Submitted:
29 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
Clinical Perspective
- ▪ Low flow-low gradient (LF-LG) aortic stenosis (AS) patients consistently exhibit poorer clinical outcomes up to 5 years of follow-up after transcatheter aortic valve implantation (TAVI) compared to other subtypes of AS patients.
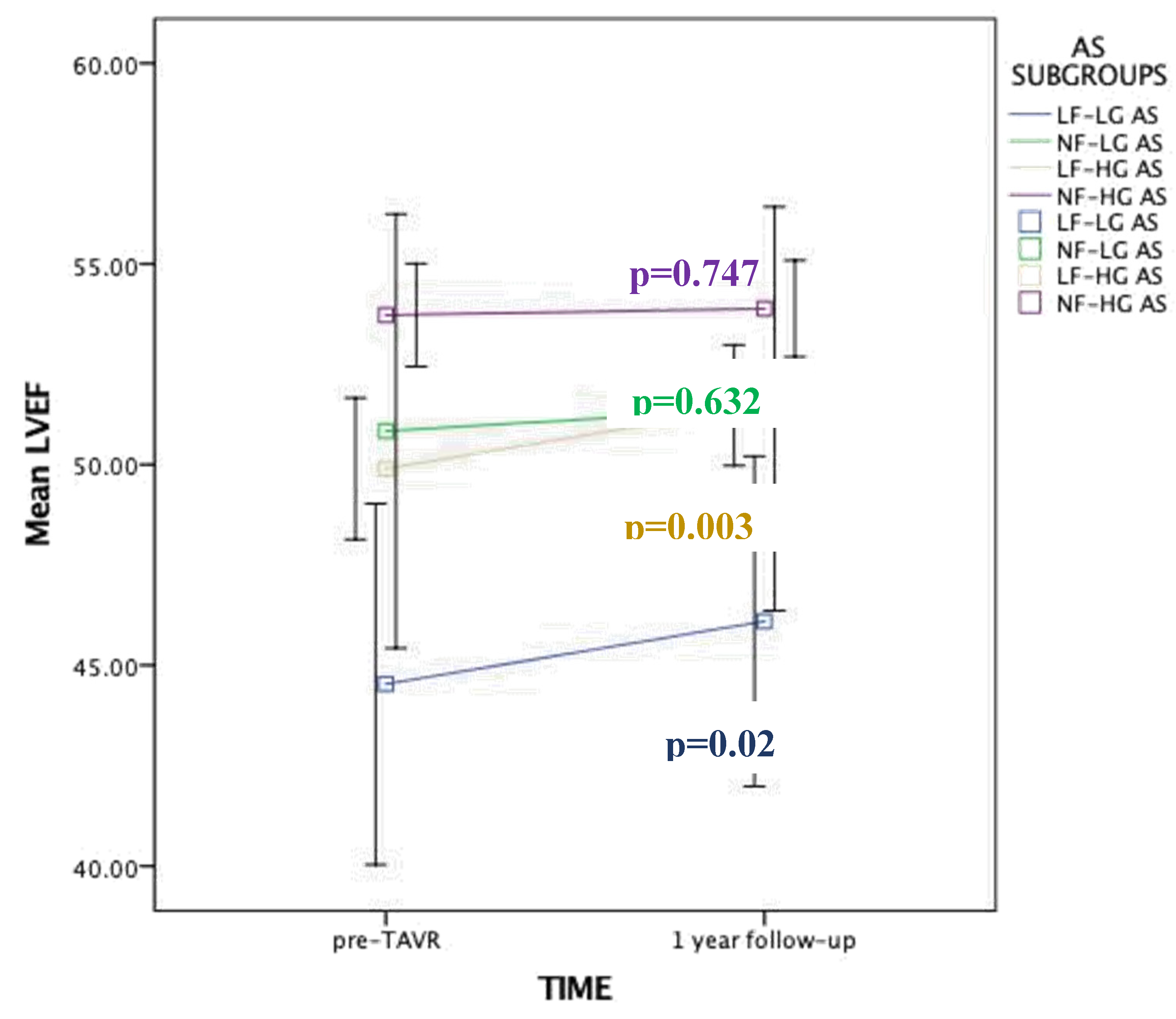
- ▪ Low flow AS patients derive the greatest benefit in terms of left ventricular ejection fraction (LVEF) improvement at 1 year following TAVI.
- ▪ Tricuspid regurgitation at baseline and post-TAVI paravalvular leak (PVL) have detrimental long-term prognostic value and could dictate a closer surveillance of TAVI patients.
1. Introduction
2. Methods
2.1. Study Population
2.2. Clinical Data
2.3. Doppler Echocardiography
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
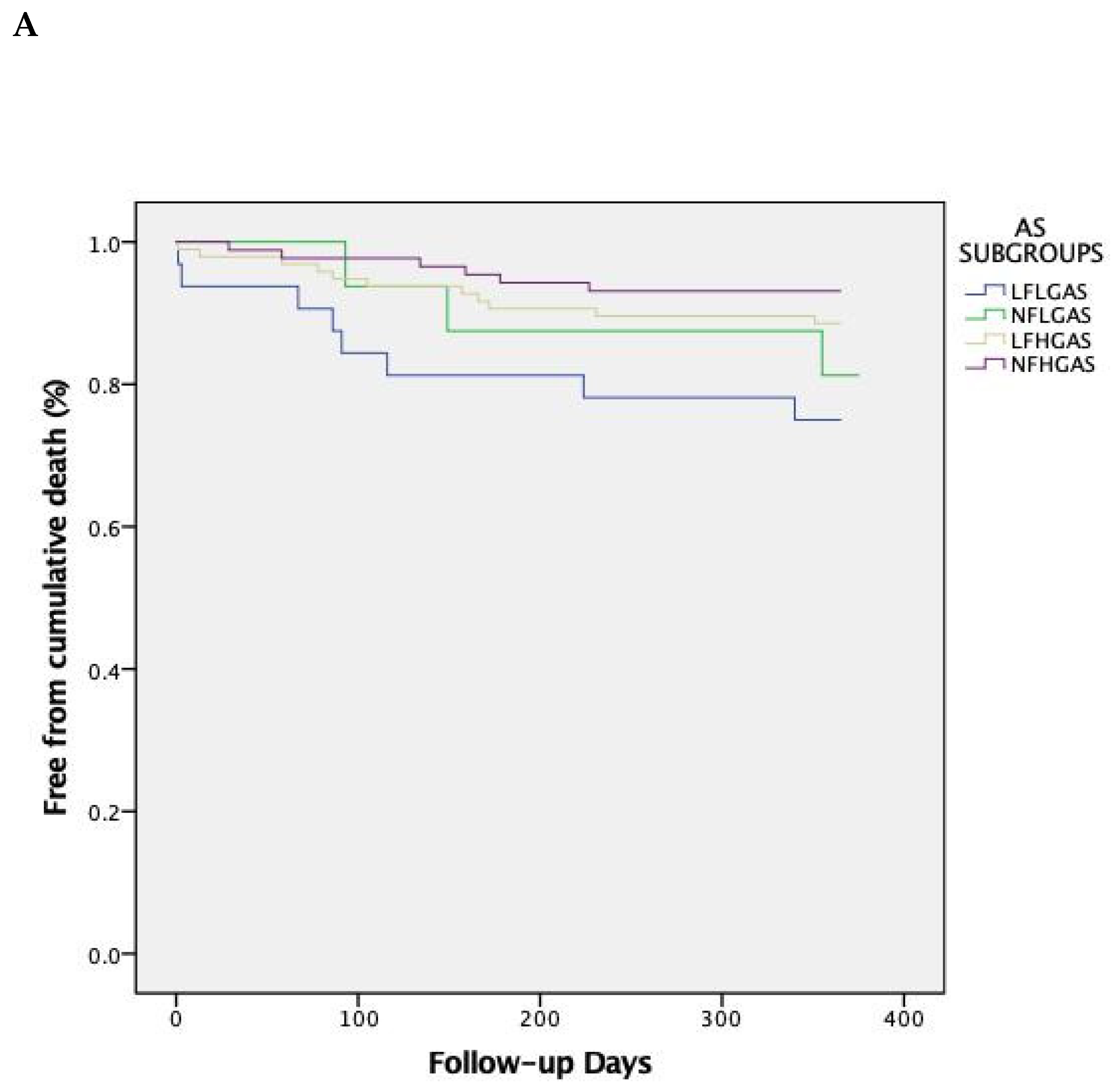
