Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
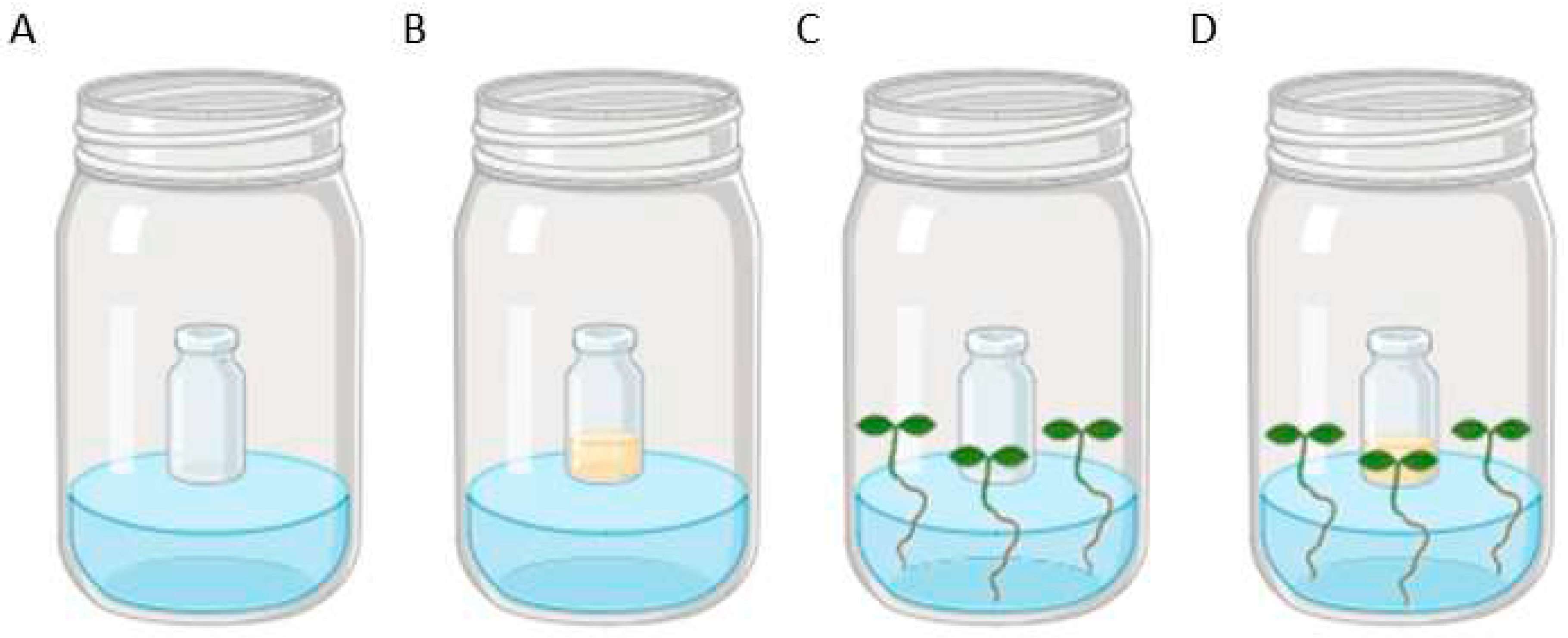
2.1. Yeast and plant conditions
2.2. Plant exposure to S. aeria VOCs
2.3. Headspace VOCs collection
2.4. Analysis of VOCs by GC-MS
2.5. Statistical analysis
3. Results
3.1. Effect of yeast VOCs on plant growth
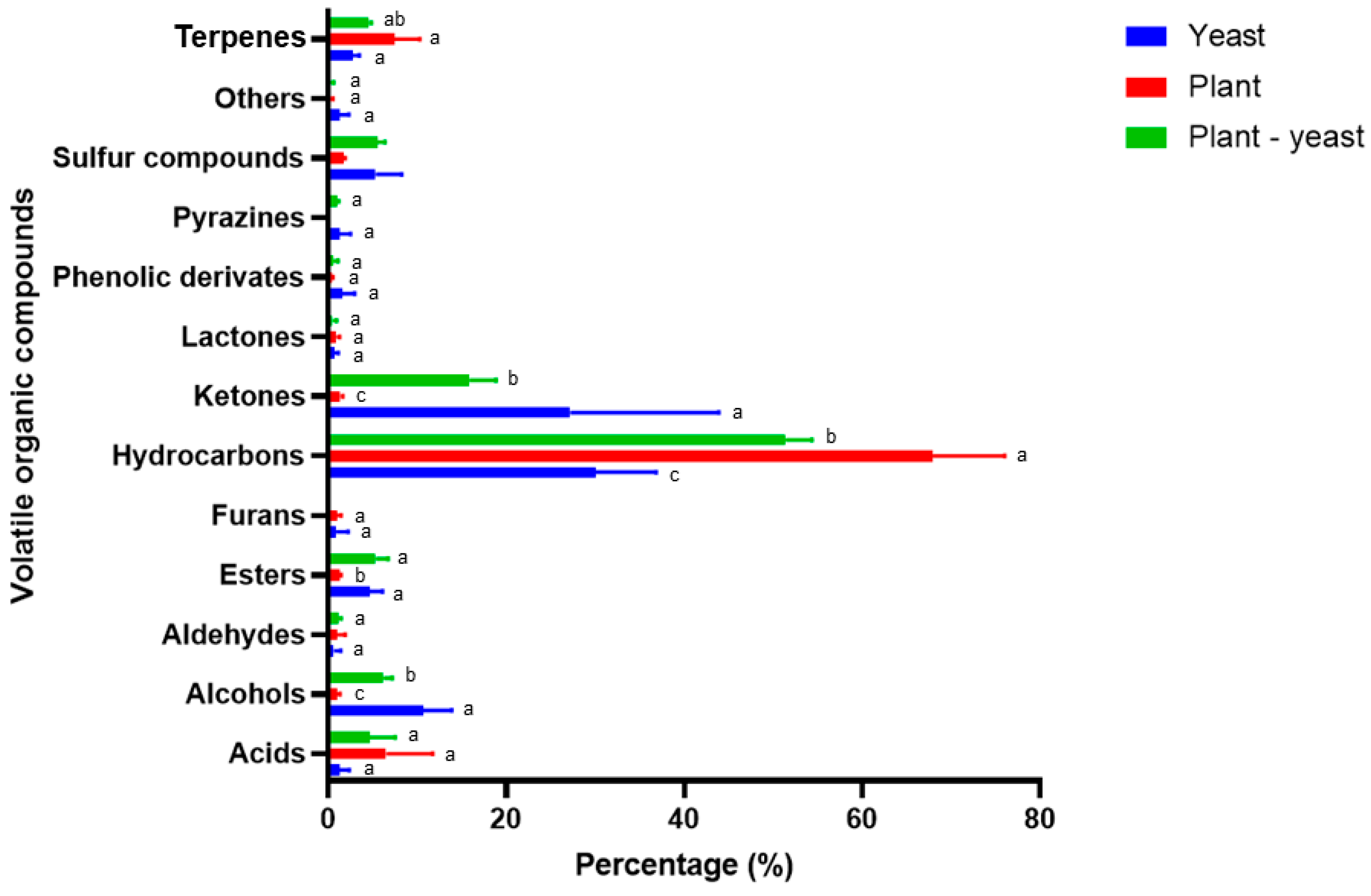
3.2. Effects of treatments on VOC profiles
4. Discussion
4.1. Synthesis of VOCs by microorganisms
4.2. Solicoccozyma aeria VOCs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruins, M. A Closer Look into the World of Biostimulants. Available online: https://european-seed.com/2021/09/a-closer-look-into-the-world-of-biostimulants/. (accessed on 26 January 2024).
