Background
Infantile hemangiomas (IHs) are the most common benign pediatric vascular tumors. The incidence varies between 1 and 3% in newborns, and 3 and 10% in infants under 12 months of age. (3:1). The prevalence is significantly higher in female, preterm and low-birth-weight infants. They are often not present at birth and pale pink and indistinctly small lesions at the beginning, which then enter the rapid growth phase and become evident within weeks.
The majority of IHs are not present at birth. Precursor lesions (pale area of vasoconstriction, an erythematous macule, a telangiectatic red macule or blue bruise-like patches) can be sometimes observed since birth. Clinically, a proliferative phase,
plateau and involution phase are observed. After rapid postnatal growth of the lesion, growth stops, and the lesion spontaneously regresses and heals, leaving a burn-like scar (fatty fibrous residue) [
1,
2].
The majority of IHs regress spontaneously. The need for medical treatment is determined by the location of the IHs, complications, organ dysfunction caused by the lesion, and functional losses. The surgical approach is not a priority in IHs that are expected to regress spontaneously or that can be controlled with medical treatment. Surgery is often performed for reconstruction after the fatty fibrous residue phase [
1,
3].
Vascular malformations are important in the differential diagnosis of IHs. Mulliken and Glowacki [
4] first classified vascular anomalies in 1982. In 1996, this classification was adopted and expanded by the International Society for the Study of Vascular Anomalies (ISSVA), which is modified annually, and was lastly updated in 2018 [
5,
6,
7,
8]. Infantile hemangiomas are mainly classified as focal, multifocal, and segmental according to its distribution. They are also classified as superficial, deep, and mixed type according to the localization [
2,
8]. Approximately 60% of IHs are located in the head and neck region, followed by the trunk, extremities, and genitalia [
9].
In this study, we aimed to evaluate the clinical features of the patients diagnosed with head and neck IHs and followed and to assess their treatment indications, complications, and treatment responses.
Materials and Methods
The records of a total of 410 patients who were admitted to XXX, Oncology Institute, Department of Pediatric Oncology between January 1st, 2010 and January 1st, 2022 and followed with a