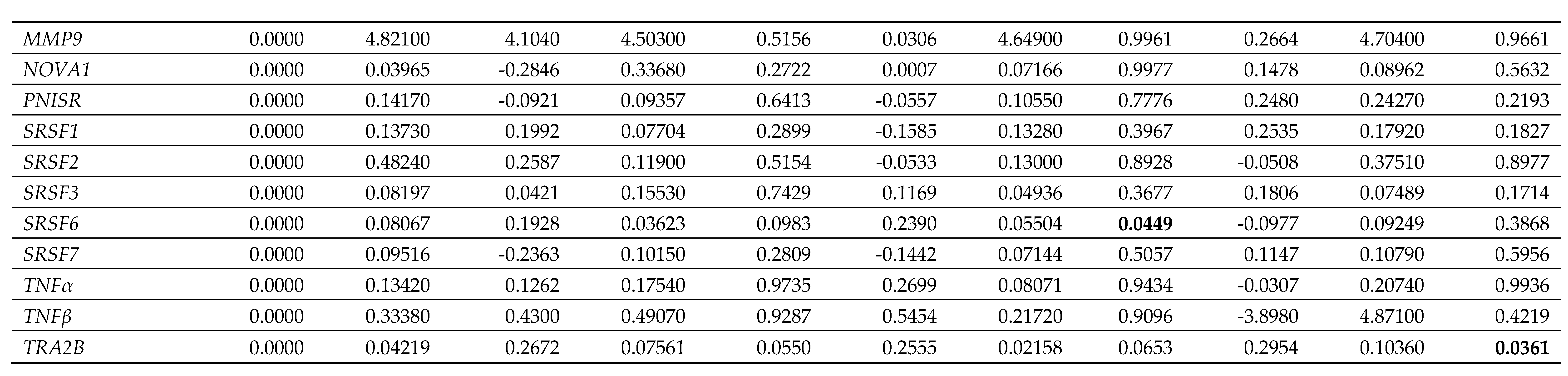
|
| Drug Name | Dose (µM) | Fold change in CDKN2A |
|---|---|---|
| Tucidinostat | 10 | 2.048 |
| Doxifluridine | 10 | 1.559 |
| Doxorubicin (hydrochloride) | 10 | 1.498 |
| Bromhexine (hydrochloride) | 10 | 1.167 |
| Homoharringtonine | 10 | 1.160 |
| Chlorambucil | 10 | 1.133 |
| Aspirin | 10 | 1.072 |
| Amoxapine | 10 | 1.034 |
| Doxorubicin (hydrochloride) | 1 | 0.969 |
| Imatinib | 10 | 0.948 |
| Montelukast (sodium) | 10 | 0.888 |
| Atorvastatin (hemicalcium salt) | 10 | 0.822 |
| Ribociclib | 10 | 0.820 |
| Baricitinib (phosphate) | 10 | 0.820 |
| Irinotecan (hydrochloride) | 10 | 0.804 |
| Levoleucovorin (calcium) | 10 | 0.798 |
| Epirubicin (hydrochloride) | 10 | 0.790 |
| Cobimetinib | 10 | 0.773 |
| Homoharringtonine | 1 | 0.765 |
| Decitabine | 10 | 0.744 |
| Sunitinib | 10 | 0.722 |
| Temozolomide | 10 | 0.700 |
| Silibinin | 10 | -0.686 |
| Diacerein | 10 | -0.694 |
| Vinorelbine (ditartrate) | 1 | -0.713 |
| Alpelisib | 10 | -0.717 |
| Ethamsylate | 10 | -0.734 |
| Diethylstilboestrol | 1 | -0.753 |
| Altretamine | 10 | -0.782 |
| Panobinostat | 1 | -0.791 |
| Sertraline (hydrochloride) | 1 | -0.805 |
| Deferoxamine (mesylate) | 10 | -0.822 |
| Balsalazide | 1 | -0.852 |
| Pexidartinib | 1 | -0.890 |
| Bexarotene | 10 | -0.894 |
| Clofarabine | 10 | -0.897 |
| Caffeic acid | 10 | -0.903 |
| Pazopanib (hydrochloride) | 10 | -0.909 |
