1. Introduction
Calcium ions (Ca
2+), as one ubiquitous messenger in cells, are one of the most important biochemical signals involved in many cellular physiological processes. Changes in Ca
2+ concentration often lead to changes in downstream related kinase levels, thereby regulating physiological activities, such as cell differentiation[
1,
2], apoptosis[
3], gene expression[
4], etc. The calcium signal transduction in cells is precise and diverse, and similar trends may lead to different outcomes. The interaction between organelles and the action of calcium channels jointly maintains the dynamic balance of intracellular calcium levels. The intracellular Ca
2+ homeostasis is an important foundation for maintaining normal physiological activities of cells. Abnormal calcium signaling can lead to the occurrence of diseases. Asthma, a chronic airway disease, often accompanied by shortness of breath and airway remodeling during onset[
5,
6]. At the same time, airway epithelial cells and inflammatory cells secrete PDGF to promote the contraction and proliferation of airway smooth muscle (ASM) cells[
7,
8]. The regulation of ASM cells largely relies on the conduction of calcium signals[
9].
In fact, the distribution of Ca
2+ within cells is not uniform, and there is a certain concentration gradient. Among them, the endoplasmic reticulum (ER) is the largest intracellular organelle related to Ca
2+ storage[
10]. Under normal physiological conditions, the level of Ca
2+ in the ER can reach over a thousand times of the Ca
2+ concentration in cytoplasm[
11]. Excessive or insufficient calcium levels in ER can promote unfolded protein reaction response and cause cell apoptosis[
12]. Mitochondria also have the function of storing and releasing calcium, and play an important role in the conduction of calcium signals[
13]. The enrichment of calcium into the mitochondrial matrix can promote mitochondrial ATP production, but excessive calcium concentration can also induce cell apoptosis[
14]. In cytoplasm, the increase in calcium levels is mainly due to the action of calcium channels on the plasma membrane and the release of calcium stored in organelles. The uptake of extracellular calcium by cells mainly depends on several calcium channels for controlling calcium influx on the plasma membrane, such as L-type calcium channels[
15], and store-operated Ca
2+(SOC) channels[
16].
The regulation of calcium signaling by cells receiving extracellular cytokine stimulation is inseparable from the G protein coupled receptor (GPCR) on the plasma membrane. GPCR can capture the stimulation of cytokines and convert them into the activation of intracellular phospholipase C (PLC)[
17]. Phosphorylated PLC can further promote the production of inositol-1,4,5 tris-phosphonate (IP
3)[
18]. IP
3 receptor (IP
3R) is predominantly located on the ER, and induction by IP
3 can promote the release of calcium from the ER, leading to an upregulation of cytoplasmic calcium levels[
19,
20]. In addition, there is another calcium release channel on the ER, the ryanodine receptor [
21], and the ER organelles maintain high calcium level through SERCA pump uptake of calcium from cytoplasm[
22]. The uptake of calcium by mitochondria depends on related calcium transporters, such as mitochondrial calcium uniporter (MCU)[
23]. In addition, studies have shown that mitochondrial associated membranes (MAMs), the contact part between the outer and ER membranes of mitochondria, are involved in calcium signaling between the ER and mitochondria[
24,
25].
The changes in intracellular calcium signals are a rapidly changing process, sometimes recovering from calcium oscillations to a resting state in just a few tens of seconds. Traditional chemical fluorescent dyes for calcium verification often have inaccuracies, significant errors, and cannot reflect changes in subcellular calcium levels in a timely manner. Fluorescence resonance energy transfer (FRET) is an emerging technique in recent decades, with timeliness and the ability to observe changes in kinase levels at the cellular or subcellular level in a targeted manner[
26,
27]. FRET-based calcium biosensors enable real-time visualization of changes in intracellular and subcellular calcium levels. Calmodulin (CaM) is the core part of the construction of calcium biosensors[
28]. CaM has affinity for calcium ions, which can cause certain conformational changes after binding to calcium ions, resulting in spatial positional changes in the fluorescence fragments at both ends of the sensor[
29], and generating FRET changes. By adding localization peptides[
30], FRET biosensors can achieve calcium detection at subcellular levels.
In this paper, we used FRET technique for real-time monitoring calcium levels in cytoplasm, ER, mitochondria outer membrane (Out-Mito) and mitochondrial matrix (mito-matrix) by four versions of FRET biosensors. They are cytoplasmic calcium (Cyto-Ca
2+), ER calcium (ER-Ca
2+), mitochondrial outer membrane calcium (Out-Mito-Ca
2+), and mito-matrix calcium (Mito-Ca
2+) biosensors. These FRET calcium biosensors were proteins consisting of four core parts including ECFP, CaM, M13 peptide and YPet[
31], while specific signals peptides can help target the biosensor proteins to subcellular locations (diagram shown in
Figure 1A). When the calmodulin (CaM) in FRET biosensors bind to calcium in cells, CaM can interact with M13 to change the spatial position of the two fluorescence fragments, hence the FRET changes were generated[
32]. In this work, by combining FRET biosensors with several common calcium channels’ inhibitors, the PDGF-induced flow mechanism of calcium signaling at different compartments of ASM cells was explored. Our work shows that PDGF stimulation promotes calcium influx through the plasma membrane and calcium release from the ER, leading to increase of cytosolic calcium level and mitochondrial uptake of calcium. Moreover, the extracellular calcium influx through plasma membrane affected ER calcium release, which is not entirely dependent on the PLC-IP
3R pathway.
2. Materials and Methods
2.1. Cell culture
The airway smooth muscle (ASM) cells used in our experiments were purchased from Beina Biotech Co., which are derived from the ASM of female Sprague Dawley rats aged 6-8 weeks. Primary rat ASM cells were cultured in a low sugar medium (DMEM, Sigma Aldrich) containing 10% fetal bovine serum (FBS, Thermo), and maintained in a humid incubator at 37℃ with 5% CO2.
2.2. Chemical reagents
Dimethyl sulfoxide (DMSO) and platelet derived growth factor (PDGF) were purchased from Beyotime Biotechnology; 2-Amino-ethoxydiphenyl borate (2-APB), and nifedipine were from Sigma-Aldrich; Thapsigargin and U73122 from MedChemExpress; Ruthenium red (RuR) from Macklin; Fibronectin and calcium free culture medium from Thermo Fisher Scientific.