- Carvajal, M.; Godoy, L.; Gebauer, M.; Catrileo, D.; Albornoz, F. Screening for Indole-3-Acetic Acid Synthesis and 1-Aminocyclopropane-Carboxylate Deaminase Activity in Soil Yeasts from Chile Uncovers Solicoccozyma Aeria as an Effective Plant Growth Promoter. Plant Soil 2023. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. (Amsterdam) 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Farran, I.; Larraya, L.; Ancin, M.; Arregui, L.M.; Veramendi, J. Plant Growth-Promoting Traits of Yeasts Isolated from Spanish Vineyards: Benefits for Seedling Development. Microbiol. Res. 2020, 237, 126480. [Google Scholar] [CrossRef]
- Hernández-Fernández, M.; Cordero-Bueso, G.; Ruiz-Muñoz, M.; Cantoral, J.M. Culturable Yeasts as Biofertilizers and Biopesticides for a Sustainable Agriculture: A Comprehensive Review. Plants 2021, 10, 822. [Google Scholar] [CrossRef]
- Poveda, J. Beneficial Effects of Microbial Volatile Organic Compounds (MVOCs) in Plants. Appl. Soil Ecol. 2021, 168, 104118. [Google Scholar] [CrossRef]
- Lee, S.; Hung, R.; Yap, M.; Bennett, J.W. Age Matters: The Effects of Volatile Organic Compounds Emitted by Trichoderma Atroviride on Plant Growth. Arch. Microbiol. 2015, 197, 723–727. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Xie, Y.-S.; Zhu, K.; Wang, N.; Li, Z.-J.; Yu, G.-J.; Guo, J.-H. Volatile Organic Compounds Emitted by Bacillus Sp. JC03 Promote Plant Growth through the Action of Auxin and Strigolactone. Plant Growth Regul. 2019, 87, 317–328. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.-S.; Krishnamachari, V.; Payton, P.; Sun, Y.; Grimson, M.; Farag, M.A.; Ryu, C.-M.; Allen, R.; Melo, I.S.; et al. Rhizobacterial Volatile Emissions Regulate Auxin Homeostasis and Cell Expansion in Arabidopsis. Planta 2007, 226, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.A.C.; de Araujo, N.O.; Mulato, A.T.N.; Persinoti, G.F.; Sforça, M.L.; Calderan-Rodrigues, M.J.; Oliveira, J.V. de C. Bacterial Volatile Organic Compounds (VOCs) Promote Growth and Induce Metabolic Changes in Rice. Front. Plant Sci. 2022, 13, 1056082. [Google Scholar] [CrossRef] [PubMed]
- Choińska, R.; Piasecka-Jóźwiak, K.; Chabłowska, B.; Dumka, J.; Łukaszewicz, A. Biocontrol Ability and Volatile Organic Compounds Production as a Putative Mode of Action of Yeast Strains Isolated from Organic Grapes and Rye Grains. Antonie Van Leeuwenhoek 2020, 113, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Di Foggia, M.; Corbetta, M.; Baldo, D.; Ratti, C.; Baraldi, E. Biocontrol Activity and Plant Growth Promotion Exerted by Aureobasidium Pullulans Strains. J. Plant Growth Regul. 2021, 40, 1233–1244. [Google Scholar] [CrossRef]
- Contarino, R.; Brighina, S.; Fallico, B.; Cirvilleri, G.; Parafati, L.; Restuccia, C. Volatile Organic Compounds (VOCs) Produced by Biocontrol Yeasts. Food Microbiol. 2019, 82, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Volatile Organic Compounds from Wickerhamomyces Anomalus, Metschnikowia Pulcherrima and Saccharomyces Cerevisiae Inhibit Growth of Decay Causing Fungi and Control Postharvest Diseases of Strawberries. Int. J. Food Microbiol. 