diagnosis of IHs were retrospectively analyzed. The study protocol was approved by the institutional Ethics Committee (2022/35-22). A written informed consent was obtained from parents and/or legal guardians of the patients. The study was conducted in accordance with the principles of the Declaration of Helsinki.
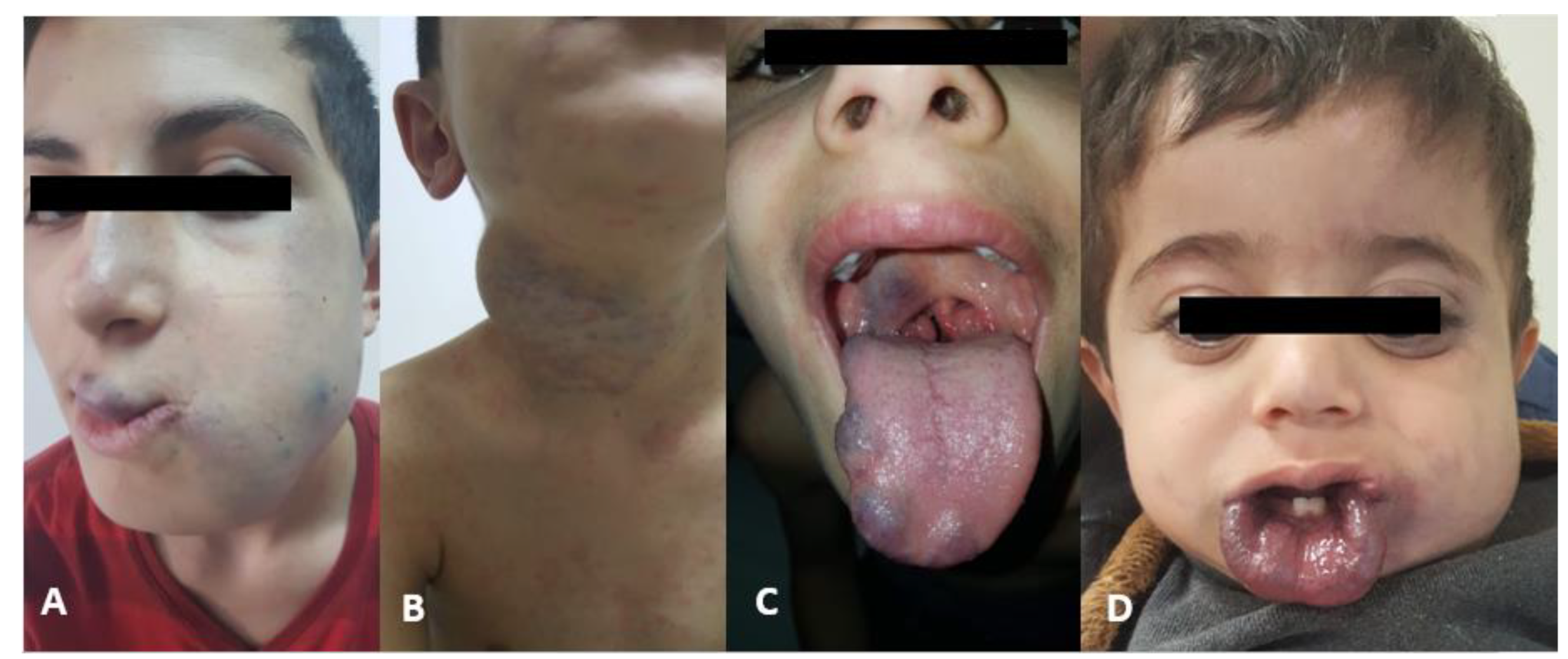
Cases referred to our center for the treatment with the diagnosis of hemangioma but consistent with vascular malformation were not included (
Figure 1). Propranolol was the first choice for treatment and it was used in tablet form between 2010 and 2021, but as of 2021, we also initiated using the suspension form. We administered the drug orally in two doses, starting at a low dose of 0.25 mg/kg/dose and gradually increasing to 2 mg/kg/day, while all the patients were hospitalized for 48 to 72 h. We followed the patients for side effects such as hypotension and hypoglycemia. After leaving the hospital, we performed monthly check-up calls within 10-day intervals in the first month. Infantile hemangioma was localized in the head and neck region in 60.5% (248/410) of these cases. The age at the time of admission, sex, history of prematurity, hemangioma type, location, number, complications, indications for treatment, treatment methods (medical and/or surgical), treatment responses, and follow-up times were recorded.