2.3. Constructions of calcium FRET plasmids
The four versions of calcium FRET biosensor plasmids include cytoplasmic calcium (Cyto-Ca
2+), ER calcium (ER-Ca
2+), mitochondria outer membrane calcium (Out-Mito-Ca
2+) and mito-matrix calcium (Mito-Ca
2+) biosensors (listed in
Figure 1A). For Cyto-Ca
2+ biosensor, the FRET expression plasmid was using pcDNA3.1-Ca
2+-YPet version reported in our previous work[
31].
For ER-Ca
2+ biosensor, the expression plasmid was generated using the pcDNA3.1-Ca
2+-YPet and pRSETb-Ca
2+-YPet plasmids as templates. By the PCR primer design, the DNA sequence of ER-targeting signal peptide (MLLPVLLLGLLGAAAD) [
33] was added to the 5' end of the forward primer behind
Hind III restriction enzyme site while the ER retention peptide (KDEL) sequence was added to the 5' end of the reverse primer before
EcoR I site. The DNA fragment, which contains Ca
2+-YPet portion along with the two signal peptides, was amplified by PCR using pRSETb-Ca
2+-YPet plasmid as the template. After the PCR product was purified using the gel extraction kit (Vazyme, DC301-01), the fragment “ER-ECFP-CaM-M13-YPet-KDEL” and pcDNA3.1-Ca
2+-YPet plasmid were both double digested using
Hind III and
EcoR I. The digested PCR fragment and pcDNA3.1 vector were ligated together to generate ER-Ca
2+ biosensor.
Similarly, for Out-Mito-Ca
2+ biosensor, the signal peptide DAKAP1 encoding sequence[
34] was added to the 5' end of the forward primer behind
Hind III. The DAKAP1-ECFP-CaM-M13-YPet fragment was amplified by PCR, and ligated into pcDNA3.1 vector after digestion with
Hind III and
EcoR I to generate the Out-Mito-Ca
2+ biosensor.
For Mito-Ca
2+ biosensor, we obtained the mitochondrial matrix-targeting signal DNA fragment by gene synthesis service, which contains the signal peptide sequence as four folds of COX8 (4xMSVLTPLLLRGLTGSARRLPVPRAKIHSLGDP) and a linker peptide (RSGSAKDPT)[
35]. The 4xCOX8-linker fragment and pcDNA3.1-ECFP-CaM-M13-YPet were double digested with
Nhe I and
Hind III, and ligated together to generate the Mito-Ca
2+ biosensor.
2.4. FRET plasmid transfection
According to the instruction of Lipofectamine 3000 (Invitrogen), the appropriate number of ASM cells were incubated into a 12-well plate overnight in advance, and then FRET plasmids (1.5 μg per well) were transfected into cells by using Lipofectamine 3000 regents. After 8-10 hours, culture medium was replaced with a new low sugar DMEM medium containing 10% FBS. After 36 hours of transfection, ASM cells were digested with Accutase Cell Dissociation Reagent (Thermo) and inoculated into a confocal dish (NEST) precoated with fibronectin. Then the cells were subjected to starvation treatment in medium containing 1% FBS for 16-20 hours. Subsequently, FRET imaging experiments were conducted.
2.5. Intracellular FRET imaging of the calcium biosensors
The Zeiss microscope imaging system has a multi-position function and is equipped with a cell culture chamber (Zeiss). In the FRET microscope system, the filter parameters of the ECFP and FRET channels are excitation (436 ± 10 nm), dichroic mirror (455 nm), and emission (480 ± 20 nm) for ECFP, and emission (535 ± 15 nm) for YPet. In our imaging experiments, an x100 Oil objective was chosen to acquire the FRET images, and the ASM cell samples were placed in the cell culture chamber. By controlling the fast switch between ECFP and FRET channels through Zeiss software system, the image data of ASM cells from both channels were collected almost simultaneously. During the experimental process, fluorescence images were collected at interval of 1 min for 20 minutes duration.
Inhibitor pretreatments include adding appropriate concentrations of reagents such as DMSO (<0.1% v/v), 2-APB (100 μM), nifedipine (10 μM), and thapsigargin (10 μM), or RuR (10 μM) in advance, followed by incubation for 1 hour before cell imaging. To add PDGF stimulation (50 ng/ mL) in the middle, 1 mL medium containing PDGF was injected into the dish through a guiding microtube without disruption of the imaging process. In the experimental group with calcium free culture, L-glutamine (0.4 mL) and sodium pyruvate (0.2 mL) were added to a calcium free medium (19.4 mL) in advance. Before performing live cell imaging, removed the culture medium, washed three times with PBS (phosphate buffered saline), and then added pre-prepared calcium-free culture medium immediately before placed on the microscope.
2.6. Data processing
Quantitative analysis of image data was conducted using the FRET image analysis software FluoCell 6.0.0[
36]. Principally, after background subtraction, the ratio of the fluorescence intensity was calibrated between ECFP and FRET channels by pixel to pixel, and ratiometric images were acquired along with FRET/ECFP ratio data. The data statistical analysis was conducted in GraphPad Prism 6.0 software. The time-course curves of FRET ratio (Mean ± S.E.M.), and the graphs with scattering dots (Mean ± S.D.) along with the differences between each two data groups were analyzed.
Student’s t-tests were conducted between the control group and an experimental group, and multiple rounds of t-tests were conducted on variable experimental conditions. *, **, ***, and **** indicate p value < 0.05, 0.01, 0.001, and 0.0001 for significant difference, while 'ns' indicates no significant difference.
4. Discussion
The intracellular calcium homeostasis is extremely important and closely related to many cellular physiological activities [
43,
44,
45]. PDGF can also regulate physiological activities such as cell proliferation, migration, and differentiation by altering intracellular calcium levels[
9]. We investigated PDGF-induced shuttles of intracellular calcium among different cellular compartments by generating calcium FRET biosensors tagged with subcellular-targeted peptides.
Previous studies have shown that PDGF stimulation increases calcium influx[
46]. We confirmed that PDGF-induced cytosolic calcium increase is also related to the regulation of the ER. Our experiments demonstrated that extracellular calcium flow through the plasma membrane can affect the calcium regulations of cytoplasm, ER, and mitochondria. When the calcium in the extracellular environment is emptied, PDGF-induced [Ca
2+]
C increase in cytoplasm, and the release of calcium from the ER are significantly inhibited (
Figure 1). After decreased ER calcium release, the cytoplasm lose important sources of calcium signaling (
Figure 2 and
Figure 3). Therefore, the calcium signaling changes caused by PDGF are mainly provided by the extracellular environment and ER, flowing towards the cytoplasm including mitochondria.
