Submitted:
29 January 2024
Posted:
30 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
2. Materials and methods
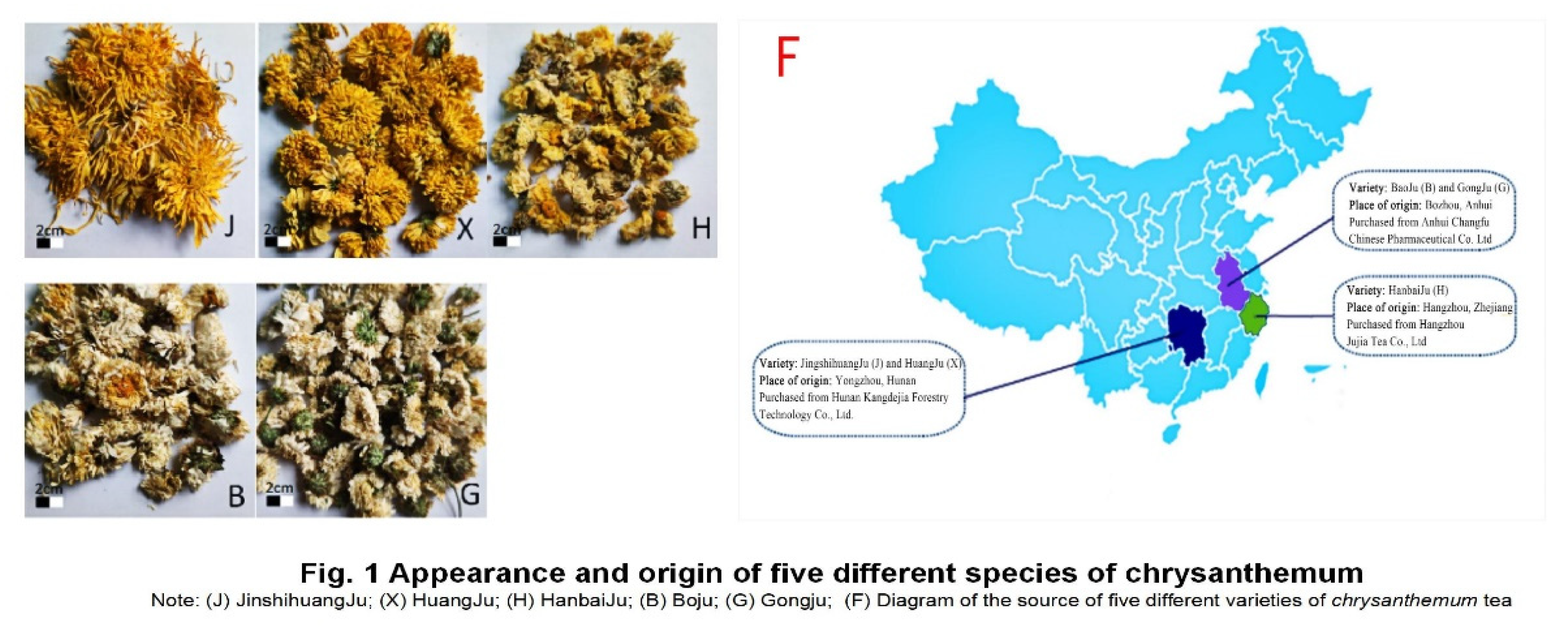
2.1. Chrysanthemum tea samples
2.2. Sensory Evaluation for chrysanthemum tea samples
2.3. Electronic tongue analysis for chrysanthemum tea samples
2.4. HPLC Analysis for chrysanthemum tea samples
2.5. LC-MS/MS-Based untargeted metabolomics analysis
2.6. Metabolomics data acquisition and analysis
2.7. Bioinformatics analysis
3. Results & Discussion
3.1. Sensory quality of the five different varieties of chrysanthemum tea
| Chrysanthemum varieties | |||||
|---|---|---|---|---|---|
| Taste characteristics | J | X | H | B | G |
| Sourness | -25.02±0.00d | -21.36±0.09a | -24.06±0.09c | -21.38±0.08a | -23.36±0.05b |
| Bitterness | 11.11±0.00b | 7.36±0.01e | 11.34±0.01a | 9.33±0.01d | 9.63±0.02c |
| Astringency | 13.60±0.00d | 15.79±0.07b | 14.89±0.05c | 16.79±0.04a | 14.96±0.03c |
| Aftertaset-b | 1.89±0.00a | 0.58±0.03e | 0.90±0.05c | 1.03±0.05b | 0.69±0.02d |
| Aftertaset-a | 2.79±0.00c | 3.36±0.02a | 2.03±0.06e | 3.00±0.07b | 2.60±0.02d |
| Umami | 11.88±0.00a | 11.19±0.02b | 9.55±0.02e | 10.25±0.01d | 10.30±0.02c |
| Richness | 2.17±0.00c | 3.19±0.06a | 1.54±0.06e | 2.33±0.09b | 1.87±0.01d |
| Saltiness | -7.32±0.00b | -2.97±0.03a | -13.25±0.01e | -7.74±0.01c | -9.82±0.06d |
