1. Introduction
The cranial cruciate ligament (CCL) is an intraarticular structure in the stifle. It prevents the cranial subluxation and internal rotation of the tibia and the hyperextension of the stifle [
1].
CCL rupture is the most common acquired orthopedic disease and the first cause of hindlimb lameness in dogs [
2,
3]. The cause and pathogenesis of this disorder remain elusive. Abnormal anatomical conformation of the limb, increased tibial plateau angle, overweight, reduction of elasticity and resistance to stress due to the patient’s age, presence of immune-mediated inflammatory diseases, breed predisposition, excessive acute limb loading, traumatic hyperextension, and excessive internal rotation of the tibia that may overload the CCL are all pathogenetic hypotheses that have been advanced [
4,
5].
Following the failure of the cranial cruciate ligament, a cranial tibial thrust (CTT) is generated at every step of the subject affected by this pathology. This continuous cranial sliding of the tibia and the internal rotation of the tibia on the femur stress the other stifle’s anatomical structures, such as the meniscus and the medial collateral and patellar ligaments [
6,
7].
It is reported in the literature that 32% to 77% of dogs that have a rupture of the cranial cruciate ligament also have meniscal injury [
8,
9,
10]. Overweight, the chronicity of the injury to the cranial cruciate ligament, and the complete, not just partial, rupture of the ligament are factors that increase the incidence of meniscal damage [
8,
9,
11]. The mechanism of meniscal tear associated with cranial cruciate ligament insufficiency relates to abnormal motion of the cranial cruciate ligament–deficient joint [
12,
13]. The medial meniscus is usually affected by tears because it is firmly attached to the tibia and is compressed against the medial femoral condyle during the cranial tibial thrust, while the lateral meniscus maintains a more neutral position [
12].
Injury of the medial collateral ligament (MCL) in dogs is usually seen in conjunction with CCL rupture [14,15]. The rotational stability of the stifle is guaranteed not only by the cranial cruciate ligament, but also by the medial and lateral collateral ligaments. In particular, internal rotation of the stifle is limited by the MCL and the cranial cruciate ligament itself. While rupture of the MCL alone is rare, following frequent rupture of the cranial cruciate ligament in dogs, an abnormal force acts on the MCL during flexion and internal rotation of the tibia [17,18].
The patellar ligament is the tendon of the quadriceps femoris muscle that runs from the patella to the tibial tuberosity. It is anatomically a ligament because it connects two bone structures (patella and tibial tuberosity); however, its histological structure (86% collagen fiber type III) is more similar to a tendon than a ligament [19–21].
The patellar ligament is an important stabilizer of the stifle, and it is highly stressed in patients suffering from rupture of the cranial cruciate ligament. To date, however, there are no studies describing the establishment of anatomical or functional damage to this ligament after CCL rupture.
Elastosonography is an imaging technique complementary to ultrasound that evaluates the mechanical properties of tissue. In human medicine, it is used principally to diagnose thyroid, breast, and prostate cancer and steatosis or liver fibrosis. Tissue stiffness is usually considered as a biomarker of pathological lesion [22]. The biomechanical properties of tendons are associated with their function. For example, the Achilles tendon (common calcaneal tendon) is substantially hard; in contrast, it is known that the patellar ligaments in clinically healthy dogs show highly elastic biomechanical properties [19]. The Achilles tendon acts as a stabilizer, while the patellar ligament allows the patella to glide in the trochlear groove in the proximal-distal direction [23].
Recently, a study evaluated the elasticity and softness of the patellar ligament in dogs using ultrasonographic and elastosonographic examinations. Overall, 89.3% of the 30 patellar ligaments examined were graded as soft or mostly soft, while the remaining 10.7% were classified as intermediate [24].
Two elastosonographic techniques are mainly described: strain and shear elastosonography. The first is also known as quasi-static or compression elastosonography, and it measures the deformation of tissue caused by the operator with the probe. The second is called dynamic elastosonography, and it evaluates the deformation of tissue caused by shear waves. They are both described for assessment of the patellar ligament [25].
Several studies have also proven that, following tibial plateau leveling osteotomy (TPLO) or tibial tuberosity advancement (TTA), between 50% and 61% of cases showed clinical and radiographic signs referable to thickening and tendinopathy of the patellar ligament. It is not yet clear whether the ligament, even before the tibia is treated with TPLO or TTA, is damaged with respect to the physiological picture [26,27].
The purpose of this study is to assess the appearance of the patellar ligament by ultrasound and elastosonography in dogs affected by rupture of the cranial cruciate ligament but not yet treated in order to understand if signs of thickening and reduction of elasticity increase over time between the day of ligament rupture and the day of diagnosis and, therefore, persist even before being treated with the surgical procedure of tibial plateau leveling osteotomy or tibial tuberosity advancement.
Understanding this aspect could have implications for the choice of timing to treat patients suffering from rupture of the cranial cruciate ligament, but also for the contextualization of the radiographic and ultrasound pictures of patellar ligament desmitis that are frequently observed in subjects undergoing TPLO and TTA.
2. Materials and Methods
2.1. Ethics Statement
The Ethics Committee for Clinical Study in Animal Patients of the University of Camerino has given its authorization to carry out this clinical study (protocol number. 01/24). Informed consent was obtained from the owners of all the dogs.
2.2. Animals
Thirty-three dogs of various breeds with unilateral naturally occurring CCL disease were prospectively enrolled in the study. An orthopedic examination allowed the diagnosis of a rupture of the cranial cruciate ligament to be formulated. All dogs underwent general, orthopedic, and neurological physical examinations in order to exclude other concomitant pathologies. In addition, the hematologic and biochemistry profiles were acquired. Dogs with concurrent orthopedic, neurological, or metabolic disease were excluded from the study, as well as dogs with contralateral CCL disease. They were divided into three groups based on the time elapsed from the onset of lameness to diagnosis: Group 1 (1–15 days), Group 2 (16–60 days), and Group 3 (over 60 days).
Dogs were subjected to radiographic examination of the stifle and ultrasonographic and elastosonographic examinations of the patellar ligament.