Cells have a certain self-protection mechanism, and when calcium overload occurs in the ER, certain pathways are activated to maintain intracellular calcium homeostasis[
47,
48]. In addition, we also observed changes in intracellular calcium signal flow after inhibiting IP
3R, L-type calcium pathway, and SERCA calcium pump. The inhibition of IP
3R can reduce the upregulation of cytoplasmic and mitochondrial calcium caused by PDGF. However, there is not much inhibitory effect on the calcium release from the ER. This may be regulated by another calcium release channel ryanodine receptor on ER membrane. Additionally, our recent work reported that L-type calcium channel is not relevant with mechanical stretch-activated ERK via calcium signals[
49]. Hence, chemical and biomechanical activations of intracellular calcium may be through different sets of calcium channels on the plasma membrane.
After a certain inhibition of calcium influx in cells, the average calcium changes in cytoplasm, and mitochondria decreased significantly, which is consistent with the experimental results in calcium free culture medium. Surprisingly, PDGF-induced ER calcium release was attenuated with reserved higher [Ca
2+]
ER after immediate switch to calcium free culture medium (
Figure 1I&J). This indicates that extracellular calcium influx may enhance ER release of calcium. This observation can be attributed to calcium-dependent activation of ryanodine receptor (RyR) resulting in ER calcium release[
50]. Our data proved that extracellular calcium influx effects ER calcium release and is essential for PDGF-induced full activation of cytosolic calcium signals in cells.
The inhibition of SERCA calcium pump initially leads to an increase in cytoplasmic calcium levels, but gradually decreases over time and becomes unresponsive to PDGF stimulation (
Figure 2 and
Figure 3) [
51]. Our experimental data confirmed the process for calcium release from ER into the cytosol and further exported into the medium when addition of SERCA inhibitor (
Figure 5C-F). Hence, the dynamic and active calcium shuttling between ER storage and cytoplasm is essential to maintain calcium homeostasis in cells.
The calcium level near the outer mitochondrial membrane shows similar responses to PDGF-induced cytosolic calcium, which was not influenced by the mitochondrial MCU channel inhibition with RuR (
Figure 4). In addition, treatment with RuR inhibitor didn’t interfere with PDGF-induced cytosolic calcium increase, but significantly reduce ER's release of calcium (
Figure 5G-L), likely through RuR inhibition of ryanodine receptors on ER[
42]. Hypothetically, the extracellular calcium might have a compensative role in the cytosolic calcium from the less ER release with RuR treatment.
Figure 1.
PDGF-induced calcium changes in cell cytoplasm and ER storage. The calcium concentrations were measured by calcium FRET biosensors. (A) The depictions of the four versions of calcium FRET biosensors, as described in the Methods. (B, C) Ratiometric FRET images of Cyto-Ca2+ biosensor induced with PDGF (50 ng/mL) in ASM cells pretreated with DMSO (as control) or U73122 (10 μM) in normal culture medium (B), or in calcium-free culture medium (C). (D) Quantified time-course curves of cytoplasmic calcium FRET ratio (FRET/ECFP) in the ASM cells under the (B, C) conditions in normal or Ca2+-free medium. (E, F) Statistical comparisons of peak values of FRET/ECFP ratio (E), and the FRET change rates (F) from the quantified curves in (D). The sample sizes of Cyto-Ca2+ FRET measurements for DMSO, U73122, (-)Ca2+/DMSO, and (-)Ca2+/U73122 are 55, 40, 72, 66, respectively. (G, H) Ratiometric FRET images of ER-Ca2+ biosensor induced with PDGF in ASM cells pretreated with DMSO or U73122 in normal medium (G) or Ca2+-free medium (H). (I, J, K) Quantified time-course curves of ER calcium FRET ratio (I), and the statistical comparisons of peak values of FRET/ECFP ratio (J) and the FRET change rates (K) under the various conditions of (G, H). The sample sizes of ER-Ca2+ FRET measurements for DMSO, U73122, (-)Ca2+/DMSO, and (-)Ca2+/U73122 are 58, 47, 45, 47, respectively.
Figure 1.
PDGF-induced calcium changes in cell cytoplasm and ER storage. The calcium concentrations were measured by calcium FRET biosensors. (A) The depictions of the four versions of calcium FRET biosensors, as described in the Methods. (B, C) Ratiometric FRET images of Cyto-Ca2+ biosensor induced with PDGF (50 ng/mL) in ASM cells pretreated with DMSO (as control) or U73122 (10 μM) in normal culture medium (B), or in calcium-free culture medium (C). (D) Quantified time-course curves of cytoplasmic calcium FRET ratio (FRET/ECFP) in the ASM cells under the (B, C) conditions in normal or Ca2+-free medium. (E, F) Statistical comparisons of peak values of FRET/ECFP ratio (E), and the FRET change rates (F) from the quantified curves in (D). The sample sizes of Cyto-Ca2+ FRET measurements for DMSO, U73122, (-)Ca2+/DMSO, and (-)Ca2+/U73122 are 55, 40, 72, 66, respectively. (G, H) Ratiometric FRET images of ER-Ca2+ biosensor induced with PDGF in ASM cells pretreated with DMSO or U73122 in normal medium (G) or Ca2+-free medium (H). (I, J, K) Quantified time-course curves of ER calcium FRET ratio (I), and the statistical comparisons of peak values of FRET/ECFP ratio (J) and the FRET change rates (K) under the various conditions of (G, H). The sample sizes of ER-Ca2+ FRET measurements for DMSO, U73122, (-)Ca2+/DMSO, and (-)Ca2+/U73122 are 58, 47, 45, 47, respectively.
