1. Introduction
Soil contamination is mainly caused by the presence of human-made chemicals, including sewage, industrial municipal waste, and agricultural chemicals such as heavy metals, petroleum hydrocarbons, solvents, fertilizers and pesticides. These chemicals are applied to farmland including crops. In this case, the toxic substances can exceed the soil’s bearing capacity, leading to the destruction of soil health. This, in turn, affects the quality of groundwater, plant growth, animal health and ultimately, human health. However, suitable plants can remediate soil through processes such as absorption, metabolism, and interaction between roots and microorganisms [
1].
Various approaches, including physical remediation techniques like surface capping, landfilling, and encapsulation, chemical methods such as stabilization, solidification, soil flushing, soil washing, and electrokinetics, as well as bioremediation involving the use of plants and microorganisms, have been applied to eliminate heavy metals from contaminated sites. The effectiveness of these techniques is significantly influenced by factors such as toxicity, soil types, soil conditions, cost, and the intended use of the land. However, it's worth noting that these remediation methods, employed for the removal of heavy metals from soil and water, are often disruptive, costly, labor-intensive, and can potentially give rise to additional issues [
2].
Utilizing hyperaccumulator plant species that exhibit high tolerance to heavy metals present in the environment or soil, phytoremediation emerges as a practical and promising approach for the removal of heavy metals from polluted areas [
3].
The effort is focused on identifying new plant species that are resistant to various pollutants. One such plants that can be cultivated for this purpose is Helianthus, commonly known as the sunflower. Helianthus and belongs to the family of Asteraceae [
4]. It is a highly adaptable plant that can thrive in a wide range of latitudes and soil types, including sandy and clayey soils, even those lacking in nutrients. Additionally, it can be grown in slightly acidic to neutral soils [
5]. One of its most notable characteristics is its ability to produce high biomass and accumulate heavy metals [
4]. Sunflower stands out as a crucial oilseed crop, serving as a significant supplier of biomass and vegetable oil applicable in energy and industrial sectors. Recognized for its resilience, sunflower is known to withstand relatively elevated levels of heavy metals. Acknowledged for its heavy metal tolerance, the sunflower is distinguished by its heightened biomass, rapid growth, higher translocation factor, and inclination to accumulate significant levels of heavy metals in its fatty tissues. Research suggests that sunflowers can effectively eliminate heavy metals, including lead, chromium, copper, cadmium, and zinc [
2].
The cultivation of sunflower for phytoremediation of contaminated soils, particularly for the removal of trace metals, is an alternative technique that offers significant advantages, such as low cost and high efficiency [
6]. Moreover, it is considered a relatively environmentally friendly and aesthetically appropriate method [
7], [
8]. The removal of pollutants is achieved through a process involving the extraction of metals from the soil, their incorporation into plant tissues and subsequent processing of the harvested parts rich in accumulated contaminants, which can be accomplished through methods such as drying, ashing, or composting [
6].
Nonetheless, effectively handling the plant biomass acquired throughout and after the process continues to pose a significant hurdle in the adoption of phytoremediation techniques, given its potential fluctuating levels of heavy metals and metalloids. The recommended techniques for recycling residues from phytoremediation plants include drying, incineration, gasification, pyrolysis, acid extraction, composting, and anaerobic digestion. Securing valuable products from the biomass, such as bioenergy, could contribute to the commercial viability of phytoremediation technologies for soils contaminated with trace elements [
7].
Several studies have confirmed that Helianthus annuus L. exhibits a higher metal bioconcentration translocation factor and demonstrates strong stress tolerance. Additionally, it has a relatively short growth cycle and is easy to manage, making it a favorable choice for phytoremediation purposes. Moreover, sunflowers are known for their high biomass production, further enhancing their effectiveness in remediation efforts [
9].
Phytoremediation utilizes plant-based technology, employing either natural or genetically modified plant species, to restore land and water sources that have been contaminated [
10]. It involves utilizing plants to absorb heavy metals and mitigate their harmful impact on the environment [
2]. It is a method that holds significant potential for the rehabilitation of contaminated soils. Through phytoremediation, plants play a crucial role in improving the soil structure, creating a favorable growth environment for microorganisms that aid in reducing soil toxicity. This process promotes plant growth and metabolism while facilitating the removal of organic pollutants from the soil [
1].
Despite the significant advantages of phytoremediation, such as its cost-effectiveness, environmental friendliness, and effectiveness in treating a wide range of environmental pollutants [
11], there are also some disadvantages compared to conventional technologies. One of the drawbacks is the long-term recovery time required for remediation, during which the cultivated soil remains unproductive [
12]. Furthermore, many plant species are unable to survive in contaminated environments, and even if they are resistant to pollutants, their biomass production is typically reduced [
13]. The specific type and extent of contamination, as well as the length of the growing season, can also influence the duration of the remediation process [
14].
The objective of this research was to evaluate the impact of various types of solid waste on the growth of two species of Helianthus plants, namely Helianthus annuus and Helianthus giant. The solid waste materials tested included fireplace ash, dust from oil burners, forge dust, soil from the land of Milos, and a combination of soil from the land of Milos with olive core. The study aimed to assess how these different waste materials influenced the growth and development of the Helianthus plants.
In the study, two strains of the sunflower plant were cultivated: Helianthus giant and Helianthus annuus, specifically focusing on the Neoma hybrid developed by the Syngenta company. The Neoma hybrid strain is characterized by a well-balanced plant structure with relatively low height, strong resistance to tilting and diseases, and an inflorescence that produces a large number of seeds. This strain exhibits high production potential and boasts a notably high oil content. It has shown excellent performance in fertile irrigated fields. The seeds of Helianthus giant were obtained from commercial sources for the study.
2. Materials and Methods
An experiment was conducted on a plot of land with a total area of approximately 2,000 square meters, located in Kalivia. Kalivia is situated in the northeast of the Attica prefecture in Greece, approximately 12 km southeast of Eleftherios Venizelos International Airport of Athens. The experiment took place for a period of four months, from May to August.
The plot is situated equidistant between the settlement of Kalivia and the town of Markopoulos. A completely randomized block design was employed for the experiment, utilizing the following treatments: (i) control, (ii) fireplace ash, (iii) dust from oil burners, (iv) forge dust, (v) soil from the land of Milos, and (vi) a combination of soil from the land of Milos with olive core. The experimental plots had dimensions of 1.6 m x 1.6 m, resulting in a plot surface area of 2.56 m². The soil was tilled to a depth of 35 cm.
On the same day, the experimental squares were marked out, and peat was added to the substrate. The ground surface was divided into 25 experimental plots, each measuring 1.6 m x 1.6 m. The volume of soil in each plot was 0.9 m³. Paths approximately 40 cm in length were created between the plots to allow easy access for measurements and experimental work, such as irrigation, sampling, and measurements. Each square was delineated from the others using a special tape, as depicted in the photo below (
Figure 1).
Ten kilograms of peat per square meter were added to the soil substrate and mixed using a milling machine. Peat is a product resulting from the decomposition of vegetation that saturated in waterlogged and anoxic environment [
15]. For this experiment, black peat was utilized. The following solid wastes were used:
Fireplace ash (FA), produced during the burning of wood. Wood, like other organic materials, primarily consists of carbon, oxygen, and hydrogen. When wood is burned, most of these elements are converted into water and carbon dioxide, which are released into the atmosphere. However, a small percentage remains in the form of ash, which comprises the by-products of combustion. These by-products include various salts, such as calcium carbonate (comprising approximately 24-43% of the ash), potassium carbonate (approximately 10%), and phosphates (less than 1%). Additionally, ash may contain trace amounts of elements such as iron, manganese, zinc, and copper, as well as certain heavy metals like cadmium, nickel, lead, and chromium. The quantities of these chemicals can vary depending on the temperature at which the combustion occurs.
Dust from oil burners (OB). Combustion of hydrocarbons gives ash with a high content of oxides, mainly oxides of silicon, aluminum, and iron.
Forge dust (FD). The dust was collected by an ironworks that specializes in the processing of various types of iron ores.
Soil from the land of Milos (M), known as pozzolan. It is an inorganic material possessing properties like cement. Pozzolan can be obtained from both natural sources, known as natural pozzolans, or from artificial sources like blast furnace slag, fly ash, and silica fume. Natural pozzolan is an industrial mineral of volcanic origin, specifically volcanic tuffs, and it contains a high concentration of active silica and aluminum oxide-alumina. It has been widely utilized in the cement industry [
16].
Soil from the land of Milos (M) in combination with olive core (OC). The olive core refers to the solid waste produced by mills during the separation of liquids from the solid parts of olives. The moisture content of olive core varies depending on factors such as the type of olive, the harvest season, and the mill's operation type (two-phase or three-phase).
Two days prior to sowing, the materials were applied to the plots and incorporated into the substrate using a milling machine. This process aimed to achieve homogenization of the materials to a depth of approximately 35 cm. The sowing of sunflowers followed this procedure. Within each plot, there were nine designated sowing locations spaced 40 cm apart from each other. The sowing places were arranged in a cross pattern, positioned within a square frame. The borders of the frame were approximately 40 cm away from the edges of the square. The peripheral plants of the frame were positioned 40 cm beyond the boundaries of each plot. This placement ensured that the root system of these plants would be within the solid waste dump zone and within the irrigation surface of the plot. For the sowing, Helianthus giant seeds were placed in the corners of the frame, while the other locations were reserved for Helianthus annuus (Neoma hybrid) seeds. The seeds were planted at a depth of 3 cm from the soil surface. To ensure successful germination, 2-3 seeds were placed in each sowing position. In instances where multiple sunflowers sprouted, any excess plants were removed a few days after germination. The soil temperature at the time of sowing was approximately 24 °C. Around 15 days after germination, the insecticide chlorpyrifos agrodan 48 ec was sprayed to protect the young plants from chewing and sucking insects.
Figure 2 illustrates the experimental plot arrangements, while
Table 1 provides the concentrations of the solid wastes that were added.
Initially, the experimental field was irrigated daily in response to the elevated environmental temperatures, reaching approximately 32 °C, which prevailed during the sowing period. Subsequently, the irrigation frequency was adjusted to every second day to maintain the necessary moisture levels for seed germination and seedling establishment. After the sprouting phase, irrigation was adjusted based on environmental conditions, such as temperature and rainfall, with the goal of ensuring adequate soil moisture for the optimal growth of the plants.
The parameters of the plant that were studied included the following: the distance between the acetabulum (leaf attachment point) and the 1st leaf, the distance between the 1st and the 2nd leaf, the length and width of the 5th leaf, the surface area of the 5th leaf, the height of the plant, and the diameter of the flower head. These measurements commenced approximately one month after sowing and were conducted on a weekly basis.
3. Results
3.1. Determination of the physicochemical characteristics of the materials
Table 2 provides the physicochemical characteristics of the solid waste used in the experiment, while
Table 3 presents the soil analysis results of the experimental field. It is important to mention that soil sampling and analysis were conducted one month prior to sowing, allowing for the calculation of the soil's chemical composition based on the fundamental parameters.
The analysis results revealed a potassium deficiency in the substrate. As a remedy, a total of 7 kg of Complezal fertilizer was added to the entire experimental area, covering approximately 100 m2.
3.2. Effects of Fireplace ash (FA)
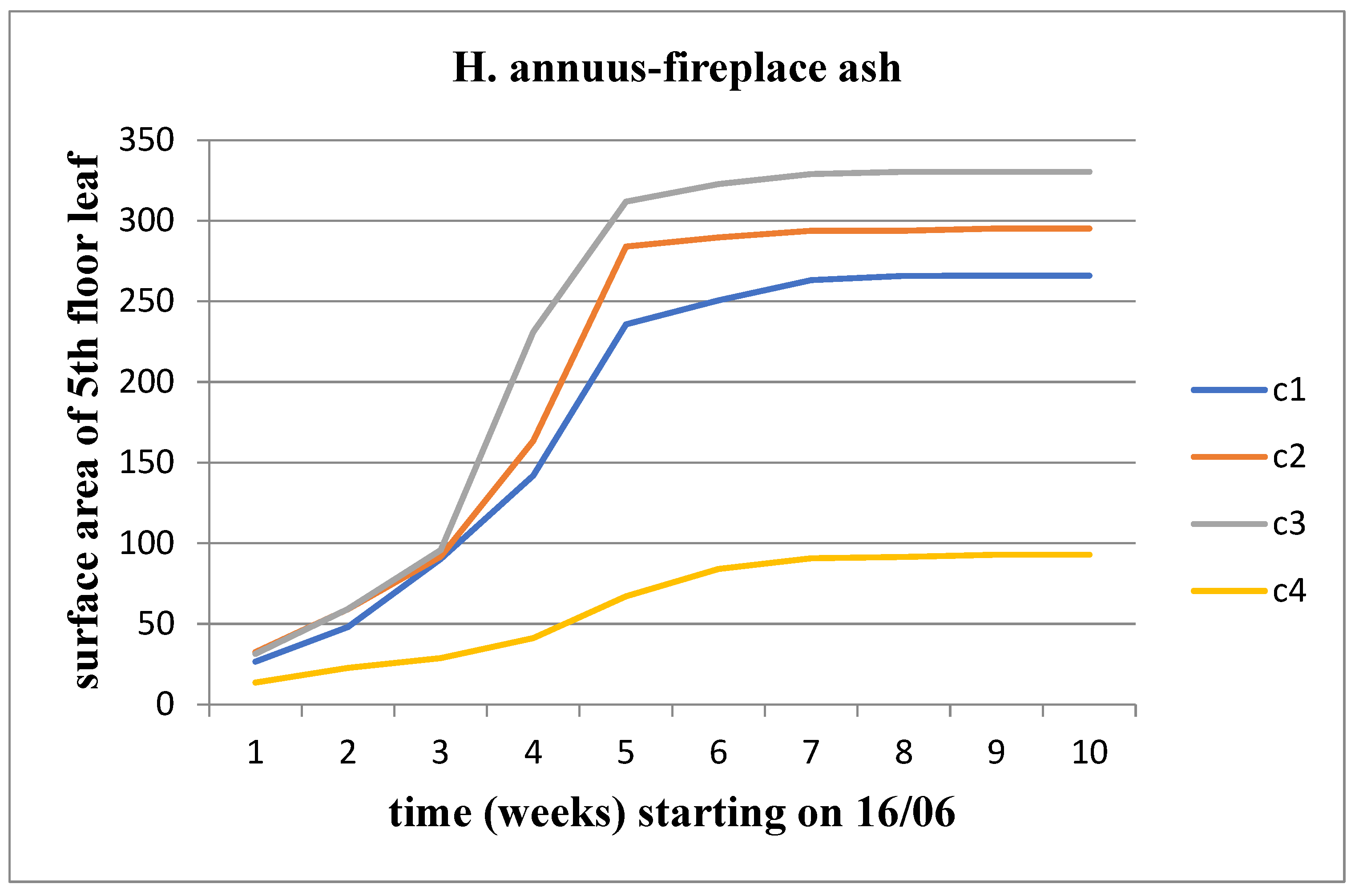
It was observed that for Helianthus annuus, all three parameters (length, width, area) increased over time for all concentrations of waste. When comparing the concentrations, an increase in the value of the aforementioned parameters was observed up to the C3 waste concentration, followed by a decrease from the C4 concentration onwards (
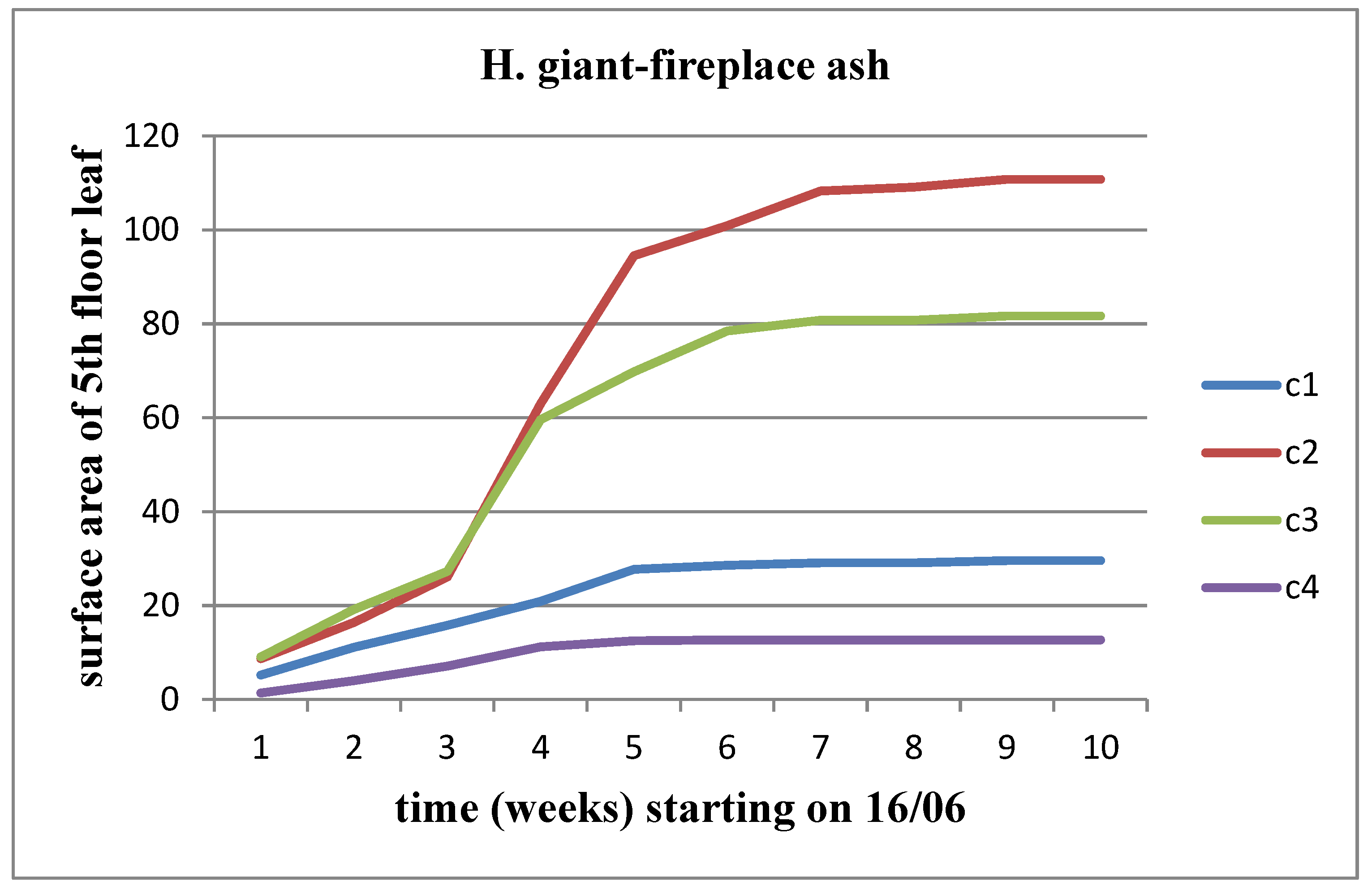
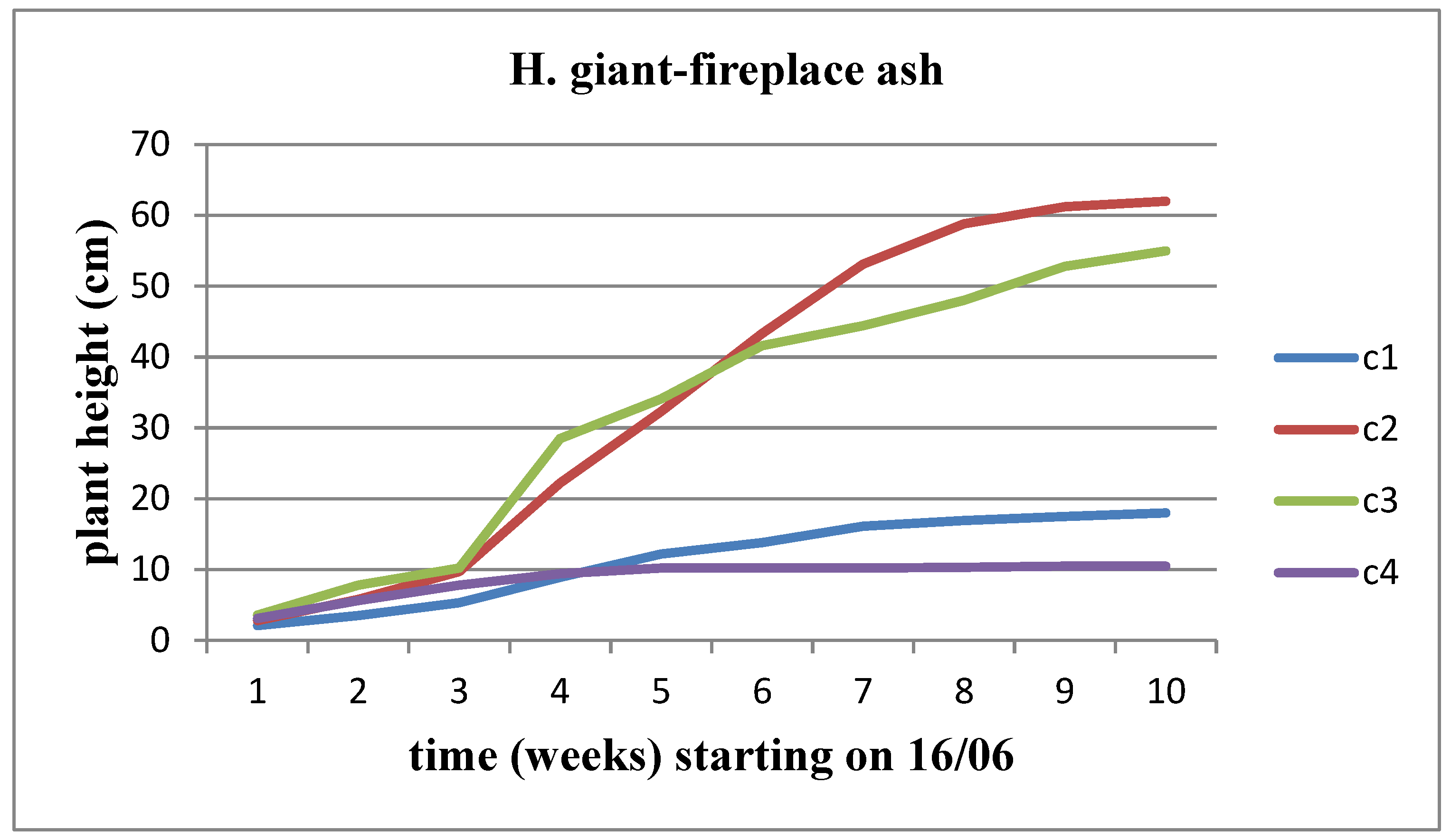
Figure 3). The same observation applies to Helianthus giant, except that the decline begins from the C3 concentration onwards (
Figure 4). However, this growth rate appears to be decreasing as we approach the time of flower head emergence.