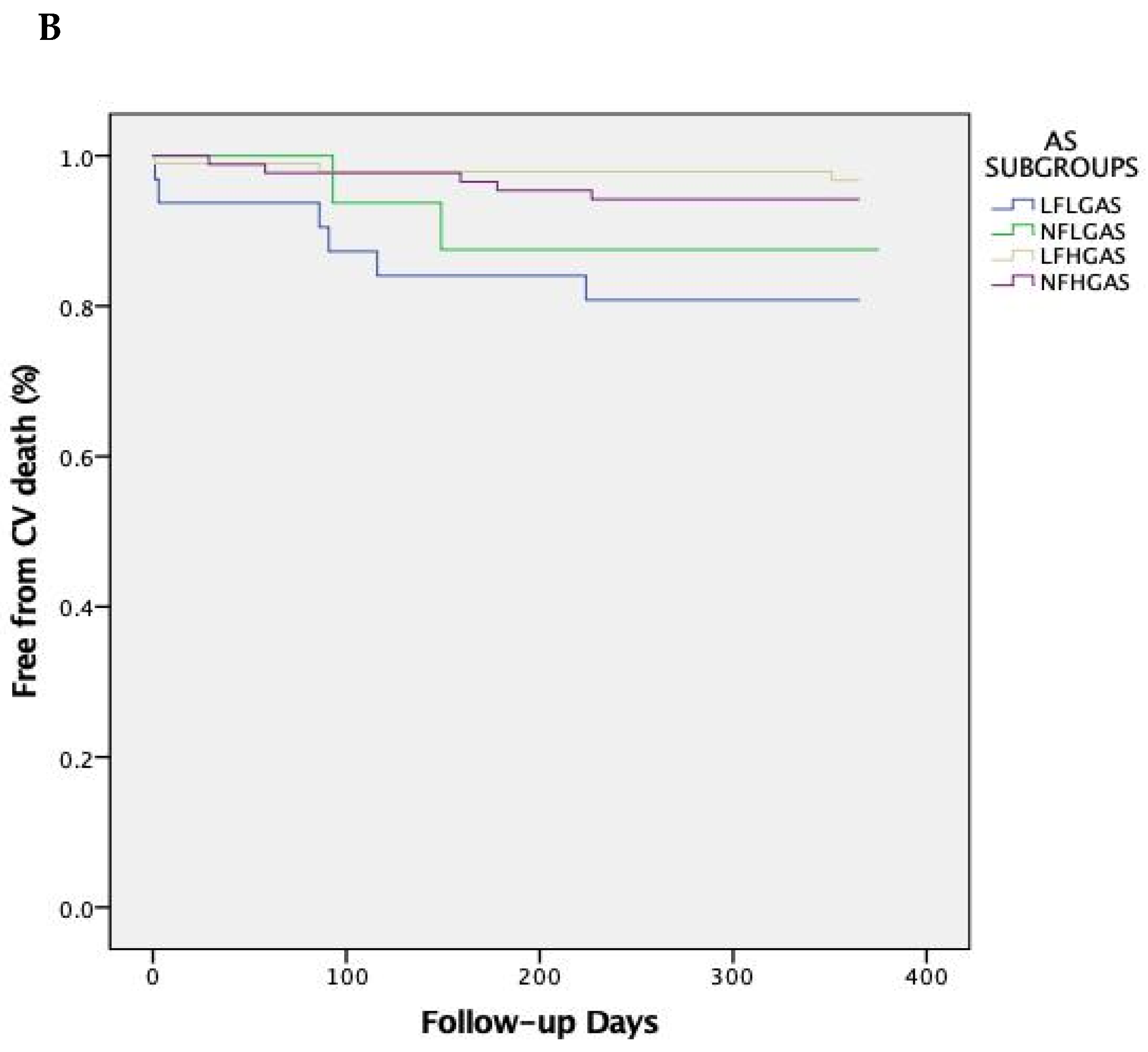
3.1. One-Year and Late Clinical Outcomes
3.2. Predictors of Late Global Mortality
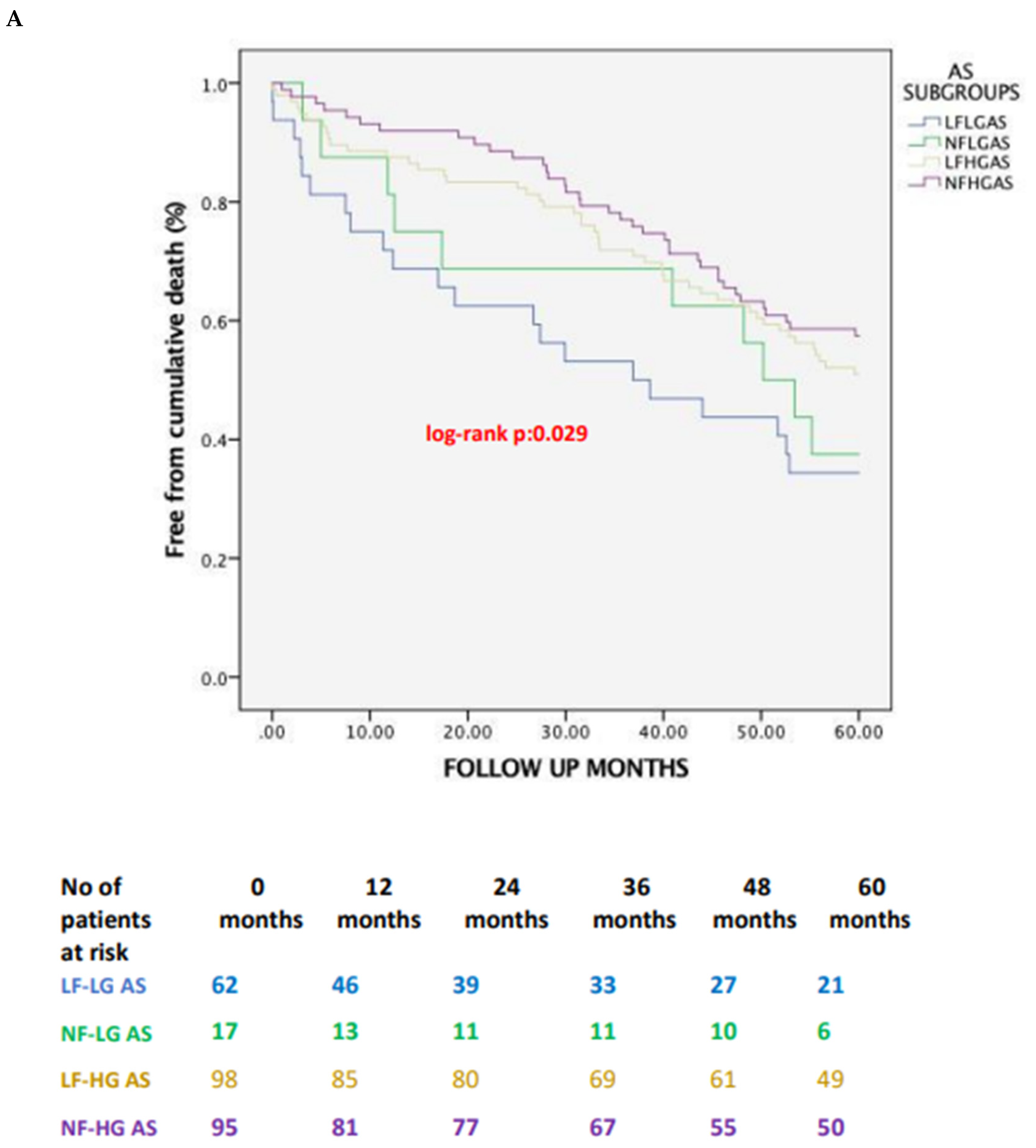
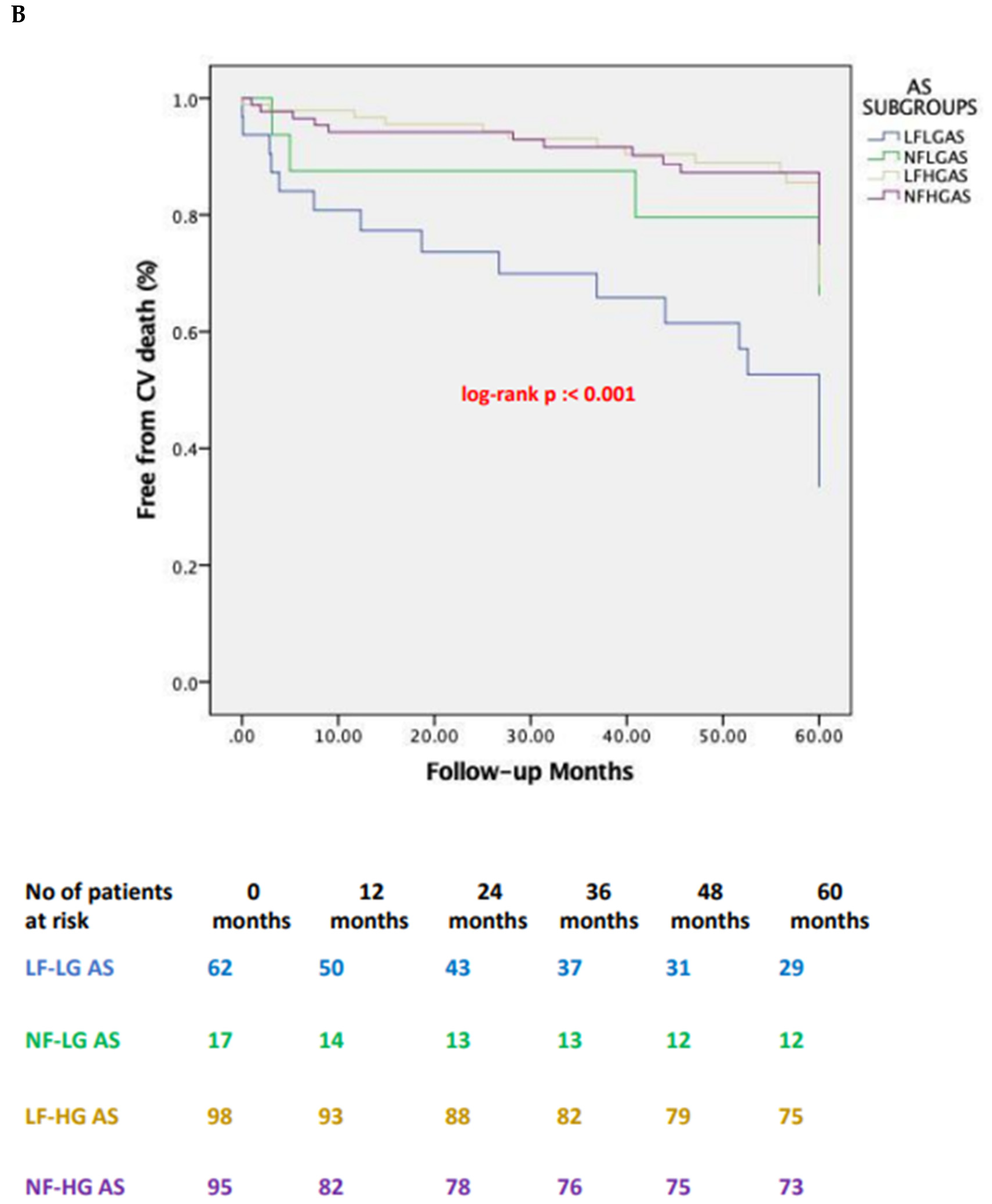
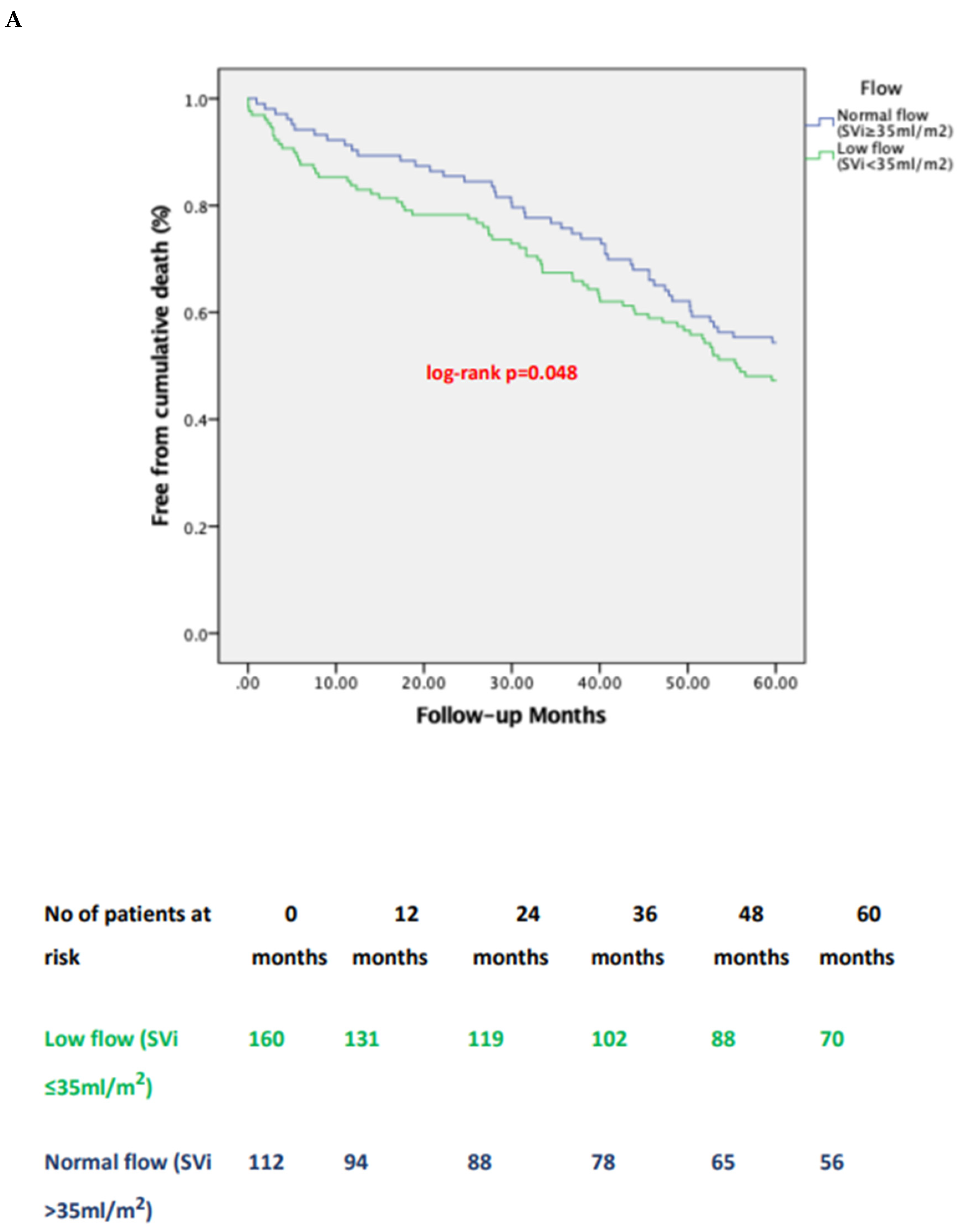
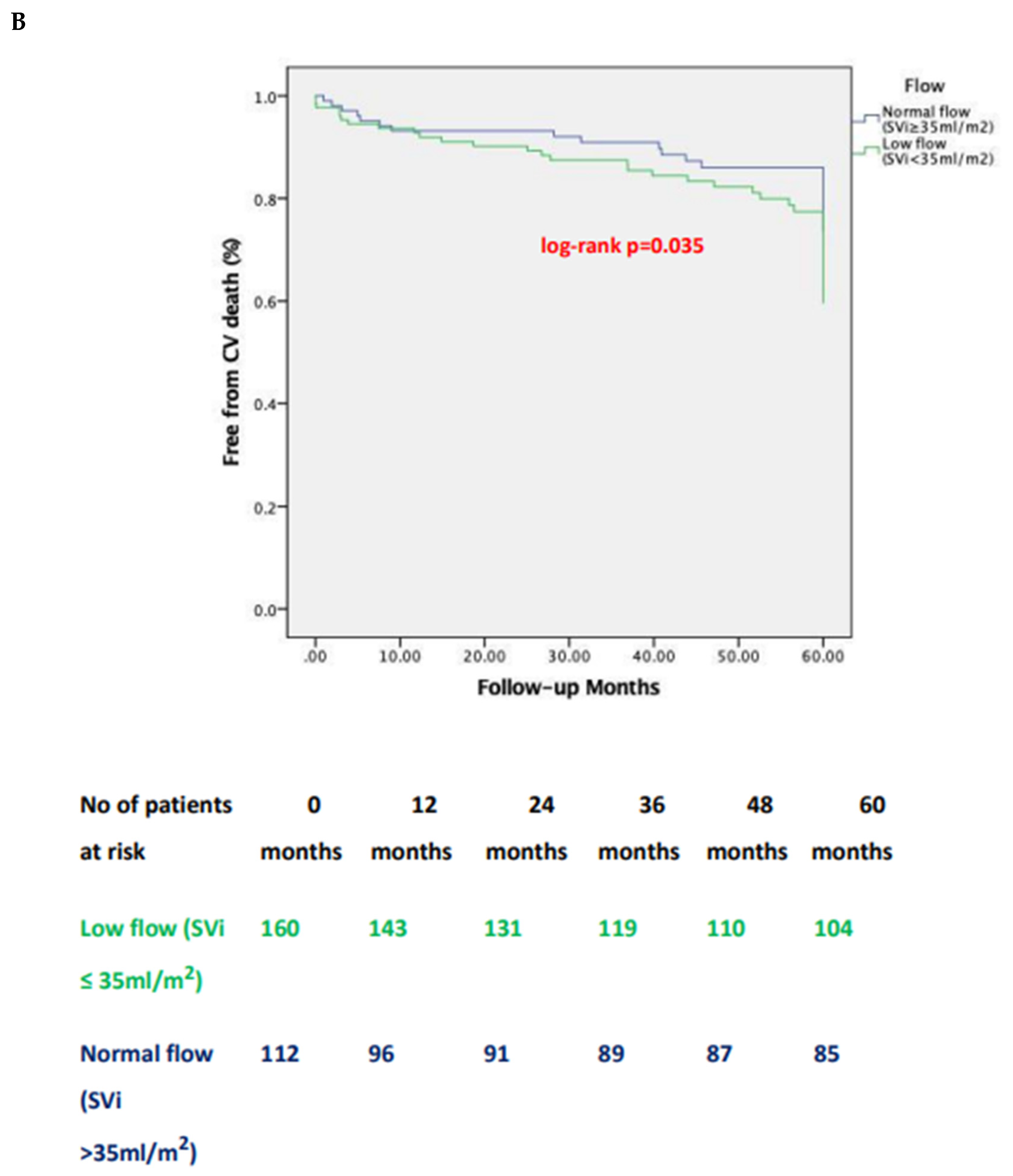
3.3. Impact of Pre-Tavi Flow on Late Outcomes
3.4. Changes in LVEF over Time
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Abbreviations
References
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger E V., Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143. [CrossRef]
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W, Neumann F-J, Myers P, Abdelhamid M, Achenbach S, Asteggiano R, Barili F, Borger MA, Carrel T, Collet J-P, Foldager D, Habib G, Hassager C, Irs A, Iung B, Jahangiri M, Katus HA, Koskinas KC, Massberg S, Mueller CE, Nielsen JC, Pibarot P, Rakisheva A, Roffi M, Rubboli A, Shlyakhto E, Siepe