Statistical analysis
Statistical analysis was performed using the SPSS version 22.0 software (IBM Corp., Armonk, NY, USA). Descriptive data were expressed in median (min-max) or number and frequency, where applicable.
Results
A total of 248 patients with IHs in the head and neck region were followed in our center. The median age at the time of admission was 4 months (0–13 years), and female/male ratio (170/78) was 2.17. Among these cases, 64.1% were under six months of age. Age distribution of the cases is shown in
Table 1. Prematurity history was present in 28% of patients.
In 72% of the cases (179/248), there was only head and neck IHs, while the remaining 28% had additional trunk and/or extremity IHs. The characteristics of hemangiomas on admission are shown in
Table 2.
Figure 1 shows examples of vascular malformations that are important for the differential diagnosis.
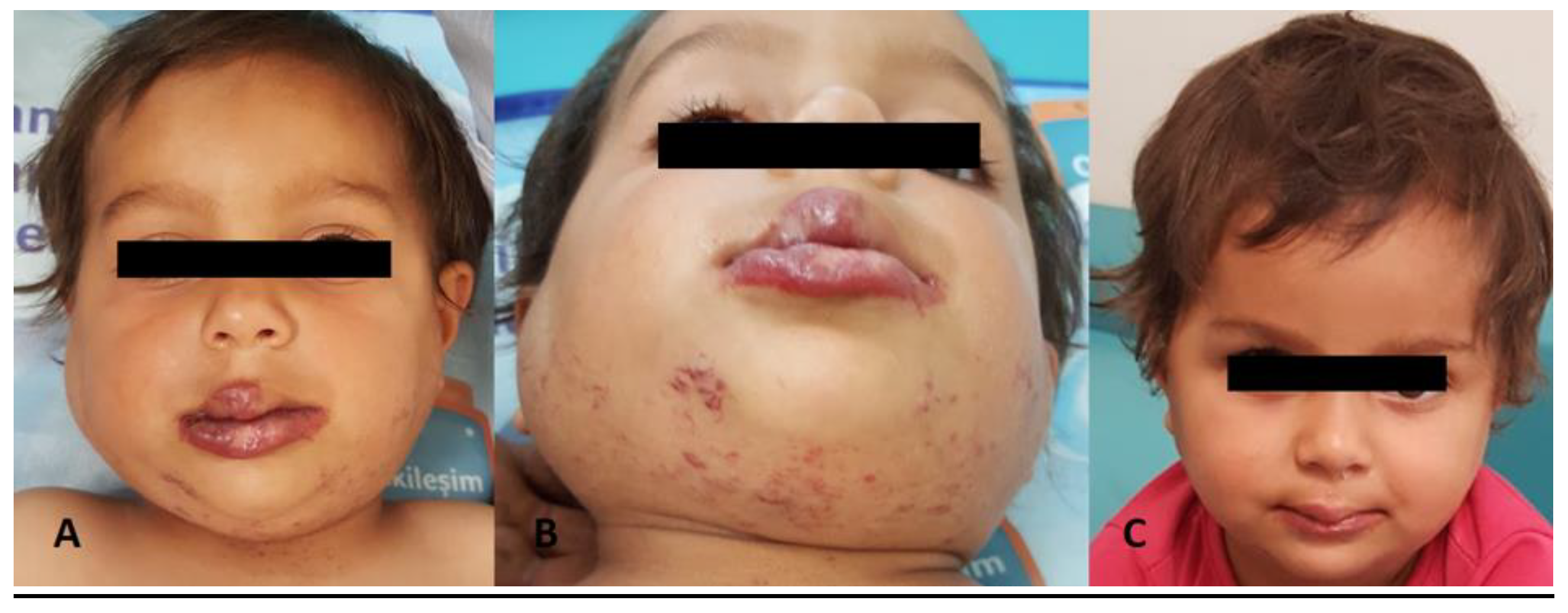
Figure 2,
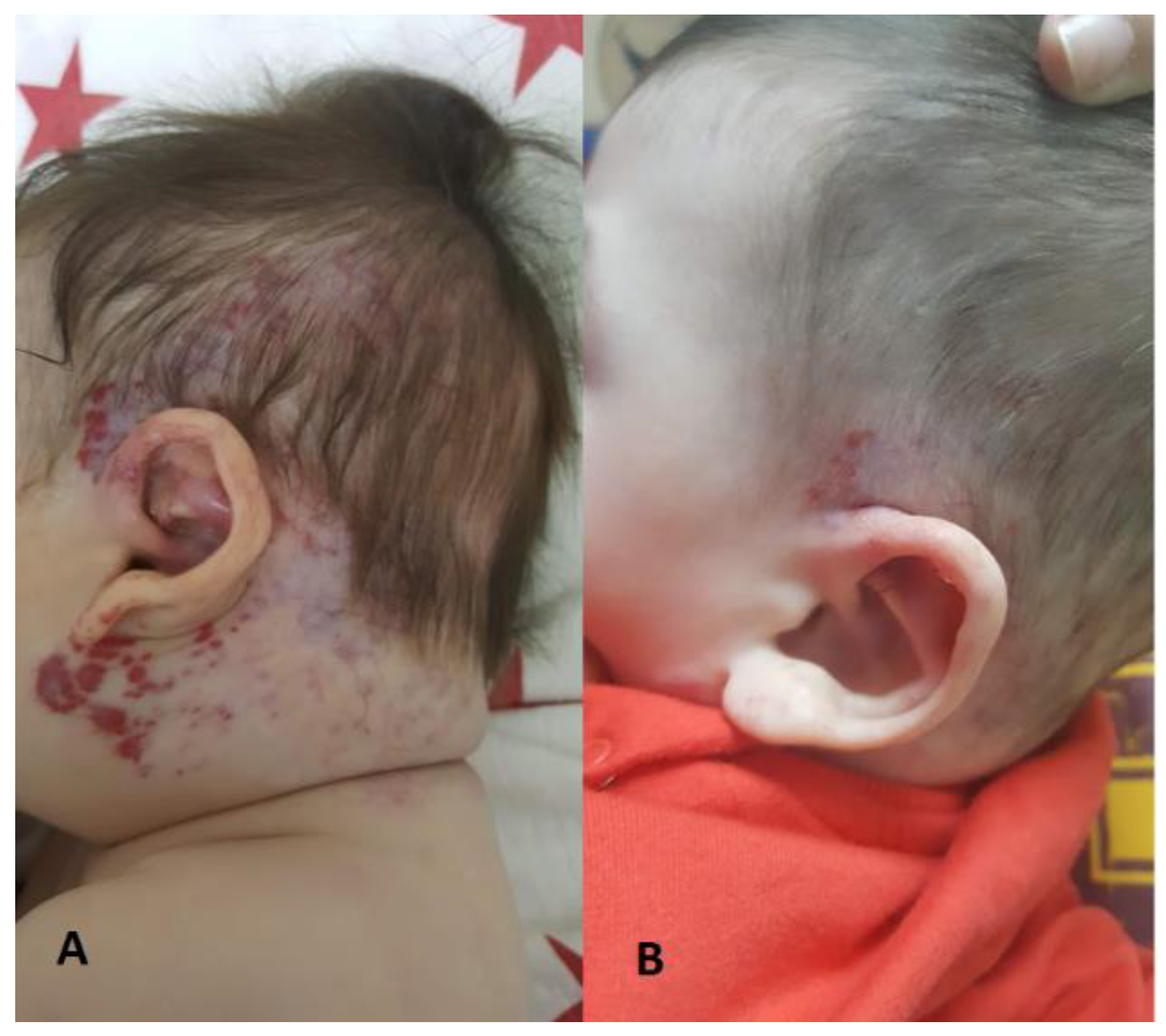
Figure 3, and
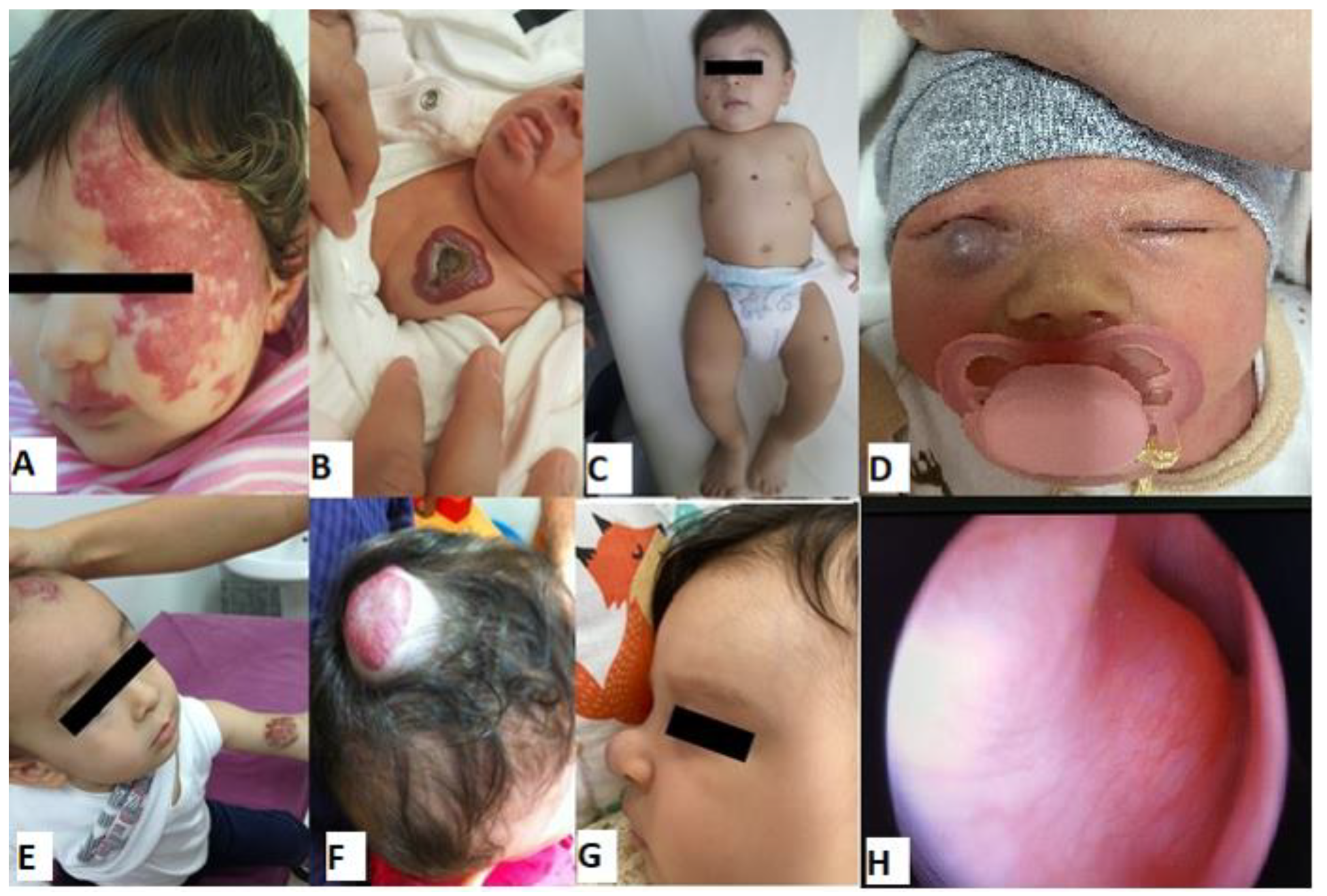
Figure 4 show IH patients.
