Submitted:
28 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
| Data of tested patients | |||
|---|---|---|---|
| Serum | |||
| Control patients (n=15) | Parkinson’s Disease patients (n=30) | ||
| Age | |||
| Mean | 39 | Mean | 62 |
| Median | 37 | Median | 66 |
| Min-Max | 19-54 | Min-Max | 40-75 |
| Gender | |||
| Females | 4 | Females | 12 |
| % of females tested | 27% | % of females tested | 40% |
| Males | 11 | Males | 18 |
| % of males tested | 73% | % of males tested | 60% |
| Data of tested patients | |||
| CSF | |||
| Control patients (n=5) | Parkinson’s Disease patients (n=13) | ||
| Age | |||
| Mean | 52 | Mean | 57 |
| Median | 56 | Median | 61 |
| Min-Max | 35-69 | Min-Max | 37-75 |
| Gender | |||
| Females | 3 | Females | 6 |
| % of females tested | 60 | % of females tested | 46% |
| Males | 2 | Males | 7 |
| % of males tested | 40 | % of males tested | 54% |
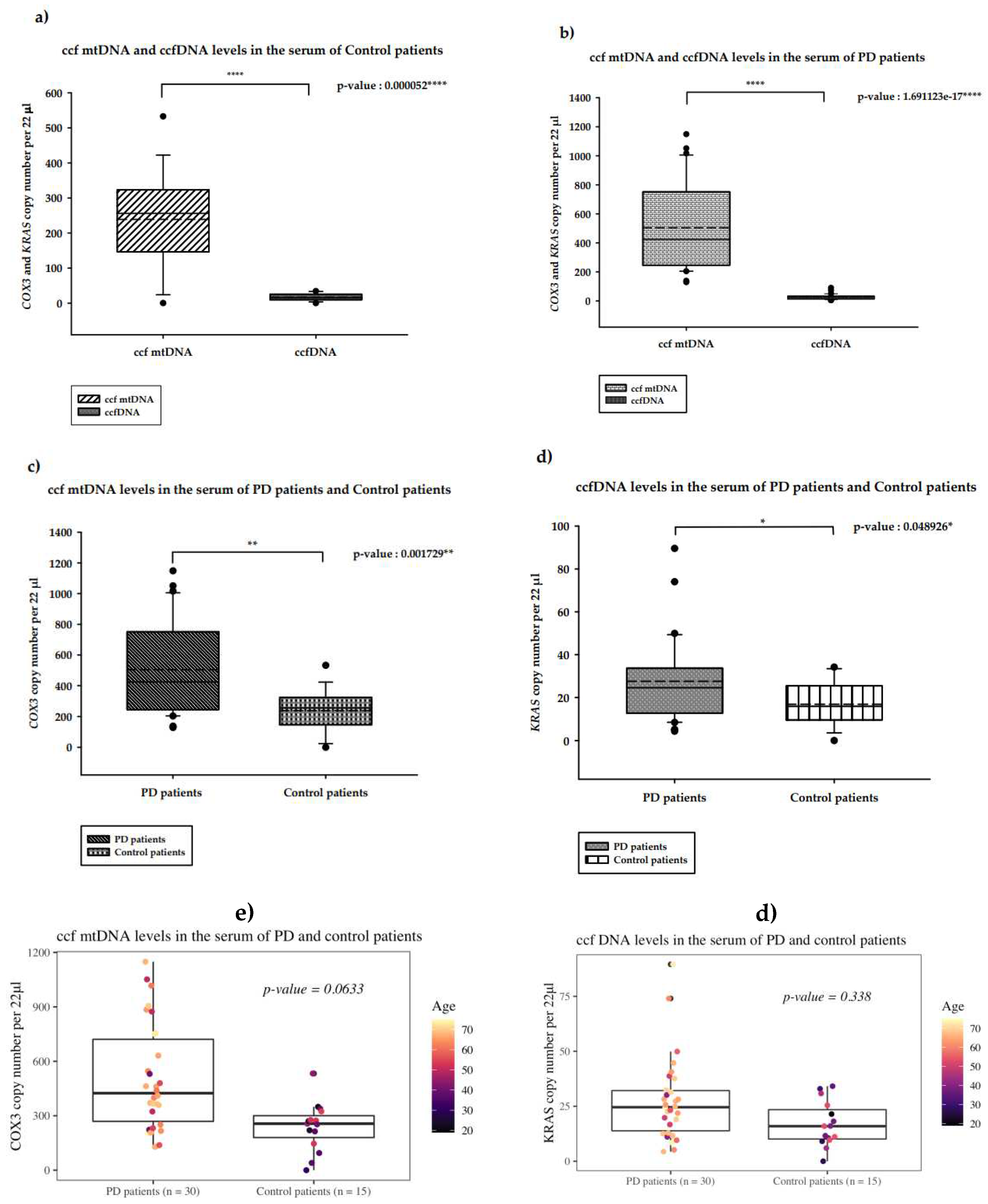
2.1. Quantification of serum ccf-DNA in PD patients
2.1.1. Serum ccf mtDNA and ccfDNA in PD patients versus control patients
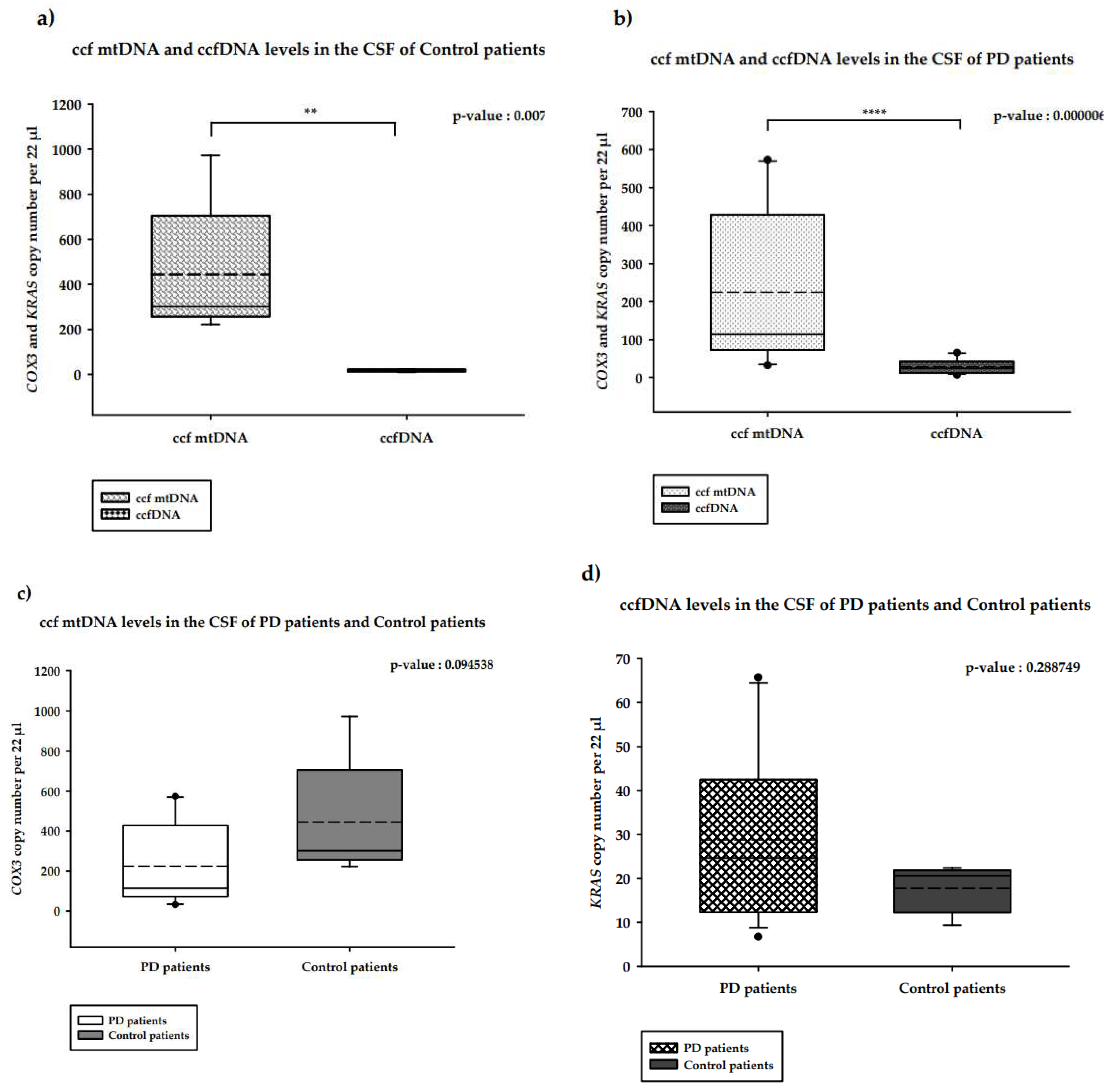
2.2. Quantification of CSF ccf-DNA in PD patients
2.2.1. CSF ccf mtDNA and ccfDNA in PD patients versus healthy controls
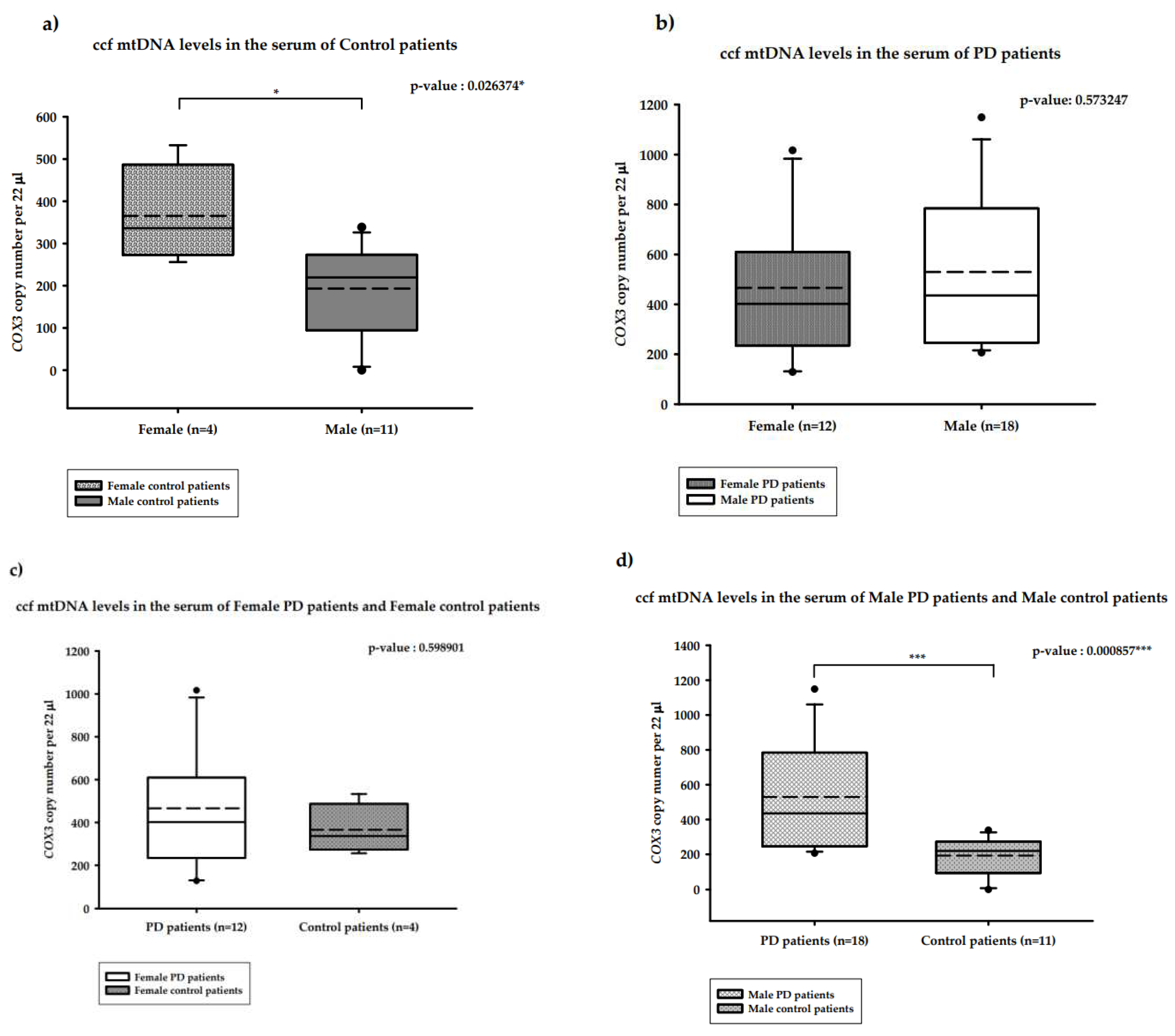
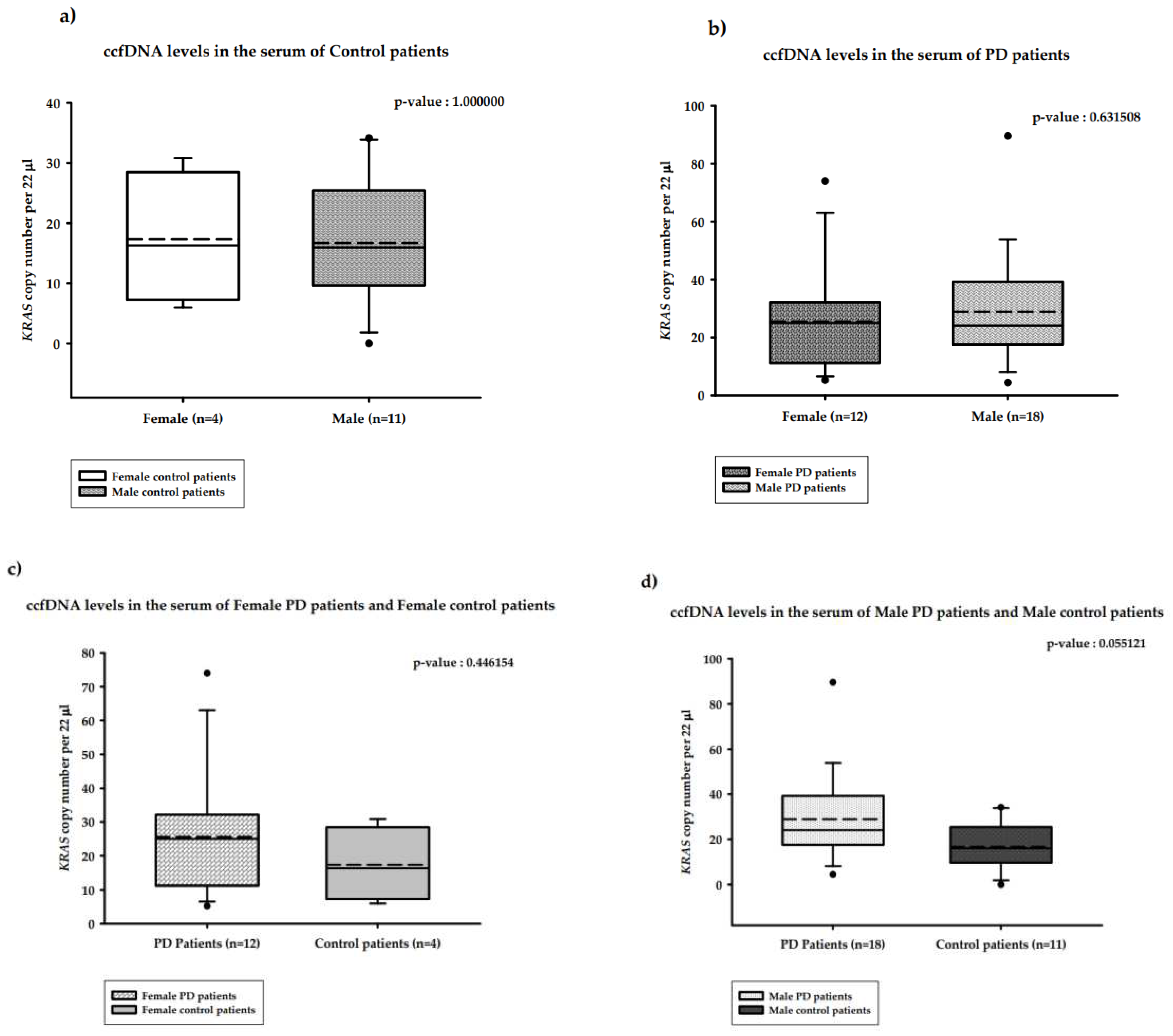
2.3. Distribution of the copy number of serum ccf-DNA and gender in PD patients
2.3.1. Distribution of the copy number of serum ccf mtDNA and gender in PD versus healthy controls
2.3.2. Distribution of the copy number of serum ccfDNA and gender in PD versus healthy controls
3. Discussion
| source of sample | gender male/ female |
type of PD | Ccf mtDNA/ healthy control |
Ccf mtDNA/ ccfDNA |
Ccf DNA/ healthy control |
number of PD/number of control |
method of analysis | reference |