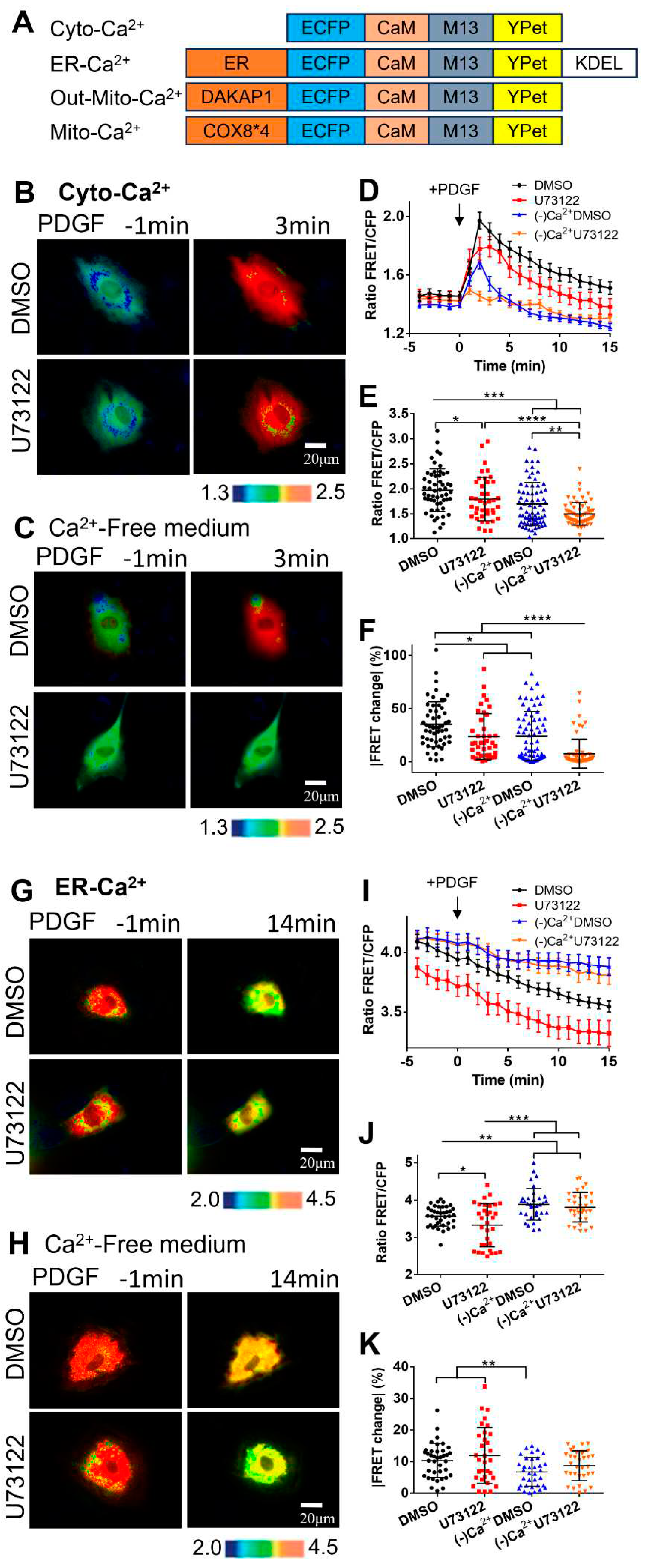
Figure 2.
PDGF-induced calcium changes in cell cytoplasm and ER storage with one-hour pre-incubation of calcium channel inhibitors. (A) Ratiometric FRET images of Cyto-Ca2+ biosensor induced with PDGF in ASM cells pretreated for one hour with DMSO (as control), 2-APB (100 μM), nifedipine (10 μM), and thapsigargin (10 μM). (B) Quantified time-course curves of cytoplasmic calcium FRET ratio (FRET/ECFP) in the ASM cells under the (A) conditions. (C, D) Statistical comparisons of peak values of FRET/ECFP ratio (C), and the FRET change rates (D) from the quantified curves in (B). The sample sizes of Cyto-Ca2+ FRET measurements for DMSO, 2-APB, nifedipine, and thapsigargin are 66, 53, 49, 51, respectively. (E) Ratiometric FRET images of ER-Ca2+ biosensor induced with PDGF in ASM cells pretreated with DMSO, 2-APB, nifedipine, and thapsigargin. (F, G, H) Quantified time-course curves of ER calcium FRET ratio (F), and the statistical comparisons of peak values of FRET/ECFP ratio (G) and the FRET change rates (H) under the various conditions of (E). The sample sizes of ER-Ca2+ FRET measurements for DMSO, 2-APB, nifedipine, and thapsigargin are 53, 52, 50, 71, respectively.
Figure 2.
PDGF-induced calcium changes in cell cytoplasm and ER storage with one-hour pre-incubation of calcium channel inhibitors. (A) Ratiometric FRET images of Cyto-Ca2+ biosensor induced with PDGF in ASM cells pretreated for one hour with DMSO (as control), 2-APB (100 μM), nifedipine (10 μM), and thapsigargin (10 μM). (B) Quantified time-course curves of cytoplasmic calcium FRET ratio (FRET/ECFP) in the ASM cells under the (A) conditions. (C, D) Statistical comparisons of peak values of FRET/ECFP ratio (C), and the FRET change rates (D) from the quantified curves in (B). The sample sizes of Cyto-Ca2+ FRET measurements for DMSO, 2-APB, nifedipine, and thapsigargin are 66, 53, 49, 51, respectively. (E) Ratiometric FRET images of ER-Ca2+ biosensor induced with PDGF in ASM cells pretreated with DMSO, 2-APB, nifedipine, and thapsigargin. (F, G, H) Quantified time-course curves of ER calcium FRET ratio (F), and the statistical comparisons of peak values of FRET/ECFP ratio (G) and the FRET change rates (H) under the various conditions of (E). The sample sizes of ER-Ca2+ FRET measurements for DMSO, 2-APB, nifedipine, and thapsigargin are 53, 52, 50, 71, respectively.