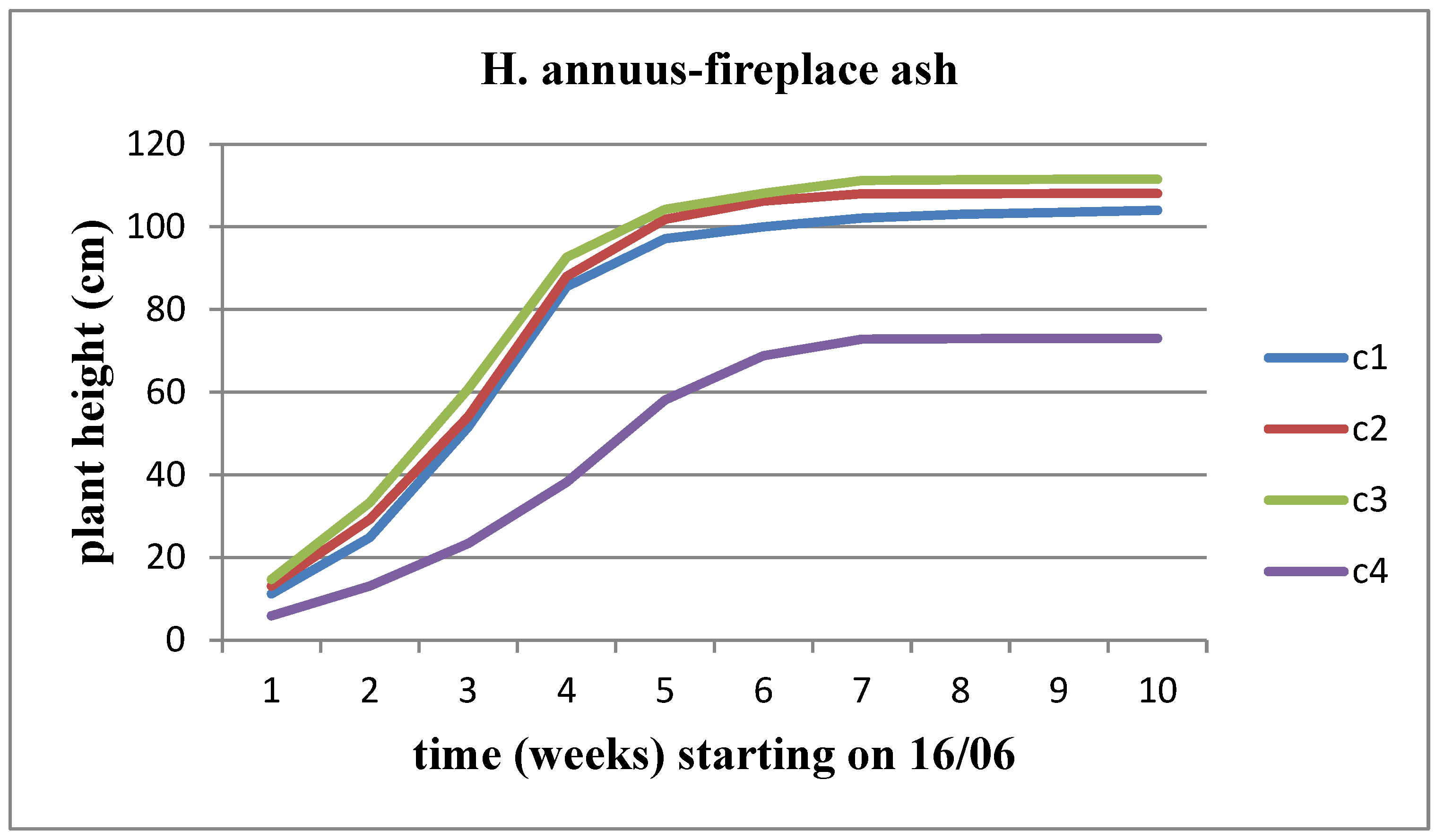
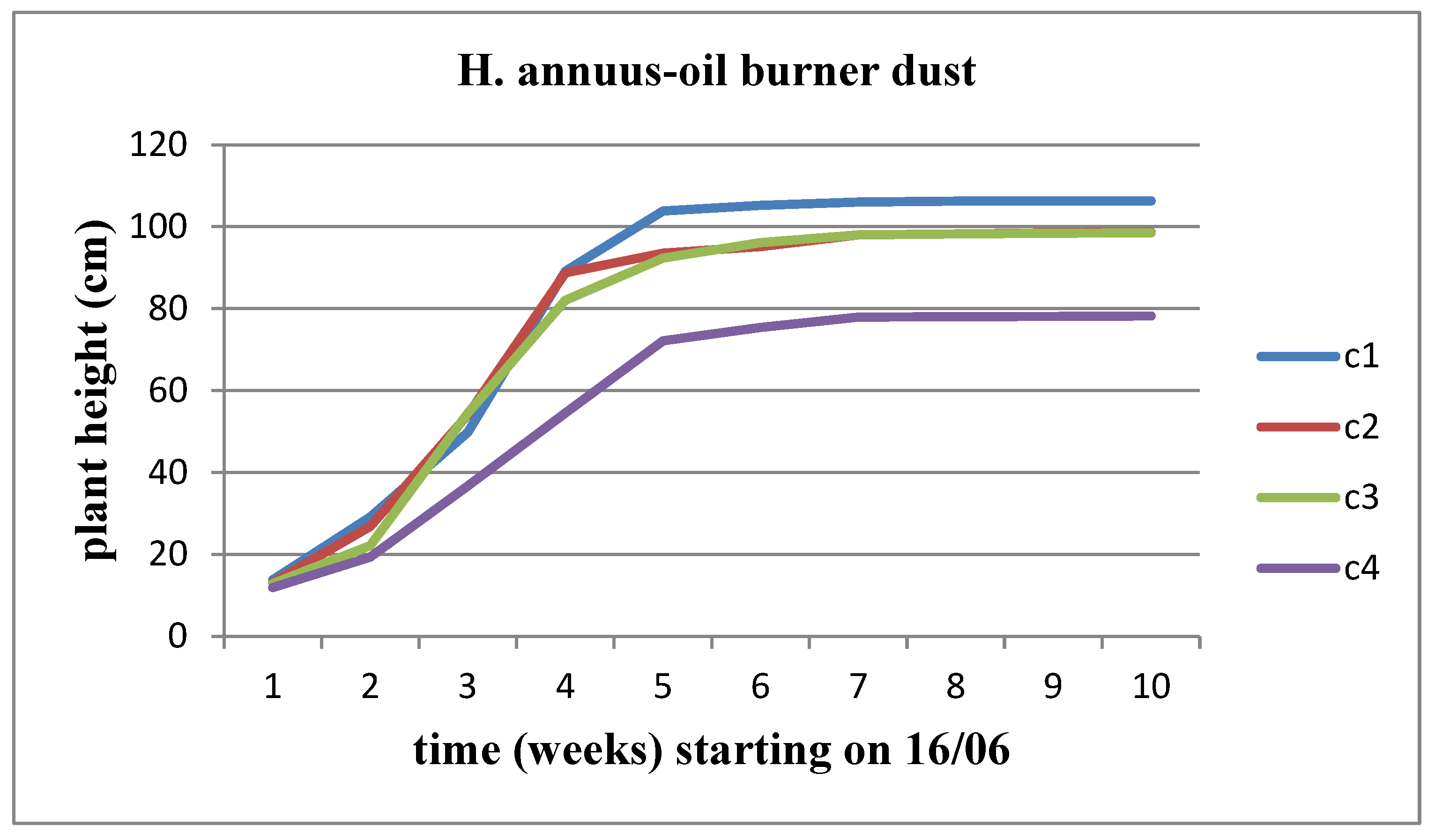
The height of the plant increases over time for both sunflower stems at all concentrations of solid waste. However, when comparing the concentrations, we observe that Helianthus annuus exhibits a significantly higher growth rate and increase in height up to the C3 concentration, after which the plant height decreases. On the other hand, Helianthus giant demonstrates a slower growth rate and height increase until the C2 concentration, followed by a significant decrease in growth rate and plant height from the C3 concentration onwards (
Figure 5 and
Figure 6).