|---|---|---|---|---|---|---|---|---|
|
serum |
- | mut+/+ PD PRKN/PINK1 mut+/– PD PRKN/PINK1 |
increase increase |
- |
- | 17/57 17/55 |
ddPCR |
[21] |
| serum | - | idiopathic |
increase |
increase |
increase | 30/15 | ddPCR | this study |
| serum | male | idiopathic | increase | increase* | no difference* | 18/11 | ddPCR | this study |
| serum | female | idiopathic | no difference* | no difference* | no difference* | 12/4 | ddPCR | this study |
| CSF | - | idiopathic | reduced | - |
- |
56/10 | qPCR | [18] |
| CSF | - | EOPD | reduced | 176/87 | qPCR | [19] | ||
| CSF | - | idiopathic | reduced* | increase | increase* | 13/5 | ddPCR | this study |
4. Materials and Methods
4.1. Collection of CSF and serum samples
4.2. Study cohort and sampling procedure
4.3. ccfDNA isolation
4.4. Quantification of serum ccf mtDNA and ccfDNA levels
| Gene | Sequence of the Forward primer (5’->3’) | Sequence of the Reverse primer (5’->3’) |
|---|---|---|
| COX3 | GACCCACCAATCACATGC | TGAGAGGGCCCCTGTTAG |
| KRAS | CCTTGGGTTTCAAGTTATATG | CCCTGACATACTCCCAAGGA |
4.5. Statistical analysis
4.6. Data presentation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson's disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease. JAMA. 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, R. Cell-Free DNA: Applications in Different Diseases. Methods Mol Biol. 2019, 1909, 3–12. [Google Scholar] [PubMed]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 216–231. [Google Scholar] [CrossRef] [PubMed]