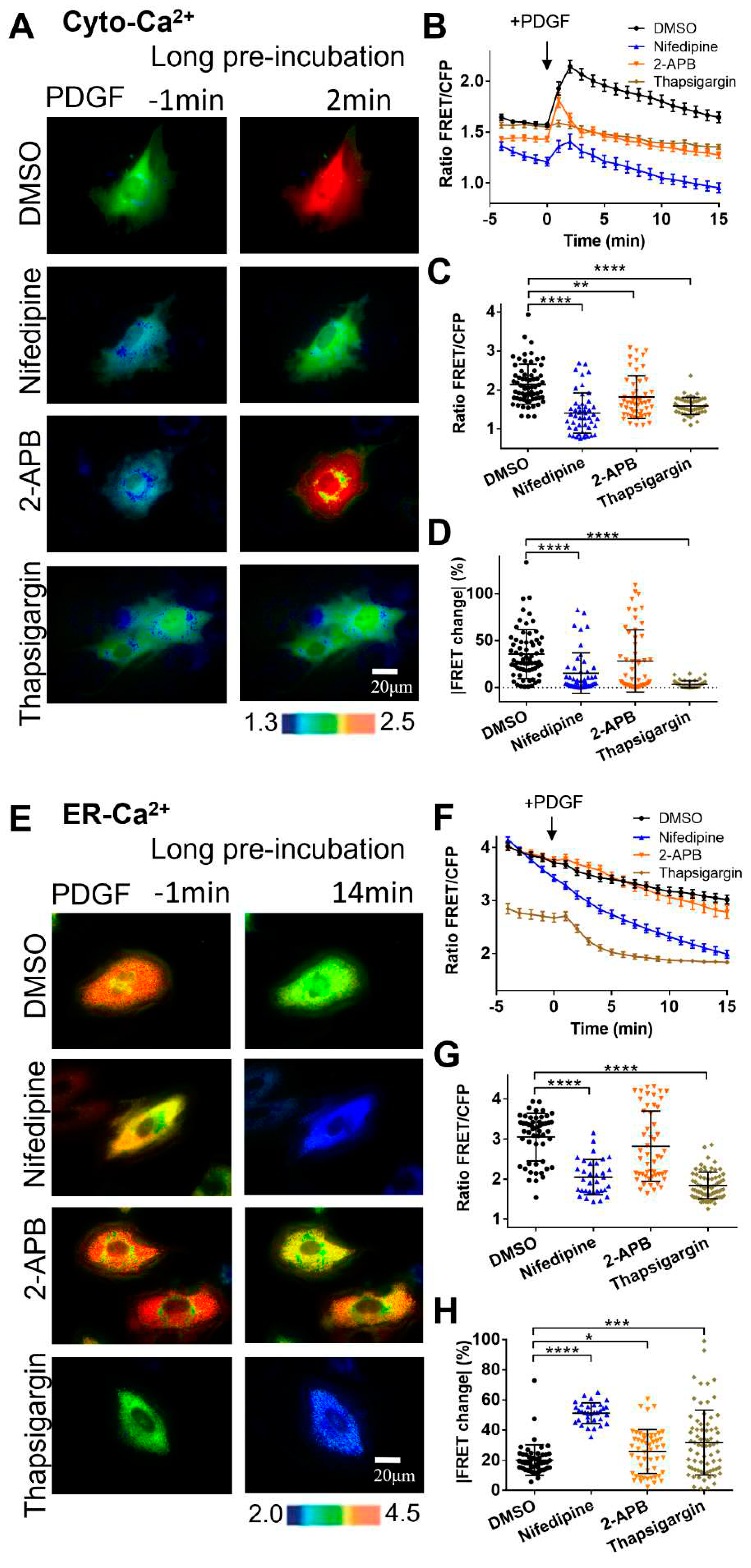
Figure 3.
PDGF-induced calcium changes in cell cytosol and ER storage under immediate addition of calcium channel inhibitors before microscopic imaging. (A-D) Ratiometric FRET images of Cyto-Ca2+ biosensor induced with PDGF in ASM cells with immediate addition of DMSO (as control), 2-APB (100 μM), nifedipine (10 μM), and thapsigargin (10 μM) (A), the corresponding quantified time-course curves of cytoplasmic calcium FRET ratio (FRET/ECFP) (B), and statistical comparisons of peak values of FRET ratio (C), and the FRET change rates (D). The sample sizes of Cyto-Ca2+ FRET for DMSO, 2-APB, nifedipine, and thapsigargin are 37, 36, 32, 32, respectively. (E-H) Ratiometric FRET images of ER-Ca2+ biosensor induced with PDGF in ASM cells with immediate addition of DMSO, 2-APB, nifedipine, and thapsigargin (E), quantified time-course curves of ER calcium FRET ratio (F), and the statistical comparisons of peak values of FRET/ECFP ratio (G) and the FRET change rates (H). The sample sizes of ER-Ca2+ FRET for DMSO, 2-APB, nifedipine, and thapsigargin are 37, 42, 38, 38, respectively.
Figure 3.
PDGF-induced calcium changes in cell cytosol and ER storage under immediate addition of calcium channel inhibitors before microscopic imaging. (A-D) Ratiometric FRET images of Cyto-Ca2+ biosensor induced with PDGF in ASM cells with immediate addition of DMSO (as control), 2-APB (100 μM), nifedipine (10 μM), and thapsigargin (10 μM) (A), the corresponding quantified time-course curves of cytoplasmic calcium FRET ratio (FRET/ECFP) (B), and statistical comparisons of peak values of FRET ratio (C), and the FRET change rates (D). The sample sizes of Cyto-Ca2+ FRET for DMSO, 2-APB, nifedipine, and thapsigargin are 37, 36, 32, 32, respectively. (E-H) Ratiometric FRET images of ER-Ca2+ biosensor induced with PDGF in ASM cells with immediate addition of DMSO, 2-APB, nifedipine, and thapsigargin (E), quantified time-course curves of ER calcium FRET ratio (F), and the statistical comparisons of peak values of FRET/ECFP ratio (G) and the FRET change rates (H). The sample sizes of ER-Ca2+ FRET for DMSO, 2-APB, nifedipine, and thapsigargin are 37, 42, 38, 38, respectively.