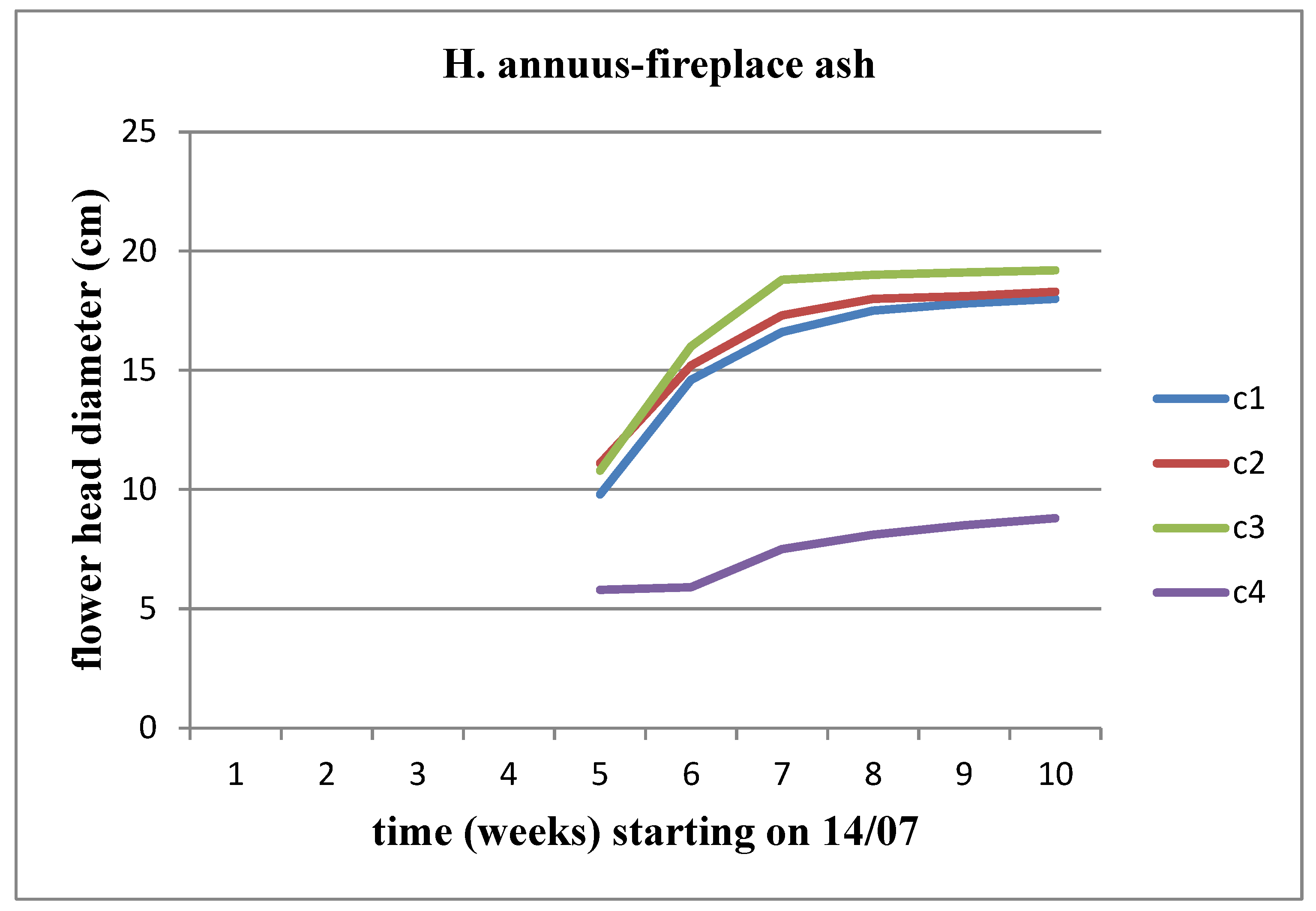
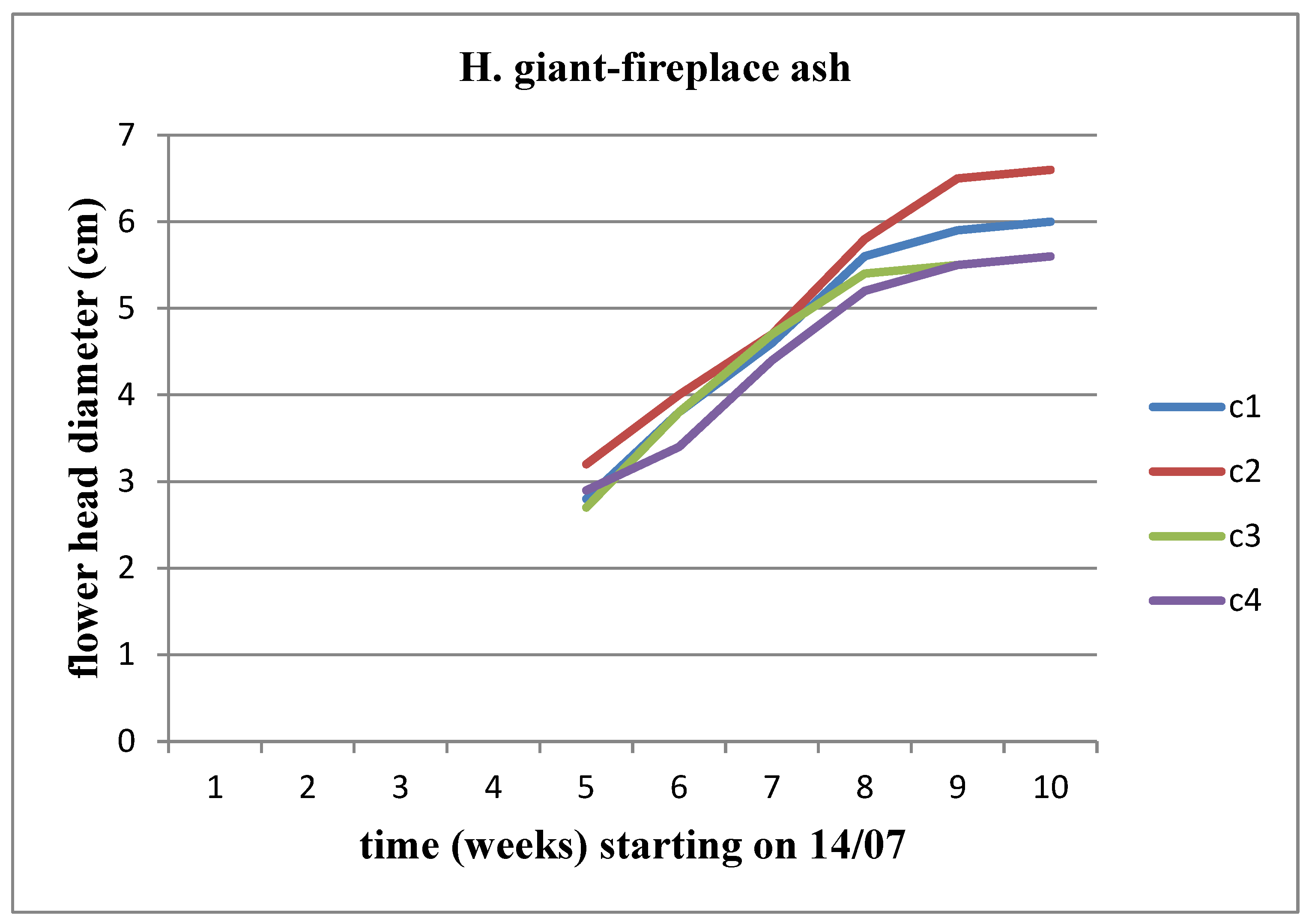
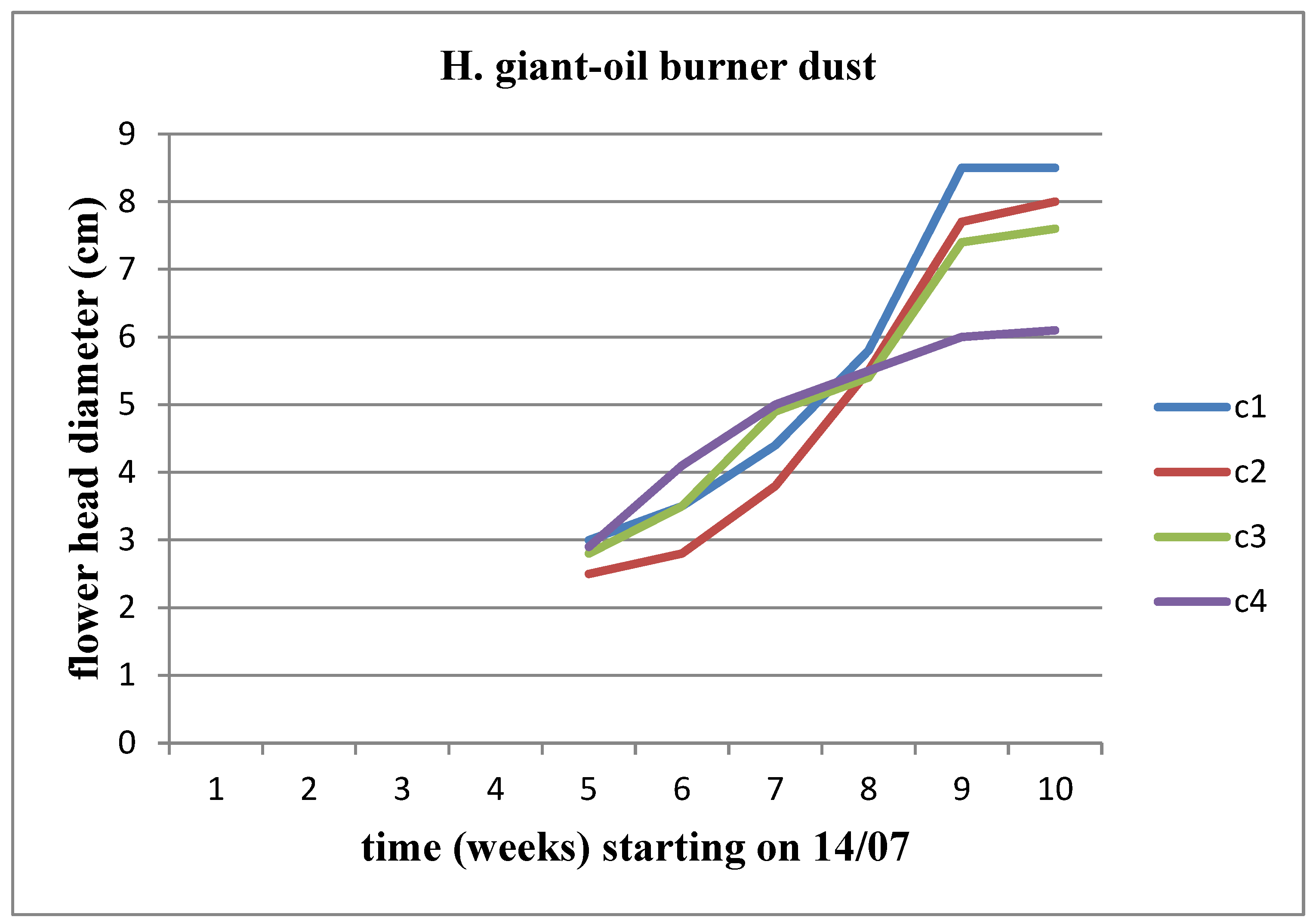
The flower head appeared on both sunflower stems during the middle of the 5th week (
Figure 7 and
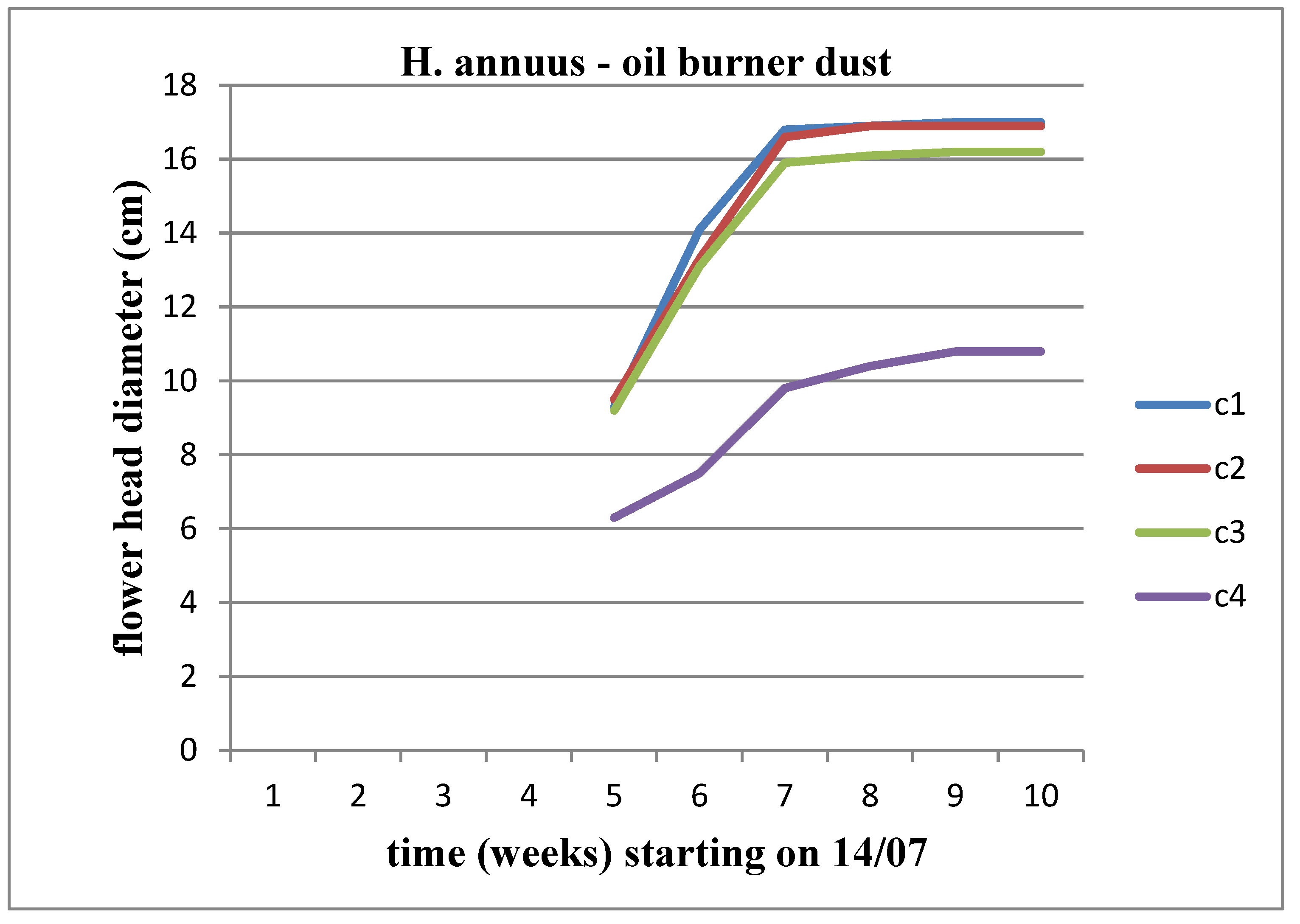
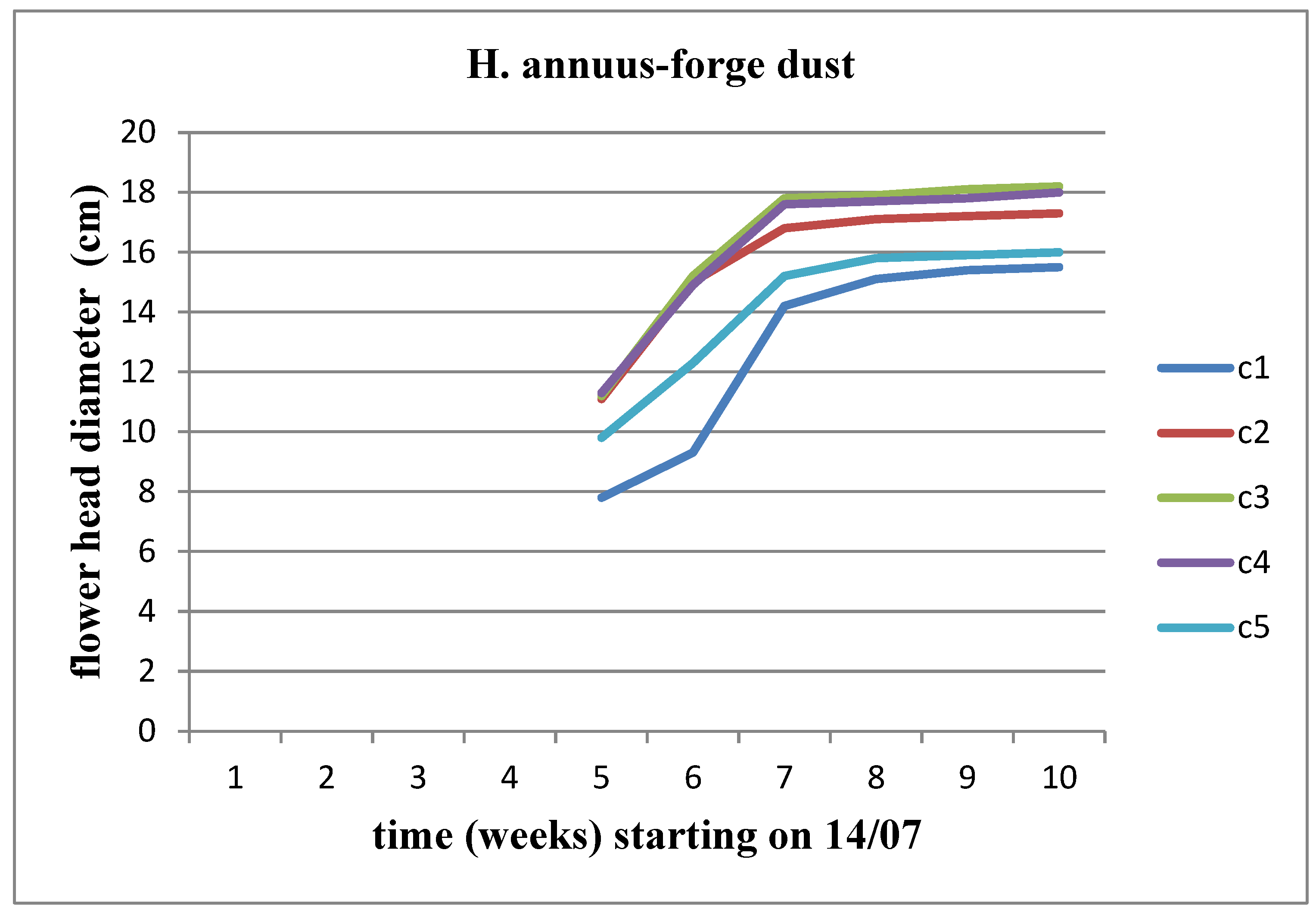
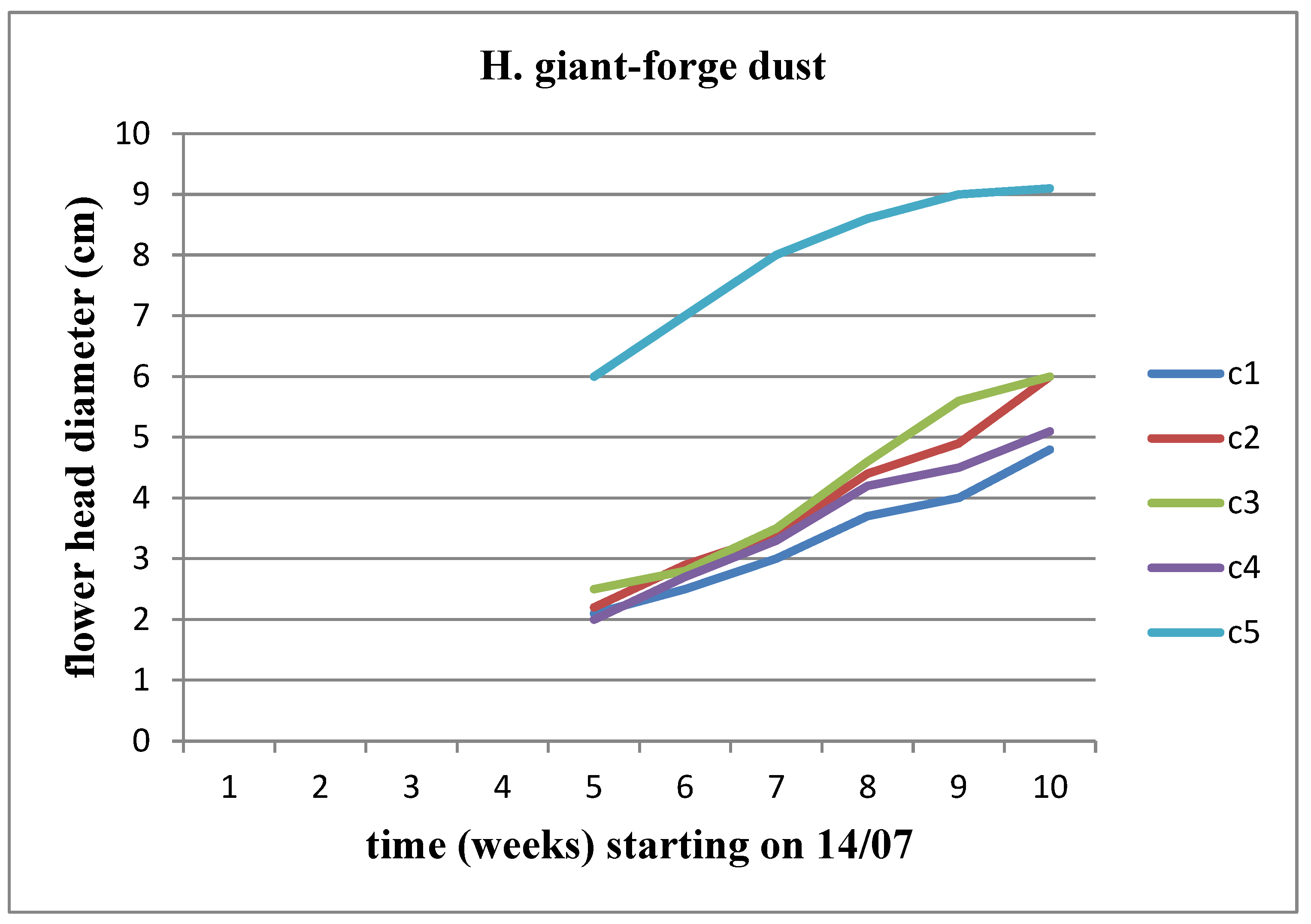
Figure 8). We observe an increasing trend in the diameter of the flower head over time, reaching its peak at the C3 concentration for Helianthus annuus and at the C2 concentration for Helianthus giant.
It is worth noting that neither of the sunflower stems developed at the C5 (17.8 kg/m3) concentration.