2.3. Ultrasound and elastosonographic examination
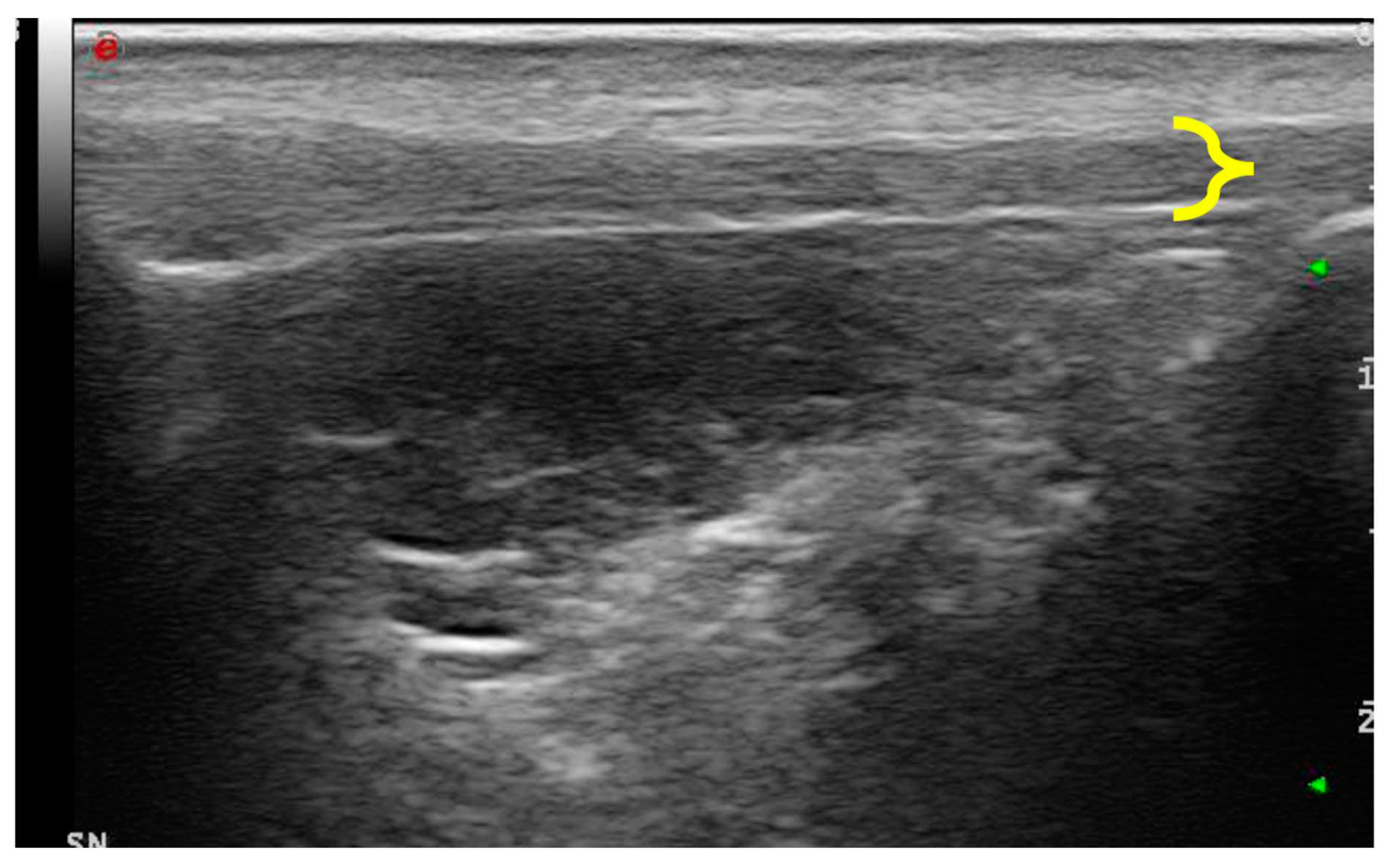
Patients were positioned in lateral recumbency with the affected stifle up and in maximal passive flexion. This position allowed us to avoid the anisotropy of the fibrillar structures of the patellar ligament. With awake dogs, a conventional B-mode ultrasonographic examination of the patellar ligament was performed using a My Laboratory Class C ultrasound machine (Esaote, Genova, Italy) equipped with a 12–18 MHz linear transducer (LA 435; Esaote, Genova, Italy). The cranial region was shaved from 2 cm distal to the tibial tuberosity to 1 cm proximal to the patella, and coupling gel was applied. A patellar ligament was considered normal when the fibrillar echotexture was homogeneous, parallel, and slightly broadened at the origin. Ligaments that exhibited ultrasonographic evidence of pathologies, such as disrupted patterns, increased cross-sectional diameter, or internal mineralization, were considered abnormal.
The ligament thickness was calculated on the longitudinal ultrasound images of the patellar ligament. The landmark to take measures was identified in the exact middle of the ligament measured from the most distal portion of its insertion on the patella to the most proximal insertion on the tibial tuberosity.