M, Sitges M, Sondergaard L, Sousa-Uva M, Tarantini G, Zamorano JL, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W, Benchabi Y, Chilingaryan A, Metzler B, Rustamova Y, Shumavets V, Lancellotti P, Smajic E, Trendafilova-Lazarova D, Samardzic J, Karakyriou M, Palecek T, Sanchez Dahl J, Meshaal MS, Palm K, Virtanen M, Bouleti C, Bakhutashvili Z, Achenbach S, Boutsikou M, Kertész AB, Danielsen R, Topilsky Y, Golino P, Tuleutayev R, Elezi S, Kerimkulov A, Rudzitis A, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. [CrossRef]
- Clavel M-A, Dumesnil JG, Capoulade R, Mathieu P, Sénéchal M, Pibarot P. Outcome of Patients With Aortic Stenosis, Small Valve Area, and Low-Flow, Low-Gradient Despite Preserved Left Ventricular Ejection Fraction. J Am Coll Cardiol. 2012;60:1259–1267. [CrossRef]
- Lauten J, Rost C, Breithardt OA, Seligmann C, Klinghammer L, Daniel WG, Flachskampf FA. Invasive Hemodynamic Characteristics of Low Gradient Severe Aortic Stenosis Despite Preserved Ejection Fraction. J Am Coll Cardiol. 2013;61:1799–1808. [CrossRef]
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical Low-Flow, Low-Gradient Severe Aortic Stenosis Despite Preserved Ejection Fraction Is Associated With Higher Afterload and Reduced Survival. Circulation. 2007;115:2856–2864. [CrossRef]
- Dumesnil JG, Pibarot P. Low-flow, low-gradient severe aortic stenosis in patients with normal ejection fraction. Curr Opin Cardiol. 2013;28:524–530. [CrossRef]
- Pibarot P, Dumesnil JG. Low-Flow, Low-Gradient Aortic Stenosis With Normal and Depressed Left Ventricular Ejection Fraction. J Am Coll Cardiol. 2012;60:1845–1853. [CrossRef]
- Kulik A, Burwash IG, Kapila V, Mesana TG, Ruel M. Long-Term Outcomes After Valve Replacement for Low-Gradient Aortic Stenosis. Circulation. 2006;114. [CrossRef]
- Connolly HM, Oh JK, Schaff H V., Roger VL, Osborn SL, Hodge DO, Tajik AJ. Severe Aortic Stenosis With Low Transvalvular Gradient and Severe Left Ventricular Dysfunction. Circulation. 2000;101:1940–1946. [CrossRef]
- Monin J-L, Quéré J-P, Monchi M, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Tribouilloy C, Guéret P. Low-Gradient Aortic Stenosis. Circulation. 2003;108:319–324. [CrossRef]
- Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle R-P, Neumann F-J, Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart. 2010;96:1463–1468. [CrossRef]
- Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281–289. [CrossRef]
- Barasch E, Fan D, Chukwu EO, Han J, Passick M, Petillo F, Norales A, Reichek N. Severe isolated aortic stenosis with normal left ventricular systolic function and low transvalvular gradients: pathophysiologic and prognostic insights. J Heart Valve Dis. 2008;17:81–8.