- Gaitsch, H.; Franklin, R.J.M.; Reich, D.S. Cell-free DNA-based liquid biopsies in neurology. Brain. 2023, 146, 1758–1774. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.C.; Barrie, E.S.; Malinowski, J.; Jenkins, G.P.; McClain, M.R.; LaGrave, D.; Leung, M.L. ACMG Professional Practice and Guidelines Committee. Electronic address: documents@acmg.net. Systematic evidence-based review: The application of noninvasive prenatal screening using cell-free DNA in general-risk pregnancies. Genet Med. 2022, 24, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Yoshida, K.; Hashiramoto, A.; Matsui, K. Cell-Free DNA in Rheumatoid Arthritis. Int J Mol Sci. 2021, 22, 8941. [Google Scholar] [CrossRef]
- Gambardella, S.; Limanaqi, F.; Ferese, R.; Biagioni, F.; Campopiano, R.; Centonze, D.; Fornai, F. ccf-mtDNA as a Potential Link Between the Brain and Immune System in Neuro-Immunological Disorders. Front. Immunol. 2019, 10, 1064. [Google Scholar] [CrossRef]
- Aucamp, J.; Bronkhorst, A.J.; Badenhorst, C.P.S.; Pretorius, P.J. The diverse origins of circulating cell-free DNA in the human body: a critical re-evaluation of the literature. Biol. Rev. 2018, 93, 1649–1683. [Google Scholar] [CrossRef]
- Thierry, A.R.; Messaoudi, S.E.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017, 17, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Stroun, M.; Lyautey, J.; Lederrey, C.; Olson-Sand, A.; Anker, P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin. Chim. Acta Int. J. Clin. Chem. 2001, 313, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.M.; Yin, P.H.; Chi, C.W.; Hsu, C.Y.; Wu, C.W.; Lee, L.M.; Wei, Y.H.; Lee, H.C. Mitochondrial DNA mutations and mitochondrial DNA depletion in breast cancer. Genes Chromosomes Cancer 2006, 45, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Swarup, V.; Rajeswari, M.R. Circulating (cell-free) nucleic acids--a promising, non-invasive tool for early detection of several human diseases. FEBS Lett 2007, 581, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Yu, M. Circulating