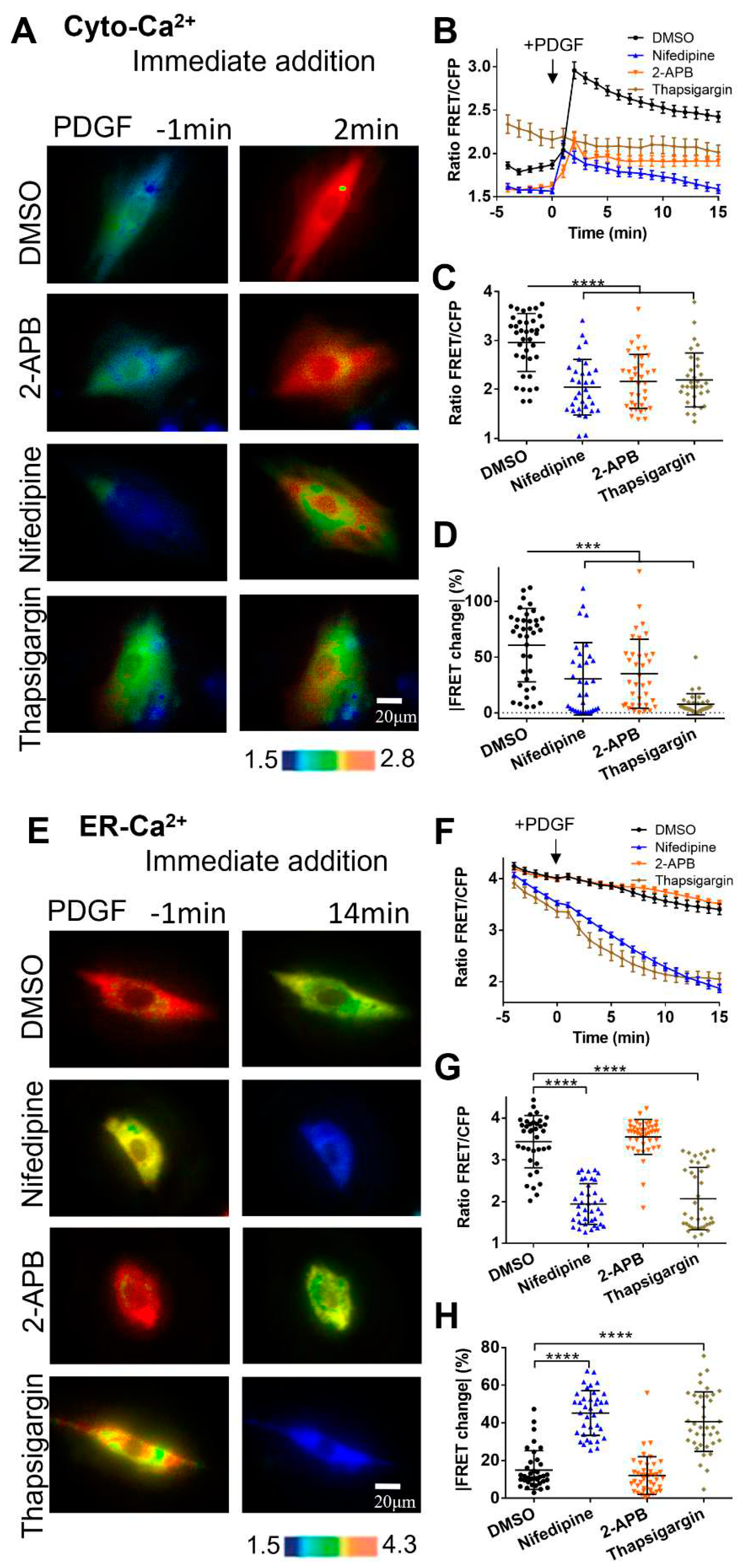
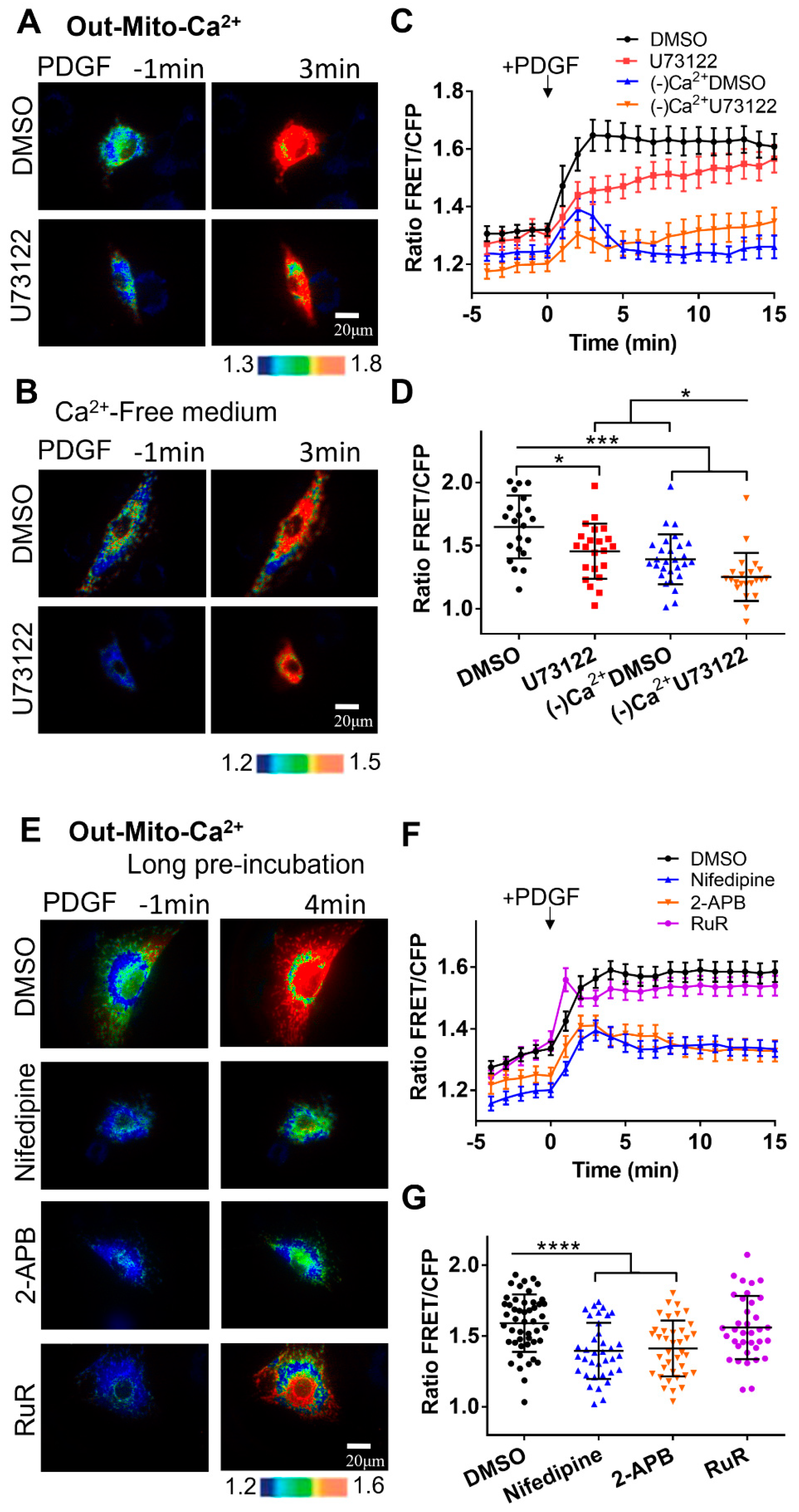
Figure 4.
PDGF-induced calcium changes at the outer mitochondrial membrane measured by Out-Mito-Ca2+ FRET biosensor. (A, B) PDGF-induced ratiometric FRET images of ASM cells pretreated with DMSO or U73122 (10 μM) in normal culture medium (A), or calcium-free medium (B). (C-D) Quantified time-course curves of Out-Mito-Ca2+ FRET ratio (C), and statistical comparisons of peak values of FRET/ECFP ratio (D) under the (A, B) conditions. The sample sizes for DMSO, U73122, (-)Ca2+/DMSO, and (-)Ca2+/U73122 are 33, 33, 40, 33, respectively. (E) Ratiometric FRET images of Out-Mito-Ca2+ biosensor induced by PDGF in ASM cells with one-hour pre-incubation of DMSO, 2-APB (100 μM), nifedipine (10 μM), and RuR (10 μM). (F, G) Quantified time-course curves of Out-Mito-Ca2+ FRET ratio (F), and statistical comparisons of peak values of FRET/ECFP ratio (G). The sample sizes of Out-Mito-Ca2+ FRET for DMSO, 2-APB, nifedipine, thapsigargin, and RuR are 48, 38, 36, 36, respectively.