- Adda J, Mielot C, Giorgi R, Cransac F, Zirphile X, Donal E, Sportouch-Dukhan C, Réant P, Laffitte S, Cade S, Le Dolley Y, Thuny F, Touboul N, Lavoute C, Avierinos J-F, Lancellotti P, Habib G. Low-Flow, Low-Gradient Severe Aortic Stenosis Despite Normal Ejection Fraction Is Associated With Severe Left Ventricular Dysfunction as Assessed by Speckle-Tracking Echocardiography. Circ Cardiovasc Imaging. 2012;5:27–35. [CrossRef]
- Pereira JJ, Lauer MS, Bashir M, Afridi I, Blackstone EH, Stewart WJ, McCarthy PM, Thomas JD, Asher CR. Survival after aortic valve replacement forsevere aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol. 2002;39:1356–1363. [CrossRef]
- Levy F, Laurent M, Monin JL, Maillet JM, Pasquet A, Le Tourneau T, Petit-Eisenmann H, Gori M, Jobic Y, Bauer F, Chauvel C, Leguerrier A, Tribouilloy C. Aortic Valve Replacement for Low-Flow/Low-Gradient Aortic Stenosis. J Am Coll Cardiol. 2008;51:1466–1472. [CrossRef]
- Tribouilloy C, Lévy F, Rusinaru D, Guéret P, Petit-Eisenmann H, Baleynaud S, Jobic Y, Adams C, Lelong B, Pasquet A, Chauvel C, Metz D, Quéré J-P, Monin J-L. Outcome After Aortic Valve Replacement for Low-Flow/Low-Gradient Aortic Stenosis Without Contractile Reserve on Dobutamine Stress Echocardiography. J Am Coll Cardiol. 2009;53:1865–1873. [CrossRef]
- Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. New England Journal of Medicine. 2016;374:1609–1620. [CrossRef]
- Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PWJC, Kappetein AP. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. New England Journal of Medicine. 2017;376:1321–1331. [CrossRef]
- Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, Kleiman NS, Chetcuti S, Hermiller JB, Heiser J, Merhi W, Zorn GL, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J V., Mumtaz M, Oh JK, Huang J, Adams DH. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J Am Coll Cardiol. 2018;72:2687–2696. [CrossRef]
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. New England Journal of Medicine. 2010;363:1597–1607. [CrossRef]
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. New England Journal of Medicine. 2011;364:2187–2198. [CrossRef]
- Pibarot P, Salaun E, Dahou A, Avenatti E, Guzzetti E, Annabi M-S, Toubal O, Bernier M, Beaudoin J, Ong G, Ternacle J, Krapf L, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Leipsic J, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Généreux P, Pershad A, Alu MC, Xu K, Rogers E, Webb JG, Smith CR, Mack MJ, Leon MB, Hahn RT. Echocardiographic Results of Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. Circulation. 2020;141:1527–1537. [CrossRef]
- Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. New England Journal of Medicine. 2019;380:1706–1715. [CrossRef]
- Saybolt MD, Fiorilli PN, Gertz ZM, Herrmann HC. Low-Flow Severe Aortic Stenosis. Circ Cardiovasc Interv. 2017;10. [CrossRef]
- Gotzmann M, Lindstaedt M, Bojara W, Ewers A, Mügge A. Clinical outcome of transcatheter aortic valve implantation in patients with low-flow, low gradient aortic stenosis. Catheterization and Cardiovascular Interventions. 2012;79:693–701. [CrossRef]
- Lauten A, Zahn R, Horack M, Sievert H, Linke A, Ferrari M, Harnath A, Grube E, Gerckens U, Kuck K-H, Sack S, Senges J, Figulla HR. Transcatheter Aortic Valve Implantation in Patients With Low-Flow, Low-Gradient Aortic Stenosis. JACC Cardiovasc Interv. 2012;5:552–559. [CrossRef]
- O’Sullivan CJ, Stortecky S, Heg D, Pilgrim T, Hosek N, Buellesfeld L, Khattab AA, Nietlispach F, Moschovitis A, Zanchin T, Meier B, Windecker S, Wenaweser P. Clinical outcomes of patients with low-flow, low-gradient, severe aortic stenosis and either preserved or reduced ejection fraction undergoing transcatheter aortic valve implantation. Eur Heart J. 2013;34:3437–3450. [CrossRef]
- Elhmidi Y, Piazza N, Krane M, Deutsch M, Mazzitelli D, Lange R, Bleiziffer S. Clinical presentation and outcomes after transcatheter aortic valve implantation in patients with low flow/low gradient severe aortic stenosis. Catheterization and Cardiovascular Interventions. 2014;84:283–290. [CrossRef]
- Kamperidis V, Joyce E, Debonnaire P, Katsanos S, van Rosendael PJ, van der Kley F, Sianos G, Bax JJ, Ajmone Marsan N, Delgado V. Left Ventricular Functional Recovery and Remodeling in Low-Flow Low-Gradient Severe Aortic Stenosis after Transcatheter Aortic Valve Implantation. Journal of the American Society of Echocardiography. 2014;27:817–825. [CrossRef]
- Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, Kapadia S, Tuzcu EM, Babaliaros V, Thourani V, Szeto WY, Bavaria JE, Kodali S, Hahn RT, Williams M, Miller DC, Douglas PS, Leon MB. Predictors of Mortality and Outcomes of Therapy in Low-Flow Severe Aortic Stenosis. Circulation. 2013;127:2316–2326. [CrossRef]
- Ben-Dor I, Maluenda G, Iyasu GD, Laynez-Carnicero A, Hauville C, Torguson R, Okubagzi P, Xue Z, Goldstein SA, Lindsay J, Satler LF, Pichard AD, Waksman R. Comparison of Outcome of Higher Versus Lower Transvalvular Gradients in Patients With Severe Aortic Stenosis and Low (<40%) Left Ventricular Ejection Fraction. Am J Cardiol. 2012;109:1031–1037. [CrossRef]
