1. Introduction
Brimonidine is a selective alpha-2 adrenergic agonist, with a 1780-fold selectivity for α2 vs α1-adrenergic receptors [
1], increasingly used for the treatment of open-angle glaucoma (OAG) in children and adults [
2]. It is reported to be up to 12-fold more alpha-2 selective than clonidine and up to 32-fold more alpha 2 selective than apraclonidine (p-aminoclonidine) [
3].
Brimonidine lowers intraocular pressure through a dual mechanism. It inhibits adenylyl cyclase with a decrease of cyclic AMP levels and noradrenaline release through alpha-2 receptor activation, thus reducing aqueous humor production [
4] and stimulates the outflow of aqueous humor via uveoscleral pathway [
5]. This latter mechanism is responsible for the drug effect following chronic treatment [
6]. Although more polar and less lipophilic than clonidine (
Figure 1) brimonidine is known to cross the blood-brain barrier [
7].
Indeed, in addition to local complications such as dermatitis, brimonidine has been shown to cause systemic toxicity, including neurological side effects [
8]. The potential central nervous system (CNS) side effects of a2-adrenergic stimulation in young children are unresponsiveness, lethargy, hypoventilation, and stupor. CNS depression may mimic opioid toxicity [
9,
10,
11,
12]. Berlin and coworkers [
13] reported a case of a 1-month-old infant affected by Peters anomaly, characterized by corneal opacification associated with glaucoma, who presented recurrent episodes of unresponsiveness, hypotension, hypotonia, hypothermia, and bradycardia. The infant was under treatment with the following ophthalmic drops: Trusopt (dorzolamide), Betoptic (be-taxolol), and Alphagan (brimonidine). During each episode, following naloxone administration, the patient dramatically but only transiently improved. The episodes disappeared only after brimonidine discontinuation [
13].
There are only a few data on brimonidine safety in children. The initial clinical trials mainly involved patients aged 2-7 years with a diagnosis of glaucoma, and no trials enrolled patients aged less than 1 year [
3]. However, as it does not cause reduced pulmonary function and heart rate (HR), unlike β-adrenergic blocking agents, it is licensed for use in children over 5 years old. [
14]. Yet, the lower weight could make the patient more susceptible to complications; thus, the pediatric group is at greater risk [
15]. In Italy, brimonidine administration to children aged 2 to 12 years is not recommended (PdfDownloadServlet (agenziafarmaco.gov.it) [
16].
We report one case of transient encephalopathy in a toddler, in whom brimonidine accidental toxicity was suspected and then confirmed by a toxicology study.
2. Case Presentation
The 8-month-old girl, first born to unrelated healthy parents, was reported with normal growth and development. She was taken to the emergency room due to the mother's home observation of a state of hyporeactivity and hypotonia. The mother also reported attempted breastfeeding with difficulty in sucking due to incoordination. The little girl attempted to stand but fell backwards, hitting her occiput: immediate crying, no loss of consciousness, no vomiting. No fever or any other significant symptoms in the previous days.
At physical examination, the child was drowsy and arousable for brief moments with persistent crying, hypotonia, and bilateral miosis. On palpation of the skull no swellings or bony steps. Pinkish-pale skin. Eupnoecious, diffusely harsh vesicular murmur on the chest with good air penetration. Valid and rhythmic cardiac activity. Abdomen was treatable. She was afebrile, with SpO2: 99% in room air, ABP 99/51 mmHg, and heart rate 113/min.
The laboratory work-up was not informative. PCR on pharyngeal swab for detection of SARS-CoV-2 genome was negative. ECG and EEG were normal. CT scan of the skull and brain was normal. Toxicological study on blood and urine was completely negative. Flumazenil administration was not effective in reducing the state of drowsiness of the child. Starting from hour +4, the child progressively improved, and by hour +6, she recovered to a normal state of consciousness.
In an attempt to identify a possible toxic agent, the parents were asked to check the home environment at the child's reach. Later on, the mum reported the vials of brimonidine-based eye drops used by the grandfather, found empty on the sofa near where the little girl had been playing.