2018, 265, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Li, X.; Zhang, S.; Yao, Y.; Zhang, Y.; Liu, Y.; Peng, X.; Huang, J.; Peng, F. Identification and Functional Studies of Microbial Volatile Organic Compounds Produced by Arctic Flower Yeasts. Front. Plant Sci. 2022, 13, 941929. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Robnett, C.J. Phylogenetic Relationships among Yeasts of the “Saccharomyces Complex” Determined from Multigene Sequence Analyses. FEMS Yeast Res. 2003, 3, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Mitchell, T.R.; Gold, S.E. Volatiles Produced by Bacillus Mojavensis RRC101 Act as Plant Growth Modulators and Are Strongly Culture-Dependent. Microbiol. Res. 2018, 208, 76–84. [Google Scholar] [CrossRef]
- Raza, W.; Wang, J.; Wu, Y.; Ling, N.; Wei, Z.; Huang, Q.; Shen, Q. Effects of Volatile Organic Compounds Produced by Bacillus Amyloliquefaciens on the Growth and Virulence Traits of Tomato Bacterial Wilt Pathogen Ralstonia Solanacearum. Appl. Microbiol. Biotechnol. 2016, 100, 7639–7650. [Google Scholar] [CrossRef] [PubMed]
- Amprayn, K.-O.; Rose, M.T.; Kecskés, M.; Pereg, L.; Nguyen, H.T.; Kennedy, I.R. Plant Growth Promoting Characteristics of Soil Yeast (Candida Tropicalis HY) and Its Effectiveness for Promoting Rice Growth. Appl. Soil Ecol. 2012, 61, 295–299. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennett, J.W. Arabidopsis Thaliana as a Model System for Testing the Effect of Trichoderma Volatile Organic Compounds. Fungal Ecol. 2013, 6, 19–26. [Google Scholar] [CrossRef]
- Cheng, X.; Etalo, D.W.; van de Mortel, J.E.; Dekkers, E.; Nguyen, L.; Medema, M.H.; Raaijmakers, J.M. Genome-Wide Analysis of Bacterial Determinants of Plant Growth Promotion and Induced Systemic Resistance by Pseudomonas Fluorescens. Environ. Microbiol. 2017, 19, 4638–4656. [Google Scholar] [CrossRef]
- Baroja-Fernández, E.; Almagro, G.; Sánchez-López, Á.M.; Bahaji, A.; Gámez-Arcas, S.; De Diego, N.; Dolezal, K.; Muñoz, F.J.; Climent Sanz, E.; Pozueta-Romero, J. Enhanced Yield of Pepper Plants Promoted by Soil Application of Volatiles from Cell-Free Fungal Culture Filtrates Is Associated with Activation of the Beneficial Soil Microbiota. Front. Plant Sci. 2021, 12, 752653. [Google Scholar] [CrossRef]
- Gutiérrez-Luna, F.M.; López-Bucio, J.; Altamirano-Hernández, J.; Valencia-Cantero, E.; de la Cruz, H.R.; Macías-Rodríguez, L. Plant Growth-Promoting Rhizobacteria Modulate Root-System Architecture in Arabidopsis Thaliana through Volatile Organic Compound Emission. Symbiosis 2010, 51, 75–83. [Google Scholar] [CrossRef]
- Waisel, Y.; Eshel, A. Functional Diversity of Various Constituents of a Single Root System. In Plant Roots; CRC Press, 2002; pp. 157–174. ISBN 9780824706319. [Google Scholar]
- Zhang, X.; Wu, F.; Gu, N.; Yan, X.; Wang, K.; Dhanasekaran, S.; Gu, X.; Zhao, L.; Zhang, H. Postharvest Biological Control of Rhizopus Rot and the Mechanisms Involved in Induced Disease Resistance of Peaches by Pichia Membranefaciens. Postharvest Biol. Technol. 2020, 163, 111146. [Google Scholar] [CrossRef]
- Nieto, K.F.; Frankenberger, W.T., Jr. Influence of Adenine, Isopentyl Alcohol and Azotobacter Chroococcum on the Growth of Raphanus Sativus. Plant Soil 1990, 127, 147–156. [Google Scholar] [CrossRef]