Figure 4.
PDGF-induced calcium changes at the outer mitochondrial membrane measured by Out-Mito-Ca2+ FRET biosensor. (A, B) PDGF-induced ratiometric FRET images of ASM cells pretreated with DMSO or U73122 (10 μM) in normal culture medium (A), or calcium-free medium (B). (C-D) Quantified time-course curves of Out-Mito-Ca2+ FRET ratio (C), and statistical comparisons of peak values of FRET/ECFP ratio (D) under the (A, B) conditions. The sample sizes for DMSO, U73122, (-)Ca2+/DMSO, and (-)Ca2+/U73122 are 33, 33, 40, 33, respectively. (E) Ratiometric FRET images of Out-Mito-Ca2+ biosensor induced by PDGF in ASM cells with one-hour pre-incubation of DMSO, 2-APB (100 μM), nifedipine (10 μM), and RuR (10 μM). (F, G) Quantified time-course curves of Out-Mito-Ca2+ FRET ratio (F), and statistical comparisons of peak values of FRET/ECFP ratio (G). The sample sizes of Out-Mito-Ca2+ FRET for DMSO, 2-APB, nifedipine, thapsigargin, and RuR are 48, 38, 36, 36, respectively.
Figure 5.
Characterization of calcium levels at cellular compartments. (A, B) Statistical comparisons of calcium FRET levels at cellular cytoplasm (Cyto-Ca2+), ER (ER-Ca2+), and outer mitochondrial membrane (Out-Mito Ca2+in resting ASM cells (A) or with PDGF stimulation (at 5 min) (B). The ratio values and sample sizes for without/with PDGF stimulations: Cyto-Ca2+ (1.6 ± 0.022, N=87)/(2.05 ± 0.05, N=87), ER-Ca2+ (3.9 ± 0.046, N=78)/(3.7 ± 0.043, N=78), and Out-Mito-Ca2+ (1.3 ± 0.021, N=48)/(1.58 ± 0.032, N=48), respectively. (C-F) The time courses (average values) and FRET ratio comparisons of cytosolic calcium (peak values) (C, D) and ER (at 25 min) (E, F) calcium levels in ASM cells treated with DMSO, nifedipine, 2-APB, and thapsigargin. (G-I) Ratiometric FRET images of Cyto-Ca2+ biosensor induced with PDGF (G), the time courses of cytosolic calcium FRET ratio (H), and statistical comparison of peak value of FRET/ECFP ratio (I) in ASM cells pretreated with DMSO, or RuR (10 μM). (J-L) Ratiometric FRET images of ER-Ca2+ biosensor induced with PDGF (J), the time courses (K) and statistical comparison (at 14 min) of FRET/ECFP ratio (L) in ASM cells pretreated with DMSO, or RuR (10 μM).
Figure 5.
Characterization of calcium levels at cellular compartments. (A, B) Statistical comparisons of calcium FRET levels at cellular cytoplasm (Cyto-Ca2+), ER (ER-Ca2+), and outer mitochondrial membrane (Out-Mito Ca2+in resting ASM cells (A) or with PDGF stimulation (at 5 min) (B). The ratio values and sample sizes for without/with PDGF stimulations: Cyto-Ca2+ (1.6 ± 0.022, N=87)/(2.05 ± 0.05, N=87), ER-Ca2+ (3.9 ± 0.046, N=78)/(3.7 ± 0.043, N=78), and Out-Mito-Ca2+ (1.3 ± 0.021, N=48)/(1.58 ± 0.032, N=48), respectively. (C-F) The time courses (average values) and FRET ratio comparisons of cytosolic calcium (peak values) (C, D) and ER (at 25 min) (E, F) calcium levels in ASM cells treated with DMSO, nifedipine, 2-APB, and thapsigargin. (G-I) Ratiometric FRET images of Cyto-Ca2+ biosensor induced with PDGF (G), the time courses of cytosolic calcium FRET ratio (H), and statistical comparison of peak value of FRET/ECFP ratio (I) in ASM cells pretreated with DMSO, or RuR (10 μM). (J-L) Ratiometric FRET images of ER-Ca2+ biosensor induced with PDGF (J), the time courses (K) and statistical comparison (at 14 min) of FRET/ECFP ratio (L) in ASM cells pretreated with DMSO, or RuR (10 μM).