| Chrysanthemum varieties | |||||
|---|---|---|---|---|---|
| Sensory indicators | J | X | H | B | G |
| Bitterness | 2.90±0.72b | 4.13±0.53a | 2.70±0.60bc | 3.13±0.67b | 2.23±0.65c |
| Astringency | 2.47±0.69b | 3.37±0.95a | 2.33±0.50b | 2.50±0.45b | 2.27±0.68b |
| Umami | 1.93±0.75a | 1.47±0.85a | 2.06±0.93a | 1.57±0.39a | 2.10±0.75a |
| Sweetness | 1.60±0.54ab | 1.07±0.14c | 1.77±0.57a | 1.23±0.42bc | 1.93±0.66a |
| Aroma | 2.17±0.57c | 3.43±0.83a | 3.07±0.72ab | 2.23±0.72c | 2.43±0.97bc |
3.2. Comparison of the contents of main active compounds
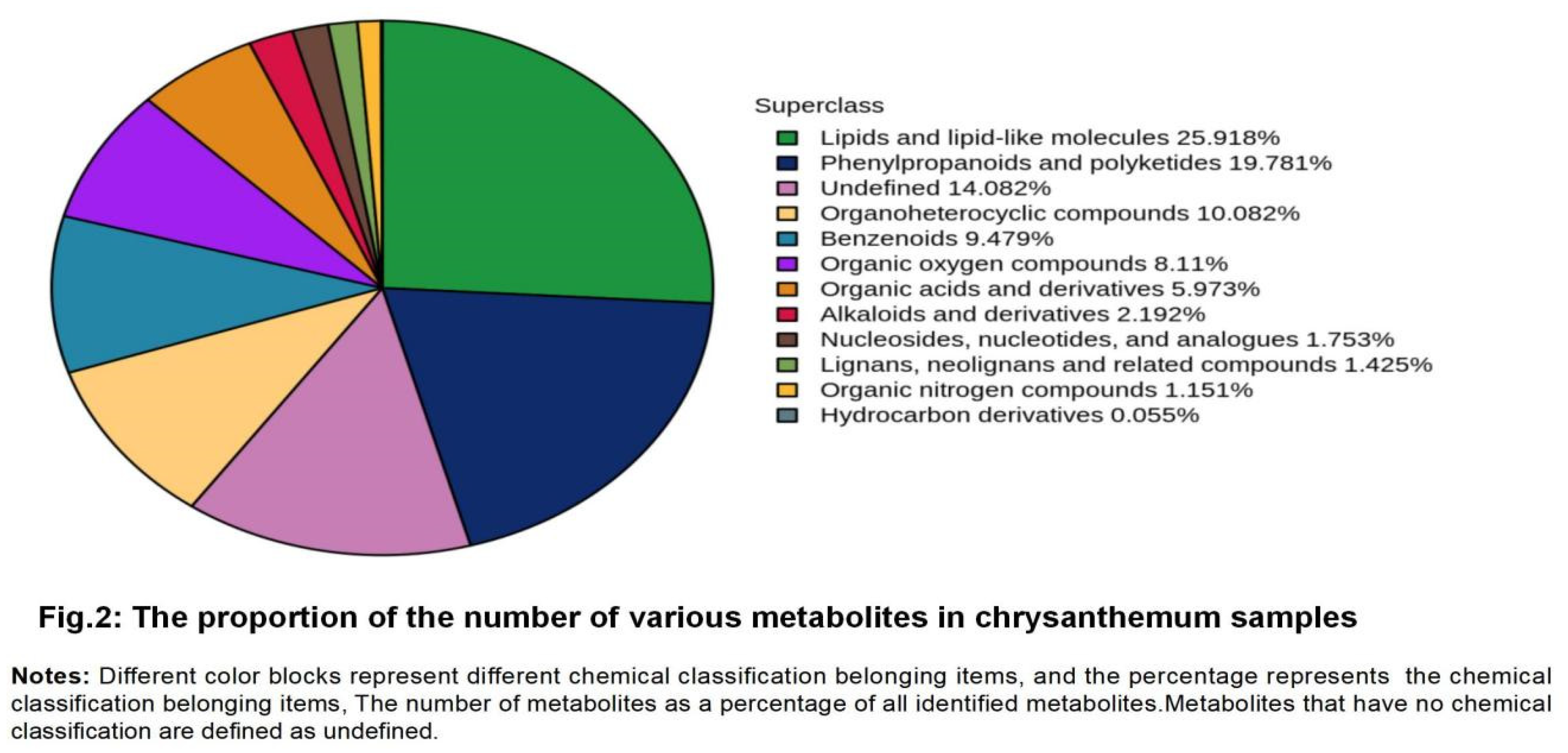
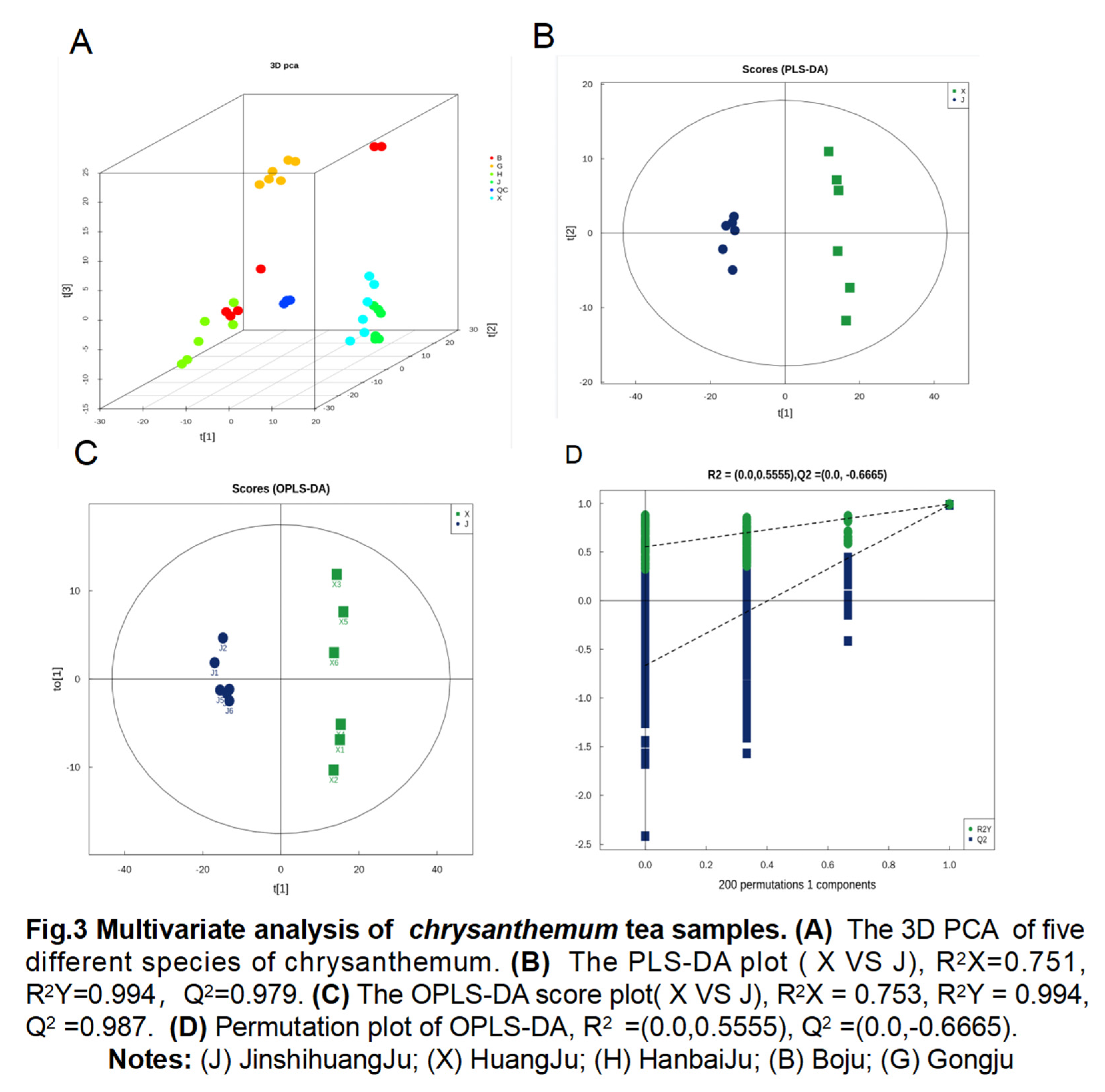
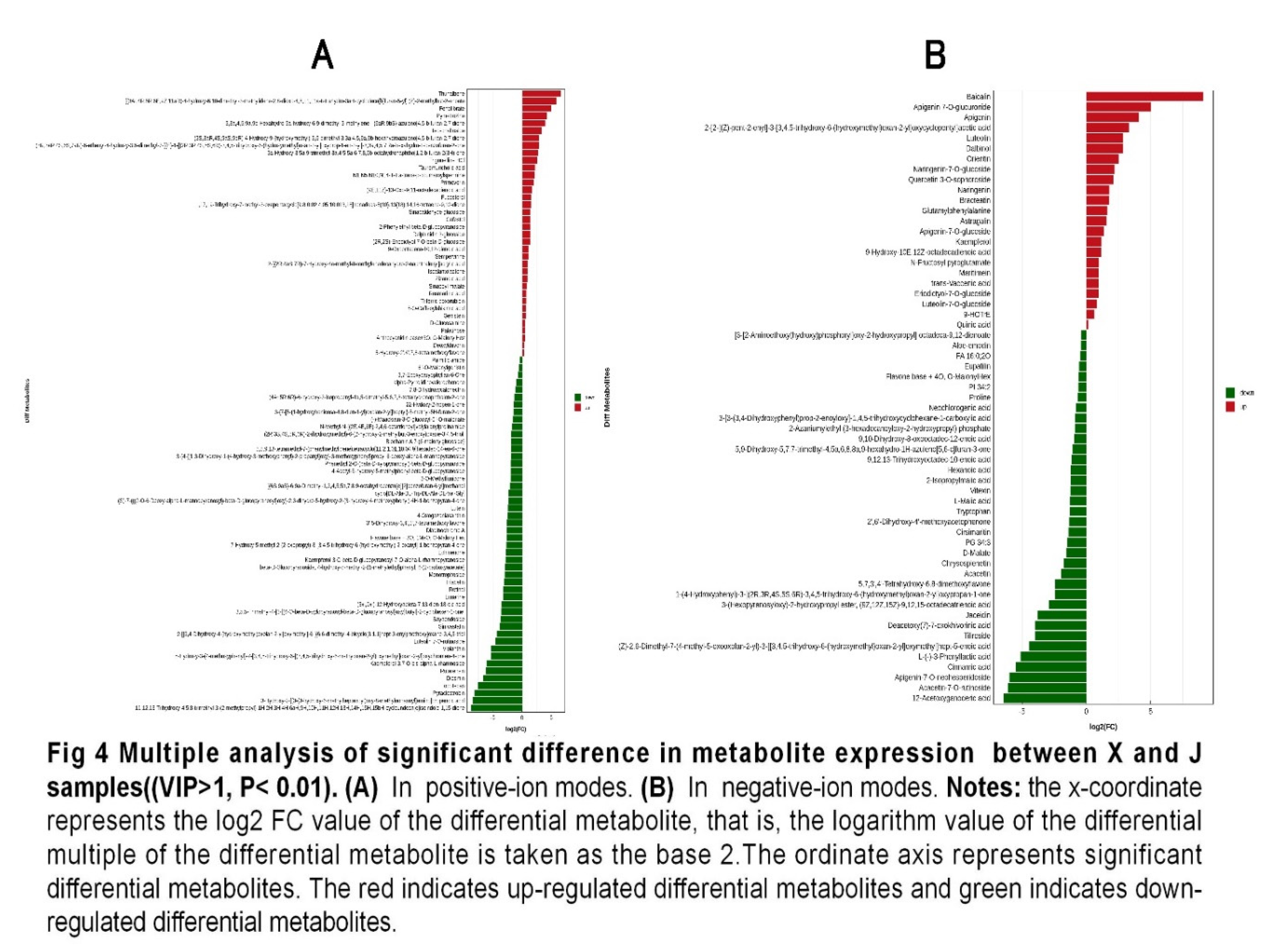
3.3. Nontargeted metabolomics analysis
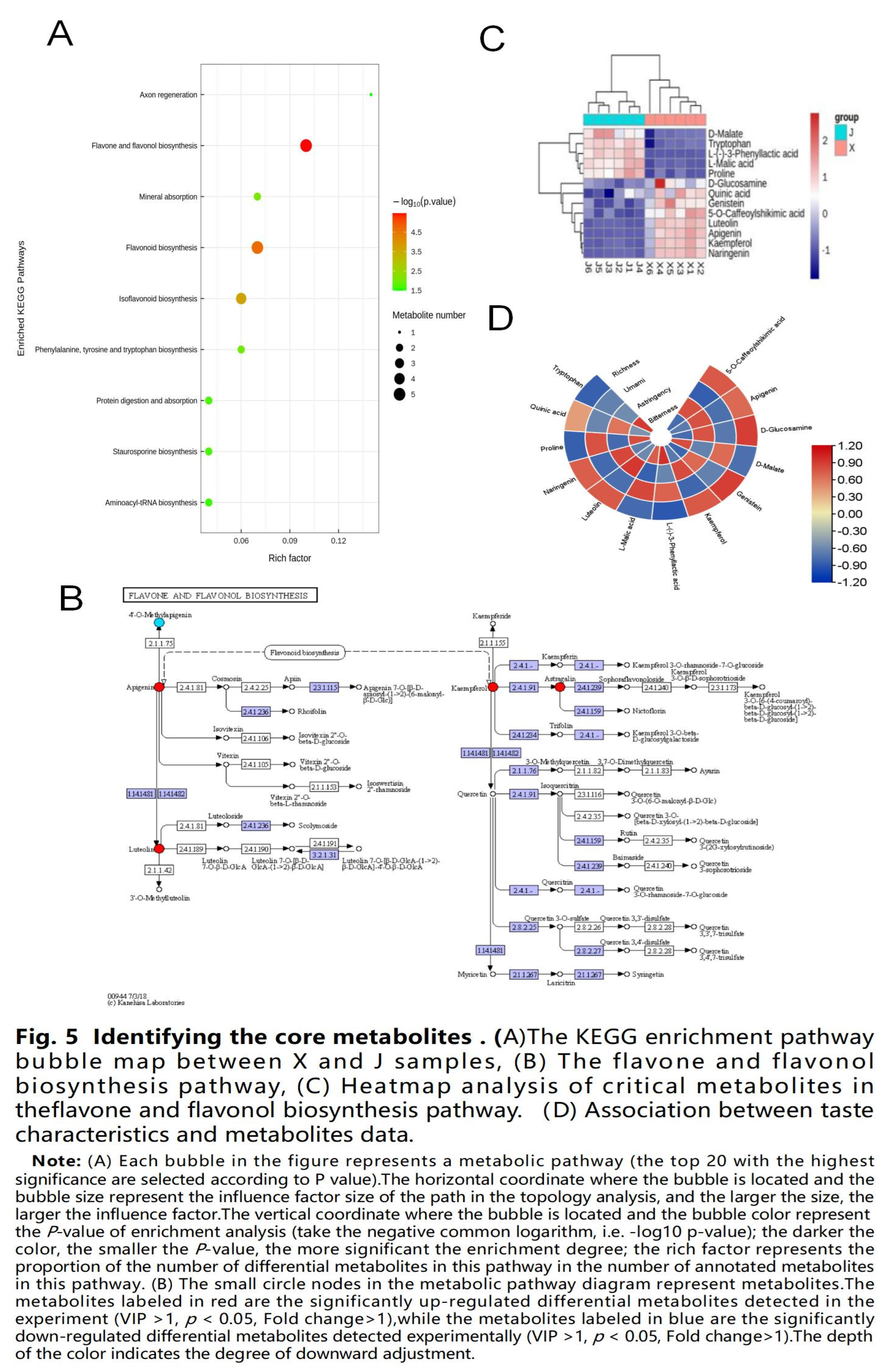
3.4. Identifying the core metabolites
4. Conclusion
Supplementary Materials
Acknowledgments
References
- Li, Y.; Quan, H.; Liang, L.; Yang, T.; Feng, L.; Mao, X.; Wang, Y. Nontargeted metabolomics reveals the discrimination of Cyclocarya paliurus leaves brewed by different methods. Food Res. Int.. 2021, 142, 110221. [Google Scholar] [CrossRef]
- Yuan, H.; Jiang, S.; Liu, Y.; Daniyal, M.; Jian, Y.; Peng, C.; Shen, J.; Liu, S.; Wang, W. The flower head of Chrysanthemum morifolium Ramat. (Juhua): A paradigm of flowers serving as Chinese dietary herbal medicine. J Ethnopharmacol. 2020, 261, 113043. [Google Scholar] [CrossRef]