- Song, G.C.; Choi, H.K.; Ryu, C.-M. Gaseous 3-Pentanol Primes Plant Immunity against a Bacterial Speck Pathogen, Pseudomonas Syringae Pv. Tomato via Salicylic Acid and Jasmonic Acid-Dependent Signaling Pathways in Arabidopsis. Front. Plant Sci. 2015, 6, 821. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, P.; Ali, N.; Saini, S.; Pati, P.K.; Pati, A.M. Physiological and Molecular Insight of Microbial Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2023, 14, 1041413. [Google Scholar] [CrossRef] [PubMed]
- Ladino-Orjuela, G.; Gomes, E.; da Silva, R.; Salt, C.; Parsons, J.R. Metabolic Pathways for Degradation of Aromatic Hydrocarbons by Bacteria. Rev. Environ. Contam. Toxicol. 2016, 237, 105–121. [Google Scholar]
- Oikawa, P.Y.; Lerdau, M.T. Catabolism of Volatile Organic Compounds Influences Plant Survival. Trends Plant Sci. 2013, 18, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Lerdau, M.; Keller, M. Controls on Isoprene Emission from Trees in a Subtropical Dry Forest. Plant Cell Environ. 1997, 20, 569–578. [Google Scholar] [CrossRef]
- Funk, J.L.; Mak, J.E.; Lerdau, M.T. Stress-induced Changes in Carbon Sources for Isoprene Production in Populus Deltoides. Plant Cell Environ. 2004, 27, 747–755. [Google Scholar] [CrossRef]
- Pérez-Corral, D.A.; Ornelas-Paz, J.d.J.; Olivas, G.I.; Acosta-Muñiz, C.H.; Salas-Marina, M.Á.; Berlanga-Reyes, D.I.; Sepulveda, D.R.; Mares-Ponce de León, Y.; Rios-Velasco, C. Growth Promotion of Phaseolus Vulgaris and Arabidopsis Thaliana Seedlings by Streptomycetes Volatile Compounds. Plants 2022, 11, 875. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Avendaño, E.; Bejarano-Bolívar, A.A.; Kiel-Martínez, A.-L.; Ramírez-Vázquez, M.; Méndez-Bravo, A.; von Wobeser, E.A.; Sánchez-Rangel, D.; Guerrero-Analco, J.A.; Eskalen, A.; Reverchon, F. Avocado Rhizobacteria Emit Volatile Organic Compounds with Antifungal Activity against Fusarium Solani, Fusarium Sp. Associated with Kuroshio Shot Hole Borer, and Colletotrichum Gloeosporioides. Microbiol. Res. 2019, 219, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins (Basel) 2021, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-X.; Eidman, K.; Gan, X.-W.; Haefliger, O.P.; Carroll, P.J.; Pika, J. Structure Elucidation of Novel Norcysteine-Containing Dipeptides from the Chinese Vegetable Toona Sinensis. In ACS Symposium Series; ACS symposium series; American Chemical Society: Washington, DC, 2015; pp. 147–158. ISBN 9780841231146. [Google Scholar] [CrossRef]
- Wang, J.-W.; Luo, Z.-H.; Xu, W.; Ding, J.-F.; Zheng, T.-L. Transformation of Dimethyl Phthalate Esters (DMPEs) by a Marine Red Yeast Rhodotorula Mucilaginosa Isolated from Deep Sea Sediments of the Atlantic Ocean. Int. Biodeterior. Biodegradation 2016, 109, 223–228. [Google Scholar] [CrossRef]
- Luo, Z.-H.; Wu, Y.-R.; Pang, K.-L.; Gu, J.-D.; Vrijmoed, L.L.P. Comparison of Initial Hydrolysis of the Three Dimethyl Phthalate Esters (DMPEs) by a Basidiomycetous Yeast, Trichosporon DMI-5-1, from Coastal Sediment. Environ. Sci. Pollut. Res. Int. 2011, 18, 1653–1660. [Google Scholar] [CrossRef]