3. Biochemical study
Following the suspicion of brimonidine assumption by eye drop, an ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method was applied for the determination of brimonidine in urine and plasma samples, respectively [
17,
18], collected about 10 hours after exposure. Sample preparation and UPLC-MS/MS analysis are fully detailed in the
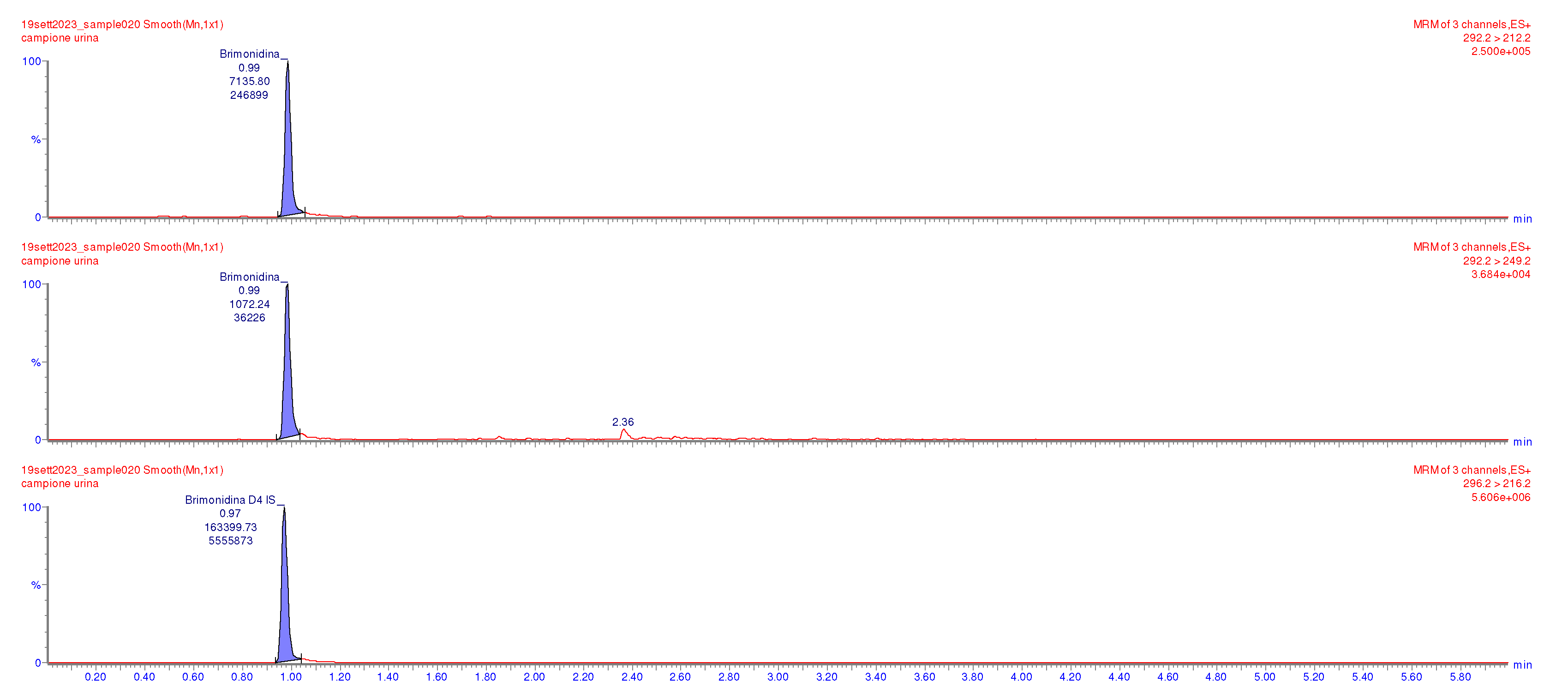
Supplementary Materials, Supplementary Tables (ST1 and ST2), and Figures (SF1-SF4). The UPLC/MS/MS analysis performed on the urine sample revealed 8.40 ng/mL brimonidine concentration levels, supporting the initial clinical suspicion. The chromatograph in
Figure 2 clearly highlights the presence of brimonidine in the urine of the patient sample.