cell-free mitochondrial DNA as a novel cancer biomarker: opportunities and challenges. Mitochondrial DNA. 2012, 23, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wu, L.R.; Yan, Y.H.; Zhang, J.X.; Chu, T.; Kwong, L.N.; Patel, A.A.; Zhang, D.Y. Limitations and opportunities of technologies for the analysis of cell-free DNA in cancer diagnostics. Nat Biomed Eng. 2022, 6, 232–245. [Google Scholar] [CrossRef]
- Bruno, D.C.F.; Donatti, A.; Martin, M.; Almeida, V.S.; Geraldis, J.C.; Oliveira, F.S.; Dogini, D.B.; Lopes-Cendes, I. Circulating nucleic acids in the plasma and serum as potential biomarkers in neurological disorders. Braz J Med Biol Res. 2020, 53, e9881. [Google Scholar] [CrossRef]
- Pyle, A.; Brennan, R.; Kurzawa-Akanbi, M.; Yarnall, A.; Thouin, A.; Mollenhauer, B.; Burn, D.; Chinnery, P.F.; Hudson, G. Reduced cerebrospinal fluid mitochondrial DNA is a biomarker for early-stage Parkinson's disease. Ann Neurol. 2015, 78, 1000–1004. [Google Scholar] [CrossRef]
- Lowes, H.; Pyle, A.; Santibanez-Koref, M.; Hudson, G. Circulating cell-free mitochondrial DNA levels in Parkinson’s disease are influenced by treatment. Mol Neurodegeneration, 2020, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Lowes, H.; Kurzawa-Akanbi, M.; Pyle, A.; Hudson, G. Post-mortem ventricular cerebrospinal fluid cell-free-mtDNA in neurodegenerative disease. Sci. Rep. 2020, 10, 15253. [Google Scholar] [CrossRef] [PubMed]
- Borsche, M.; König, I.R.; Delcambre, S.; Petrucci, S.; Balck, A.; Brüggemann, N.; Zimprich, A.; Wasner, K.; Pereira, S.L.; Avenali, M.; Deuschle, C.; Badanjak, K.; Ghelfi, J.; Gasser, T.; Kasten, M.; Rosenstiel, P.; Lohmann, K.; Brockmann, K.; Valente, E.M.; Youle, R.J.; Grünewald, A.; Klein, C. Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain. 2020, 143, 3041–3051. [Google Scholar] [CrossRef] [PubMed]