| Aspirin | 1 | -0.916 |
| Dexamethasone | 1 | -0.917 |
| Pazopanib | 10 | -0.921 |
| Rucaparib (phosphate) | 10 | -0.984 |
| Glasdegib | 1 | -1.005 |
| Aceglutamide | 10 | -1.020 |
| Trimethoprim | 10 | -1.021 |
| Crizotinib (hydrochloride) | 10 | -1.051 |
| Acalabrutinib | 1 | -1.069 |
| Zidovudine | 10 | -1.080 |
| Citalopram (hydrobromide) | 10 | -1.094 |
| Topotecan (hydrochloride) | 10 | -1.111 |
| Rucaparib (phosphate) | 1 | -1.126 |
| Alpelisib | 1 | -1.153 |
| Sertraline (hydrochloride) | 10 | -1.154 |
| Erlotinib | 1 | -1.157 |
| Triclabendazole | 10 | -1.168 |
| Nefopam (hydrochloride) | 10 | -1.174 |
| Altretamine | 1 | -1.184 |
| Bortezomib | 1 | -1.212 |
| Nefopam (hydrochloride) | 1 | -1.217 |
| Penfluridol | 10 | -1.230 |
| Clioquinol | 10 | -1.241 |
| Ethynyl estradiol | 1 | -1.259 |
| Panobinostat | 10 | -1.260 |
| Clofibrate | 1 | -1.272 |
| Mizoribine | 10 | -1.291 |
| Belinostat | 10 | -1.330 |
| Valpromide | 10 | -1.351 |
| Bosutinib | 1 | -1.354 |
| Berberine (chloride hydrate) | 10 | -1.367 |
| Nelarabine | 1 | -1.403 |
| Acalabrutinib | 10 | -1.405 |
| Tofacitinib (citrate) | 10 | -1.412 |
| Erdosteine | 1 | -1.470 |
| Bortezomib | 10 | -1.475 |
| Bosutinib | 10 | -1.478 |
| Osalmid | 1 | -1.493 |
| Topotecan (hydrochloride) | 1 | -1.515 |
| Bezafibrate | 10 | -1.523 |
| Orotic acid | 10 | -1.532 |
| Methylthiouracil | 1 | -1.551 |