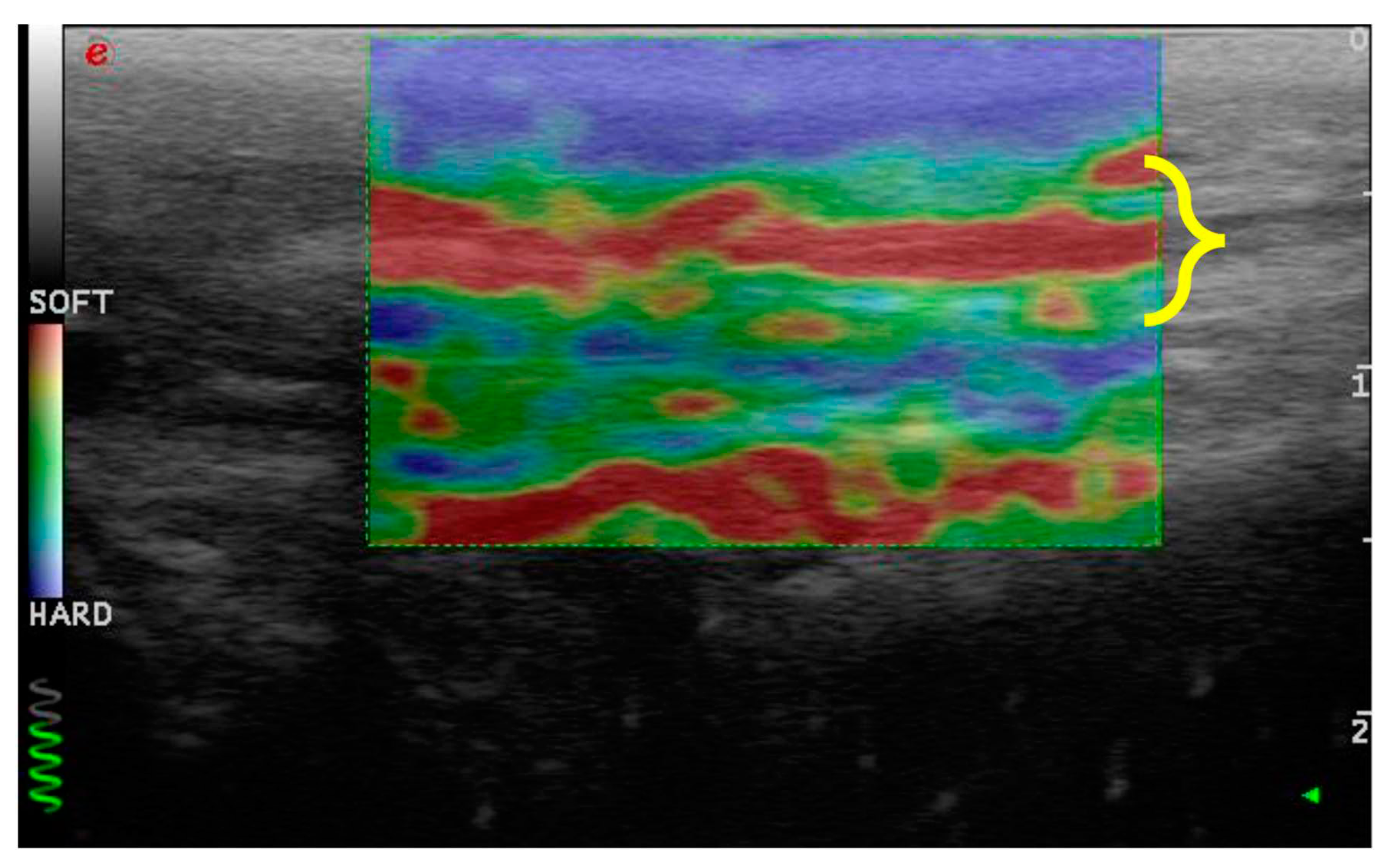
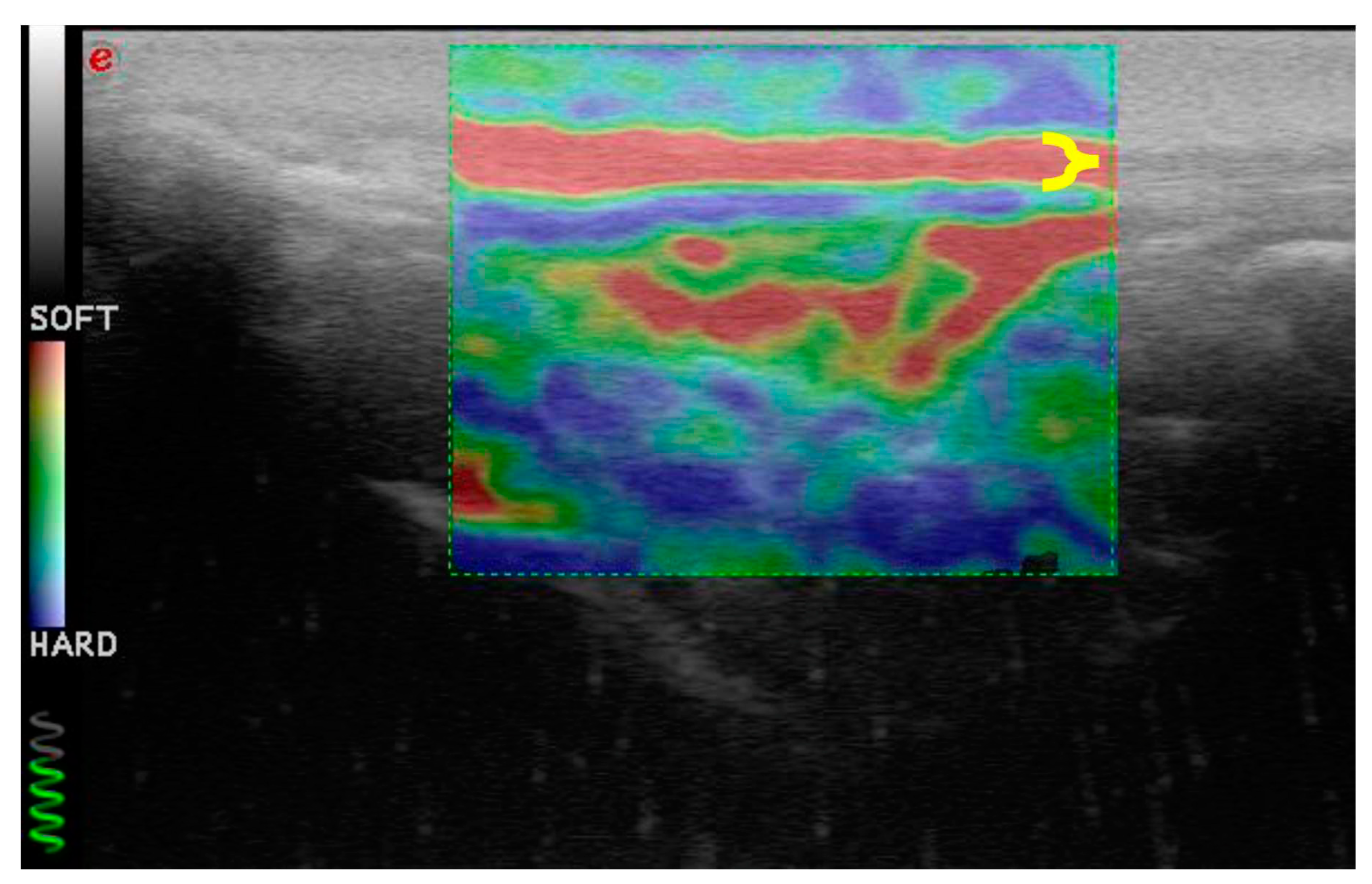
The elastosonographic images were obtained by applying light rhythmic pressure with the probe. The operator evaluated each patellar ligament twice and acquired only longitudinal sections. A color translucent map superimposed on the B-mode images characterized the images.
Each color indicated the relative elasticity of the different structures compared with the mean elasticity of the entire area: blue (mostly hard), green (intermediate), and red (soft). Only images without artifacts were evaluated. The elastosonographic images were considered assessable when they showed:
consistency between the elastogram and the underlying B-mode ultrasound image;
blue coloration of skin and dermal tissue because they are harder than the patellar ligament; and
green coloration of the marker (green coil).
Tissue elasticity was measured by calculating the percentage of softness and hardness with dedicated software (ElaXto, Esaote).
The acquired data were compared between Groups 1, 2, and 3, and the group of dogs with cranial cruciate ligament disease was compared to a group of dogs with healthy stifles that had already been studied [22].