- Lauten A, Figulla HR, Möllmann H, Holzhey D, Kötting J, Beckmann A, Veit C, Cremer J, Kuck K-H, Lange R, Zahn R, Sack S, Schuler G, Walther T, Beyersdorf F, Böhm M, Heusch G, Meinertz T, Neumann T, Welz A, Mohr FW, Hamm CW. TAVI for low-flow, low-gradient severe aortic stenosis with preserved or reduced ejection fraction: a subgroup analysis from the German Aortic Valve Registry (GARY). EuroIntervention. 2014;10:850–859. [CrossRef]
- Clavel MA, Webb JG, Rodés-Cabau J, Masson JB, Dumont E, De Larochellière R, Doyle D, Bergeron S, Baumgartner H, Burwash IG, Dumesnil JG, Mundigler G, Moss R, Kempny A, Bagur R, Bergler-Klein J, Gurvitch R, Mathieu P, Pibarot P. Comparison Between Transcatheter and Surgical Prosthetic Valve Implantation in Patients With Severe Aortic Stenosis and Reduced Left Ventricular Ejection Fraction. Circulation. 2010;122:1928–1936. [CrossRef]
- Rodés-Cabau J. Transcatheter aortic valve implantation: current and future approaches. Nat Rev Cardiol. 2012;9:15–29. [CrossRef]
- Ribeiro HB, Lerakis S, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez-Sarano M, Cheema AN, Nombela-Franco L, Amat-Santos I, Muñoz-García AJ, Garcia del Blanco B, Zajarias A, Lisko JC, Hayek S, Babaliaros V, Le Ven F, Gleason TG, Chakravarty T, Szeto WY, Clavel M-A, de Agustin A, Serra V, Schindler JT, Dahou A, Puri R, Pelletier-Beaumont E, Côté M, Pibarot P, Rodés-Cabau J. Transcatheter Aortic Valve Replacement in Patients With Low-Flow, Low-Gradient Aortic Stenosis. J Am Coll Cardiol. 2018;71:1297–1308. [CrossRef]
- Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, Kapadia S, Tuzcu EM, Babaliaros V, Thourani V, Szeto WY, Bavaria JE, Kodali S, Hahn RT, Williams M, Miller DC, Douglas PS, Leon MB. Predictors of Mortality and Outcomes of Therapy in Low-Flow Severe Aortic Stenosis. Circulation. 2013;127:2316–2326. [CrossRef]
- Baron SJ, Arnold S V., Herrmann HC, Holmes DR, Szeto WY, Allen KB, Chhatriwalla AK, Vemulapali S, O’Brien S, Dai D, Cohen DJ. Impact of Ejection Fraction and Aortic Valve Gradient on Outcomes of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2016;67:2349–2358. [CrossRef]
- Connolly HM, Oh JK, Orszulak TA, Osborn SL, Roger VL, Hodge DO, Bailey KR, Seward JB, Tajik AJ. Aortic Valve Replacement for Aortic Stenosis With Severe Left Ventricular Dysfunction. Circulation. 1997;95:2395–2400. [CrossRef]
- Clavel M-A, Berthelot-Richer M, Le Ven F, Capoulade R, Dahou A, Dumesnil JG, Mathieu P, Pibarot P. Impact of Classic and Paradoxical Low Flow on Survival After Aortic Valve Replacement for Severe Aortic Stenosis. J Am Coll Cardiol. 2015;65:645– 653. [CrossRef]
- Quere J-P, Monin J-L, Levy F, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Gueret P, Tribouilloy C. Influence of Preoperative Left Ventricular Contractile Reserve on Postoperative Ejection Fraction in Low-Gradient Aortic Stenosis. Circulation. 2006;113:1738–1744. [CrossRef]
- Monin J-L, Monchi M, Kirsch MEW, Petit-Eisenmann H, Baleynaud S, Chauvel C, Metz D, Adams C, Quere J-P, Gueret P, Tribouilloy C. Low-gradient aortic stenosis: impact of prosthesis-patient mismatch on survival. Eur Heart J. 2007;28:2620–2626. [CrossRef]
- Vaquette B. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart. 2005;91:1324–1329. [CrossRef]
- Ribeiro JM, Teixeira R, Siserman A, Puga L, Lopes J, Sousa JP, Lourenço C, Belo A, Gonçalves L. Impact of previous coronary artery bypass grafting in patients presenting with an acute coronary syndrome: Current trends and clinical implications. Eur Heart J Acute Cardiovasc Care. 2020;9:731–740. [CrossRef]
- Généreux P, Pibarot P, Redfors B, Mack MJ, Makkar RR, Jaber WA, Svensson LG, Kapadia S, Tuzcu EM, Thourani VH, Babaliaros V, Herrmann HC, Szeto WY, Cohen DJ, Lindman BR, McAndrew T, Alu MC, Douglas PS, Hahn RT, Kodali SK, Smith CR, Miller DC, Webb JG, Leon MB. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. 2017;38:3351–3358. [CrossRef]
| Groups | Mean transvalvular gradient | Stroke Volume index |
|---|---|---|
| Low-flow, high-gradient | ≥40 mm Hg | ≤35mL/m2 |
| (LF-HG) | ||
| Low-flow, low-gradient | <40 mm Hg | ≤35mL/m2 |
| (LF-LG) | ||
| Normal-flow, high- | ≥40 mm Hg | >35mL/m2 |
| gradient (NF-HG) | ||
| Normal-flow, low-gradient | <40 mm Hg | >35mL/m2 |
| (NF-LG) |
| All patients | NF-HG AS | LF-HG AS | NF-LG AS | LF-LG AS | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N=272) | 95 | (34.9%) | 98 | (36%) | 17 | (6.3%) | 62 | (22.8%) | |||
| Age, yrs | 80 | ± 7 | 80 | ± 7 | 81 | ± 7 | 77 | ± 8 | 78 | ± 9 | 0.124 |
| BMI, kg/m2 | 26.06±3.17 | 25.32±2.75 | 26.83±3.48 | 26.54±3.64 | 25.67±2.56 | 0.008 | |||||
| Male, (%) | 134 (49.2%) | 34 | (35.8%) | 50 | (51%) | 9 (52.9%) | 41(65.6%) | 0.018 | |||
| Diabetes, (%) | 87 | (32%) | 32 | (33.7%) | 29 | (29.6%) | 7 (41.2%) | 19 | (31.3%) | 0.623 | |
| Hypertension, (%) | 206 (75.7%) | 67 | (70.5%) | 76 | (77.6%) | 11 | (64.7%) | 52 | (84.4%) | 0.475 | |
| CAD, (%) | 129 (47.4%) | 41 | (43.2%) | 44 | (44.9%) | 9 (52.9%) | 35 | (56,3%) | 0.268 | ||