Only the follow-up strategy was applied in 49% (n=121) of all patients, and 23% (28/121) of these patients did not attend physical examination after the first admission. These 28 patients were considered to have no complications and were included in the study. Although no treatment was given, local complications were not observed in any of the patients who attended the outpatient clinic controls on a regular basis. The median follow-up period of these patients was 6 months (0–84 months). None of these patients with spontaneously regressed lesions underwent surgery.
There was an indication for treatment in 55% (136/248) of the cases. Treatment was initiated in 127 (51%) of these, excluding nine patients. The families of these nine patients did not accept the treatment and were followed without treatment. Propranolol was started in all patients (n=127) with indications for treatment. During follow-up, the most common drug-related side effect was abnormal liver function tests (11%, n=14), which completely regressed after reducing the dose of the drug. Hypoglycemia was detected in two cases in the acute period of propranolol treatment, which resolved following the correct use of the drug after feeding. Other possible side effects such as nightmares, sleep disorders, and diarrhea were not observed.
Only propranolol was administered in 119 of the patients. There was no adequate response to propranolol in eight patients despite its use for a median of four weeks. In these patients, steroids and other drugs were added (
Figure 2). There was a patient with hemangioma located in one half of the face and extending to the pharyngeal region, and she was treated with steroids, propranolol, and interferon. In a patient with subglottic hemangioma and severe respiratory distress, a response was obtained by administering vincristine and cyclophosphamide in the acute phase in addition to steroids and propranolol. The median duration of propranolol treatment was 6 months (3-24 months), and treatment response was achieved in 93% (8/119) patients.
Three of the patients with periorbital hemangioma (3/55) had ocular complications. These patients had poor vision and needed glasses. In one patient, reduction surgery was required due to poor vision due to the hemangioma located in the lower eyelid, and steroid was used in addition to propranolol for one month. Afterwards, partial response was obtained, and fibrous scar residue development was observed only after three years of age. Subglottic hemangiomas (n=9) were followed together with the Department of Otorhinolaryngology of our center and all received propranolol. Tracheostomy was performed in one patient due to respiratory distress at the time of diagnosis. All patients with subglottic hemangiomas had a complete response to medical treatment.
Complications were observed in 42 (17%) of the 248 patients and 62% of these patients were between three and nine months. All but one of them received treatment. Of the cases with complications, seven had capillary, seven had deep, and 28 had mixed type hemangioma. Treatment indications and complications of hemangioma are listed in
Table 3. Treatment indications were as follows: periorbital localization (40%), cosmetic problem (24%), perioral localization (14%), respiratory distress (9%), infection/bleeding/ulceration (9%), and periauricular localization (4%). The most common complication in cases receiving propranolol treatment was infection/bleeding/ulceration. Respiratory distress, feeding difficulty, vision problems and hearing problems were the other common complications. The median follow-up period of all patients was 14 months (0–118 months).
Discussion
In the present study, head and neck IHs constituted 60.5% of all hemangiomas. While IHs are seen at a rate of 8 to 12% in infants, its incidence increases up to 22% in those with a history of premature birth [
10]. In our study, the rate of prematurity was 28%. Infantile hemangiomas are seen three to five times more frequently in girls than in boys [
1,
2]. The female/male ratio of our patients was 2.23, which is consistent with the literature.