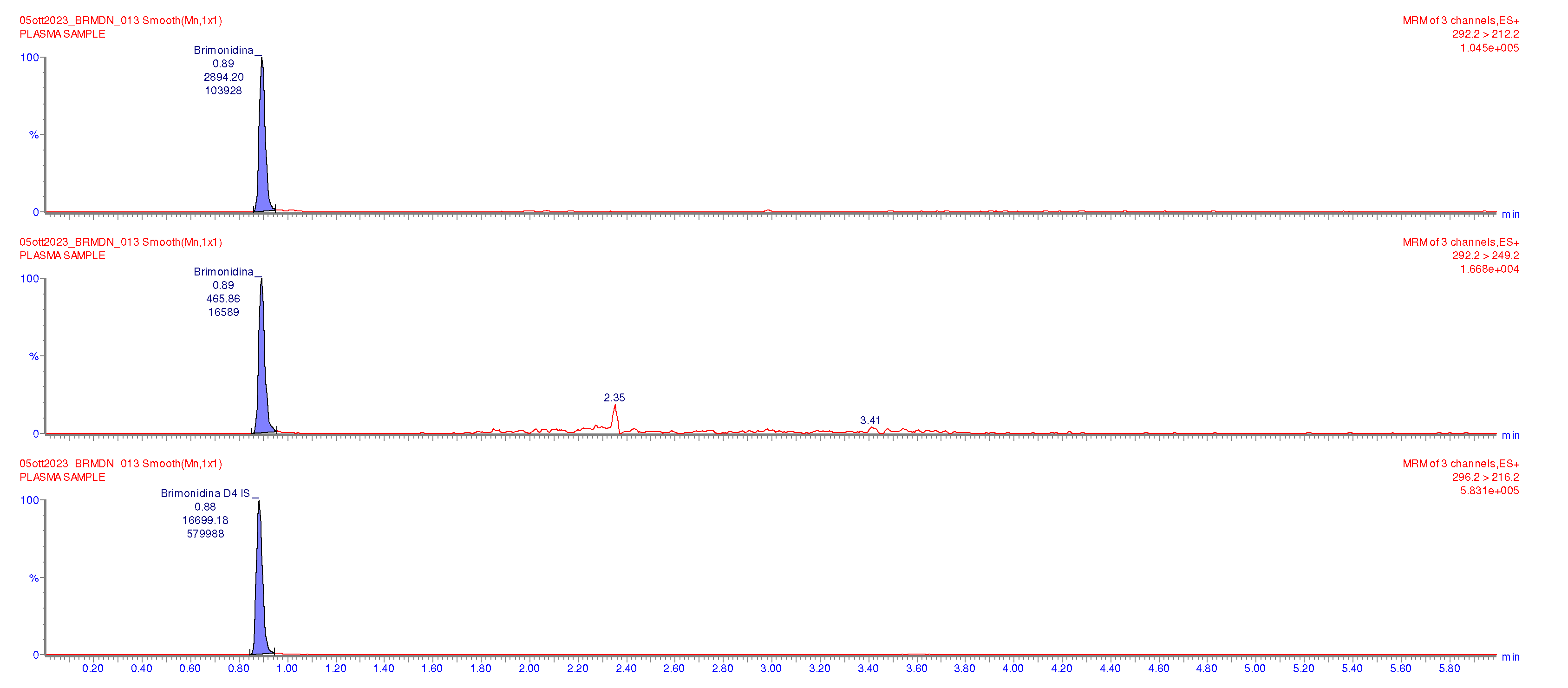
The clinical suspicion was further supported by the analysis of a plasma patient sample with brimonidine concentration levels of 0.79 ng/mL, as shown in the chromatograph reported in
Figure 3.
4. Discussion
Brimonidine is a potentially lethal medication for young infants, even as an ophthalmic drop. Studies on different animal models have shown that topical solution causes greater systemic absorption than topical gels [
19]. It has been reported that tear volumes increase with age being 0.5 μL for newborns, 2.5 μL for older infants, and 6 μL for adults [
20]; thus, topically applied drugs are much more concentrated in young infants. Based on these data, a newborn needs only one-half, and a 3-year-old child only needs two-thirds of the adult dosage to reach an equivalent ocular concentration [
20]. The eye membranes in infants are thinner than in adults, allowing faster drug absorption and corneal permeation. Moreover, the drug solution can reach the nasal cavity, where the compound can be absorbed through the nasal mucosa and reach systemic circulation, bypassing the liver, thus avoiding first-pass metabolism [
20].
In 2021, Ghaffari et al. reported a series of six cases found in the literature on systemic side effects of accidental oral or nasal ingestion of brimonidine eye drops in children, reported up to the end of 2019 [
21], together with five additional new cases from personal observation. Their age ranged between 9 days and 4 years.
Miosis may be a helpful sign to suspect brimonidine intoxication but should not be considered a must for suspecting the diagnosis.
The duration of hypotonia may be variable. Ghaffari et al. report that in all of their cases, hypotonia lasted about twice the time needed for recovery to a normal level of consciousness [
21].
In the present case, the child restored by hour +6 a normal level of consciousness and muscular tone. Duration and type of complication may likely reflect (also) the dose of brimonidine assumed [
11,
22]. Gaffari et al. acknowledge that lack of body fluid brimonidine concentration was a limitation in their study [
21]. In our case, analysis of the blood and urine samples allowed us to confirm the diagnosis of brimonidine accidental intoxication.