- Han, A.R.; Nam, B.; Kim, B.R.; Lee, K.C.; Song, B.S.; Kim, S.H.; Kim, J.B.; Jin, C.H. Phytochemical Composition and Antioxidant Activities of Two Different Color Chrysanthemum Flower Teas. Molecules. 2019, 24, 329. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, M.; Jiang, Z.; Zafar, S.; Xie, Q.; Yang, Y.; Liu, Y.; Yuan, H.; Jian, Y.; Wang, W. Chemistry and Pharmacological Activity of Sesquiterpenoids from the Chrysanthemum Genus. Molecules. 2021, 26, 3038. [Google Scholar] [CrossRef] [PubMed]
- Xiang-Wei, C.; Dan-Dan, W.; Dong-Jie, C.; Hui, Y.; Xiao-Dong, S.; Wen-Bin, Z.; Jin-Ao, D. Historical Origin and Development of Medicinal and Tea Chrysanthemum morifolium Resources. Modern Chinese Medicine. 2019, 01, 116–123. [Google Scholar]
- Liao, Y.; Zhou, X.; Zeng, L. How does tea (Camellia sinensis) produce specialized metabolites which determine its unique quality and function: a review. Crit Rev Food Sci Nutr. 2022, 62, 3751–3767. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cheng, P.; Wang, J.H.; Li, H. Analysis of Floral Volatile Components and Antioxidant Activity of Different Varieties of Chrysanthemum morifolium. Molecules. 2017, 22, 1790. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, G. Volatile flavor composition of gamguk (Chrysanthemum indicum) flower essential oils. Food Sci. Biotechnol.. 2011, 20, 319–325. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, Y.; Hong, B.; Zhao, X.; Gu, Z. Characteristic Volatile Fingerprints of Four Chrysanthemum Teas Determined by HS-GC-IMS. Molecules. 2021, 26, 7113. [Google Scholar] [CrossRef]
- Kaneko, S.; Chen, J.; Wu, J.; Suzuki, Y.; Ma, L.; Kumazawa, K. Potent Odorants of Characteristic Floral/Sweet Odor in Chinese Chrysanthemum Flower Tea Infusion. J. Agric. Food Chem.. 2017, 65, 10058–10063. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Liu, Z.; Wang, S.; Huang, B.; Hu, Z.; Liu, Y. Comparative transcriptome analysis of three chrysanthemums provides insights into flavonoid and terpenoid biosynthesis. J. Plant Bio. 2021, 64, 389–401. [Google Scholar] [CrossRef]
- Sumner, L.W.; Mendes, P.; Dixon, R.A. Plant metabolomics: large-scale phytochemistry in the functional genomics era. Phytochemistry. 2003, 62, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, W.; Chen, Y.; Zhang, X.; Wang, X.; Wang, F.; Qian, Y.; Qiu, J. Identification of markers for tea authenticity assessment: Non-targeted metabolomics of highly similar oolong tea cultivars (Camellia sinensis var. sinensis). Food Control. 2022, 142, 109223. [Google Scholar] [CrossRef]