Although most IHs are expected to regress spontaneously, the treatment approach varies according to the patient. The time of treatment onset is also important. It is known that better treatment responses are obtained when initiated in the proliferative phase. In a previous study evaluating all IHs admitted to our center between 2000 and 2007, the complication rate was found to be higher [
11]. During that period, only IHs with a high risk of complications were referred to oncology clinics. Complications developed in 35% of 37 patients with head and neck IH. With the introduction of propranolol as first-line therapy after 2008, initiation of treatment has become more frequent and the complication rate may have decreased [
12,
13,
14,
15,
16,
17]. Prior to the serendipitous discovery of propranolol [
13], steroids had an important place in the treatment of IHs, particularly in difficult and complicated cases. Its side-effect profile was broad, while its effect was limited. Particularly, in airway hemangiomas, complications after the use of propranolol, and the need for surgical intervention such as tracheostomy have almost disappeared [
3,
17,
18]. In our study, nine patients had subglottic hemangioma, and a response was achieved with propranolol in all these patients. Respiratory distress developed in only one patient in the first week of treatment, and tracheostomy was performed at the time of diagnosis. However, it resolved after the effective dose of propranolol was increased. We also had two patients who were followed in our clinic for subglottic hemangioma before 2008 and required tracheostomy. Propranolol is a synthetic beta-adrenergic receptor blocker. Although the mechanism of action in the treatment of his has not been fully elucidated yet, it is considered to be vasoconstriction, decreased renin production, inhibition of angiogenesis, and stimulation of apoptosis [
22]. A report related to the use of propranolol and its effective optimal dose was published in 2013 [
22]. The dose can be started as 0.25 mg/kg/dose and increased up to 2 to 3 mg/kg/day, divided into two or three doses. Treatment can be continued up to 12 to 18 months [
12,
19,
20,
21,
22]. In our center, treatment is started at 0.25 mg/kg/dose and gradually increased to 2 mg/kg/dose.
The major uncontrollable local complications are bleeding, ulcer, and infection. In particular, in head and neck IHs, the risk of development of local complications such as bleeding and ulcer determines the need for treatment. Periorbital, perioral, intraoral, and subglottic locations, and compression and obstruction to important organs such as the eyes, mouth, and nose are indications for treatment. Periorbital or eyelid hemangiomas can cause amblyopia, anisometropia, astigmatism, and even blindness [
22,
23,
24,
25,
26]. Conducting follow-up with the collaboration of an ophthalmologist is of utmost importance from the beginning of the diagnosis. Feeding difficulties can be also seen in perioral hemangiomas. While the complication rate of our patients was 17%, local complications accounted for half of them, consistent with the literature. This was followed by respiratory distress, feeding difficulty, and vision and hearing problems. The ages of 62% of these children were between three to nine months. Léauté-Labrèze at al. [
27] recently reported that therapy initiation before 2.5 months of age was associated with a significantly higher rate of treatment success with propranolol. In another recent study, early (before three months of age) initiation of treatment of IHs with propranolol resulted in significantly higher response rates [
28].
The most commonly reported adverse effects of propranolol are hypotension, hypoglycemia, asymptomatic bradycardia, impaired liver function tests, and hyperkalemia. Sleep disturbances, cold extremities, diarrhea, and gastroesophageal reflux are other reported side effects. To prevent these side effects, small babies (<8 weeks old) are hospitalized to start treatment. Blood glucose levels, blood pressure, and pulse are monitored every 1 to 3 hours after taking the drug. It is recommended to give the drug after feeding. Baseline electrocardiography and echography are performed before treatment. Cardiogenic shock, sinus bradycardia, hypotension, heart blocks above first degree, heart failure, and history of bronchial asthma are contraindications for propranolol treatment [
22,
26]. In the present study, hypoglycemia was detected in two cases due to propranolol treatment in the acute period, and the drug could be continued if used correctly after feeding. In the follow-up during propranolol use, we observed abnormalities in liver tests (11%) most frequently, which were controlled by dose reduction. Other than that, no side effects were observed. In some cases, propranolol treatment was interrupted during bronchiolitis periods.