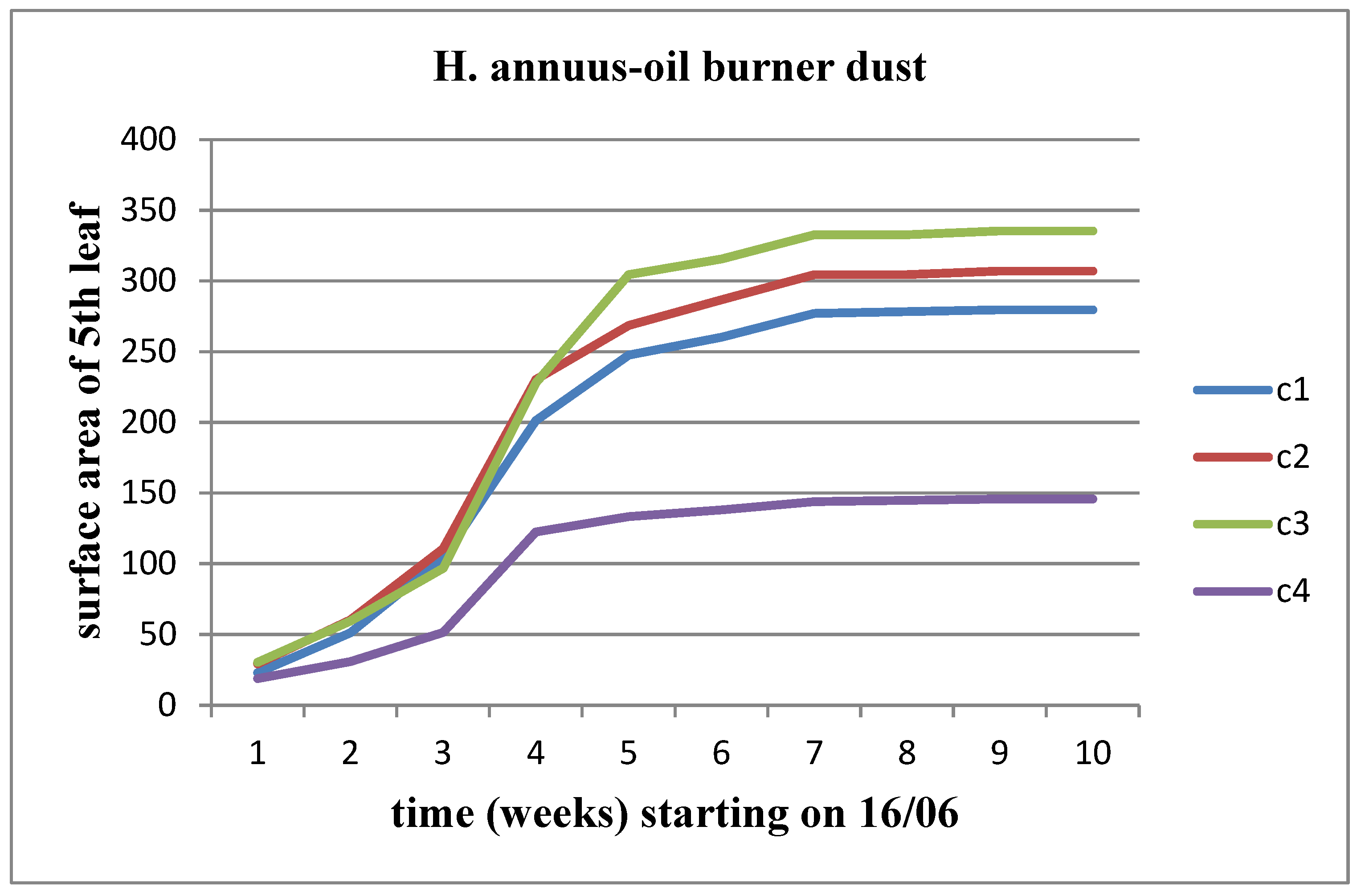
3.3. Effects of of Dust from oil burners (OB)
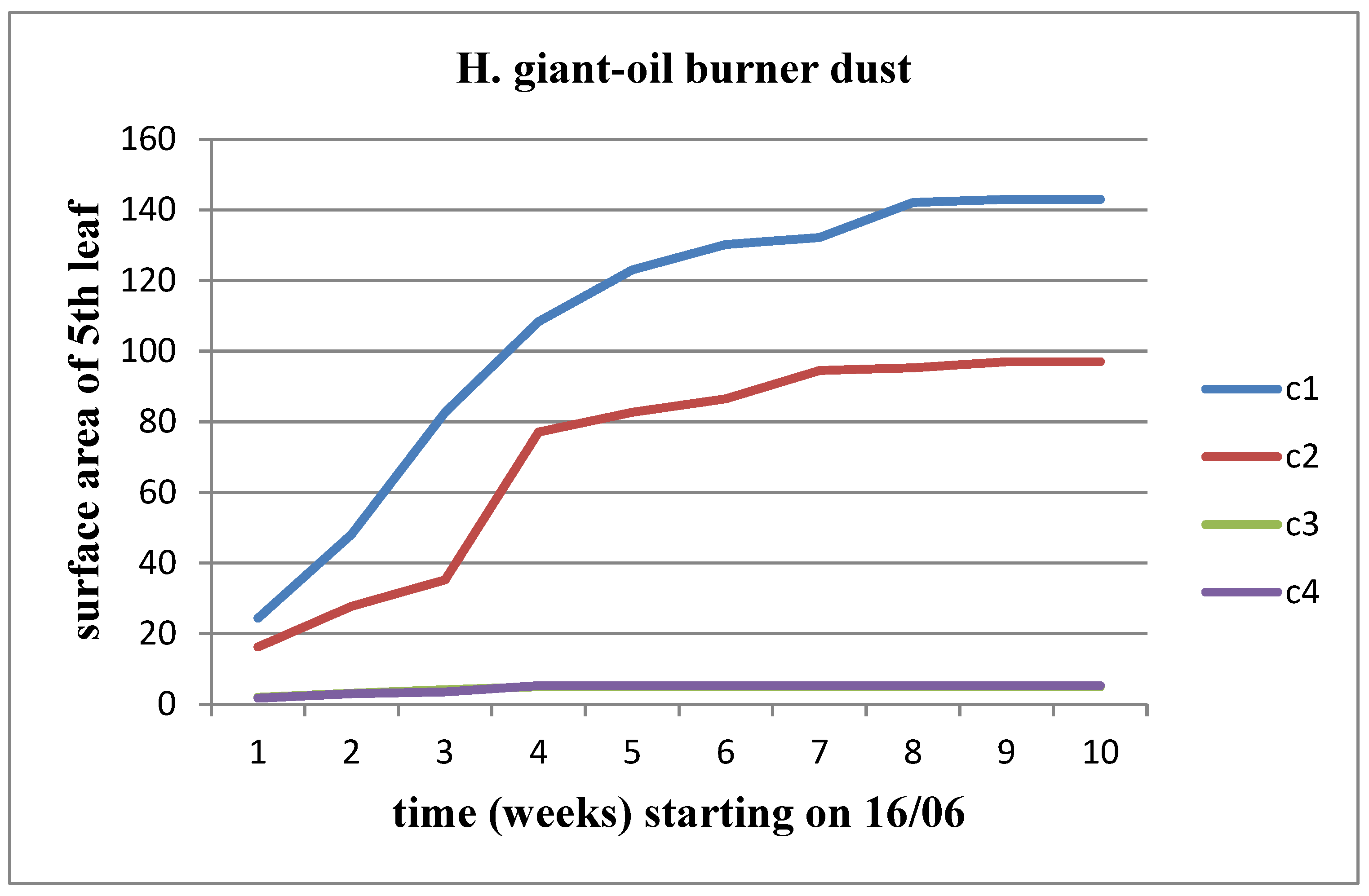
All parameters (length, width, area) increased over time for each waste concentration in both stems of the plant. The growth rate decreased as the moment of flower head appearance approached. For Helianthus annuus, the increase continued until the C3 waste concentration. However, for the Helianthus giant, the OB dust had a negative effect, resulting in measurements showing a downward trend from the control concentration onwards. In fact, at concentrations C3 and C4, there was a stagnation in the leaf surface area after the fourth week of vegetation (
Figure 9 and
Figure 10).
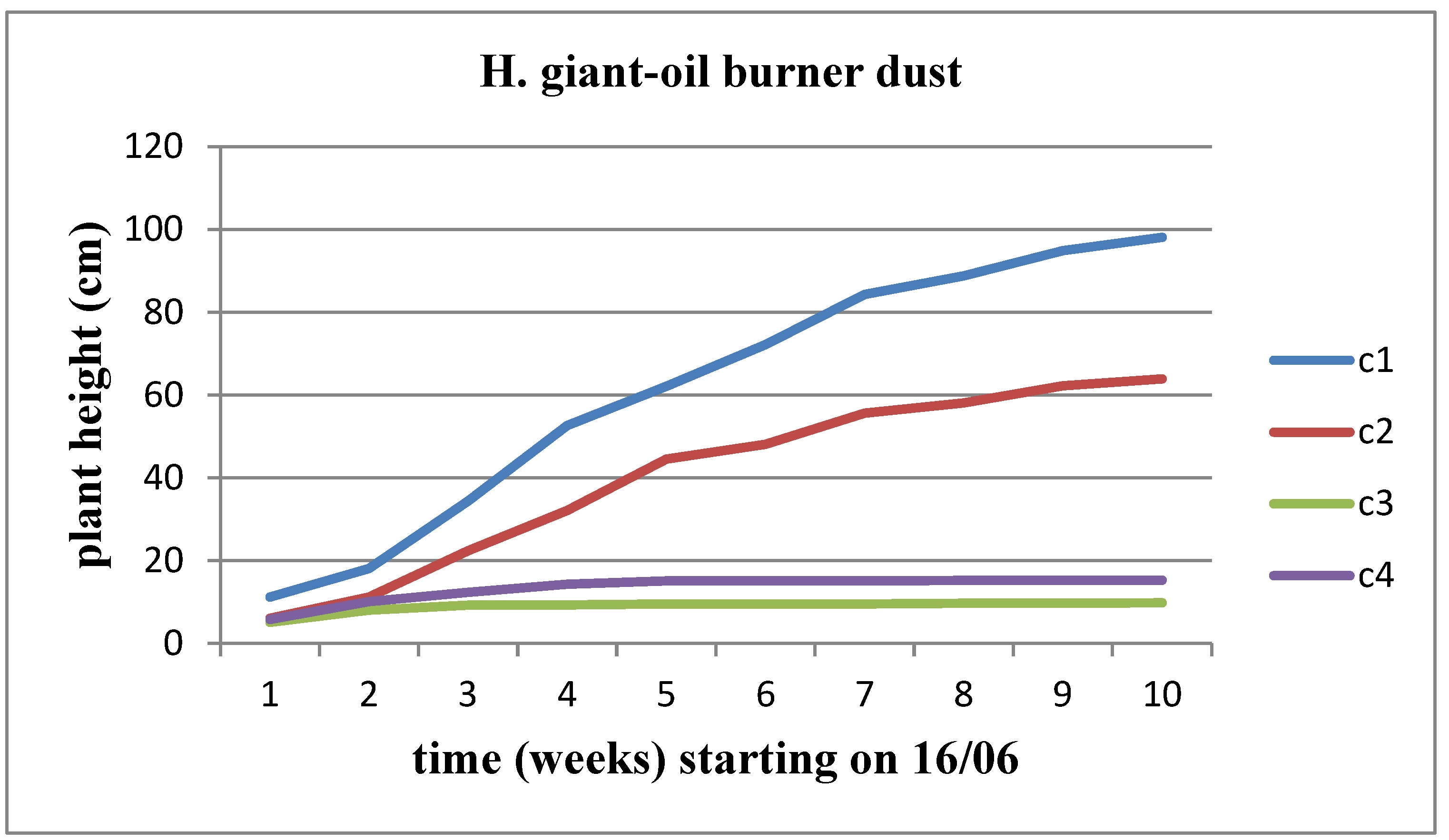
The height of the plant undergoes incremental changes over time across all waste concentrations for both sunflower stems, exhibiting a higher rate of height increase for the Helianthus annuus strain. However, Helianthus annuus maintains height stability, reaching a level comparable to the control up to concentration C3. At concentration C4, there is a decline in plant height. In contrast, the Helianthus giant demonstrates a slower growth rate, with the plant height starting to decrease immediately after removal from the control area. This decline is observed as the concentration of solid waste increases (
Figure 11 and
Figure 12).
An increase in the diameter of the flower head was observed over time for each waste concentration (
Figure 13 and
Figure 14). This increase persisted until the C2 concentration for Helianthus annuus. However, for the Helianthus giant, the increase in waste concentration had only negative effects on the diameter of the flower head.
It is also worth noting that neither of the two sunflower stems developed fully at the C5 (17.8 kg/m3) concentration.
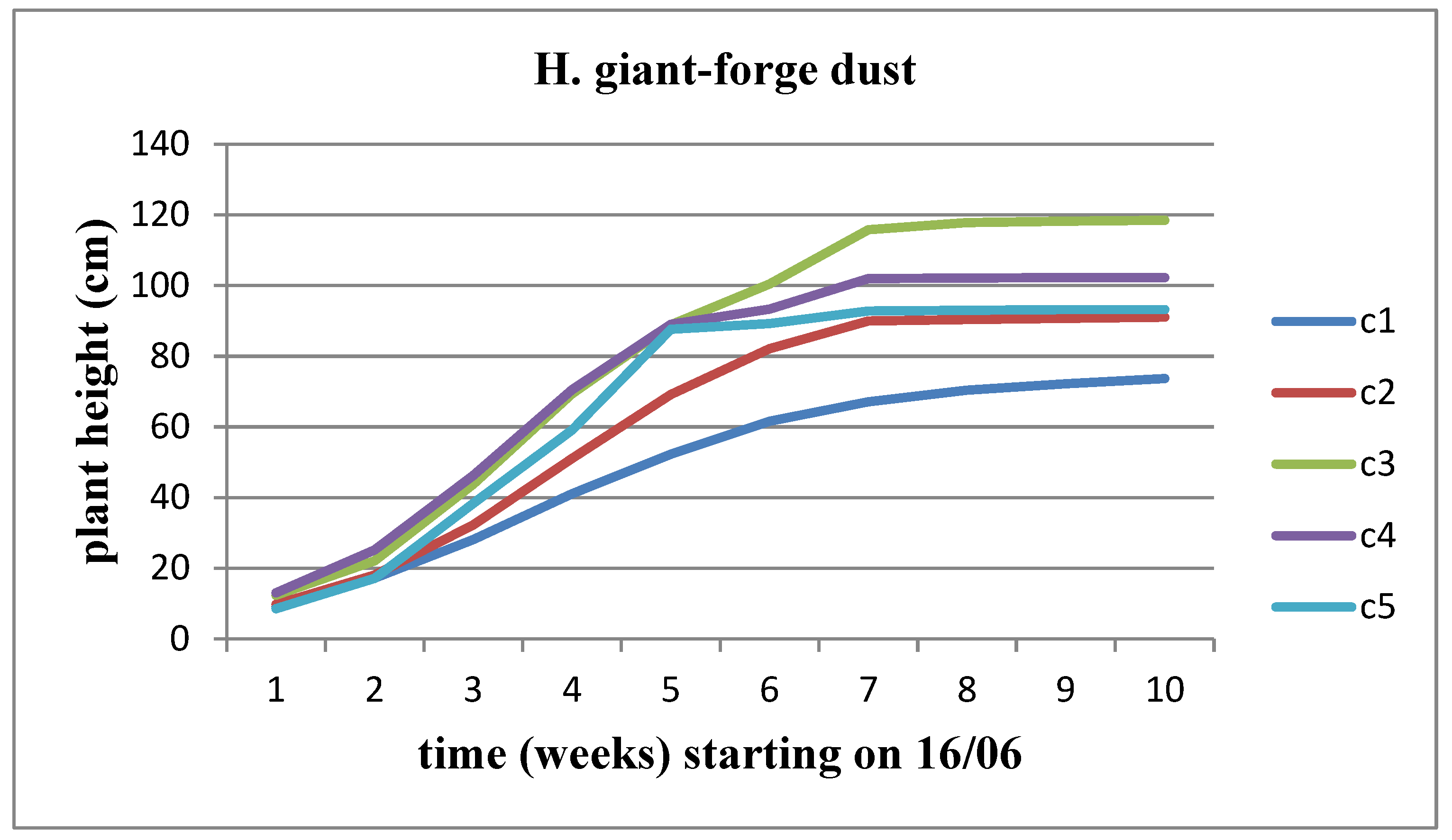
3.4. Effect of Forge dust (FD)
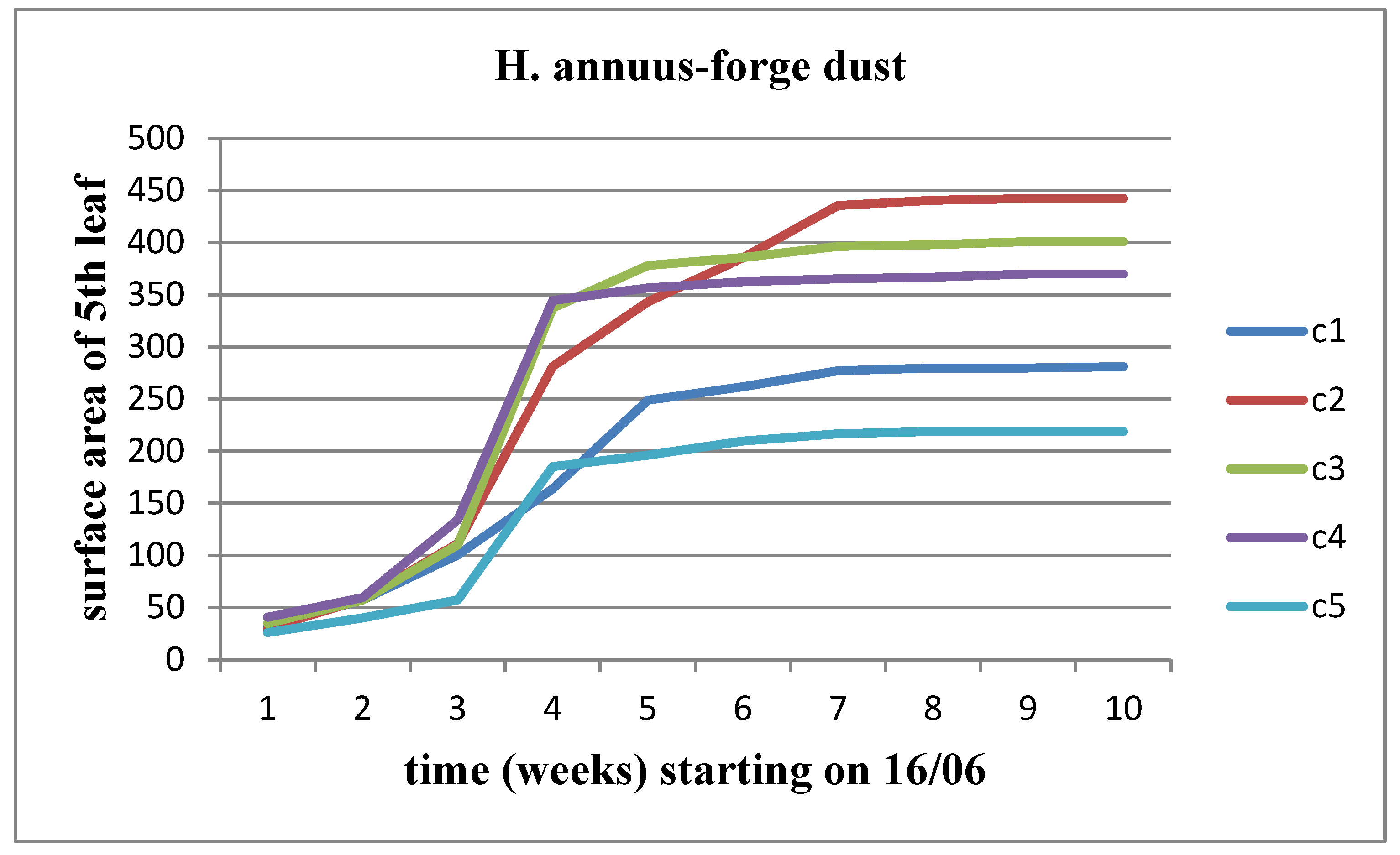
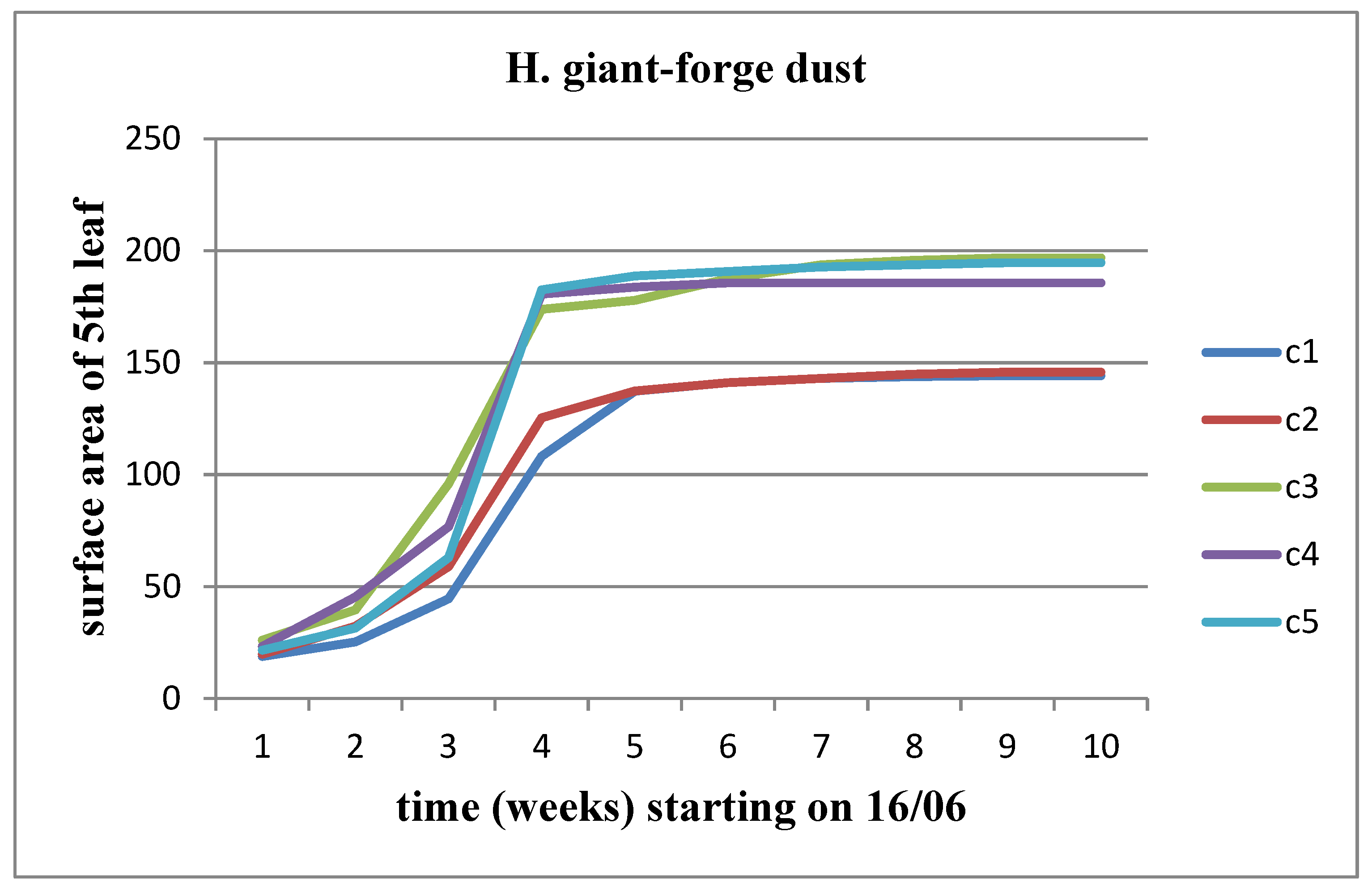
There was an observed increase in all parameters (length, width, and area) over time for each concentration, although the rate of increase was limited as the flower head's appearance time approached. When examining the variation of these parameters across different solid waste concentrations, an increase was observed up to concentration C2 compared to the control for Helianthus annuus. This increase persisted for subsequent concentrations of waste in the case of Helianthus giant. However, stagnation appeared to set in over time for Helianthus giant, particularly at concentrations C4 and C5 (
Figure 15 and
Figure 16).