- Phat, C.; Moon, B.; Lee, C. Evaluation of umami taste in mushroom extracts by chemical analysis, sensory evaluation, and an electronic tongue system. Food Chem. 2016, 192, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Hui-jie, Y.E.; Shi-feng, L.; Yao-li, O. Study on the interaction between the astringent constituents of different varieties of Chrysanthemum and saliva. Food & Mach.. 2022, 04, 42–46. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.; Granato, D.; Xu, Y.; Ho, C. Association between chemistry and taste of tea: A review. Trends Food Sci Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yao, Y.; Hua, J.; Zhou, Q.; Jiang, Y.; Deng, Y.; Yang, Y.; Wang, J.; Yuan, H.; et al. Phytochemical comparison of different tea (Camellia sinensis) cultivars and its association with sensory quality of finished tea. LWT. 2020, 117, 108595. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, Q.; Chen, Z.; Zhang, J.; Chen, Q.; Wang, Y.; Wei, X. Distinct Changes of Metabolic Profile and Sensory Quality during Qingzhuan Tea Processing Revealed by LC-MS-Based Metabolomics. J. Agric. Food Chem.. 2020, 68, 4955–4965. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Grevink, R.; Zielinski, A.A.F.; Nunes, D.S.; van Ruth, S.M. Analytical Strategy Coupled with Response Surface Methodology To Maximize the Extraction of Antioxidants from Ternary Mixtures of Green, Yellow, and Red Teas (Camellia sinensis var. sinensis). J. Agric. Food Chem.. 2014, 62, 10283–10296. [Google Scholar] [CrossRef]
- Rudge, R.E.D.; Fuhrmann, P.L.; Scheermeijer, R.; van der Zanden, E.M.; Dijksman, J.A.; Scholten, E. A tribological approach to astringency perception and astringency prevention. Food Hydrocoll. 2021, 121, 106951. [Google Scholar] [CrossRef]
- Yuan, H.; Luo, J.; Lyu, M.; Jiang, S.; Qiu, Y.; Tian, X.; Liu, L.; Liu, S.; Ouyang, Y.; Wang, W. An integrated approach to Q-marker discovery and quality assessment of edible Chrysanthemum flowers based on chromatogram–effect relationship and bioinformatics analyses. Ind. Crop Prod. 2022, 188, 115745. [Google Scholar] [CrossRef]
- Gibbins, H.L.; Carpenter, G.H. Alternative Mechanisms of Astringency – What is the Role of Saliva. J Texture Stud. 2013, 44, 364–375. [Google Scholar] [CrossRef]
- Ma, S.; Lee, H.; Liang, Y.; Zhou, F. Astringent Mouthfeel as a Consequence of Lubrication Failure. Angewandte Chemie (International ed. in English). 2016, 55, 5793–5797. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Deng, T.; Yuan, W.; Deng, H.; Jin, M. Plasma metabolomic study in Chinese patients with wet age-related macular degeneration. BMC Ophthalmol. 