Following ocular administration of one drop of brimonidine (0,2%) in adult patients in each eye twice a day, a maximal plasma drug concentration is reported to be 0.0585 ng/mL [
13,
23]. The drug absorption is known to occur within 1-4 hours with an elimination half-life of about 3 hrs. Systemic brimonidine undergoes an extensive liver metabolism, and the drug, with its metabolites, is eliminated mainly by urinary excretion [
8]. Approximately 74% of an orally administered radioactive dose of brimonidine is recovered in urine after 120 hours from treatment.
Brimonidine concentration in the plasma sample collected from our patient about ten hours after the accidental ingestion was 0,790 ng/mL. Thus, in our 8-month-old girl, plasma concentrations were about 13-fold higher than the maximum mean plasma concentration observed in adults. This level was similar to that found in an infant with Peters anomaly treated with ophthalmic drops, including 0.2% brimonidine. The young patient was brought to the emergency department, proving lethargic, hypotonic, hypothermic, and unresponsive to stimulation. These episodes were repeated 5 times during the hospitalization period. In this case, brimonidine plasma concentration resulted to be 1459 pg/mL and 700 pg/mL in plasma 0-3 and 6 hrs after instillation, respectively [
13]. These elevated plasma levels, along with the resolution of symptoms following brimonidine withdrawal, indicated the drug as the possible cause of the reported intermittent coma episodes [
13].
5. Conclusions
To our knowledge, this is the first report for determining brimonidine levels in urine and plasma by UPLC-MS/MS.
Insufficient knowledge of the family members about the potential hazards of an apparently innocuous, topical medication such as eye drops may put children at a greater risk of poisoning. Among the reasons for the higher vulnerability observed among young infants, an immature blood-brain barrier and deficiency of specific cytochrome P450 enzymes can be included [
24,
25]. Necessary warnings should be better given to parents when prescribing this medication.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Chromatographic separation of brimonidine in a urine sample used as a blank sample type; Figure S2: Chromatographic separation of brimonidine in a blank urine spiked sample. Figure S3: Chromatographic separation of brimonidine in a plasma sample used as a blank sample type; Figure S4: Chromatographic separation of brimonidine in a blank plasma spiked sample. Table S1: LC gradient; Table S2: MRM parameters for Brimonidine and internal standard Brimonidine-d4. .
Author Contributions
Conceptualization, D.T. and M.A.; biochemical methodology, M.Z. and C.R.; clinical investigation, D.T.; C.S.; P.B. and M.A.; writing—original draft preparation, D.T. and M.Z.; writing—review and editing, C.R.; P.B. and M.A.; visualization, D.T.; C.S.; P.B. and C.R.; supervision, P.B.; C.R. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
The study was conducted in accordance with the Declaration of Helsinki and written informed consent has been obtained from the parents of the patient to scientific use of the anonymized data.
Data Availability Statement
No new data were created by this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cantor, L.B. The evolving pharmacotherapeutic profile of brimonidine, an alpha 2-adrenergic agonist, after four years of continuous use. Expert Opin Pharmacother 2000, 1, 815–34. [Google Scholar] [CrossRef]
- Lusthaus, J.A. , Goldberg I. Brimonidine and brinzolamide for treating glaucoma and ocular hypertension; a safety evaluation. Expert Opin Drug Saf 2017, 16, 1071–1078. [Google Scholar] [CrossRef]
- Daubert, G.P. Is brimonidine ophthalmic a safe therapy for infants? Clin Pharm Ther. 2006, 31, 289-92. [Google Scholar] [CrossRef]
- Greenfield, D.S.; Liebmann J.M., Ritch R. Brimonidine: a new alpha2-adrenoreceptor agonist for glaucoma treatment. J Glaucoma. 1997, 6, 250-8. [Google Scholar] [CrossRef] [PubMed]