After ultrasonographic and elastosonographic evaluations, all dogs underwent X-ray examination with two projections in order to exclude concomitant stifle diseases and perform the preoperative planning. Therefore, all patients were subjected to sedation to achieve a suitable degree of muscle relaxation and immobility during the diagnostic procedure. All dogs were premedicated with the same anesthetic protocol. Specifically, 2 μg/kg of dexmedetomidine and 0.2 mg/kg of butorphanol were administered intramuscularly. Subsequently, the cephalic vein was cannulated, and propofol (1–2 mg/kg) was administered as needed. Furthermore, additional oxygenation was ensured by the administration of pure oxygen via a face mask.
The tibial plateau angle (TPA) was measured in the mediolateral view with the stifle and tarsus joints flexed at 90°. The TPA was estimated at insertion between the proximal tibial joint orientation line and the mechanical axis of the tibia in the sagittal plane. The angle between the proximal tibial joint orientation line and an axis perpendicular to the mechanical axis of the tibia was the TPA.
2.4. Statistical Analysis
Data were not normally distributed, so the Kruskal–Wallis nonparametric test was used to compare patellar ligament thickness, hardness, and softness values between groups. Hardness and softness values of the patellar ligament between normal dogs and dogs with CCL rupture were compared with the Mann–Whitney U test. Correlation between hardness and softness values and the time elapsed from the onset of the lameness (days) was evaluated with a Spearman test.
Statistical significance was set at p-value < 0.05.
3. Results
3.1. Enrolled Patients
We examined 33 patellar ligaments from 33 dogs affected by unilateral cranial cruciate rupture, 18 females and 15 males, belonging to different breeds of medium-sized and large dogs: five Labrador retrievers, four golden retrievers, four American Staffordshire terriers, three cani corsi italiani, three Siberian huskies, two rottweilers, one American pit bull terrier, one springer spaniel, one braque Saint-Germain, one boxer, one German shepherd, one bracco italiano, and six mixed-breed dogs. Eleven dogs belonged to Group 1, eight dogs to Group 2, and fourteen dogs to Group 3. The mean weight was 32.1 ± 7.89 kg (Group 1 = 30.05 ± 6.5 kg; Group 2 = 33.85 ± 5.67 kg; Group 3 = 32.70 ± 9.90 kg; p > 0.05), and the mean age was 5.1 ± 2.61 years (Group 1 = 5.09 ± 2.87 years; Group 2 = 4.75 ± 3.15 years; Group 3 = 5.35 ± 2.20 years; p > 0.05). The tibial plateau angle showed no statical differences between groups (p > 0.05) (Group 1 = 26.16 ± 1.61°; Group 2 = 25.75 ± 1.48°; Group 3 = 26 ± 1.75°).
3.2. Ultrasonographic Examination
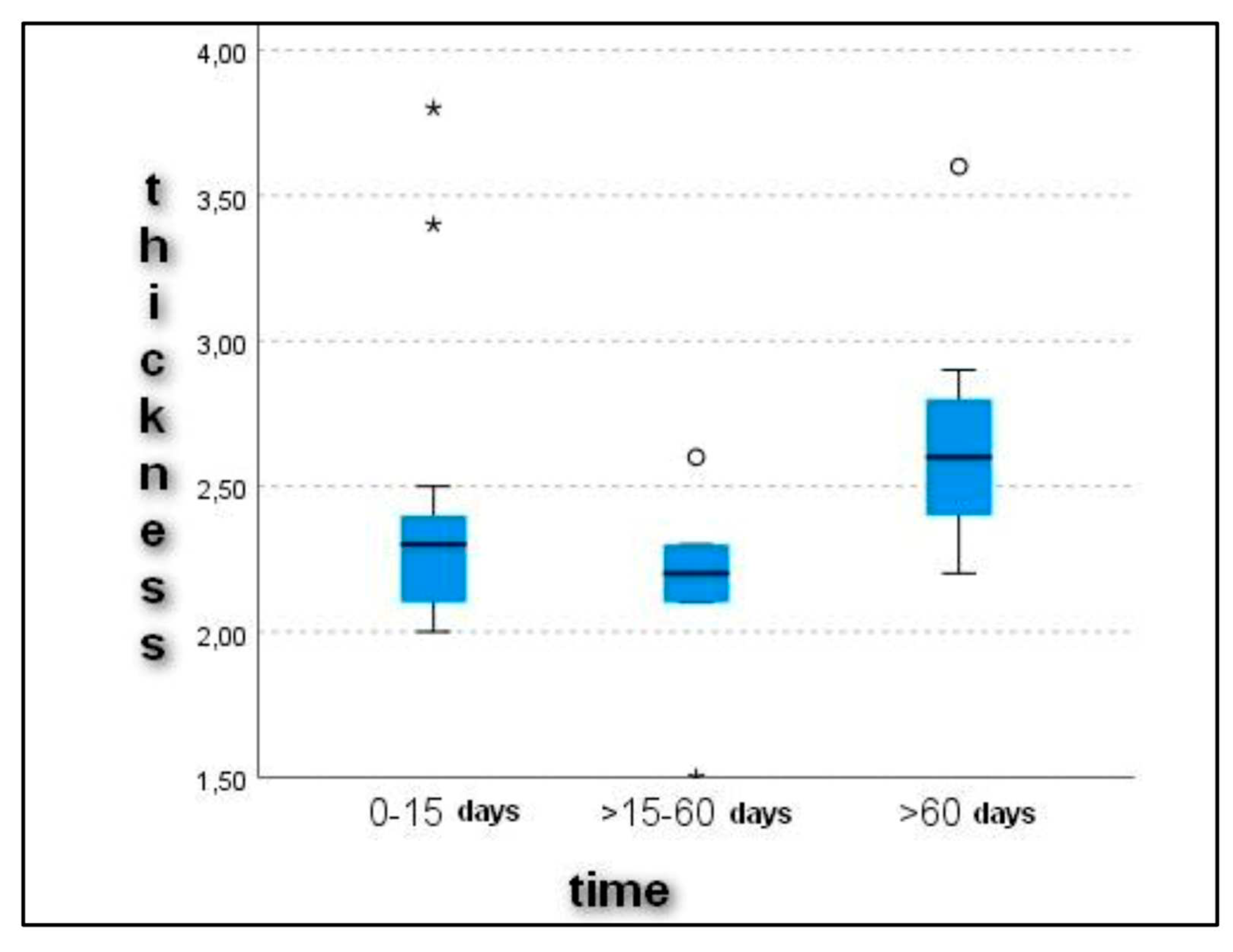
Upon ultrasonographic examination, all dogs showed alterations in the echostructure of the patellar ligament. The cross-sectional diameter of the patellar ligament was 2.4 ± 0.55 mm in Group 1, 2.1 ± 0.35 mm in Group 2, and 2.6 ± 0.41 mm in Group 3. The evaluation of the patellar ligament’s diameter revealed a progressive thickening of it with increasing time between the onset of lameness and diagnosis (
Figure 1). These data expressed statistically significant differences (
p < 0.05) in the comparison between Groups 2 (16–60 days) and 3 (>60 days) (
p = 0.007) and between Groups 1 (0–15) and 3 (>60 days) (
p = 0.037) (
Figure 2).