| Chlorpheniramine (maleate) | 10 | -1.559 |
| Nitisinone | 1 | -1.561 |
| Teniposide | 10 | -1.577 |
| Sulfasalazine | 10 | -1.584 |
| Pemetrexed (disodium hemipenta hydrate) | 1 | -1.702 |
| Nifuroxazide | 10 | -1.705 |
| Osalmid | 10 | -1.716 |
| Nicotinamide | 1 | -1.717 |
| Erlotinib | 10 | -1.741 |
| Bendazol | 1 | -1.820 |
| Bexarotene | 1 | -1.835 |
| 5-Azacytidine | 1 | -1.837 |
| Nelarabine | 10 | -1.893 |
| Clofarabine | 1 | -1.905 |
| Niraparib | 10 | -1.927 |
| Mycophenolic acid | 10 | -1.963 |
| 5-Azacytidine | 10 | -2.022 |
| Chlorzoxazone | 1 | -2.045 |
| Metyrapone | 1 | -2.066 |
| Dimethyl fumarate | 10 | -2.099 |
| Dexamethasone | 10 | -2.209 |
| Dimethyl fumarate | 1 | -2.227 |
| Chromocarb | 10 | -2.277 |
| Penfluridol | 1 | -2.460 |
| Bendazol | 10 | -2.486 |
| Methylthiouracil | 10 | -2.527 |
| Ethynyl estradiol | 10 | -2.684 |
| Abemaciclib (methanesulfonate) | 10 | -2.768 |
| Conivaptan (hydrochloride) | 10 | -2.908 |
| Sunitinib | 1 | -2.926 |
| Diethylstilbestrol | 10 | -3.068 |
| Dronedarone | 1 | -4.099 |
| Sodium 4-phenylbutyrate | 10 | -4.861 |
| Cabozantinib | 10 | -7.875 |
| Metyrapone | 10 | -8.532 |
| Abemaciclib (methanesulfonate) | 1 | -11.417 |
| Cabozantinib | 1 | -11.571 |
| Carmofur | 10 | -11.805 |
| Balsalazide | 10 | -11.887 |
| Chlorzoxazone | 10 | -12.035 |
| Treatment | Mean | SEM | P | Significance |