- Toris, C.B. , Gleason M.L., Camras C.B.., Yablonski M.E. Effects of brimonidine on aqueous humor dynamics in human eyes. Arch Ophthalmol. 1995, 113(12), 1514–7. [Google Scholar] [CrossRef] [PubMed]
- Toris, C.B. , Camras C.B., Yablonski M.E. Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am J Ophthalmol. 1999, 128(1), 8–14. [Google Scholar] [CrossRef]
- Rangan, C. , Everson G., Cantrell F.L. Central alpha-2 adrenergic eye drops: case series of 3 pediatric systemic poisonings. Pediatr Emerg Care. 2008, 24(3), 167-9. [Google Scholar] [PubMed]
- Bowman, R.J.; Cope, J.; Nischal, K.K. Ocular and systemic side effects of brimonidine 0.2% eye drops (Alphagan) in children. Eye (Lond). 2004, 18(1), 24-6.. [CrossRef]
- Shagalov D.R., Taylor D., Schleichert R., Weiss J., Weiss E. Association of central nervous system depression with topical brimonidine when used for hemostasis: a serious adverse event. JAMA Dermatol 2017, 153, 575-77. [CrossRef]
- Montague, A.J. , Bangh S., Cole J.B. Bradycardia in a child after using brimonidine as toothpaste. JAMA Dermatol 2017, 153(4), 330–31. [Google Scholar] [CrossRef]
- Montague, A.; Bangh, S.; Cole, J. (Eds.) A Toddler’s toxic taste: bradycardia after brushing with brimonidine. American College of Medical Toxicology (ACMT) Annual Scientific Meeting 2017. [Google Scholar]
- Lai Becker, M.; Huntington, N.; Woolf, A. D. Brimonidine tartrate poisoning in children: frequency, trends, and use of naloxone as an antidote. Pediatrics 2002, 123, e305–e311. [Google Scholar] [CrossRef]
- Berlin, R.J. , Lee U.T., Samples J.R., Rich L.F., Tang-Liu D.D., Sing K.A., Steiner R.D. Ophthalmic drops causing coma in an infant. J Pediatr 2001, 138(3), 441–43. [Google Scholar] [CrossRef] [PubMed]
- Juzych, M.S. Alpha-2 agonists in glaucoma therapy. Textbook of Ocular, Pharmacology. 1997. [Google Scholar]
- Fudemberg, S.J.; Batiste C., Katz LJ. Efficacy, safety, and current applications of brimonidine. Expert Opin Drug Saf 2008, 7, 795-99. [Google Scholar] [CrossRef] [PubMed]
- PdfDownloadServlet (agenziafarmaco.gov.it) accessed on December 15, 2023. 15 December.
- Jiang, S.; Chappa A.K., Proksch J.W. A rapid and sensitive LC/MS/MS assay for the quantification of brimonidine in ocular fluids and tissues. J Chromatogr B Analyt Technol Biomed Life Sci 2009, 877, 107-14. [Google Scholar] [CrossRef] [PubMed]
- del Amo, E.M. , Hammid A., Tausch M., Toropainen E., Sadeghi A., Valtari A., Puranen J., Reinisalo M., Ruponen M., Urtti A., Sauer A., Honkakoski P. Ocular metabolism and distribution of drugs in the rabbit eye: Quantitative assessment after intracameral and intravitreal administrations. Int J Pharm 2022, 613, 1–13. [Google Scholar]
- Pang, X. , Li J., Pi J., Qi D., Guo P., Li N., Wu Y., Liu Z. Increasing efficacy and reducing systemic absorption of brimonidine tartrate ophthalmic gels in rabbits. Pharm Dev Technol 2018, 23(3), 231–39. [Google Scholar] [CrossRef] [PubMed]
- Farkouh, A.; Frigo P., Czejka M. Systemic side effects of eye drops: a pharmacokinetic perspective. Clin Ophthalmol 2016, 10, 2433–2441. [Google Scholar] [CrossRef]
- Ghaffari, Z.; Zakariaei, Z.; Ghazaeian, M.; Jafari, R.; Ezoddin, N.; Yousefi Nouraee, H.; Navaeifar, M.R. Adverse effects of brimonidine eye drop in children: A case series. Journal of clinical pharmacy and therapeutics 2021, 46, 1469–1472. [Google Scholar] [CrossRef]
- Navaeifar, M.R. , Alipour A., Shokooh S.S. Accidental ingestion of local anesthetic solutions in children. J Pediatrics Rev 2019, 7(3), 169–76. [Google Scholar]
- Angelov, O.V. , Wiese A.G., Tang-Liu D.D., Acheampong A.A., Ismail I.M.., Brar BS. Preclinical safety profile of brimonidine. Eur J Ophthalmol. 1996, 6(1), 21–5. [Google Scholar] [CrossRef]
- Azarcon, C.P. , Santiago D.E. Prolonged central nervous system and respiratory depression in preterm neonates after exposure to brimonidine tartrate and timolol maleate ophthalmic drops. GMS Ophthalmol Cases. 2020, 6, 1–5. [Google Scholar]
- Saghir SA, Khan SA, McCoy AT. Ontogeny of mammalian metabolizing enzymes in humans and animals used in toxicological studies. Crit Rev Toxicol. 2012, 42(5), 323–57. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).