- Rivas, E.-M.; Wrent, P.; de Silóniz, M.-I. Rapid PCR Method for the Selection of 1,3-Pentadiene Non-Producing Debaryomyces Hansenii Yeast Strains. Foods 2020, 9, 162. [Google Scholar] [CrossRef]

| Compound | Sa | T | TSa |
|---|---|---|---|
| Acids | |||
| 1. Butanoic acid | + | - | - |
| 2. Octanoic acid | - | + | + |
| Alcohols | |||
| 3. (S)2-Pentanol | + | - | + |
| 4. (E)-2-octen-1-ol | + | - | - |
| 5. 1-dodecanol | + | - | + |
| 6. 1-nonanol | + | - | - |
| 7. 2-ethoxyethyl alcohol | + | + | + |
| 8. 2-octanol | + | - | - |
| 9. 3-octanol | + | - | - |
| 10. 5-methyl-2-hexanol | + | - | - |
| 11. 6-methyl-2-heptanol | + | - | - |
| 12. E-2-tridecen-1-ol | - | + | + |
| 13. Glycerol | + | + | + |
| 14. Isobutanol | + | - | + |
| 15. Isopentyl alcohol | + | - | + |
| 16. Isopropyl alcohol | - | - | + |
| 17. Nonadecanol | - | + | + |
| Esters | |||
| 18. Butyl hept-4-yl-ester-phthalic acid | - | - | + |
| 19. Ethyl decanoate | + | + | + |
| 20. Ethyl dodecanoate | + | + | + |
| 21. Ethyl octanoate | + | - | + |
| 22. Ethyl salicylate | + | - | + |
| 23. Ethylene formate | + | + | + |
| 24. Isopropyl palmitate | + | + | - |
| Furans | |||
| 25. 2-pentylfuran | - | - | + |
| Hydrocarbons | |||
| 26. (E)1,3-Pentadiene | - | - | + |
| 27. 2-methylheptane | - | + | + |
| 28. (E)-3-octadecene | + | + | + |
| 29. 1,2,3,4-tetramethylfulvene | + | + | + |
| 30. 2,2,11,11-tetramethyldodecane | + | + | - |
| 31. 2,3-dimethoxy-2-methylbutane | + | + | - |
| 32. 2,4,6-trimethyloctane | + | - | - |
| 33. 2,5-dimethyloctane | + | + | + |
| 34. 2,6,10-trimethyldodecane | - | + | + |
| 35. 2,7,10-trimethyldodecane | - | + | + |
| 36. 2E-5-methyl-2-undecene | + | + | + |
| 37. 3,4-dimethylundecane | - | + | + |
| 38. 6-methyloctadecane | + | - | + |
| 39. Heptane | + | + | + |
| Ketones | |||
| 40. 2-pentanone | + | - | + |
| 41. 3-methylpentan-2-one | + | - | - |
| 42. 5,9,9-trimethylspiro[3.5]nona-5,7-dien-1-one | + | - | - |
| 43. 5-hexen-2-one | - | + | - |
| 44. 5-methyl-2-heptanone | + | - | - |
| 45. Methyl isobutyl ketone | + | - | + |
| Phenolic Derivatives | |||
| 46. m-acetylphenol | + | - | + |
| 47. m.formylphenol | + | - | + |
| 48. p-isopropenylphenol | + | + | + |
| Pyrazines | |||
| 49. 2,5-dimethylpyrazine | + | - | + |
| 50. Methylpyrazine | + | - | + |
| Sulfur Compounds | |||
| 51. 1-propenylthiol | - | - | + |
| 52. Dimethyl trisulfide | + | - | - |
| 53. Methanethiol | + | - | - |
| 54. Methyl disulfide | + | - | - |
| 55. Methyl thiolacetate | + | - | - |
| Others | |||
| 56. 2-(methylmercapto)-benzothiazole | + | - | + |
| Terpenes | |||
| 57. 2-carene | - | + | + |
| 58. α-phellandrene | - | + | + |
| 59. α-Sabinene | - | + | - |
| Treatment | Order of abundance | ||||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | |
| Sa | 2-pentanone (8.2%) |
Isopentyl alcohol (3.6%) |
Methyl disulfide (2.8%) |
(S)2-pentanol (2.3%) |
Ethyl decanoate (2.1%) |
| T | 1-propenylthiol (2.3%) |
α-sabinene (1.9%) |
Heptane (1.3%) |
2,3-dimethoxy-2-methylbutane (1.0%) |
α-phellandrene (0.7%) |
| TSa | 1-propenylthiol (4.4%) |
2-pentanone (3.4%) |
(S)2-pentanol (2.5%) |
α-sabinene (1.0%) |
Heptane (1.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