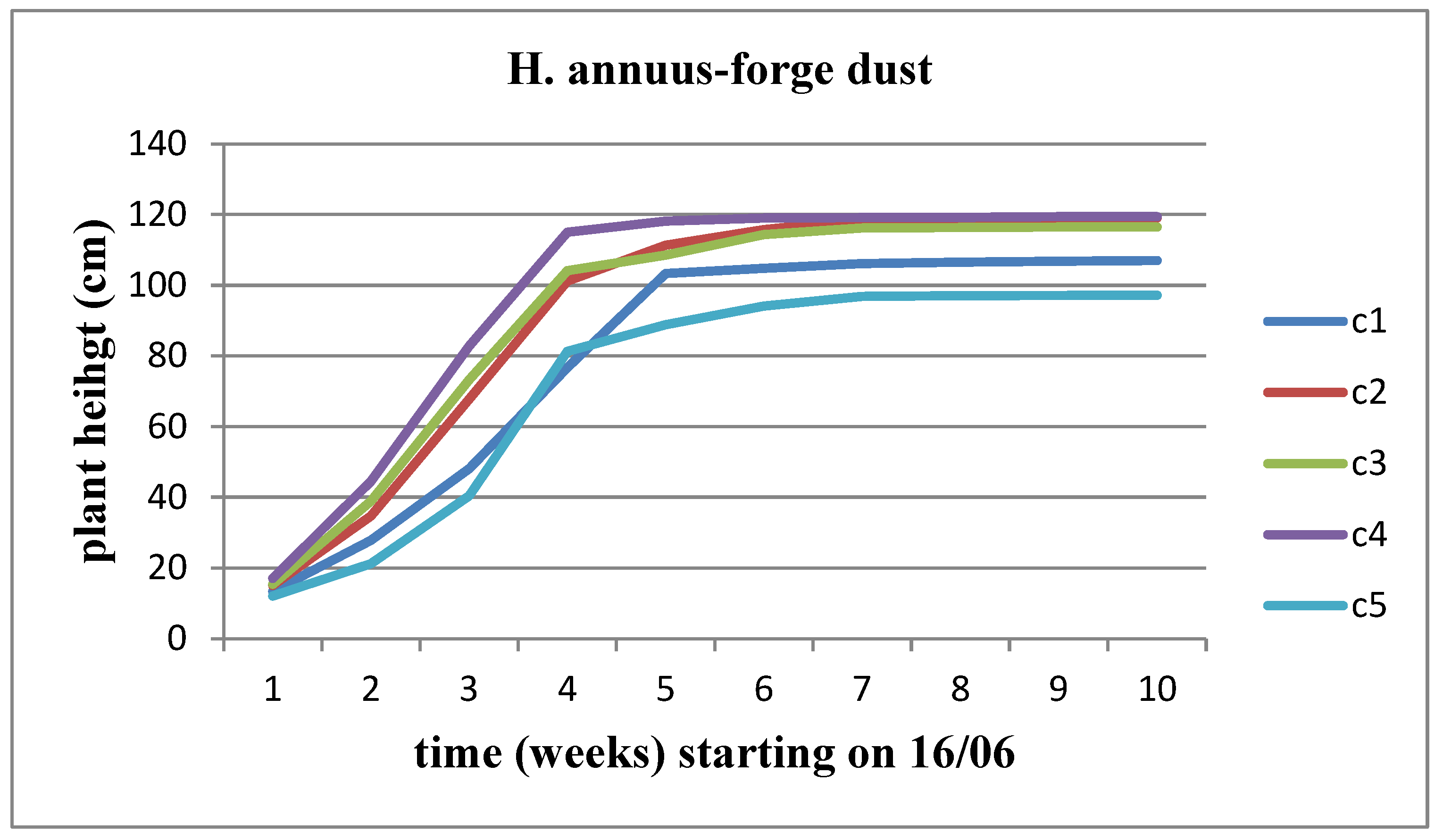
The plant's height undergoes incremental changes over time across all waste concentrations for both sunflower stems, with the Helianthus annuus strain exhibiting a higher rate of height increase. This growth rate appears to slow down as the flower head's development time approaches. When comparing the concentrations, it's observed that Helianthus annuus maintains a height of 119 cm up to the C4 concentration, but at the C5 concentration, the plant height decreases to 96.8 cm. On the other hand, the Helianthus giant demonstrates a significantly slower growth rate, stabilizing at a height of 116 cm until the C3 concentration and then slightly decreasing to 92.8 cm at the C5 concentration of solid waste (
Figure 17 and
Figure 18).
An increase in the diameter of the flower head is observed for both stems at all concentrations. Among these concentrations, we observe an increase in the diameter of the flower head for Helianthus annuus up to the C4 concentration, whereas the increase for Helianthus giant continues until the C5 concentration (
Figure 19 and
Figure 20).
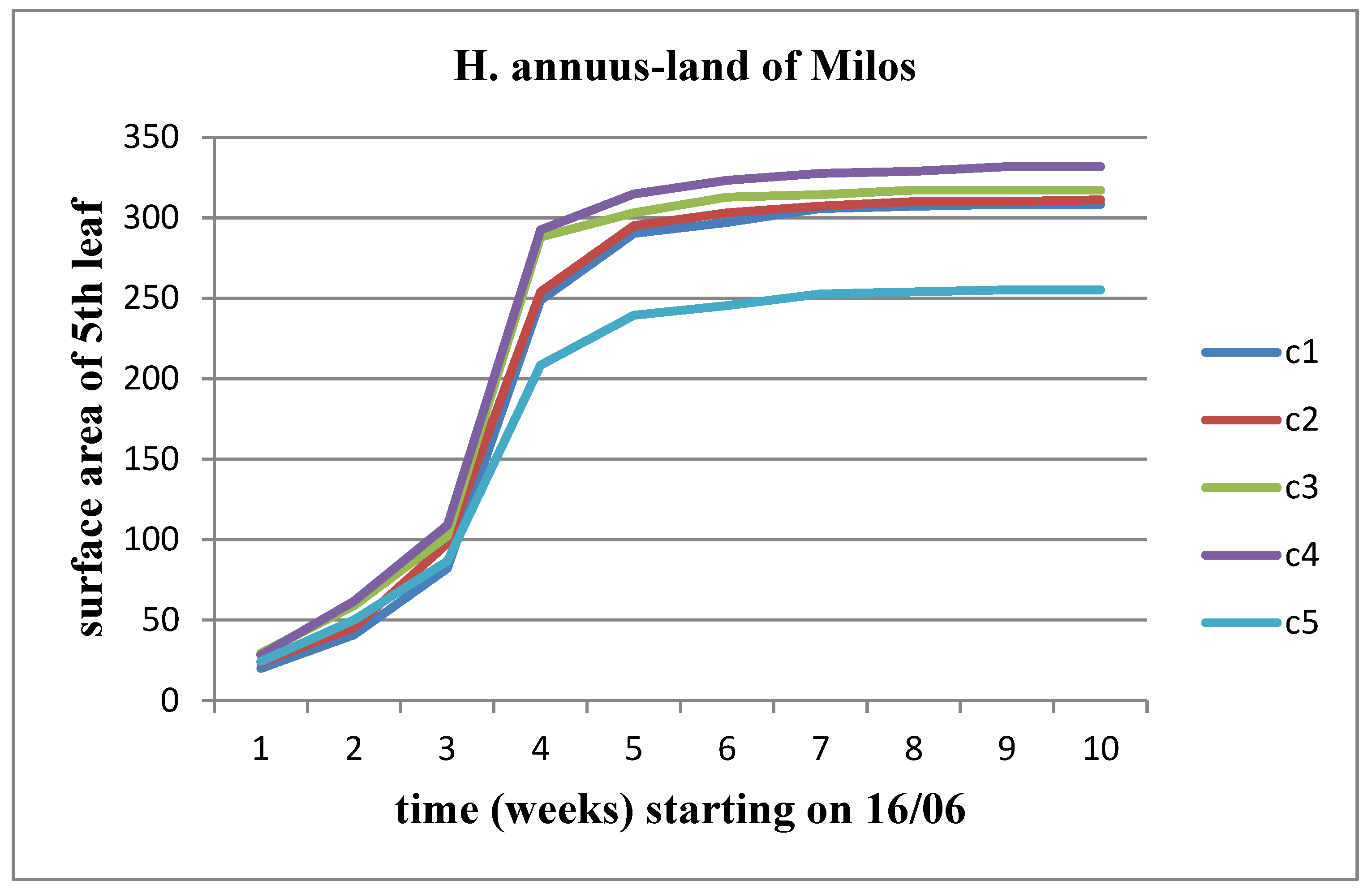
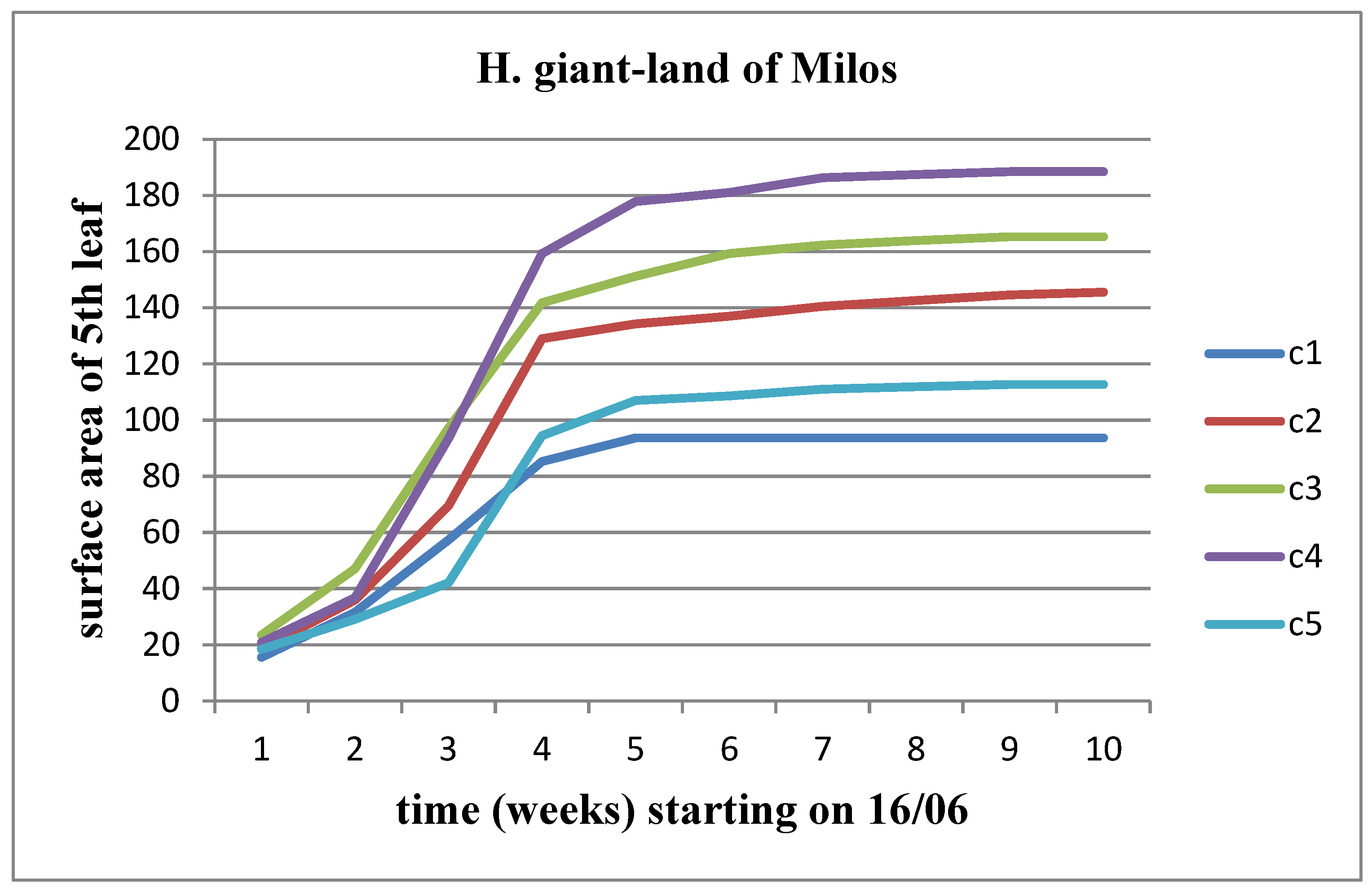
3.5. Effect of soil from the land of Milos
We observe an increase in all three parameters (length, width, and area) over time for both stems of the plant. However, the rate of this growth decreases significantly as we approach the time of flower head appearance. When studying the change in these parameters between different concentrations of Malay soil, we note a slight increase up to the C4 concentration compared to the control for both plant stems together. However, this increase does not continue at the C5 concentration.
Figure 21 and
Figure 22 depict the change in leaf surface after the application of land of Milos.
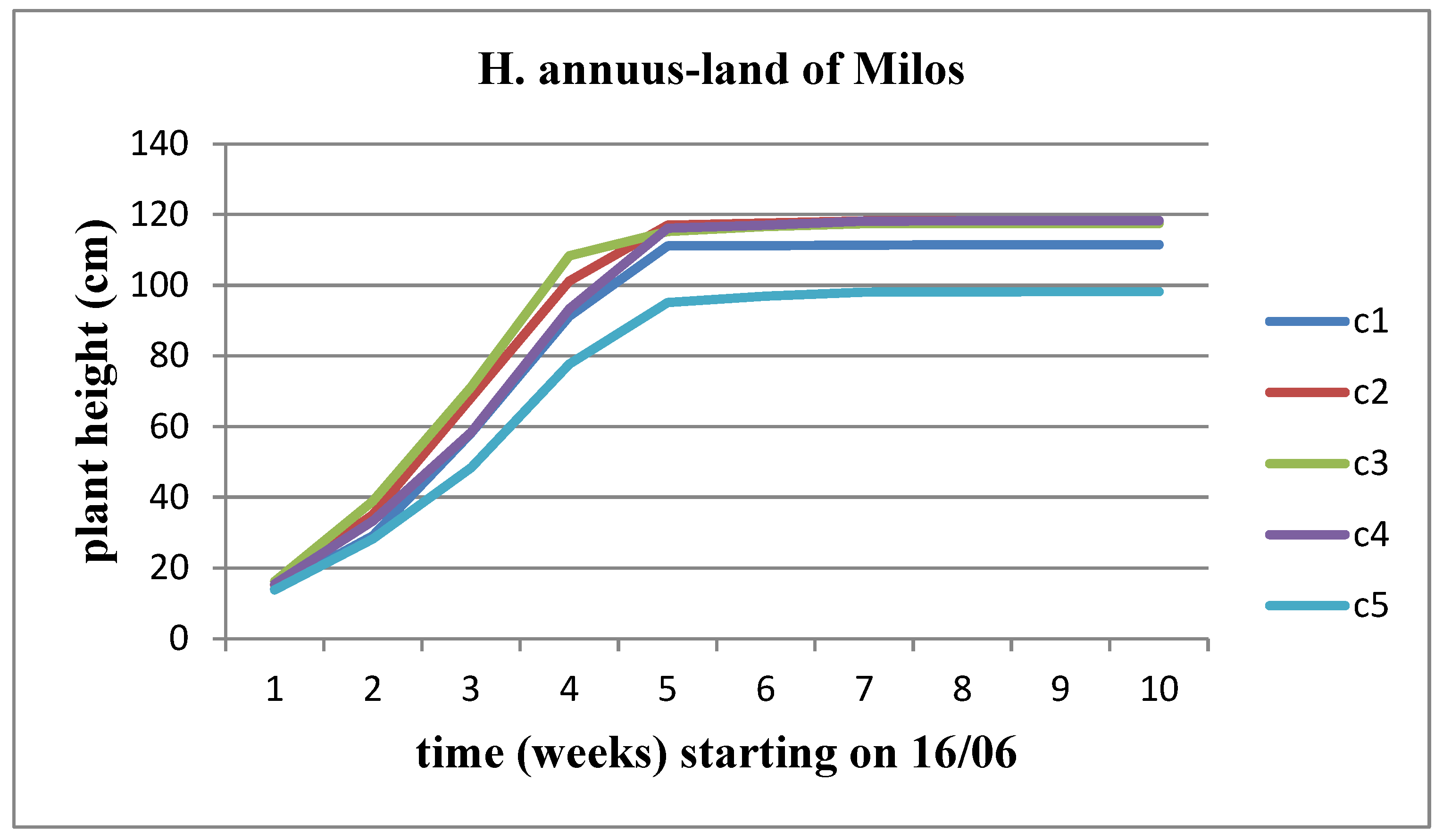
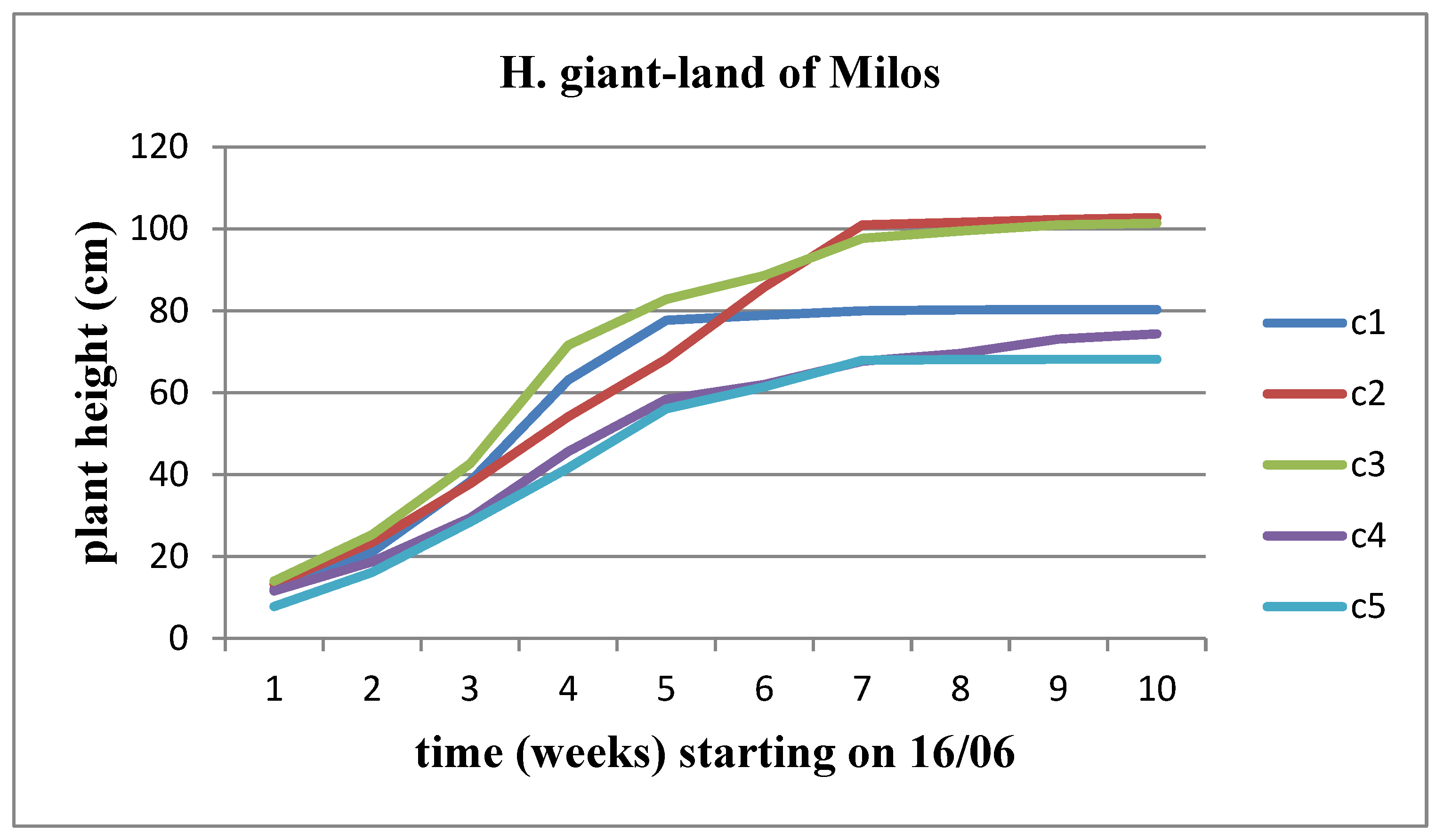
The height of the plants changes progressively over time in all concentrations of land of Milos for both sunflower stems, with a higher rate of height increase observed in the Helianthus annuus strain. However, when comparing the concentrations between them, we observe that Helianthus annuus increases in height up to the C4 concentration, whereas Helianthus giant increases its height up to the C3 concentration (Figure 23 and Figure 24).