|---|---|---|---|---|
| Assay 1 Control 10µM | 44.17 | 7.506 | - | - |
| 5-Azacytidine 10µM | 36.99 | 6.191 | 0.2496 | ns |
| Caffeic Acid 10µM | 31.67 | 3.34 | 0.0553 | ns |
| Chlorpheniramine (maleate) 10µM | 29.33 | 3.805 | 0.0264 | * |
| Diethylstilboestrol 10µM | 30.98 | 1.507 | 0.0445 | * |
| Ethynyl estradiol 10µM | 30.1 | 1.317 | 0.0337 | * |
| Levonorgestrel 10µM | 21.54 | 0.8541 | 0.002 | ** |
| Assay 2 Control 10µM | 40.05 | 9.082 | - | - |
| Amoxapine 10µM | 28.92 | 9.597 | 0.4353 | ns |
| Bendazol 10µM | 23.67 | 6.348 | 0.2568 | ns |
| Citalopram (hydrobromide) 10µM | 33.56 | 11.83 | 0.6466 | ns |
| Methylthiouracil 10µM | 33.69 | 12.09 | 0.6531 | ns |
| Sertraline (hydrochloride) 10µM | 26.81 | 10.88 | 0.3556 | ns |
| Valpromide 10µM | 23.84 | 7.242 | 0.2615 | ns |
| Assay 3 Control 10µM | 27.52 | 3.686 | - | - |
| Balsalazide 10µM | 22.99 | 2.48 | 0.3251 | ns |
| Carmofur 10µM | 16.7 | 2.985 | 0.0288 | * |
| Chlorzoxazone 10µM | 19.91 | 2.438 | 0.109 | ns |
| Conivaptan (hydrochloride) 10µM | 22.33 | 2.988 | 0.2627 | ns |
| Metyrapone 10µM | 15.36 | 0.7593 | 0.0161 | * |
| Sodium-4-Phenylbutyrate 10µM | 16.16 | 4.995 | 0.0228 | * |
| Assay 4 Control 1µM | 19.19 | 4.546 | - | - |
| Abemaciclib (methanesulfonate) 1µM | 12.09 | 1.888 | 0.1025 | ns |
| Cabozantinib 1µM | 8.13 | 0.2987 | 0.0159 | * |
| Dronedarone 1µM | 14.12 | 2.768 | 0.234 | ns |