2017, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.; Lane, G.A.; Otter, D.E.; Hemar, Y.; Quek, S.; Harrison, S.J.; Rasmussen, S. Analysis of metabolic markers of tea origin by UHPLC and high resolution mass spectrometry. Tea – from bushes to mugs: composition, stability and health aspects. Food Res. Int.. 2013, 53, 827–835. [Google Scholar] [CrossRef]
- Yang, C.; Hu, Z.; Lu, M.; Li, P.; Tan, J.; Chen, M.; Lv, H.; Zhu, Y.; Zhang, Y.; Guo, L.; et al. Application of metabolomics profiling in the analysis of metabolites and taste quality in different subtypes of white tea. Food Res. Int.. 2018, 106, 909–919. [Google Scholar] [CrossRef]
- Lin, N.; Liu, X.; Zhu, W.; Cheng, X.; Wang, X.; Wan, X.; Liu, L. Ambient Ultraviolet B Signal Modulates Tea Flavor Characteristics via Shifting a Metabolic Flux in Flavonoid Biosynthesis. J. Agric. Food Chem.. 2021, 69, 3401–3414. [Google Scholar] [CrossRef] [PubMed]
- Roland, W.S.U.; van Buren, L.; Gruppen, H.; Driesse, M.; Gouka, R.J.; Smit, G.; Vincken, J. Bitter Taste Receptor Activation by Flavonoids and Isoflavonoids: Modeled Structural Requirements for Activation of hTAS2R14 and hTAS2R39. J. Agric. Food Chem.. 2013, 61, 10454–10466. [Google Scholar] [CrossRef]