Figure 23.
The effect of land of Milos on the height of Helianthus annuus.
Figure 23.
The effect of land of Milos on the height of Helianthus annuus.
Figure 24.
The effect of land of Milos on the height of Helianthus giant.
Figure 24.
The effect of land of Milos on the height of Helianthus giant.
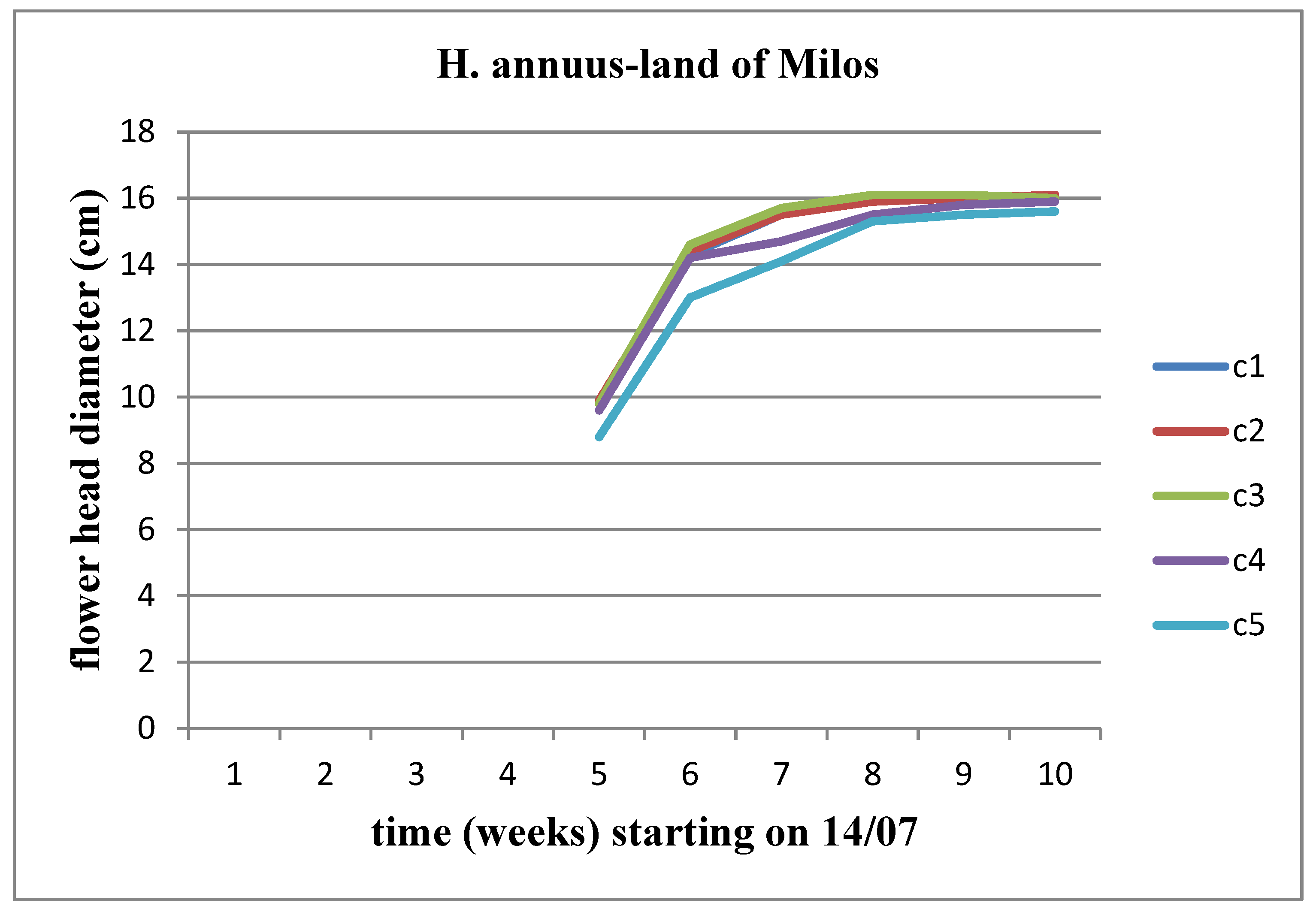
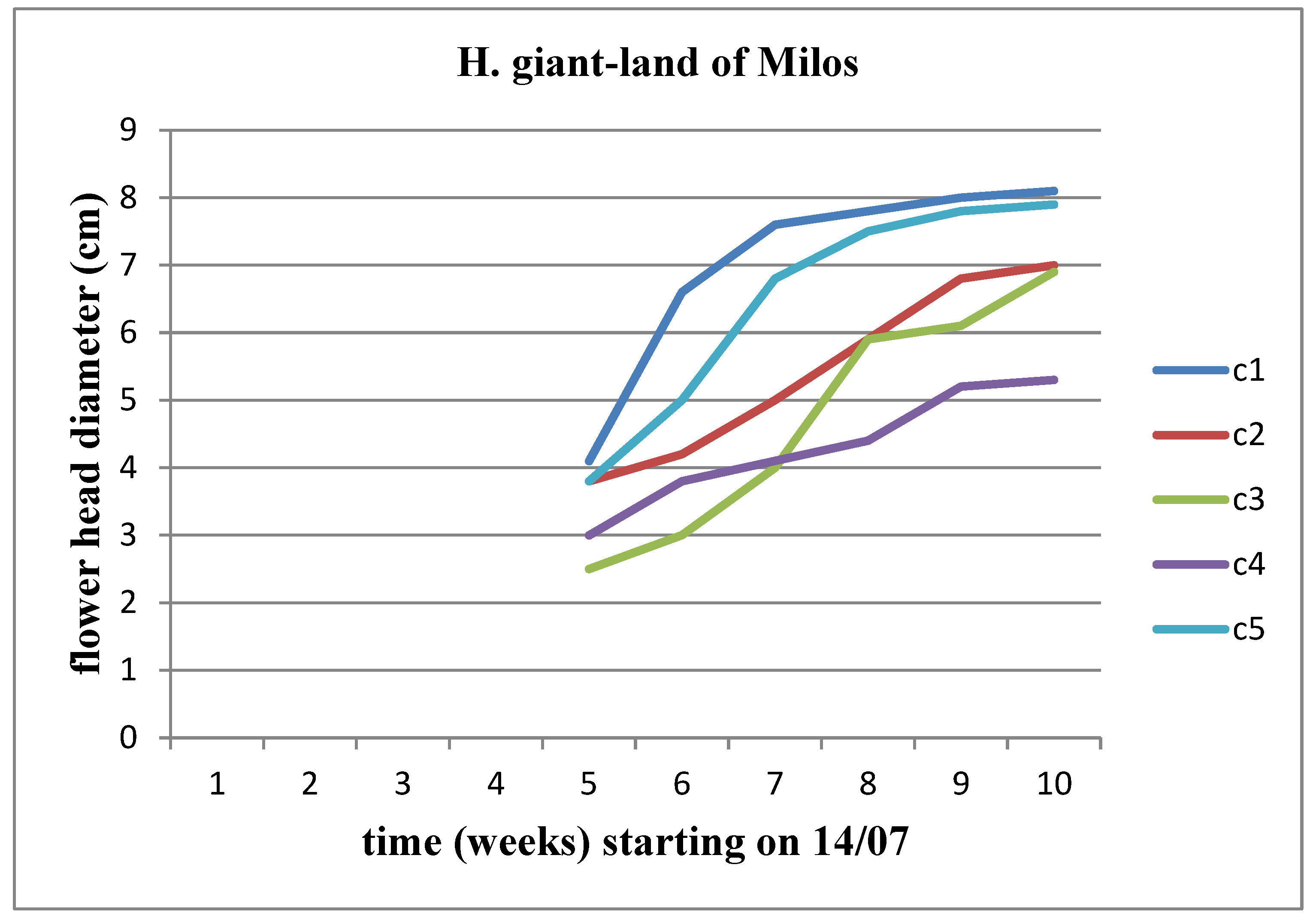
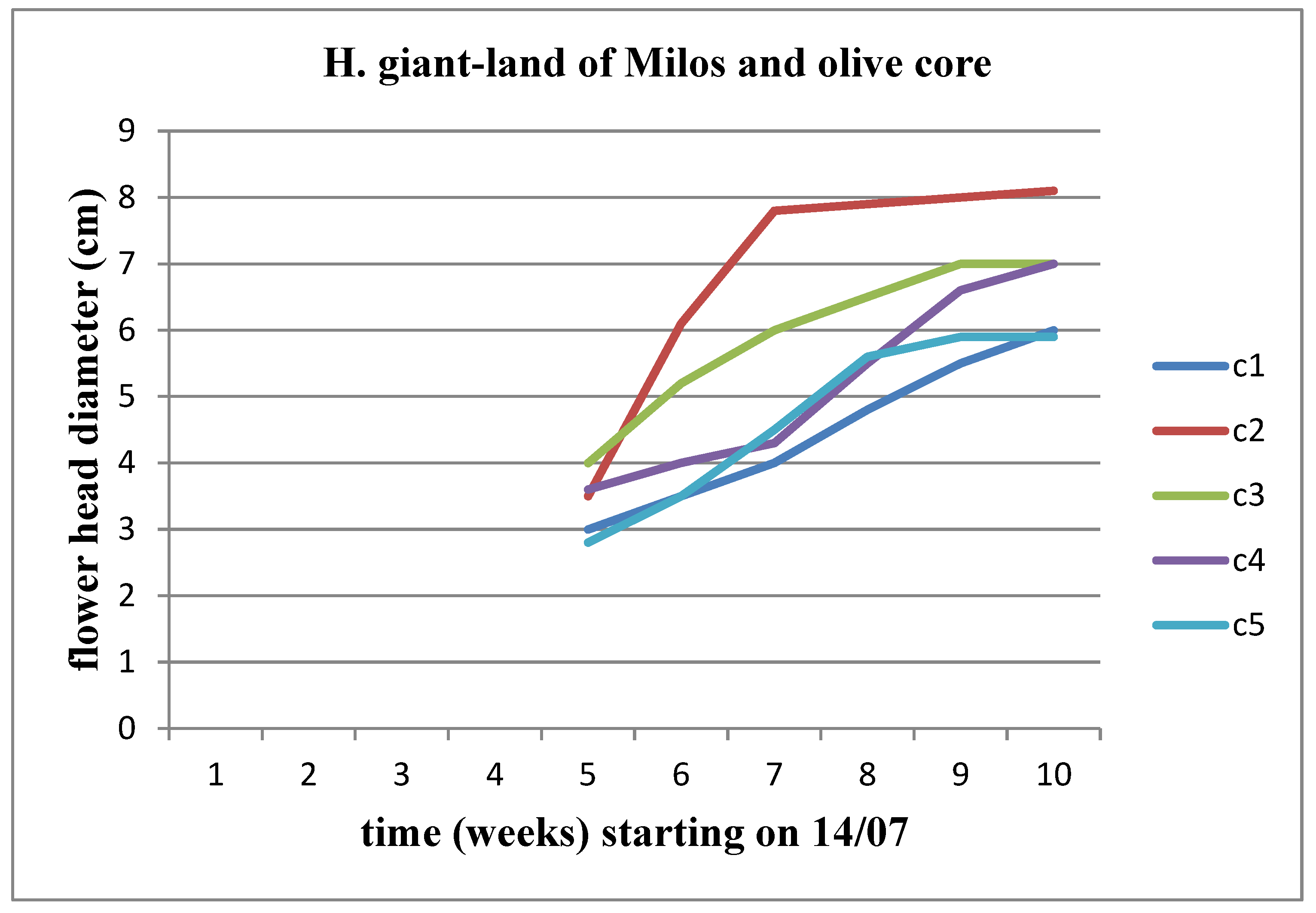
There is an increasing trend in the diameter of the flower head across all concentrations. When comparing concentrations, this upward trend persists up to the C3 concentration for Helianthus annuus. However, the flower heads of Helianthus giant did not surpass the diameter of the flower head of the control plant in any concentration of the land of Milos (Figure 25 and Figure 26).
Figure 23.
The effect of land of Milos on the diameter of the flower head for Helianthus annuus.
Figure 23.
The effect of land of Milos on the diameter of the flower head for Helianthus annuus.
Figure 24.
The effect of land of Milos on the diameter of the flower head for Helianthus giant.
Figure 24.
The effect of land of Milos on the diameter of the flower head for Helianthus giant.
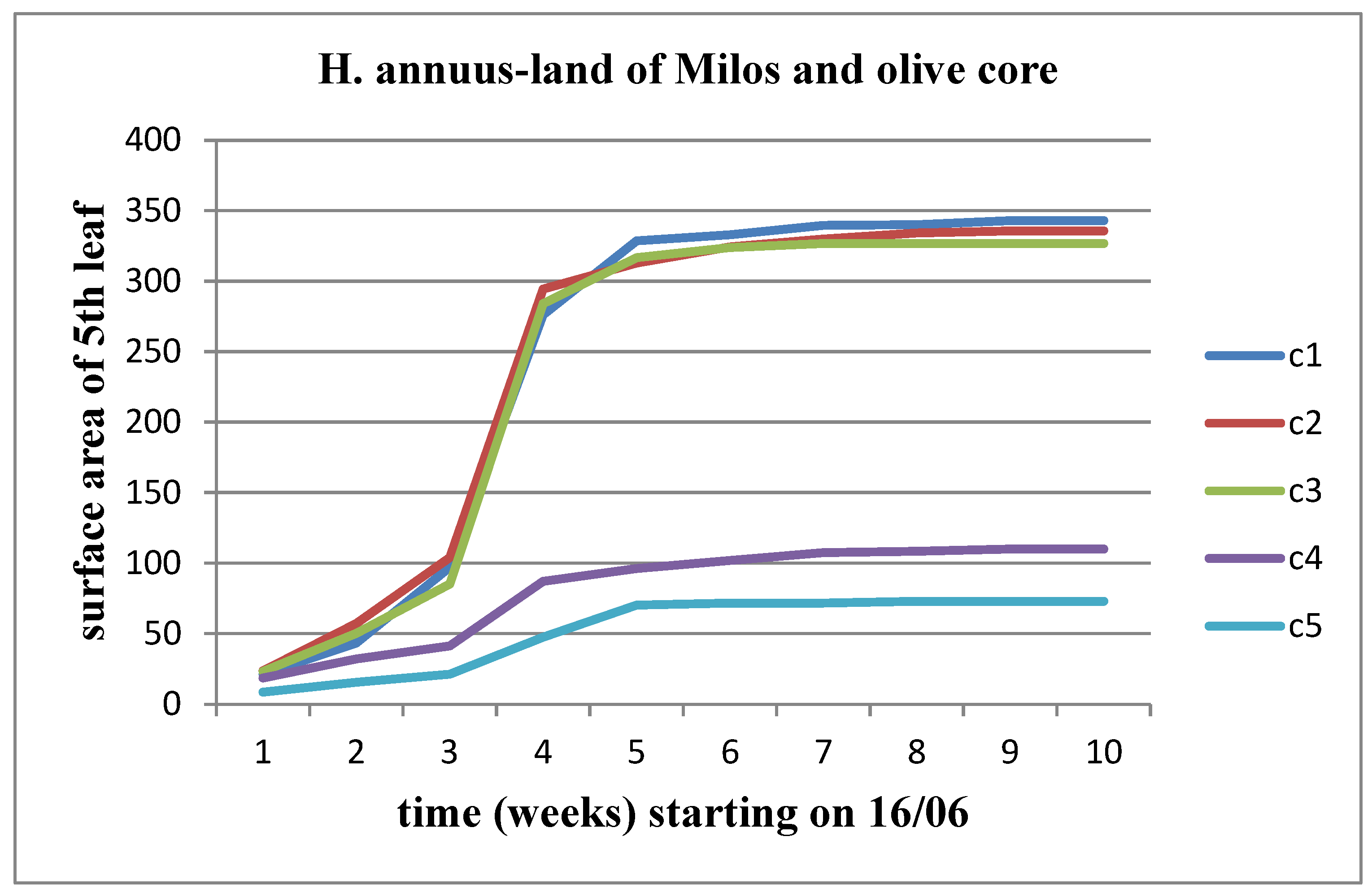
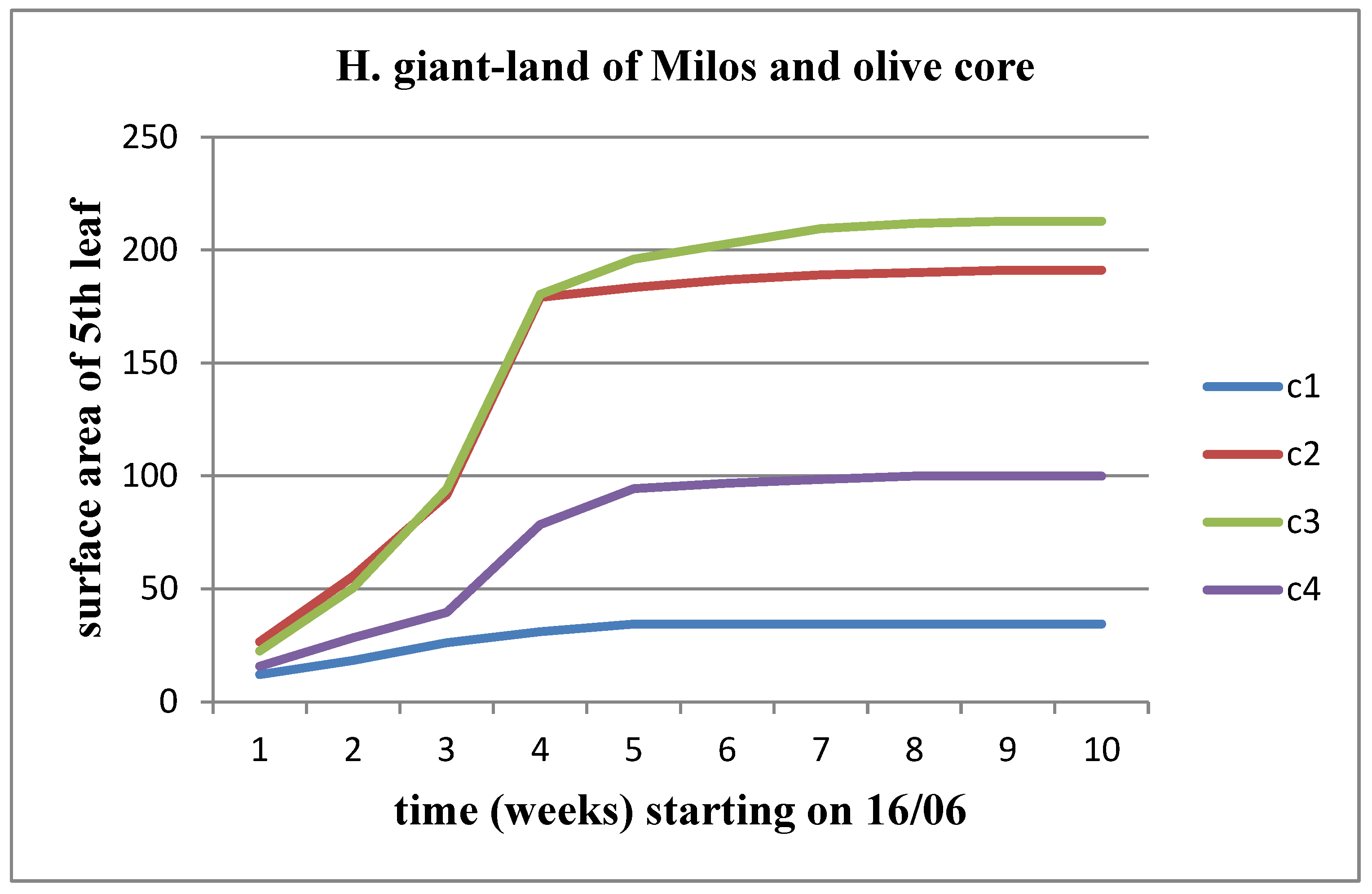
3.5. Effect of soil from the land of Milos in combination with olive core
In the five experimental plots the land of Milos was mixed with olive core at concentrations of C1 = 0 kg/m3 (control), C2 = 1.1 kg/m3, C3 = 2.2 kg/m3, C4 = 4.4 kg/m3 and C5 = 8.8 kg/m3, respectively, for each of the materials.