| Nicotinamide 1µM | 12.11 | 1.795 | 0.1034 | ns |
| Penfluridol 1µM | 10.48 | 1.586 | 0.0495 | * |
| Assay 4 Control 10µM | 16.11 | 4.794 | - | - |
| Dexamethasone 10µM | 10.26 | 2.553 | 0.1728 | ns |
| Assay 5 Control 1µM | 39.1 | 8.275 | - | - |
| Aspirin 1µM | 32.61 | 2.587 | 0.3269 | ns |
| Sunitinib 1µM | 40.42 | 1.816 | 0.8398 | ns |
| Assay 5 Control 10µM | 36.06 | 3.345 | - | - |
| Aspirin 10µM | 21.39 | 4.997 | 0.0396 | * |
| Sunitinib 10µM | 2.563 | 2.563 | 0.0002 | *** |
| Assay 6 Control 1µM | 3.163 | 0.5069 | - | - |
| Aspirin 1µM | 4.287 | 0.5053 | 0.4963 | ns |
| Sunitinib 1µM | 3.493 | 1.016 | 0.2333 | ns |
| Assay 6 Control 10µM | 4.223 | 0.6868 | - | - |
| Aspirin 10µM | 6.227 | 1.665 | 0.8404 | ns |
| Sunitinib 10µM | 0 | 0 | 0.0199 | * |
| Imatinib 10µM | 18.67 | 2.064 | <0.0001 | **** |
| Assay 7 Control 10µM | 4.07 | 0.8632 | - | - |
| Bromhexine (hydrochloride) 10µM | 5.65 | 1.818 | 0.3679 | ns |
| Doxifluridine 10µM | 6.04 | 1.637 | 0.2654 | ns |
| Doxorubicin (hydrochloride) 10µM | 15.84 | 0.1804 | <0.0001 | **** |
| Ethynyl estradiol 10µM | 5.33 | 0.8184 | 0.4704 | ns |
| Homoharringtonine 10µM | 13.11 | 1.609 | 0.0001 | *** |
| Tucidinostat 10µM | 3.13 | 0.27 | 0.5886 | ns |
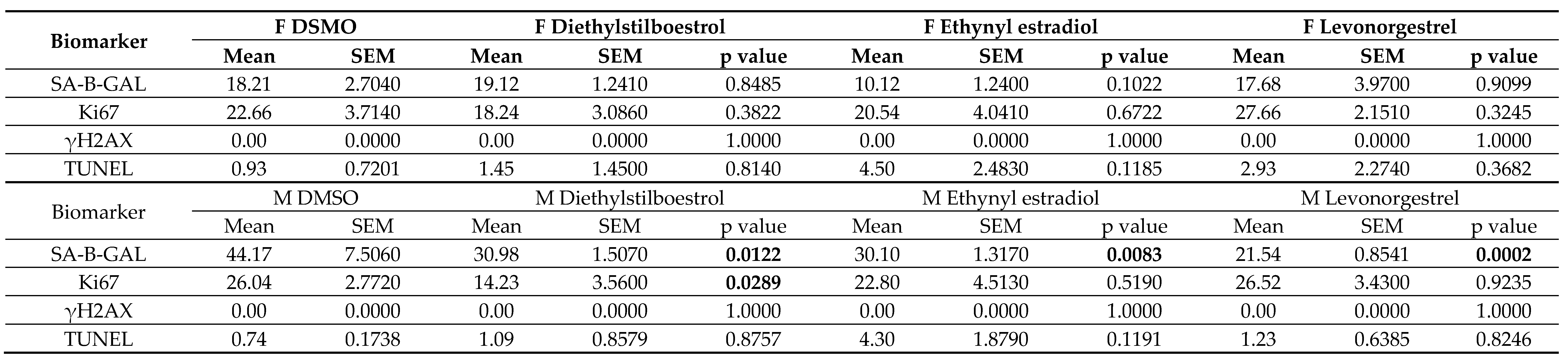 |
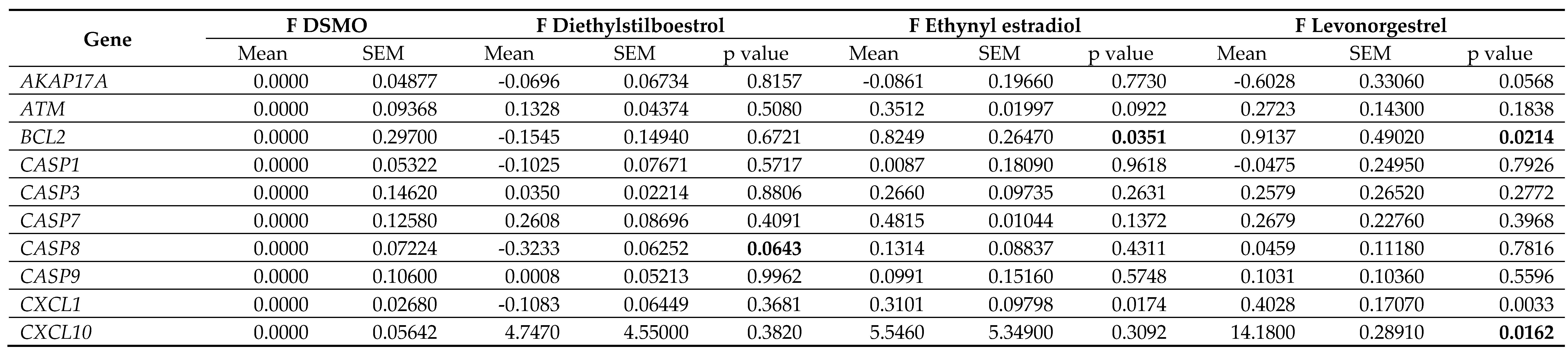 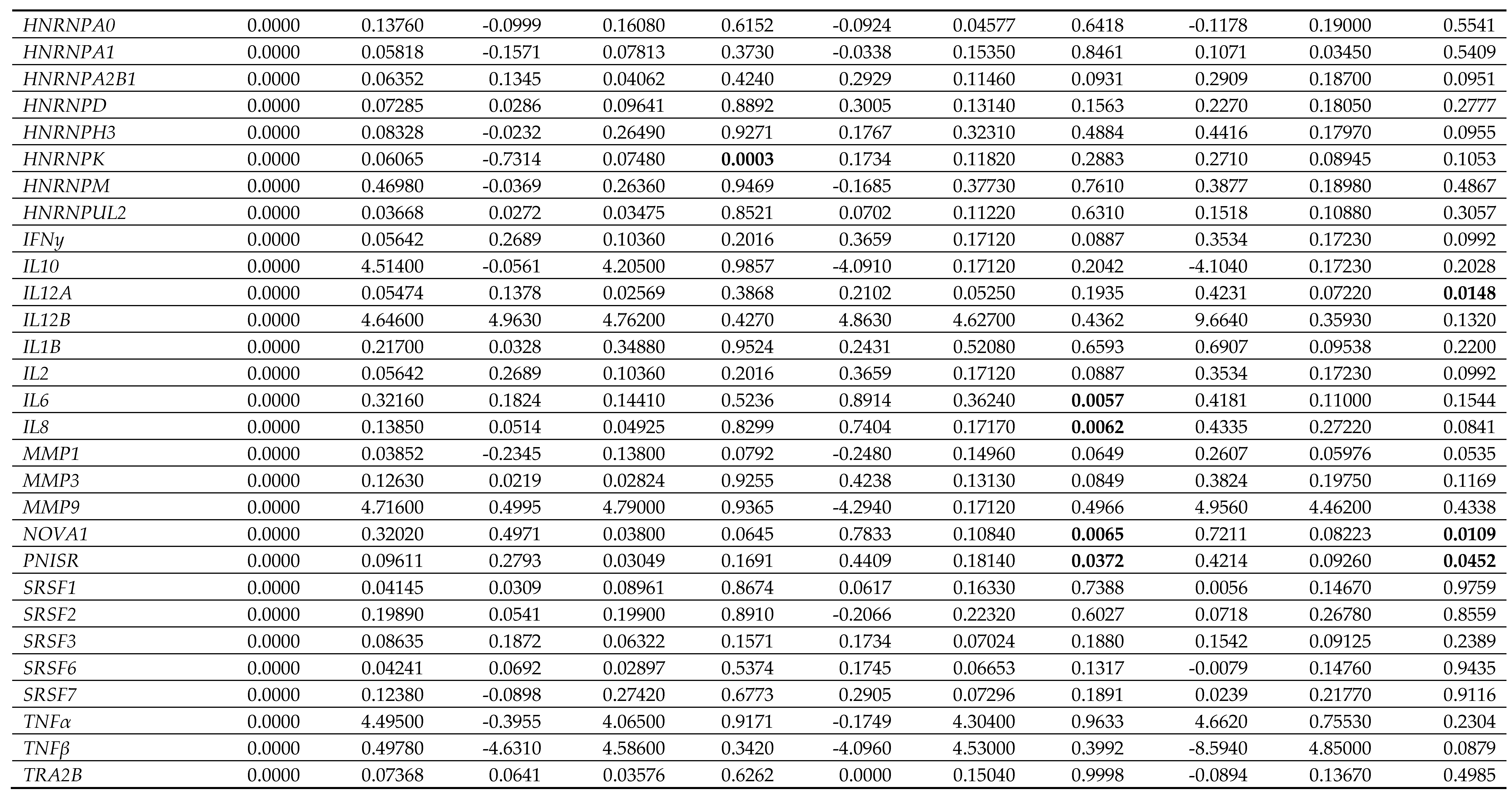
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