- Meddeb, R.; Dache, Z.A.A.; Thezenas, S.; Otandault, A.; Tanos, R.; Pastor, B.; Sanchez, C.; Azzi, J.; Tousch, G.; Azan, S.; Mollevi, C.; Adenis, A.; El Messaoudi, S.; Blache, P.; Thierry, A.R. Quantifying circulating cell-free DNA in humans. Sci Rep 2019, 9, 5520. [Google Scholar] [CrossRef] [PubMed]
- Al Amir Dache, Z.; Otandault, A.; Tanos, R.; Pastor, B.; Meddeb, R.; Sanchez, C.; Arena, G.; Lasorsa, L.; Bennett, A.; Grange, T.; El Messaoudi, S.; Mazard, T.; Prevostel, C.; Thierry, A.R. Blood contains circulating cell free respiratory competent mitochondria. The FASEB Journal. 2020, 34, 3616–3630. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, A.T.; Robin, J.D.; Sayed, M.; Litterst, C.M.; Shelton, D.N.; Shay, J.W.; Wright, W.E. Quantitative telomerase enzyme activity determination using droplet digital PCR with single cell resolution. Nucleic Acids Res 2014, 42, e104. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.E.; Tedone, E.; O’Hara, R.; Cornelius, C.; Lai, T.P.; Ludlow, A.; Wright, W.E.; Shay, J.W. The maintenance of telomere length in CD28+ T cells during T lymphocyte stimulation. Sci Rep, 2017, 7, 6785. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; Kitano, T.K.; Hodel, M.R.; Petersen, J.F.; Wyatt, P.W.; Steenblock, E.R.; Shah, P.H.; Bousse, L.J.; Troup, C.B.; Mellen, J.C.; Wittmann, D.K.; Erndt, N.G.; Cauley, T.H.; Koehler, R.T.; So, A.P.; Dube, S.; Rose, K.A.; Montesclaros, A.; Wang, S.; Stumbo, D.P.; Hodges, S.P.; Romine, S.; Milanovich, F.P.; White, H.o.u.s.e.E.; Regan, J.F.; Karlin-Neumann, G.A.; Hindson, C.M.; Saxonov, S.; Colston, B.W. Highthroughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem 2011, 83, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.B.; Coleman, V.A.; Hindson, C.M.; Herrmann, J.; Hindson, B.J.; Bhat, S.; Emslie, K.R. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal Chem 2012, 84, 1003–1011. [Google Scholar] [CrossRef]