We observe an increase in all three parameters (length, width, area) over time, for both stems of the plant. Additionally, there is a tendency for these parameters to stabilize as we approach the time of flower head appearance. When studying the variations of these parameters between material concentrations, we notice a slight decrease up to the C3 concentration compared to the control for Helianthus annuus, which worsens from the C4 concentration onward. For Helianthus giant, we observe an increasing trend in the change of these parameters up to the C3 concentration, followed by a decrease. The change in surface area for the 5
th leaf is depicted in
Figure 25 and
Figure 26.
The height of the plant changes progressively over time in all material concentrations for both stems of the plant. When comparing the concentrations with each other, we observe that both Helianthus annuus and Helianthus giant increase in height up to the C3 concentration of the materials. However, the height of the plants decreases from the C4 concentration onwards (
Figure 27 and
Figure 28).
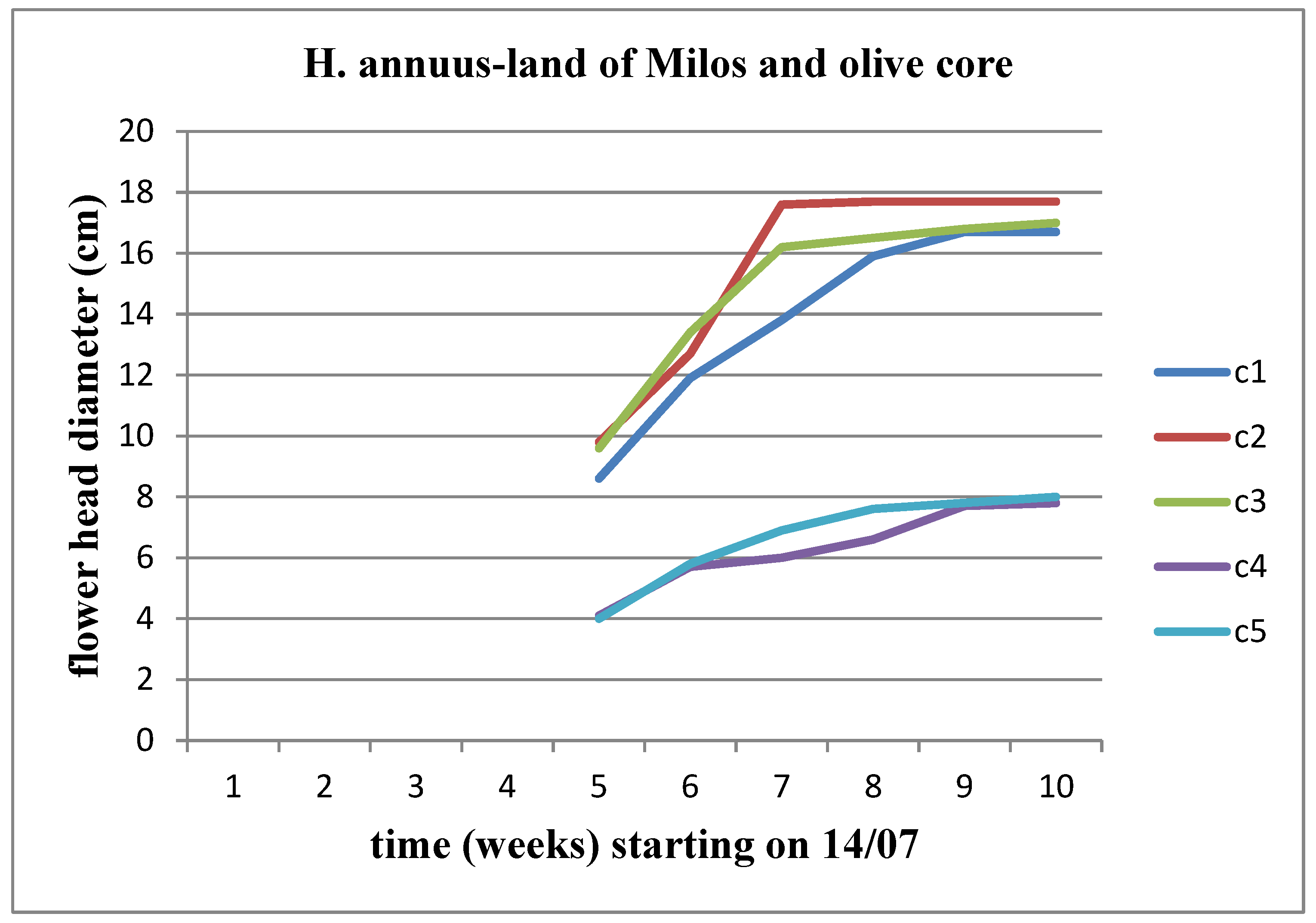
We observe a progressive increase in the diameter of the flower head for each concentration of materials over time, for both stems. When comparing the concentrations of the materials with each other, it becomes apparent that the upward trend in the diameter of the flower head appears to be interrupted at the C2 concentration for both Helianthus annuus and Helianthus giant. These results are also depicted in
Figure 27 and
Figure 28.
In all the aforementioned tests, approximately 10-15 days after the appearance and full growth of the flower head, it sheds all of its peripheral flowers as it has withered in the inflorescence (
Figure 29).
While Helianthus annuus plants displayed a single inflorescence on each plant with a large diameter, Helianthus giant plants exhibited multiple inflorescences on each plant, each with a significantly smaller diameter (
Figure 30 and
Figure 31).
In none of the experiments and experimental squares, chlorosis effects were observed in the studied plants. Furthermore, both Helianthus annuus and Helianthus giant plants completed their biological cycle, irrespective of their final size and the qualitative or quantitative composition of the soil substrate. The only exceptions were the experimental squares with the C5 concentration of ash from the fireplace and dust from the oil burner, in which no plants grew.
4. Discussion
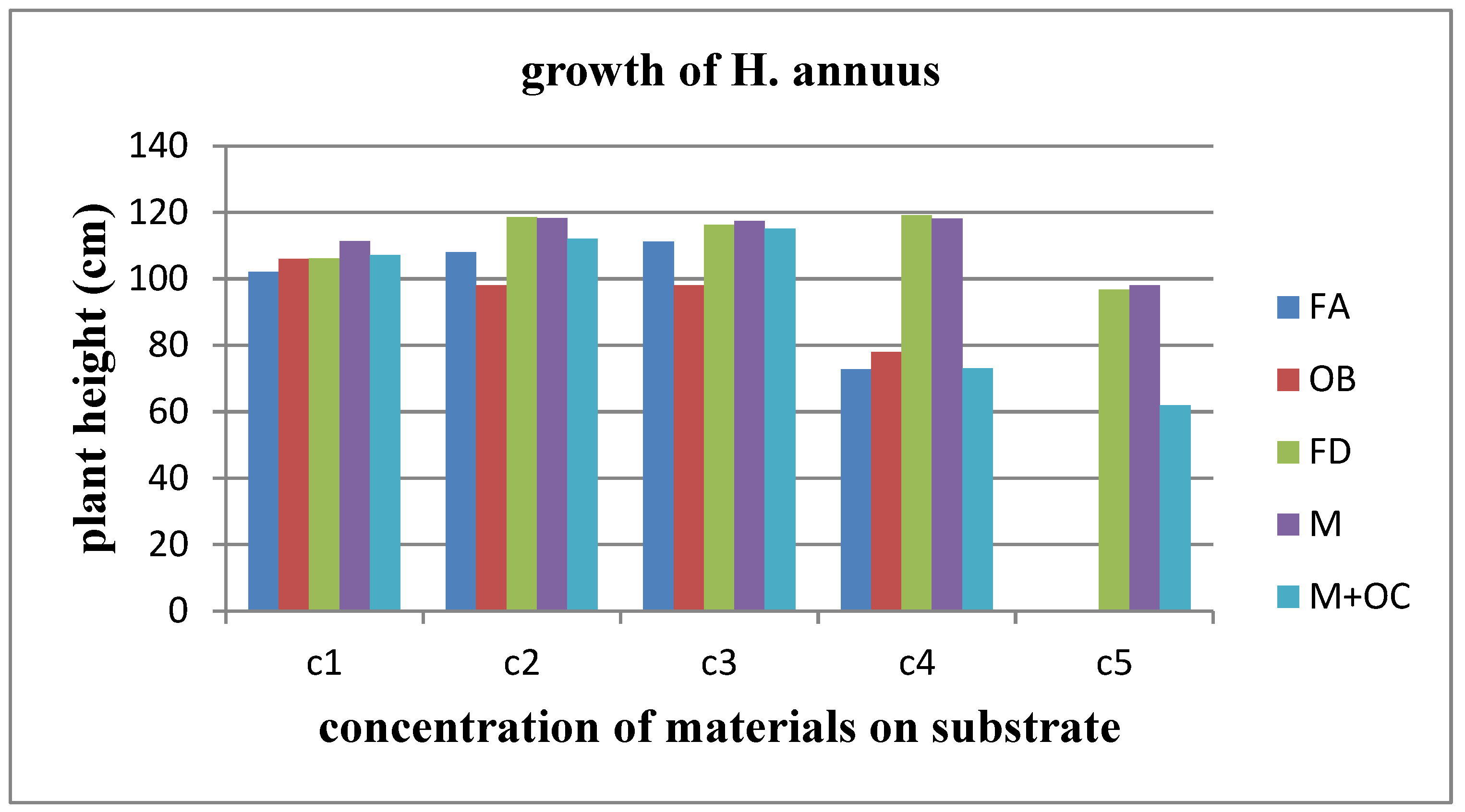
The most effective materials for the growth of Helianthus annuus are forge dust and land of Milos. As
Figure 32 illustrates, when both forge dust and land of Milos are added to the soil substrate, sunflowers at all concentrations achieve the greatest height. However, at the highest concentration, C5, although the growth rate remains the highest among these two materials, it is significantly lower than at lower concentrations. If we consider the impact of other materials on plant growth, the next most favorable material for sunflower growth is the combination of land of Milos and olive core, up to concentration C3. Following this, fireplace ash also supports growth, up to concentration C3. Oil burner dust, on the other hand, is less conducive to growth; it is the only material that has a negative effect on the growth rate of Helianthus annuus when compared to the control (C1 concentration). In fact, at concentration C5 of burner dust and fireplace ash, the plants failed to germinate.
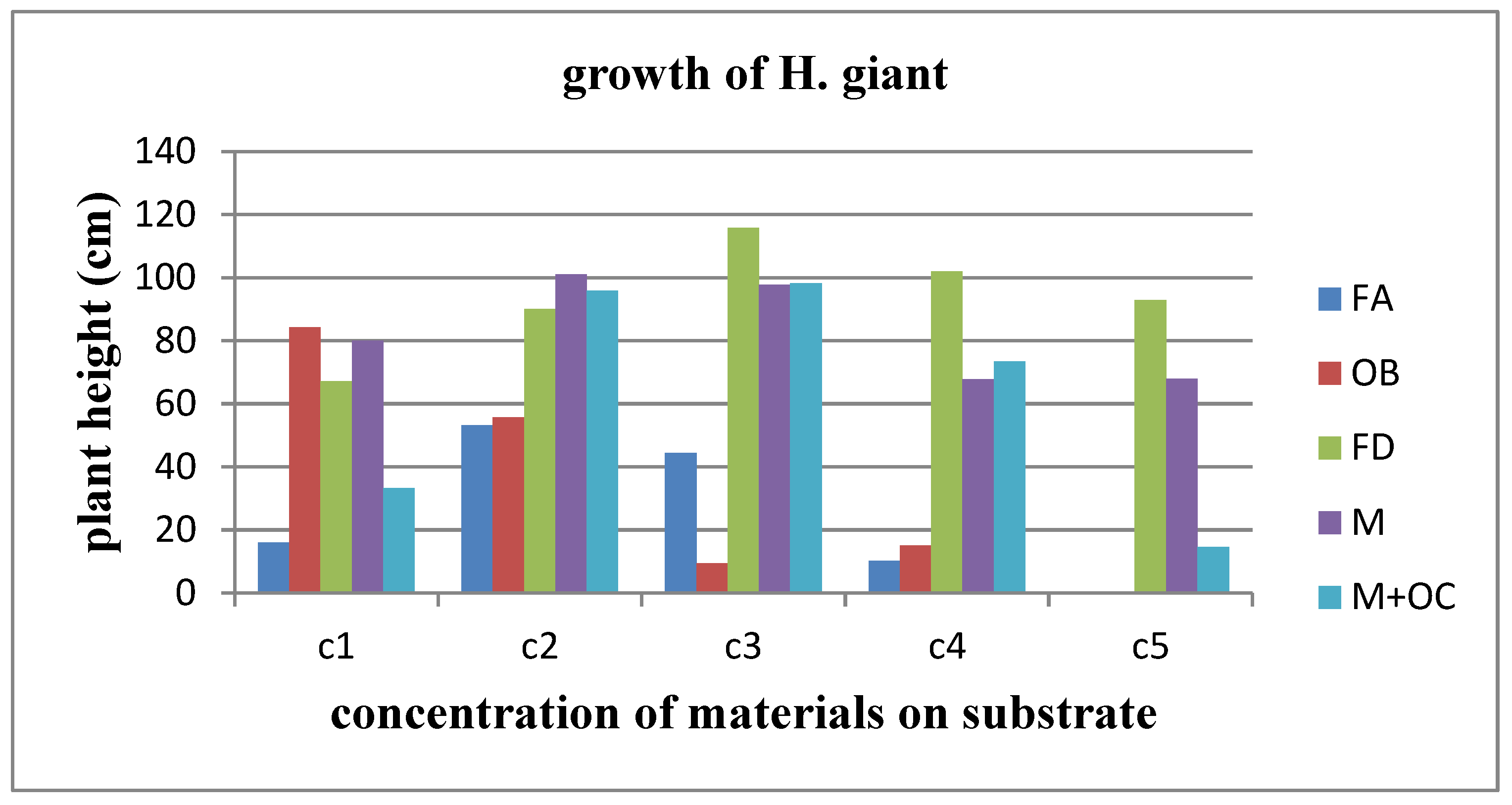
Regarding Helianthus giant, the most effective material for its growth is the dust from the forge. As
Figure 32 illustrates, the addition of forge dust to the soil substrate has a significant impact on the sunflower's growth at all concentrations, resulting in the tallest growth. However, at the highest concentrations of C4 and C5, although the growth rate is still the highest compared to other materials, it is notably lower than that observed at lower concentrations.
Following this, land of Milos exhibits a similar growth pattern to that observed with forge dust, ranking third in terms of effectiveness for plant growth. In second place for growth effectiveness is the combination of land of Milos and olive core. On the other hand, fireplace ash occupies the fourth position.
In contrast, the dust from the oil burner has a less favorable effect on growth. It stands out as the only material that, when compared to the control group (C1 concentration), negatively affects the growth rate of Helianthus giant.
In fact, at C5 concentration of both oil burner dust and fireplace ash, the plants fail to germinate entirely.
Comparing the maximum values from the two diagrams, we observe that Helianthus annuus exhibits higher growth rates when contrasted with Helianthus giant. Specifically, at the C3 and C4 concentrations of fireplace ash and burner dust, as well as at the C4 and C5 concentrations of land of Milos combined with olive core, the Helianthus giant plants experienced significant stunting. This suggests that Helianthus annuus is more efficient and resilient in the face of unfavorable environmental and soil conditions encountered during the experiment. It's worth noting that the seeds for Helianthus annuus are hybrids that have undergone special treatment and modification to thrive, even under the most challenging cultivation conditions.