3.3. Elastosonographic Examination
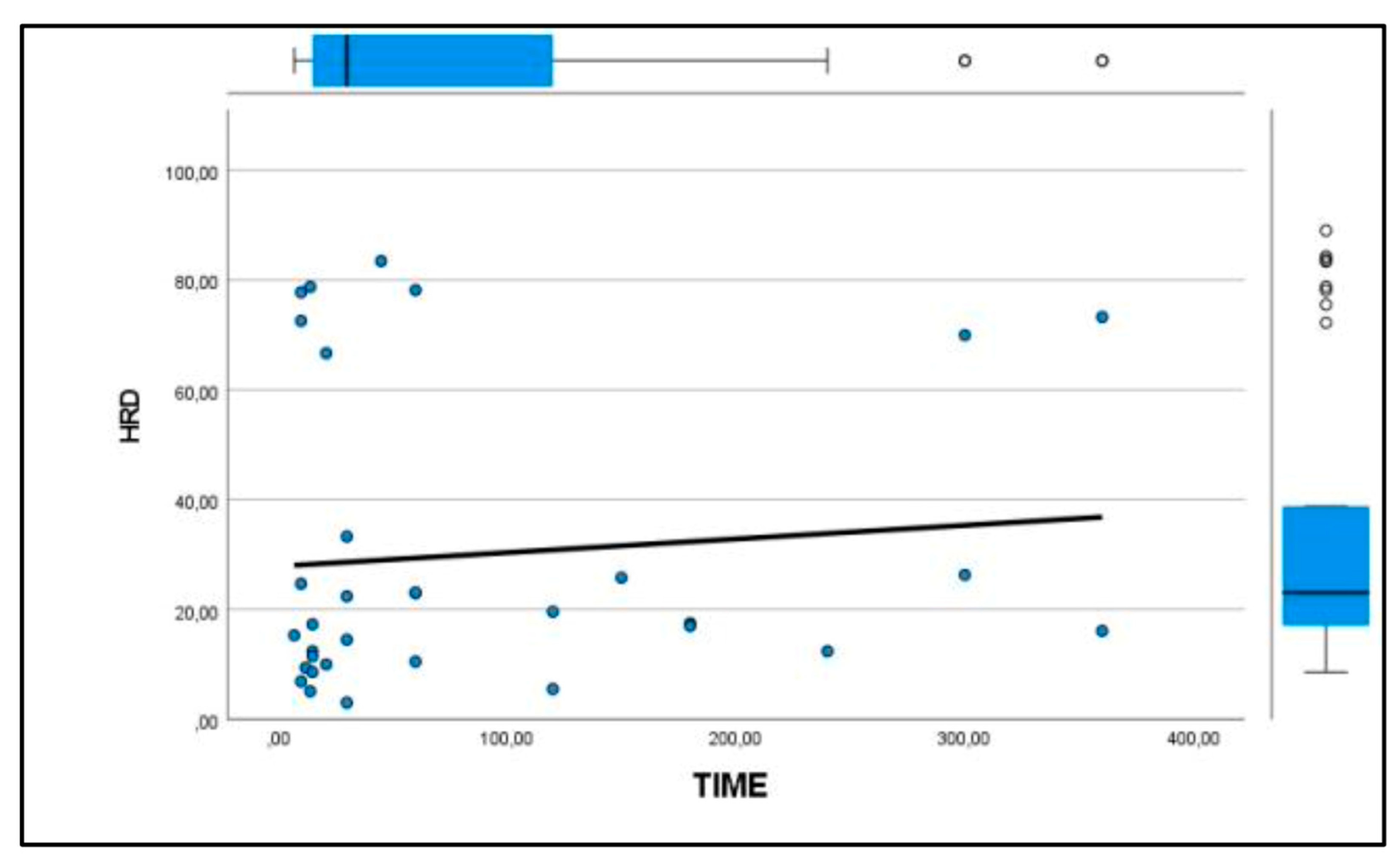
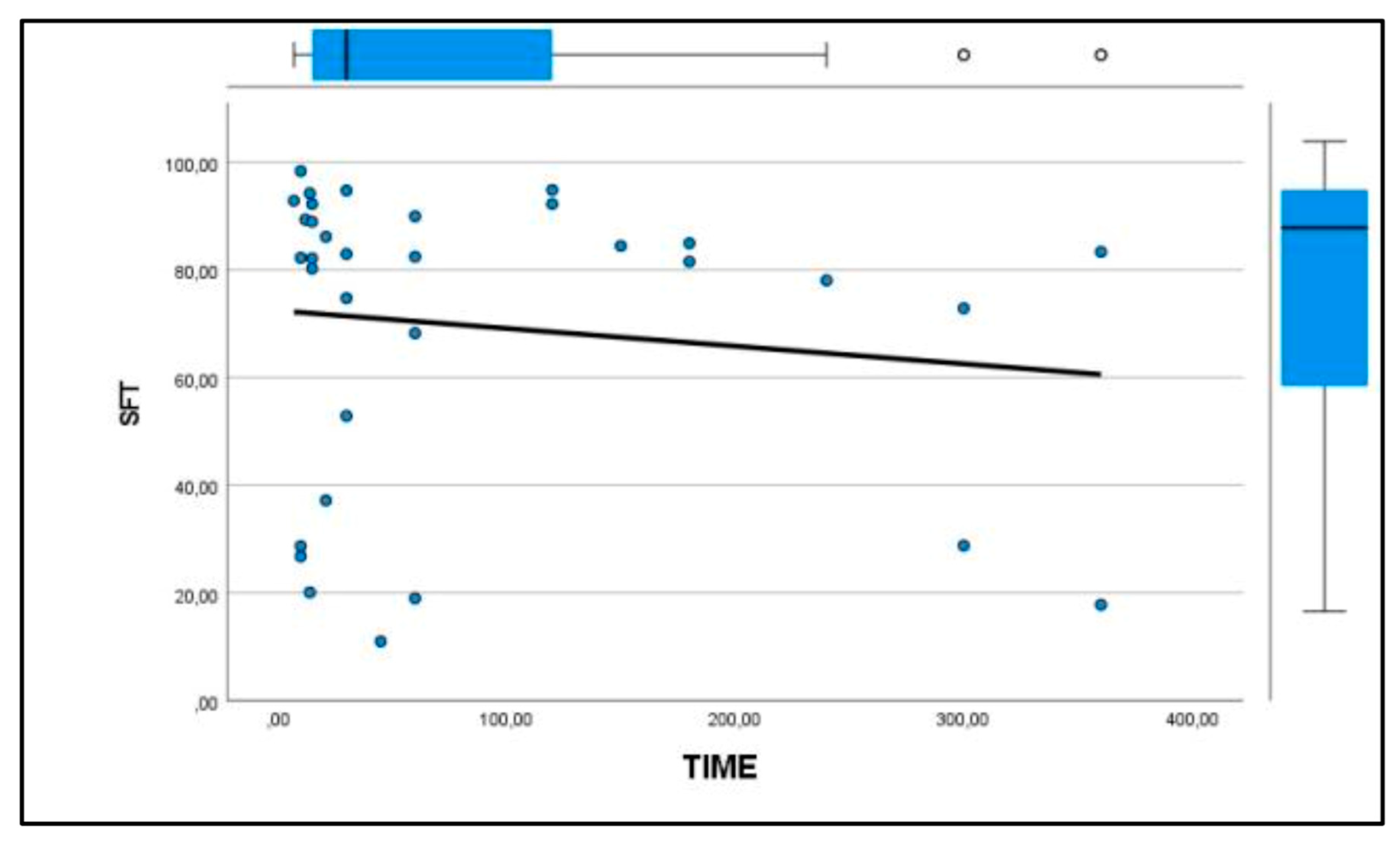
Similarly, the patellar ligament tended to become harder with increasing days after rupture, although there were no significant differences between groups (
Figure 3 and
Figure 4). After comparison with the study group of healthy dogs, it was found that the patellar ligament of dogs with cranial cruciate ligament rupture is harder than that of dogs with healthy stifles [24]. Specifically, the degree of hardness (HRD) was 25.5% in healthy dogs and 30.1% in CCL rupture–affected dogs, while the softness (SFT) was 74.5% in healthy dogs and 69.9% in the affected group of dogs (
Figure 5 and
Figure 6).
4. Discussion
This is the first study to evaluate the alteration of the patellar ligament in subjects affected by cranial cruciate ligament rupture. The results showed increased patellar ligament thickness and rigidity in dogs with chronic CCL rupture. Specifically, there were significant differences between Groups 1 and 3 and between Groups 2 and 3 in the thickness of the patellar ligament. This means that alteration occurs gradually and is not related to acute trauma. Desmitis of the patellar ligament is an overstress condition. The pathogenesis and precise identification of intrinsic risk factors, in both humans and dogs, are not yet fully understood. Patellar ligament disease is a rare disorder in dogs and horses, while it may have an incidence of 53% in human athletes [26]. This condition is commonly associated with thickening of the ligament without symptoms. In some cases, however, desmitis of the patellar ligament may appear with pain and lameness. Human and veterinary histological studies have reported the inconstant and variable presence of inflammatory cells in subjects affected by patellar ligament thickening. For this reason, the term desmopathy or tendinopathy is more appropriate because the patellar ligament undergoes a degenerative process [28].
The diagnosis of patellar ligament desmopathy is mainly clinical but should be investigated by means of instrumental investigations aimed at detecting the extent of the damage. Some different diagnostic imaging techniques have been described to evaluate the alteration of the patellar ligament. X-ray examination allows one to see the general aspect of the ligament; however, it has limitations in subjects with a moderate or severe grade of inflammation. Ultrasound and magnetic resonance imaging (MRI) are primarily used for the evaluation of the patellar ligament [29]. The first is cost effective and widely used and has the advantage of being dynamic, unlike MRI. B-mode ultrasound has high sensitivity and specificity in detecting patellar tendinopathy, and it clearly shows ligament, paratenon, and periligamentous tissue. MRI, through different sequences, is able to precisely identify the damage [30]. The advantages of MRI in patellar ligament desmopathy are related to its greater ability to pathologically image associated structures, such as the infrapatellar fat pad, that can coexist [31].