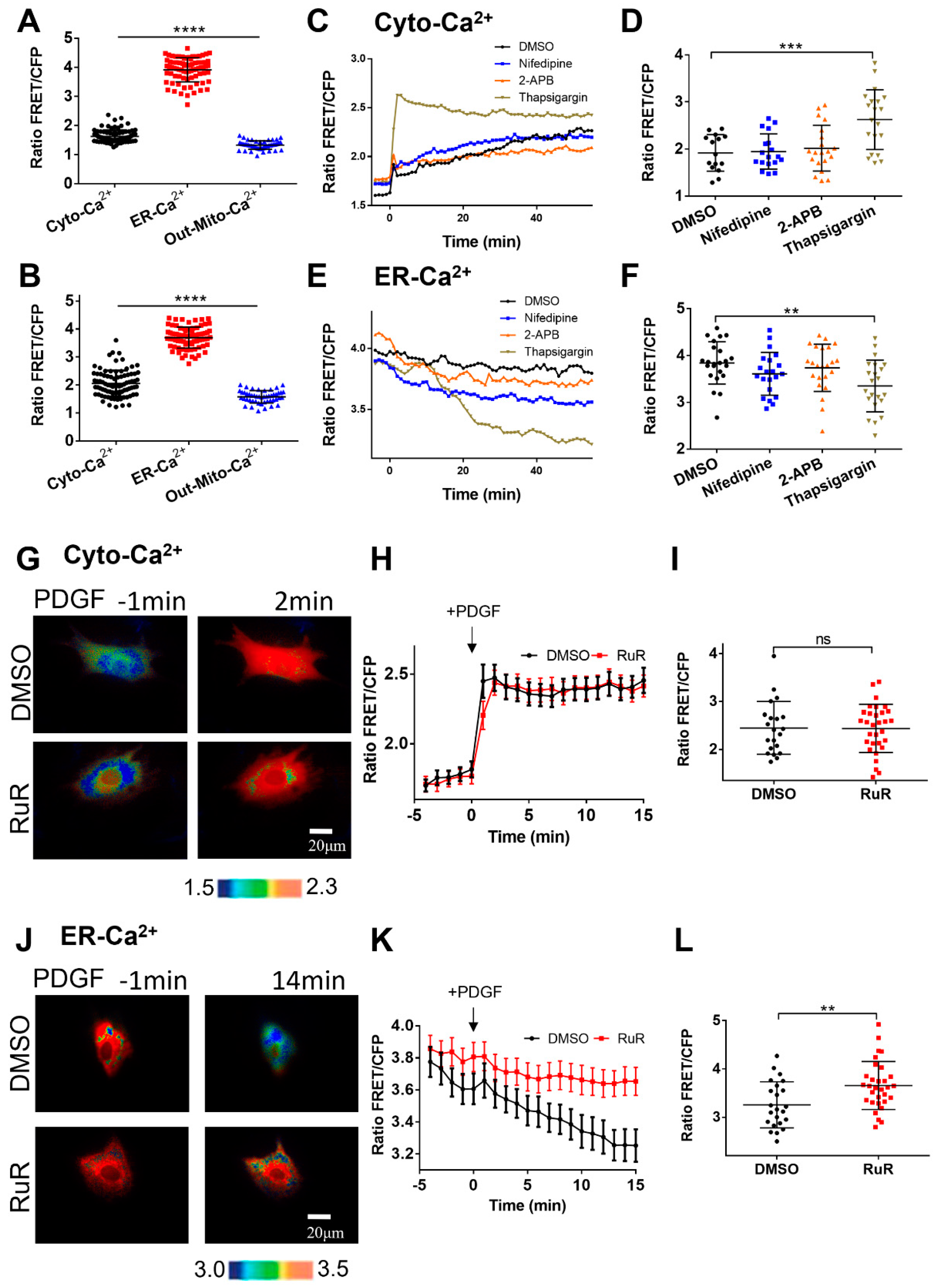
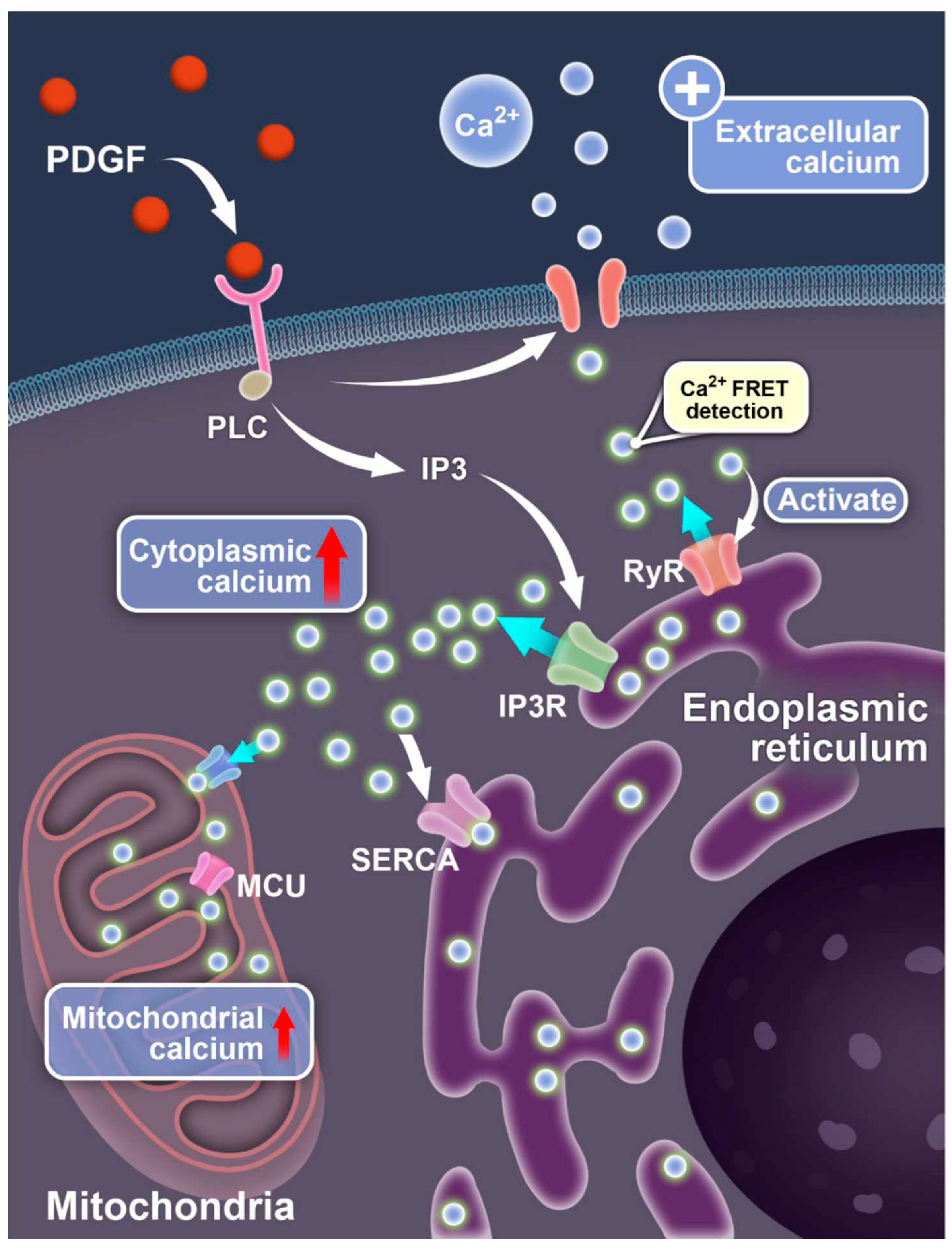
Figure 6.
Illustration for PDGF-induced calcium exchanges between different compartments of the cells. The diagrams show the signaling-mediated Ca2+ ion flow pathways between the extracellular medium, cell cytosol, endoplasmic reticulum, and mitochondria.
Figure 6.
Illustration for PDGF-induced calcium exchanges between different compartments of the cells. The diagrams show the signaling-mediated Ca2+ ion flow pathways between the extracellular medium, cell cytosol, endoplasmic reticulum, and mitochondria.