When examining the results obtained from the experimental blocks of the control group, one would anticipate a certain degree of uniformity among the materials used, given that no solid waste has been introduced into these blocks. Indeed, this expected uniformity is observed in the experimental blocks of the control group for Helianthus annuus. This consistent pattern extends to the measurements of the other parameters studied, including the distance of the 1st leaf from the cotyledon, the distance between the 1st and 2nd leaf, as well as the length and width of the 5th leaf. You can also see this uniformity depicted in
Figure 32 (C1 concentration). Conversely, such uniformity was not evident in the case of Helianthus giant. Throughout the measurements, nearly all parameters under study exhibited fluctuations among the experimental blocks of the control group for the materials used. This behavior is notably illustrated in
Figure 33 (concentration C1).
At this juncture, it's worth noting that the seeds employed for Helianthus giant were commercially sourced. Consequently, there exists the possibility that the seeds utilized originated from different varieties or related species of Helianthus giant. This hypothesis could account for the observed variations among Helianthus giant plants, including disparities in some of their fundamental morphological traits. For instance, leaf morphology displayed notable differences among some of the plants, and there were variations in the number of inflorescences. In contrast, the seeds employed for Helianthus annuus were procured from the Syngenta company, specifically the Neoma hybrid. Helianthus annuus plants exhibited a remarkable degree of uniformity in both key parameter measurements and morphological characteristics.
It's noteworthy that the plants successfully grew and completed their biological cycles in all experimental blocks, except for the ones containing the C5 concentration of fireplace ash and burner dust. An examination of the measured parameters reveals a slow initial growth rate during the early stages of cultivation. The newly planted seedlings required approximately fifteen days to acclimate to the environmental conditions of the area and the soil conditions in the experimental blocks. Subsequently, a significant surge in the growth rate of the plants was observed. Some of these plants even exhibited a doubling of their leaf area (specifically for the 5th leaf) or height between successive measurements. This robust growth continued until the plants reached their maximum height, a stage that almost coincided with the appearance of the flower head. However, it's important to note that certain parameters, such as the distance of the 1st leaf from the cotyledon and the distance between the 1st and 2nd leaf, reached a constant value earlier in the cultivation process.
These parameters reached their maximum values approximately four weeks after the commencement of the measurements. The 5th leaf completed its growth in terms of length, width, and surface area approximately seven weeks after the measurements began. The appearance of the flower head followed about six weeks from the onset of the measurements, and its diameter value stabilized approximately 15 days after its emergence. The plants achieved their maximum height roughly 70 days after the start of the measurements. It becomes evident that upon the appearance of the flower head, the plant reallocates its energy resources, slowing down other developmental processes to prioritize the proper development of the flower head. This shift is driven by the flower head's crucial role in fertilization and ensuring the species' continuation. Consequently, the appearance of the flower head marked a decrease in the growth rate, eventually leading to stagnation, as depicted in the measurements of the studied parameters and their corresponding figures.
5. Conclusions
The impact of solid waste on the growth of Helianthus annuus and Helianthus giant in the context of phytoremediation of contaminated soils is quite significant. Although solid waste can potentially have adverse effects on plant growth, these sunflower species possess attributes that render them well-suited for phytoremediation. Given their capacity to accumulate and remove contaminants from the soil, Helianthus annuus and Helianthus giant present a promising avenue for the sustainable remediation of polluted sites. However, further research and experimentation are imperative to comprehend the precise dynamics between solid waste contaminants, plant physiology, and phytoremediation efficiency, aiming to fully harness their potential in environmental cleanup endeavors.
The present investigation has revealed the following:
Helianthus annuus and Helianthus giant plants completed their biological cycles regardless of the qualitative or quantitative composition of the soil substrate. The only exceptions were the experimental blocks with the concentrations of fireplace ash and oil burner dust.
In none of the trials did the crops exhibit any signs of chlorosis.
The growth rate of the crops was initially slow during the first 15 days. Afterward, this rate increased significantly until the plants reached their maximum height. However, upon the emergence of the inflorescence, the growth rate of the plants slowed down considerably.
Across all tests, Helianthus annuus demonstrated greater resistance and performed better in the measurements when compared to Helianthus giant.
The results show that Helianthus annuus is suitable for phytoremediation of polluted soils:
with fireplace ash when the concentration of this material is not exceeds 4.4 kg/m3
with dust from the oil burner, where the concentration of this material the concentration of this material does not exceed 2.2 kg/m3
with dust from the forge, where the concentration of this material is not more than 1.1 kg/m3
with land of Milos and olive core, where the concentrations of these two does not exceed 2.2 kg/m3 for each material
Helianthus annuus could be used for phytoremediation soils (e.g. mines) which, however, contain land of Milos at a concentration of less than 4.4 kg/m3
- 6.
Additionally, the experiment suggests that Helianthus giant is suitable for phytoremediation of polluted soils:
with fireplace ash, provided the concentration of this material does not exceed 2.2 kg/m³
with forge dust, where the concentration of this material does not exceed 0.55 kg/m³
with land of Milos and olive core, as long as the concentrations of these two substances do not exceed 2.2 kg/m³ each
Helianthus giant could be used for phytoremediation of soils, such as mines, as long as the concentration of land of Milos does not exceed 2.2 kg/m³
Helianthus giant is considered unsuitable for pesticide application and phytoremediation of soils contaminated with dust from the oil burner combustor
In any case, the utilization of sunflowers for both plant protection and the phytoremediation of soils contaminated with solid waste necessitates the implementation of appropriate measures to safeguard the respective sites. It is imperative that no chemical elements present in the contaminated soil, whether they have bioaccumulated within the plant's tissues (leaves, stem) or its products (seeds, oil), be transferred to higher organisms through the food chain. The excessive accumulation of these chemical elements can potentially result in concentrations that are toxic to consumers incorporating sunflower and its derivatives into their diet. Therefore, it is crucial to take the necessary steps to shield or oversee these crops, preventing the intrusion of animals and humans not involved in the application within the affected area. Additionally, careful attention must be given to the secure disposal of the plants and their products (seeds, deceased organic matter) following the completion of their biological cycle.
Author Contributions
Conceptualization, S.P.V.; methodology, S.P.V; validation S.P.V. and E.C.; formal analysis, S.P.V. and E.C.; investigation, E.C.; resources, E.C.; data curation, S.K.G.; writing—original draft preparation, S.K.G.; writing—review and editing, S.K.G. and S.P.V.; visualization, S.K.G. and E.C.; supervision, S.P.V, I.K.K. and S.K.G.; project administration, S.P.V., I.K.K. and S.P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors express their gratitude to the Soil and Water Resources Institute Laboratory for conducting the soil analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wei, Z.; Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A Review on Phytoremediation of Contaminants in Air, Water and Soil. Journal of Hazardous Materials 2020, 403, 123658. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Khilji, S.A.; Tariq, S.; Jamal, A.; Alomrani, S.O.; Javed, T. Phytoremediation of Heavy Metals from Industrially Contaminated Soil Using Sunflower (Helianthus Annus L.) by Inoculation of Two Indigenous Bacteria. Plant Stress 2024, 11, 100297. [Google Scholar] [CrossRef]
- Bhat, S.A.; Bashir, O.; Ul Haq, S.A.; Amin, T.; Rafiq, A.; Ali, M.; Américo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of Heavy Metals in Soil and Water: An Eco-Friendly, Sustainable and Multidisciplinary Approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef] [PubMed]
- Rathi, M.; K N, Y. Brevundimonas Diminuta MYS6 Associated Helianthus Annuus L. for Enhanced Copper Phytoremediation. Chemosphere 2021, 263, 128195. [Google Scholar] [CrossRef] [PubMed]
- Kalavrouziotis, I.; Soultanopoulos, A.; Varnavas, S. Effect of Olive Core and Olive Core Ash on the Growth of Helianthus Annuus and Soil Quality. Global Nest Journal 2018, 20, 169–172. [Google Scholar]
- Adesodun, J.; Atayese, M.; Agbaje, T.; Osadiaye, B.; Mafe, O.; Soretire, A. Phytoremediation Potentials of Sunflowers (Tithonia Diversifolia and Helianthus Annuus) for Metals in Soils Contaminated with Zinc and Lead Nitrates. Water, Air, and Soil Pollution 2010, 207, 195–201. [Google Scholar] [CrossRef]
- Hunce, S.Y.; Clemente, R.; Bernal, M.P. Energy Production Potential of Phytoremediation Plant Biomass: Helianthus Annuus and Silybum Marianum. Industrial Crops and Products 2019, 135, 206–216. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil and Sediment Contamination: An International Journal 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Wang, D. Phytoremediation of Uranium and Cadmium Contaminated Soils by Sunflower (Helianthus Annuus L.) Enhanced with Biodegradable Chelating Agents. Journal of Cleaner Production 2020, 263, 121491. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, Mu.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of Heavy Metals: Mechanisms, Methods and Enhancements. Environmental Chemistry Letters 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Atia, F.; Al-Ghouti, M.; Al-Naimi, F.; Abu-Dieyeh, M.; Ahmed, T.; Almeer, S. Removal of Toxic Pollutants from Produced Water by Phytoremediation: Applications and Mechanistic Study. Journal of Water Process Engineering 2019, 32, 100990. [Google Scholar] [CrossRef]
- Chauhan, P.; Rajguru, A.B.; Dudhe, M.; Mathur, J. Efficacy of Lead (Pb) Phytoextraction of Five Varieties of Helianthus Annuus L. from Contaminated Soil. Environmental Technology & Innovation 2020, 18, 100718. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, V.; Usmani, Z.; Gupta, P.; Chandra, A. Influence of Plant Growth Promoting Rhizobacterial Strains Paenibacillus Sp. IITISM08, Bacillus Sp. PRB77 and Bacillus Sp. PRB101 Using Helianthus Annuus on Degradation of Endosulfan from Contaminated Soil. Chemosphere 2019, 225. [Google Scholar] [CrossRef] [PubMed]
- Marchiol, L.; Fellet, G.; Perosa, D.; Zerbi, G. Removal of Trace Metals by Sorghum Bicolor and Helianthus Annuus in a Site Polluted by Industrial Wastes: A Field Experience. Plant physiology and biochemistry : PPB / Société française de physiologie végétale 2007, 45, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Dekoninck, M.; Deforce, K.; Kaal, J.; Out, W.A.; Van Thienen, V.; Buyse, F.; Kubiak-Martens, L.; Tack, P.; Vincze, L.; Lycke, S.; et al. Fuelling the Roman Salt Industry. Developing a New Multiproxy Approach to Identify Peat Fuel from Archaeological Combustion Residue. Journal of Archaeological Science 2024, 161, 105892. [Google Scholar] [CrossRef]
- Amran, M.; Al-Fakih, A.; Chu, S.H.; Fediuk, R.; Haruna, S.; Azevedo, A.; Vatin, N. Long-Term Durability Properties of Geopolymer Concrete: An in-Depth Review. Case Studies in Construction Materials 2021, 15, e00661. [Google Scholar] [CrossRef]
Figure 1.
The demarcation of blocks.
Figure 1.
The demarcation of blocks.
Figure 2.
Location and composition of the experimental plots.
Figure 2.
Location and composition of the experimental plots.
Figure 3.
Effect of FA on the surface area of the 5th floor leaf area for Helianthus annuus.
Figure 3.
Effect of FA on the surface area of the 5th floor leaf area for Helianthus annuus.
Figure 4.
Effect of FA on the surface area of the 5th floor leaf area for Helianthus giant.
Figure 4.
Effect of FA on the surface area of the 5th floor leaf area for Helianthus giant.
Figure 5.
Effect of FA on the height of the Helianthus annuus.
Figure 5.
Effect of FA on the height of the Helianthus annuus.
Figure 6.
Effect of FA on the height of the Helianthus giant.
Figure 6.
Effect of FA on the height of the Helianthus giant.
Figure 7.
Effect of FA on the diameter of the flower head of the Helianthus annuus.
Figure 7.
Effect of FA on the diameter of the flower head of the Helianthus annuus.
Figure 8.
Effect of FA on the diameter of the flower head of the Helianthus giant.
Figure 8.
Effect of FA on the diameter of the flower head of the Helianthus giant.
Figure 9.
Effect of dust from OB on the surface area of the 5th leaf or Helianthus annuus.
Figure 9.
Effect of dust from OB on the surface area of the 5th leaf or Helianthus annuus.
Figure 10.
Effect of dust from OB on the surface area of the 5th leaf for Helianthus giant.
Figure 10.
Effect of dust from OB on the surface area of the 5th leaf for Helianthus giant.
Figure 11.
The effect of dust from OB on the height of Helianthus annuus.
Figure 11.
The effect of dust from OB on the height of Helianthus annuus.
Figure 12.
The effect of dust from OB on the height of Helianthus giant.
Figure 12.
The effect of dust from OB on the height of Helianthus giant.
Figure 13.
The effect of dust from OB on the diameter of the flower head of Helianthus annuus.
Figure 13.
The effect of dust from OB on the diameter of the flower head of Helianthus annuus.
Figure 14.
The Effect of dust from OB on the diameter of the flower head of Helianthus giant.
Figure 14.
The Effect of dust from OB on the diameter of the flower head of Helianthus giant.
Figure 15.
The effect of FD on the surface area of the 5th leaf for Helianthus annuus.
Figure 15.
The effect of FD on the surface area of the 5th leaf for Helianthus annuus.
Figure 16.
The effect of FD on the surface area of the 5th leaf for Helianthus giant.
Figure 16.
The effect of FD on the surface area of the 5th leaf for Helianthus giant.
Figure 17.
The effect of FD on the height of Helianthus annuus.
Figure 17.
The effect of FD on the height of Helianthus annuus.
Figure 18.
The effect of FD on the height of Helianthus giant.
Figure 18.
The effect of FD on the height of Helianthus giant.
Figure 19.
The effect of FD on flower head diameter for Helianthus annuus.
Figure 19.
The effect of FD on flower head diameter for Helianthus annuus.
Figure 20.
The effect of FD on flower head diameter for Helianthus giant.
Figure 20.
The effect of FD on flower head diameter for Helianthus giant.
Figure 21.
The effect of land of Milos on the area of 5th leaf for Helianthus annuus.
Figure 21.
The effect of land of Milos on the area of 5th leaf for Helianthus annuus.
Figure 22.
The effect of land of Milos on the area of 5th leaf for Helianthus giant.
Figure 22.
The effect of land of Milos on the area of 5th leaf for Helianthus giant.
Figure 25.
The effect of land of Milos and olive core on surface area for the 5th leaf of Helianthus annuus.
Figure 25.
The effect of land of Milos and olive core on surface area for the 5th leaf of Helianthus annuus.
Figure 26.
The effect of land of Milos and olive core on surface area for the 5th leaf of Helianthus giant.
Figure 26.
The effect of land of Milos and olive core on surface area for the 5th leaf of Helianthus giant.
Figure 27.
The effect of land of Milos and olive core on diameter of the flower head for Helianthus annuus.
Figure 27.
The effect of land of Milos and olive core on diameter of the flower head for Helianthus annuus.
Figure 28.
The effect of land of Milos and olive core on diameter of the flower head for Helianthus giant.
Figure 28.
The effect of land of Milos and olive core on diameter of the flower head for Helianthus giant.
Figure 29.
Inflorescence at the end of flowering when pollination of the flowers has been completed.
Figure 29.
Inflorescence at the end of flowering when pollination of the flowers has been completed.
Figure 30.
Helianthus annuus inflorescence.
Figure 30.
Helianthus annuus inflorescence.
Figure 31.
Helianthus giant inflorescence.
Figure 31.
Helianthus giant inflorescence.
Figure 32.
Illustration of Helianthus annuus growth in different materials.
Figure 32.
Illustration of Helianthus annuus growth in different materials.
Figure 33.
Illustration of Helianthus giant growth in different materials.
Figure 33.
Illustration of Helianthus giant growth in different materials.
Table 1.
Concentrations of solid wastes added.
Table 1.
Concentrations of solid wastes added.
| Materials |
|
Concentration levels (kg/m3) |
| |
C1 |
C2 |
C3 |
C4 |
C5 |
| Fireplace ash (FA) |
0 |
2.42 |
4.4 |
8.9 |
17.8 |
| Dust from oil burners (OB) |
0 |
2.42 |
4.4 |
8.9 |
17.8 |
Forge dust (FD)
Soil from the land of Milos (M) |
0
0 |
0.28
1.1 |
0.55
2.2 |
1.1
4.4 |
2.2
8.8 |
| Olive core (OC) |
0 |
1.1 |
2.2 |
4.4 |
8.8 |
Table 2.
Physicochemical characteristics of the solid waste used in the experiment.
Table 2.
Physicochemical characteristics of the solid waste used in the experiment.
| Parameters |
FA |
OB |
FD |
M |
OC |
| pH |
12.6 |
3.1 |
7.5 |
7.6 |
7.9 |
| EC (mmhos) |
30 |
6.6 |
0.2 |
0.8 |
2.9 |
| Total solids (%)
|
100.0 |
97.6 |
100.0 |
97.1 |
69.0 |
| Humidity (%)
|
0 |
2.4 |
0 |
2.9 |
31.0 |
| P (mg/l)
|
0 |
0 |
0 |
0 |
0.1 |
K (mg/l)
N (mg/l)
|
3.2
0.06 |
0.11
0.26 |
0
0 |
0.05
0.13 |
0.33
1.52 |
| Mg (mg/l)
|
0.87 |
0.3 |
0.16 |
0.19 |
0.03 |
Ca (mg/l)
Cu (mg/l)
Mn (mg/l)
Fe (mg/l)
Zn (mgl) |
14.7
66.0
1639.0
0.3
73.0 |
8.7
156.0
546.0
3.6
167.0 |
0.04
402.0
1295.0
26.1
452.0 |
0.28
13.0
118.0
0.9
14.0 |
0.13
3.4
4.0
0.02
4.2 |
Table 3.
Soil analysis of the experimental field.
Table 3.
Soil analysis of the experimental field.
| Parameters |
Before
lubrication |
After
lubrication |
Normal prices |
| pH |
7.42 |
7.48 |
slightly alkaline |
| EC (mmhos) |
6.193 |
6.196 |
< 4.0 normal |
| CaCO3 (%) |
9.51 |
9.55 |
2.0 – 20.0 abundant |
| Active CaCO3 (%) |
3.61 |
3.64 |
<7.5 no chlorosis |
| P (mg/l) |
22.28 |
22.38 |
16.0 – 25.0 sufficient |
| K (mg/l) |
0.311 |
0.684 |
<0.384 mοderate
>0.639 excessive |
| Mg (mg/l) |
0.85 |
0.93 |
0.83 – 1.44 sufficient |
| Ca (mg/l) |
1689.4 |
1705.5 |
200.0-2000.0 |
| NO3-N (mg/l) |
20.3 |
63.3 |
>30.0 very high |
| Cu (mg/l) |
4.9 |
5.1 |
>3.0 very high |
| Mn (mg/l) |
5.1 |
5.0 |
5.0-14.0 low |
| Fe (mg/l)
|
10.62 |
11.42 |
11.0-24.0 moderate |
| Zn (mg/l) |
4.7 |
4.9 |
3.0-5.0 moderate |
| Β (mg/l) |
1.12 |
1.16 |
0.8-1.2 moderate |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).