Warden et al. reported that ultrasonography was more accurate than MRI in confirming clinically diagnosed patellar tendinopathy. Ultrasound and MRI are both unable to provide indications of the mechanical and functional properties of the structures examined [31].
Elastosonography is a feasible imaging modality to evaluate tissue strain and softness/stiffness in the tendons and ligaments. In this study, we used real-time strain elastosonography to study changes in the mechanical properties of the patellar ligament through the strain distribution resulting from tissue compression, even though it was reported that shear elastosonography is less operator-dependent than strain elastosonography in evaluation of the patellar ligament [32]. Elastosonography is a quick and safe procedure, taking little time, often without the use of sedation, contrast medium, or both [24,33,34]. According to previous studies, we performed the elastosonography in a few minutes. In our prior study, we showed that real-time elastosonography of the patellar ligament in healthy dogs was repeatable and reproducible. The ultrasonographic and elastosonographic evaluations were performed by a single expert radiologist in this study.
McCagherty et al. described the elastosonographic findings of the patellar ligament in healthy dogs in four different positions. The authors concluded that the most appropriate stifle angle to perform elastosonography was the natural standing angle [35]. We used maximal passive flexion, as reported in our previous study and in humans. The elastosonographic features of the patellar ligament in dogs affected by CCL rupture were compared with those of healthy dogs, using the same stifle position to avoid inaccurate results [24].
Similar to the patellar ligament in humans, the normal canine patellar ligament shows a very soft elastogram [35,36]. Our study found, in dogs affected by CCL rupture, significant progressive thickening of the patellar ligament and its increase in hardness and decrease in softness with increasing time between the onset of lameness and diagnosis. Prior to our study, it was unclear whether and how the patellar ligament changes after cranial cruciate ligament rupture.
Veterinary studies have shown increased patellar ligament thickening in a large percentage of dogs undergoing tibial osteotomy (such as tibial plateau leveling osteotomy) following cranial cruciate ligament rupture [37,38]. Some of them have correlated this occurrence with the invasiveness of the surgical procedure itself, which could damage the ligament itself, or to the joint inspection methods [39].