In the first retrospective study related to propranolol, the response rate was reported as 97% [
17]. In our study, the response rate to treatment was 98% and, in another study, the response rate to treatment was 97.63% [
23]. In another single-center study, all hemangiomas treated with 2 mg/kg/day propranolol were evaluated, and the treatment response was reported as 96% [
15]. In a multi-center, double-blind, randomized study with 495 cases, on the optimal dose of propranolol, the response rate to treatment with 2 to 3 mg/kg/dose was reported as >90% [
14]. In this study, 1 mg/kg/dose was found to be less effective. In patients taking propranolol, it may be necessary to add a short-term steroid to control growth at first [
3,
25]. In our study, an adequate response was not obtained with propranolol in a patient with periorbital hemangioma, and an effective response was achieved by using it in combination with steroids for one month. Tissue diagnosis may be required for hemangiomas, when no response is obtained despite propranolol treatment. In one of our cases with IH located in the root of the nose, there was no response to propranolol, and the patient was diagnosed with rhabdomyosarcoma after biopsy [
29,
30].
Conclusion
In conclusion, more than half of IHs occur in the head and neck region. Although most of the hemangiomas are expected to regress spontaneously, the main determinant in the treatment decision is the location of the hemangioma and the vital functions it bears the risk. The time to start treatment is critical. Complications may occur, particularly during the proliferation period. In selected patients, it is of paramount importance to initiate treatment early and refer them to special centers for evaluation. Propranolol alone is an effective treatment option, and early treatment initiation increases the success rates.
Funding
This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Darrow, D.H.; Greene, A.K.; Mancini, A.J.; Nopper, A.J. Section On Dermatology SOO-H, Neck S, et al. Diagnosis and Management of Infantile Hemangioma. Pediatrics 2015, 136, e1060–104. [Google Scholar] [CrossRef]
- Krowchuk, D.P.; Frieden, I.J.; Mancini, A.J.; Darrow, D.H.; Blei, F.; Greene, A.K., et al. Clinical Practice Guideline for the Management of Infantile Hemangioma. Pediatrics 2019, 143. [CrossRef]
- Adams, D.M.; Ricci, K.W. Infantile Hemangiomas in the Head and Neck Region. Otolaryngol Clin North Am 2018, 51, 77–87. [Google Scholar] [CrossRef]
- Mulliken, J.B.; Glowacki, J. Hemangiomas and Vascular Malformations in Infants and Children: A Classification Based on Endothelial Characteristics. Plast Reconstr Surg 1982, 69, 412–22. [Google Scholar] [CrossRef]
- Chang, M.W. Updated Classification of Hemangiomas and Other Vascular Anomalies. Lymphat Res Biol 2003, 1, 259–65. [Google Scholar] [CrossRef]
- Enjolras, O.; Mulliken, J.B. Vascular Tumors and Vascular Malformations (New Issues). Adv Dermatol 1997, 13, 375–423. [Google Scholar] [PubMed]
- Kunimoto, K.; Yamamoto, Y.; Jinnin, M. Issva Classification of Vascular Anomalies and Molecular Biology. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, et al. Vascular Anomalies Classification: Recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics 2015, 136, e203–14. [CrossRef] [PubMed]
- Fowell, C.; Monaghan, A.; Nishikawa, H. Infantile Haemangiomas of the Head and Neck: Current Concepts in Management. Br J Oral Maxillofac Surg 2016, 54, 488–95. [Google Scholar] [CrossRef]
- Grantzow R, Schmittenbecher P, Cremer H, Hoger P, Rossler J, Hamm H, et al. Hemangiomas in Infancy and Childhood. S 2k Guideline of the German Society of Dermatology with the Working Group Pediatric Dermatology Together with the German Society for Pediatric Surgery and the German Society for Pediatric Medicine. J Dtsch Dermatol Ges 2008, 6, 324–9. [CrossRef]
- Gunes D, Cecen E, Ozguven AA, Kocaoglu S, Kenan Y, Gul S, et al. Hemangiomas of Childhood: Analysis of 116 Cases. Turk Klin Tip Bilim 2009, 29, 1137–48.
- Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, et al. Initiation and Use of Propranolol for Infantile Hemangioma: Report of a Consensus Conference. Pediatrics 2013, 131, 128–40. [CrossRef]
- Leaute-Labreze, C.; Dumas de la Roque, E.; Hubiche, T.; Boralevi, F.; Thambo, J.B.; Taieb, A. Propranolol for Severe Hemangiomas of Infancy. N Engl J Med 2008, 358, 2649–51. [Google Scholar] [CrossRef]
- Leaute-Labreze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, et al. A Randomized, Controlled Trial of Oral Propranolol in Infantile Hemangioma. N Engl J Med 2015, 372, 735–46. [CrossRef] [PubMed]
- Solman, L.; Murabit, A.; Gnarra, M.; Harper, J.I.; Syed, S.B.; Glover, M. Propranolol for Infantile Haemangiomas: Single Centre Experience of 250 Cases and Proposed Therapeutic Protocol. Arch Dis Child 2014, 99, 1132–6. [Google Scholar] [CrossRef]
- Mariani, L.G.; Ferreira, L.M.; Rovaris, D.L.; Bonamigo, R.R.; Kiszewski, A.E. Infantile Hemangiomas: Risk Factors for Complications, Recurrence and Unaesthetic Sequelae. An Bras Dermatol 2022, 97, 37–44. [Google Scholar] [CrossRef]
- Buckmiller, L.M.; Munson, P.D.; Dyamenahalli, U.; Dai, Y.; Richter, G.T. Propranolol for Infantile Hemangiomas: Early Experience at a Tertiary Vascular Anomalies Center. Laryngoscope 2010, 120, 676–81. [Google Scholar] [CrossRef]
- Buckmiller, L.; Dyamenahalli, U.; Richter, G.T. Propranolol for Airway Hemangiomas: Case Report of Novel Treatment. Laryngoscope 2009, 119, 2051–4. [Google Scholar] [CrossRef]
- Chinnadurai S, Fonnesbeck C, Snyder KM, Sathe NA, Morad A, Likis FE, et al. Pharmacologic Interventions for Infantile Hemangioma: A Meta-Analysis. Pediatrics 2016, 137, e20153896. [CrossRef]
- Rotter, A.; de Oliveira, Z.N.P. Infantile Hemangioma: Pathogenesis and Mechanisms of Action of Propranolol. J Dtsch Dermatol Ges 2017, 15, 1185–90. [Google Scholar] [CrossRef]
- Solman L, Glover M, Beattie PE, Buckley H, Clark S, Gach JE, et al. Oral Propranolol in the Treatment of Proliferating Infantile Haemangiomas: British Society for Paediatric Dermatology Consensus Guidelines. Br J Dermatol 2018, 179, 582–9. [CrossRef]
- Tiemann, L.; Hein, S. Infantile Hemangioma: A Review of Current Pharmacotherapy Treatment and Practice Pearls. J Pediatr Pharmacol Ther 2020, 25, 586–99. [Google Scholar] [CrossRef]
- Dong JY, Ning JX, Li K, Liu C, Wang XX, Li RH, et al. Analysis of Factors Affecting the Therapeutic Effect of Propranolol for Infantile Haemangioma of the Head and Neck. Sci Rep 2017, 7, 342. [CrossRef]
- Ji Y, Chen S, Wang Q, Xiang B, Xu Z, Zhong L, et al. Intolerable Side Effects During Propranolol Therapy for Infantile Hemangioma: Frequency, Risk Factors and Management. Sci Rep 2018, 8, 4264. [CrossRef]
- Keller, R.G.; Patel, K.G. Evidence-Based Medicine in the Treatment of Infantile Hemangiomas. Facial Plast Surg Clin North Am 2015, 23, 373–92. [Google Scholar] [CrossRef]
- Leaute-Labreze C, Baselga Torres E, Weibel L, Boon LM, El Hachem M, van der Vleuten C, et al. The Infantile Hemangioma Referral Score: A Validated Tool for Physicians. Pediatrics 2020, 145. [CrossRef]
- Leaute-Labreze, C.; Frieden, I.; Delarue, A. Early Initiation of Treatment with Oral Propranolol for Infantile Hemangioma Improves Success Rate. Pediatr Dermatol 2023, 40, 261–4. [Google Scholar] [CrossRef] [PubMed]
- Giachetti, A.; Diaz, M.S.; Boggio, P.; Posadas Martinez, M.L. Early Propranolol Treatment of Infantile Hemangiomas Improves Outcome. An Bras Dermatol 2023, 98, 310–5. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, F.; Hammill, A.; Patel, M.; Ricci, K.; Dasgupta, R. Malignant Tumors Misdiagnosed as Benign Vascular Anomalies. Pediatr Blood Cancer 2018, 65, e27051. [Google Scholar] [CrossRef] [PubMed]
- Steinklein, J.M.; Shatzkes, D.R. Imaging of Vascular Lesions of the Head and Neck. Otolaryngol Clin North Am 2018, 51, 55–76. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).