- Robin, J.; Wynn, J.; Moscovitch, M. The spatial scaffold: The effects of spatial context on memory for events. J Exp Psychol Learn Mem Cogn. 2016, 42, 308–315. [Google Scholar] [CrossRef]
- Podlesniy, P.; Figueiro-Silva, J.; Llado, A.; Antonell, A.; Sanchez-Valle, R.; Alcolea, D.; Lleo, A.; Molinuevo, J.L.; Serra, N.; Trullas, R. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann Neurol. 2013, 74, 655–668. [Google Scholar] [CrossRef]
- Wachsmuth, M.; Hubner, A.; Li, M.; Madea, B.; Stoneking, M. Age-related and heteroplasmy-related variation in human mtDNA copy number. PLoS Genet 2016, 12, e1005939. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Talaverón-Rey, M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Sánchez-Alcázar, J.A. From mitochondria to atherosclerosis: The inflammation path. Biomedicines 2021, 9, 258. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol 2017, 17, 363–375. [Google Scholar] [CrossRef]
- Regner, A.; Meirelles, L.D.S.; Ikuta, N.; Cecchini, A.; Simon, D. Prognostic utility of circulating nucleic acids in acute brain injuries. Expert Rev Mol Diagn. 2018, 18, 925–938. [Google Scholar] [CrossRef]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21, e49799. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; de Rivero Vaccari, J.P.; Dietrich, W.D.; Popovich, P.G.; Keane, R.W. Pattern recognition receptors and central nervous system repair. Exp Neurol. 