- Nakaya, A.; Katayama, T.; Itoh, M.; Hiranuka, K.; Kawashima, S.; Moriya, Y.; Okuda, S.; Tanaka, M.; Tokimatsu, T.; Yamanishi, Y.; et al. KEGG OC: a large-scale automatic construction of taxonomy-based ortholog clusters. Nucleic Acids Res.. 2013, 41, D353–357. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crop Prod... 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Li, Q.; Jin, Y.; Jiang, R.; Xu, Y.; Zhang, Y.; Luo, Y.; Huang, J.; Wang, K.; Liu, Z. Dynamic changes in the metabolite profile and taste characteristics of Fu brick tea during the manufacturing process. Food Chem. 2021, 344, 128576. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Cao, Q.Q.; Zou, C.; Zhang, Y.H.; Du, Q.Z.; Yin, J.F.; Shi, J.; Xue, S.; Xu, Y.Q. Improving the taste of autumn green tea with tannase. Food Chem. 2019, 277, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int J Mol Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS); Mass Spectrometry (MS);High-Performance Liquid Chromatography (HPLC); False Discovery Rate (FDR); Principal Component Analysis (PCA); Kyoto Encyclopedia of Genes and Genomes (KEGG);Traditional Chinese medicine (TCM);Gas Chromatography-Mass Spectrography(GC-MS); Headspacegas Chromatography-ion Mobility Spectrometry (HS-GC-IMS);Gas chromatography olfactometry (GC-O); Electron Spray Ionization (ESI); |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