| Previous MI, (%) | 55 | (20.2%) | 18 | (18.9%) | 15 | (15.3%) | 3 (17.6%) | 19 | (31.3%) | 0.005 | |
| Previous PCI, (%) | 36 | (13.2%) | 11 | (11.2%) | 11 | (10.8%) | 2 (14.3%) | 12 | (19.2%) | 0.689 | |
| Previous CABG, (%) | 63 | (23.2%) | 12 | (12.4%) | 24 | (25.3%) | 6 (35.7%) | 21(34.6%) | 0.025 | ||
| CKD, (%) | 114 (41.9%) | 36 | (37.9%) | 42 | (42.9%) | 5 (29.4%) | 31 | (50%) | 0.469 | ||
| PAD, (%) | 100 (36.7%) | 29 | (30.5%) | 30 | (30.6%) | 4 (23.5%) | 37 | (59.4%) | 0.063 | ||
| COPD, (%) | 71 | (26.1%) | 22 | (23.2%) | 25 | (25.5%) | 7 (41.2%) | 17 | (28.1%) | 0.694 | |
| EUROSCOREII | 5.14 {4.22- | 4.50 {3.42- | 5.15 {4.60- | 5.62 {4.95- | 8.34 {4.84- | 0.004 | |||||
| 6.48} | 6.0} | 6.48} | 6.88} | 13.58} | |||||||
| NYHA III/IV, (%) | 265 (97.4%) | 92 | (97.9%) | 96 | (98%) | 17 | (100)% | 60 | (96.9%) | 0.850 | |
| All patients | NFHGAS | LFHGAS | NFLGAS | LFLGAS | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N=272) | (N=95) | (N=98) | (N=17) | (N=62) | value | ||||||
| LVEF,% | 51 | ± 9 | 54 | ± 6 | 50 | ± 9 | 50 | ± 11 | 44 | ± 12 | <0.001 |
| Mean gradient, | 50 | ± 15 | 57 | ± 14 | 53 | ± 11 | 34 | ± 6 | 32 | ± 9 | <0.001 |
| mmHg | |||||||||||
| AVA, cm2 | 0.61 ± 0.15 | 0.68 ± 0.14 | 0.52 ± 0.11 | 0.77 ± 0.11 | 0.59 ± 0.16 | <0.001 | |||||
| Moderate-severe | 86 | (31.6%) | 19 | (20.3%) | 34 | (35%) | 7 (38.5%) | 26 | (42.9%) | 0.069 | |
| MR pre TAVi | |||||||||||
| Moderate-severe TR | 64 (23.5%) | 19 | (20%) | 28 | (28.6%) | 5 (29.4%) | 12 | (19.3%) | 0.438 | ||
| pre TAVi | |||||||||||
| Stroke volume | 35 | ± 11 | 45 | ± 9 | 29 | ± 6 | 40 | ± 4 | 26 | ± 5 | <0.001 |
| indexed, ml/m2 | |||||||||||
| Pulmonary artery | 44.43±12.50 | 44.95±12.52 | 44.98±13.46 | 41.76±8.46 | 42.61±11.22 | 0.620 | |||||
| systolic pressure | |||||||||||
| (PASP) (mmHg) | |||||||||||
| Severe AV | 172 (63.2%) | 58 | (61.4%) | 62 | (63.2%) | 14 | (80%) | 38 | (61.1%) | 0.717 | |
| calcification based | |||||||||||
| on MSCT | |||||||||||
| LF-LG AS | NF-LG AS | LF-HG AS | NF-HG | p LF-LG AS | p LF-LG AS | p LF-LG AS | P* | ||
| (N=62) | (N=17) | (N=98) | AS | vs NF-LG | vs LF-HG | vs NF-HG | |||
| (N=95) | AS | AS | AS | ||||||
| 1-year | all- | 16 (25.8) | 3 (18.8) | 11 (11.5) | 6 (6.3) | 0.729 | 0.084 | 0.011 | 0.048 |
| cause | |||||||||
| mortality, | |||||||||
| N (%) | |||||||||
| 1-year | CV | 12 (19.3) | 2 (12.5) | 3 (3.1) | 5 (5.7) | 0.701 | 0.008 | 0.067 | 0.018 |
| mortality, | |||||||||
| N (%) | |||||||||
| Stroke | at 1 | 2 (3.2%) | 1 (5.9) | 2 (2) | 2 (2.1) | 1.000 | 1.000 | 0.802 | 0.868 |
| year, N (%) | |||||||||
| 1-year | 16 (25.8) | 3 (18.8) | 12 (12.2) | 8 (8.4) | 1.000 | 0.092 | 0.029 | 0.097 | |
| MACCE | |||||||||
| (death, | non- | ||||||||
| fatal | MI, | ||||||||
| non-fatal | |||||||||
| stroke), N(%) | |||||||||
| 5-year | all- | 40 (64.5) | 10 (58.8) | 47 (47.9) | 40 (42.5) | 1.000 | 0.152 | 0.038 | 0.047 |
| cause | |||||||||
| mortality, | |||||||||
| N (%) | |||||||||
| 5-year | CV | 33 (53.2) | 4 (23.5) | 21 (21.4) | 17 (17.9) | 0.075 | 0.001 | 0.001 | 0.002 |
| mortality, | |||||||||
| N (%) | |||||||||
| LF-LG | NF-LG | LF-HG | NF-HG | p LF-LG vs | p LF-LG vs | p LF-LG vs | P* | |
|---|---|---|---|---|---|---|---|---|
| (N=62) | (N=17) | (N=98) | (N=95) | NF-LG | LF-HG | NF-HG | ||
| 30-day PPI | 31 | 7 | 41(41.8) | 34 | 0.683 | 0.419 | 0.147 | 0.561 |
| N (%) | (50) | (41.2) | (35.8) | |||||
| 30-day Major | 2 (3.2) | 1 (5.9) | 8 (8.2) | 7 (7.4) | 0.660 | 0.294 | 0.353 | 0.755 |
| vascular | ||||||||
| complications N | ||||||||
| (%) | ||||||||
| 30-day Life | 9 | 2 | 12 | 15(15.8) | 0.659 | 0.622 | 0.965 | 0.863 |
| threatening/major | (14.5) | (11.7) | (12.2) | |||||
| bleeding | ||||||||
| complications N | ||||||||
| (%) | ||||||||
| 95.0% CI for Exp(B) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | Wald | df | Sig. | Exp(B) | Lower | Upper | ||
| EUROSCORE II | |||||||||
| -.025 | .016 | 2.455 | 1 | .117 | .975 | .945 | 1.006 | ||
| CHRONIC KIDNEY | |||||||||
| DISEASE | .429 | .275 | 2.441 | 1 | .118 | 1.536 | .896 | 2.633 | |
| PRIOR MI | |||||||||
| .139 | .301 | .213 | 1 | .645 | 1.149 | .637 | 2.072 | ||
| Pre-TAVI MR ≥ | |||||||||
| moderate | .069 | .291 | .057 | 1 | .812 | 1.072 | .606 | 1.894 | |
| Pre-TAVI TR ≥ | 1.128 | .322 | 12.288 | 1 | .000 | 3.091 | 1.645 | 5.809 | |
| moderate | |||||||||
| Pre-TAVI PASP | |||||||||
| .005 | .012 | .194 | 1 | .660 | 1.005 | .983 | 1.028 | ||
| Pre-TAVI SVi | |||||||||
| -.051 | .018 | 8.316 | 1 | 0.004 | .951 | .918 | .984 | ||
| Post-TAVI PASP | .010 | .013 | .641 | 1 | .423 | 1.010 | .985 | 1.036 | |
| Post-TAVI PVL ≥ | 1.105 | 0.288 | 11.677 | 1 | 0.042 | 1.456 | 1.106 | 1.792 | |
| moderate | |||||||||
| FLOW-GRADIENT STATE*US* | 9.030 | 3 | .029 | ||||||
| LF-HG AS | -.374 | .505 | .548 | 1 | .459 | .688 | .256 | 1.852 | |
| NF-LG AS | .564 | .553 | 1.039 | 1 | .308 | 0.360 | .594 | 5.197 | |
| LF-LG AS | -1.022 | .433 | 5.572 | 1 | .018 | 1.757 | .154 | .841 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