Recently, only a few studies, mostly inspired by human medicine, have begun to hypothesize that TPLO changes the stifle biomechanics. Dan et al. described how TPLO reduces the stifle extensor mechanism’s moment arm, meaning that a greater force is required in the patellar ligament to achieve the same torque, so the patellar ligament is continuously under stress [26]. Zann et al. detected that patellofemoral kinematics in TPLO-treated stifles were subtly different from normal ones, characterized by slight cranial shifting of the patella relative to the trochlear groove. These findings may provide further understanding of the extensor mechanism abnormalities associated with TPLO [40]. Guenego et al. saw that after TPLO, patellar height decrease and patellar ligament tendinosis occurred, regardless of the osteotomy position [41]. It had not yet been hypothesized that the rupture of the cruciate ligament itself, causing continuous cranial tibial thrust in dogs (as opposed to humans), could cause constant stress on the patellar ligament and that therefore these biomechanical factors may be responsible for the thickening and increased hardness of the ligament in dogs even before undergoing surgery. An excessive angle of the tibial plateau could exacerbate the cranial tibial thrust that occurs after CCL rupture and thereby stress the patellar ligament more. In this study, the TPA showed no statistical differences between groups, and it did not represent a variable in dogs with an increase in patellar ligament thickness and hardness.
The first limitation of this study is the small sample size, although the groups are homogeneous in terms of age, weight, and angle of tibial plateau. Another limitation is related to the anatomical localization of ultrasonographic evaluations. We measured the thickness and elasticity in the middle of the ligament, however, the main alterations probably occur in the distal portion at its attachment on the tibial tuberosity.
Knowing the results of our study regarding the thickness and hardness of the patellar ligament in correlation with the chronicity of the lesion before surgery can be of great use in formulating a prognosis in subjects who must undergo TPLO. Furthermore, these data will also act as a driving force to stimulate owners and veterinary colleagues to treat animals affected by cranial cruciate ligament rupture early to avoid ligament modifications resulting from chronic aberrant biomechanical stimuli.
5. Conclusions
In conclusion, understanding that as time increases between the onset of CCL rupture and diagnosis and treatment, the patellar ligament progressively thickens and loses its elasticity may be helpful in timing treatment and providing a possible correlation between the onset of postoperative desmitis and the condition of the ligament before surgery.
Author Contributions
Conceptualization, A.P.P. and A.V.; methodology, L.P.; software, L.P. and A.V.; validation, A.P.P., L.P., and A.V.; formal analysis, L.P. and A.V.; investigation, L.P. and A.V.; resources, S.S., C.D.B., V.R., N.P., A.M.T., and F.D.; data curation, L.P., A.V., and A.P.P.; writing—original draft preparation, L.P. and A.P.P.; writing—review and editing, L.P. and A.V.; visualization, S.S., C.D.B., and V.R.; supervision, A.P.P.; project administration, A.P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Animal Welfare Body of the University of Camerino and received formal institutional approval in accordance with national and European law, with approval number prot. 01/24.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- de Rooster, H.; Comerford, E. Morphology and function of the cruciate ligaments. In Advances in the Canine Cranial Cruciate Ligament, 2nd ed.; Muir, P., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 3–11. [Google Scholar]
- Witsberger, T.H.; Villamil, J.A.; Schultz, L.G.; Hahn, A.W.; Cook, J.L. Prevalence of and risk factors for hip dysplasia and cranial cruciate ligament deficiency in dogs. J. Am. Vet. Med. Assoc. 2008, 232, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Sellon, D.C.; Marcellin-Little, D.J. Risk factors for cranial cruciate ligament rupture in dogs participating in canine agility. BMC Vet. Res. 2022, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Griffon, D.J. A review of the pathogenesis of canine cranial cruciate ligament disease as a basis for future preventive strategies. Vet. Surg. 2010, 39, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Kyllar, M.; Cížek, P. Cranial cruciate ligament structure in relation to the tibial plateau slope and intercondylar notch width in dogs. J. Vet. Sci. 2018, 19, 699–707. [Google Scholar] [CrossRef]
- Comerford, E.J.; Smith, K.; Hayashi, K. Update on the aetiopathogenesis of canine cranial cruciate ligament disease. Vet. Comp. Orthop. Traumatol. 2011, 24, 91–98. [Google Scholar] [PubMed]
- Spinella, G.; Arcamone, G.; Valentini, S. Cranial cruciate ligament rupture in dogs: review on biomechanics, etiopathogenetic factors and rehabilitation. Vet sci. 2021, 8, 186. [Google Scholar] [CrossRef]
- Bennett, D.; May, C. Meniscal damage associated with cruciate disease in the dog. J Small Anim Pract. 1991, 32, 111. [Google Scholar] [CrossRef]
- Ralphs, S.C.; Whitney, W.O. Arthroscopic evaluation of menisci in dogs with cranial cruciate ligament injuries: 100 cases (1999–2000). J Am Vet Med Assoc. 2002, 221, 1601. [Google Scholar] [CrossRef]
- Fitzpatrick, N.; Solano, M. Predictive variables for complications after tibial plateau leveling osteotomy with stifle inspection by arthrotomy in 1000 consecutive dogs. Vet Surg. 2010, 39, 460. [Google Scholar] [CrossRef]
- Hayes, G.M.; Langley-Hobbs, S.J.; Jeffery, N.D. Risk factors for medial meniscal injury in association with cranial cruciate ligament rupture. J Small Anim Pract. 2010, 51, 630–634. [Google Scholar] [CrossRef]
- Franklin, S.P.; Gilley, R.S.; Palmer, R.H. Meniscal injury in dogs with cranial cruciate ligament rupture. Compend Contin Educ. Vet. 2010, 32, E1–E10. [Google Scholar]
- Krupkova, O.; Smolders, L.; Wuertz-Kozak, K.; Cook, J.; Pozzi, A. The pathobiology of the meniscus: a comparison between the human and dog. Front. Vet. Sci 2018, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Das, S.; Matthews, G.; Cantatore, M.; Czopowicz, M.; Silva, L.; McCarthy, J.; Fernandez-Salesa, N.; Lafuente, P.; Allan, R.; Meeson, R.; Addison, E. Multiligament stifle injury, a multicenter retrospective study in 26 dogs. Vet. Med. Sci. 2023, 9, 1093–1102. [Google Scholar] [CrossRef]