2014, 258, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Dib, B.; Lin, H.; Maidana, D.E.; Tian, B.; Miller, J.B.; Bouzika, P.; Miller, J.W.; Vavvas, D.G. Mitochondrial DNA has a pro-inflammatory role in AMD. Biochim Biophys Acta. 2015, 1853, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Keeney, P.M.; Bennett, J.P., Jr. ALS spinal neurons show varied and reduced mtDNA gene copy numbers and increased mtDNA gene deletions. Mol. Neurodegener. 2010, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.O. Circulating cell-free DNA differentiates severity of inflammation. Biol Res Nurs. 2016, 18, 477–488. [Google Scholar] [CrossRef]
- Lehmann-Werman, R.; Magenheim, J.; Moss, J.; Neiman, D.; Abraham, O.; Piyanzin, S.; Zemmour, H.; Fox, I.; Dor, T.; Grompe, M.; Landesberg, G.; Loza, B.L.; Shaked, A.; Olthoff, K.; Glaser, B.; Shemer, R.; Dor, Y. Monitoring liver damage using hepatocyte-specific methylation markers in cell-free circulating DNA. JCI Insight. 2018, 3, e120687. [Google Scholar] [CrossRef] [PubMed]
- Lehmann-Werman, R.; Neiman, D.; Zemmour, H.; Moss, J.; Magenheim, J.; Vaknin-Dembinsky, A.; Rubertsson, S.; Nellgård, B.; Blennow, K.; Zetterberg, H.; Spalding, K.; Haller, M.J.; Wasserfall, C.H.; Schatz, D.A.; Greenbaum, C.J.; Dorrell, C.; Grompe, M.; Zick, A.; Hubert, A.; Maoz, M.; Fendrich, V.; Bartsch, D.K.; Golan, T.; Ben Sasson, S.A.; Zamir, G.; Razin, A.; Cedar, H.; Shapiro, A.M.; Glaser, B.; Shemer, R.; Dor, Y. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc Natl Acad Sci USA 2016, 113, E1826–34. [Google Scholar] [CrossRef] [PubMed]
- Zemmour, H.; Planer, D.; Magenheim, J.; Moss, J.; Neiman, D.; Gilon, D.; Korach, A.; Glaser, B.; Shemer, R.; Landesberg, G.; Dor, Y. Non-invasive detection of human cardiomyocyte death using methylation patterns of circulating DNA. Nat Commun. 2018, 9, 1443. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Jiang, P.; Chan, K.C.; Wong, J.; Cheng, Y.K.; Liang, R.H.; Chan, W.K.; Ma, E.S.; Chan, S.L.; Cheng, S.H.; Chan, R.W.; Tong, Y.K.; Ng, S.S.; Wong, R.S.; Hui, D.S.; Leung, T.N.; Leung, T.Y.; Lai, P.B.; Chiu, R.W.; Lo, Y.M. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc Natl Acad Sci USA 2015, 112, E5503–12. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Magenheim, J.; Neiman, D.; Zemmour, H.; Loyfer, N.; Korach, A.; Samet, Y.; Maoz, M.; Druid, H.; Arner, P.; Fu, K.Y.; Kiss, E.; Spalding, K.L.; Landesberg, G.; Zick, A.; Grinshpun, A.; Shapiro, A.M.J.; Grompe, M.; Wittenberg, A.D.; Glaser, B.; Shemer, R.; Kaplan, T.; Dor, Y. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat Commun 2018, 9, 5068. [Google Scholar] [CrossRef] [PubMed]
- Dor, Y.; Cedar, H. Principles of DNA methylation and their implications for biology and medicine. Lancet. 2018, 392, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; IRojo, A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson's Disease: Mechanisms and Therapeutic Implications. Cells. 2020, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Lazo, S.; Noren Hooten, N.; Green, J.; Eitan, E.; Mode, N.A.; Liu, Q.R.; Zonderman, A.B.; Ezike, N.; Mattson, M.P.; Ghosh, P.; Evans, M.K. Mitochondrial DNA in extracellular vesicles declines with age. Aging Cell. 2021, 20, e13283. [Google Scholar] [CrossRef]
- Sherwood, K.; Weimer, E.T. Characteristics, properties, and potential applications of circulating cell-free dna in clinical diagnostics: a focus on transplantation. J Immunol Methods. 2018, 463, 27–38. [Google Scholar] [CrossRef]
- Patel, R.; Kompoliti, K. Sex and Gender Differences in Parkinson’s Disease. Neurol Clin. 2023, 41, 371–379. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