- Bruce, W.J. Multiple ligamentous injuries of the canine stifle joint: A study of 12 cases. J Small Anim Pract. 1998, 39, 333–340. [Google Scholar] [CrossRef]
- Laing, E.J. Collateral ligament injury and stifle luxation. Vet Clin North Am Small Anim Pract. 1993, 23, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Palierne, S.; Blondel, M.; Vié, K.; Autefage, A. Morphometric assessment of the medial collateral ligament of the canine stifle joint. Res Vet Sci. 2022, 10, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Aron, D. Traumatic dislocation of the stifle joint: treatment of 12 dogs and one cat. J Am Anim Hosp Assoc. 1998, 24, 333. [Google Scholar]
- Haut, R.C.; Lancaster, R.L.; DeCamp, C.E. Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. J. Biomech. 1992, 25, 163–173. [Google Scholar] [CrossRef]
- Lin, T.W.; Cardenas, L.; Soslowsky, L.J. Biomechanics of tendon injury and repair. J Biomech. 2004, 2004 37, 865–877. [Google Scholar] [CrossRef]
- Ricciardi, M.; Lenoci, D. Comparative diagnostic imaging of a partial patellar ligament tear in a dog. Open Vet J. 2017, 8, 160–167. [Google Scholar] [CrossRef]
- Sigrist, R.M.; Liau, J.; El Kaffas, A.; Chammas, M.C.; Willmann, J.K. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017, 7, 1303. [Google Scholar] [CrossRef]
- Winnicki, K.; Ochała-Kłos, A.; Rutowicz, B.; Pękala, P.A.; Tomaszewski, K.A. Functional anatomy, histology and biomechanics of the human Achilles tendon—A comprehensive review. Ann Anat. 2020, 229, 151461. [Google Scholar] [CrossRef]
- Palumbo Piccionello, A.; Serrani, D.; Busoni, V.; Salvaggio, A.; Bonazzi, M.; Bergamino, C.; Volta, A. Sonoelastographic features of the patellar ligament in clinically normal dogs. Vet. Comp. Orthop. Traumatol. 2018, 31, 279–284. [Google Scholar] [CrossRef]
- Domenichini, R.; Pialat, J.B.; Podda, A.; Aubry, S. Ultrasound elastography in tendon pathology: state of the art. Skeletal Radiol. 2017, 46, 1643–1655. [Google Scholar] [CrossRef]
- Dan, M.J.; Crowley, J.; Broe, D.; Cross, M.; Tan, C.; Walsh, W.R. Patella tendinopathy Zoobiquity—What can we learn from dogs? The Knee. 2019, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- DeSandre-Robinson, D.M.; Tano, C.A.; Fiore, K.L.; Prytherch, B. Radiographic evaluation and comparison of the patellar ligament following tibial plateau leveling osteotomy and tibial tuberosity advancement in dogs: 106 cases (2009–2012). J Am Vet Med Ass. 2017, 250, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J.; Brukner, P. Patellar tendinopathy. Clin Sports Med. 2003, 22, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Pownder, S.L.; Hayashi, K.; Lin, B.Q.; Meyers, K.N.; Caserto, B.G.; Breighner, R.E.; Potter, H.G.; Koff, M.F. Differences in the magnetic resonance imaging parameter T2* may be identified during the course of canine patellar tendon healing: a pilot study. Quant Imaging Med Surg. 2021, 11, 1234. [Google Scholar] [CrossRef]
- Malmgaard-Clausen, N.M.; Tran, P.; Svensson, R.B.; Hansen, P.; Nybing, J.D.; Magnusson, S.P.; Kjaer, M. Magnetic Resonance T2 * Is Increased in Patients With Early-Stage Achilles and Patellar Tendinopathy. J Magn Reson Imag. 2021, 54, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J.; Kiss, Z.S.; Malara, F.A.; Ooi, A.B.; Cook, J.L.; Crossley, K.M. Comparative accuracy of magnetic resonance imaging and ultrasonography in confirming clinically diagnosed patellar tendinopathy. Am J Sports Med. 2007, 35, 427–443. [Google Scholar] [CrossRef]
- Akkaya, S.; Akkaya, N.; Agladıoglu, K.; Gungor, H.R.; Ok, N.; Özçakar, L. Real-time elastography of patellar tendon in patients with auto-graft bone–tendon–bone anterior cruciate ligament reconstruction. Arch Orthop Trauma Surg. 2016, 136, 837–842. [Google Scholar] [CrossRef]
- Serrani, D.; Volta, A.; Cingolani, F.; Pennasilico, L.; Di Bella, C.; Bonazzi, M.; Salvaggio, A.; Palumbo, A.P. Serial Ultrasonographic and Real- Time Elastosonographic Assessment of the Ovine Common Calcaneal Tendon, after an Experimentally Induced Tendinopathy. Vet Sci. 2021, 8, 54. [Google Scholar] [CrossRef]
- Del Signore, F.; De Dominicis, S.; Mastromatteo, G.; Simeoni, F.; Scapolo, P.A.; Tamburro, R.; mVignoli, M. Sonoelastography of Normal Canine Common Calcaneal Tendon: Preliminary Results. Vet. Comp. Orthop. Traum. 2020, 34, 200–205. [Google Scholar] [CrossRef]
- McCagherty, J.; Longo, M.; Pennington, C.; Liuti, T.; Morrison, L.R.; Brown, H.; Clements, D.N. Effect of Stifle Flexion Angle on the Repeatability of Real-Time Elastosonography of the Patellar Ligament in Medium-to Large-Breed Dogs. Vet Comp Orthop Traumatol. 2020, 33, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Porta, F.; Damjanov, N.; Galluccio, F.; Iagnocco, A.; Matucci-Cerinic, M. Ultrasound elastography is a reproducible and feasible tool for the evaluation of the patellar tendon in healthy subjects. Int J Rheu. 2014, 17, 762–766. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, L.; Liu, Q.; Zhang, W. Application of shear wave elastography and B-mode ultrasound in patellar tendinopathy after extracorporeal shockwave therapy. J Med Ultrason. 2020, 47, 469–476. [Google Scholar] [CrossRef]
- Carey, K.; Aiken, S.W.; Di Resta, G.R.; Herr L., G.; Monette, S. Radiographic and clinical changes of the patellar tendon after tibial plateau leveling osteotomy 94 cases (2000-2003). Vet Comp Orthop Traumatol. 2005, 18, 235–242. [Google Scholar]
- Mattern, K.L.; Berry, C.R.; Peck, J.N.; De Haan, J.J. Radiographic and ultrasonographic evaluation of the patellar ligament following tibial plateau leveling osteotomy. Vet Radiol Ultrasound. 2006, 47, 185–191. [Google Scholar] [CrossRef]
- Zann, G.J.; Kim, S.E.; Tinga, S.; Pozzi, A.; Banks, S.A. The effect of tibial plateau leveling osteotomy on patellofemoralkinematics in dogs: An in vivo study. Vet Surg. 2020, 49, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Guénégo, L.; Vezzoni, A.; Vezzoni, L. Comparison of tibial anatomical- mechanical axis angles and patellar positions between tibial plateau levelling osteotomy (TPLO) and modified cranial closing wedge osteotomy (AMA- based CCWO) for the treatment of cranial cruciate ligament disease in large dogs with tibial plateau slopes greater than 30° and clinically normal Labradors retrievers. BMC Vet Res. 2021, 17, 368. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).