Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
Purpose
2. Materials and Methods
2.1. Study Participants
2.2. CMR Protocol
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. CMR Findings
3.3. Laboratory Parameters
3.4. Clinical Symptoms
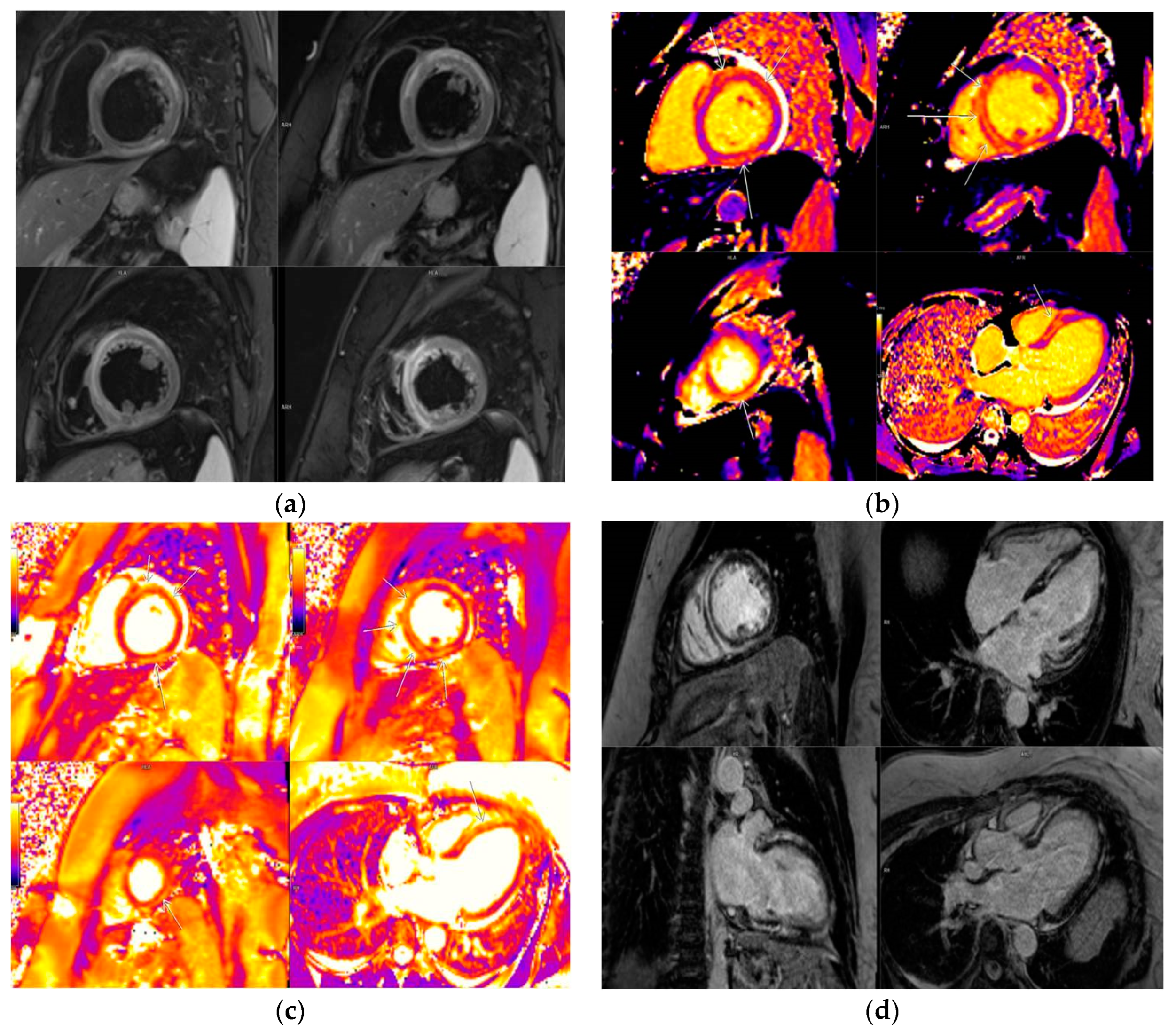
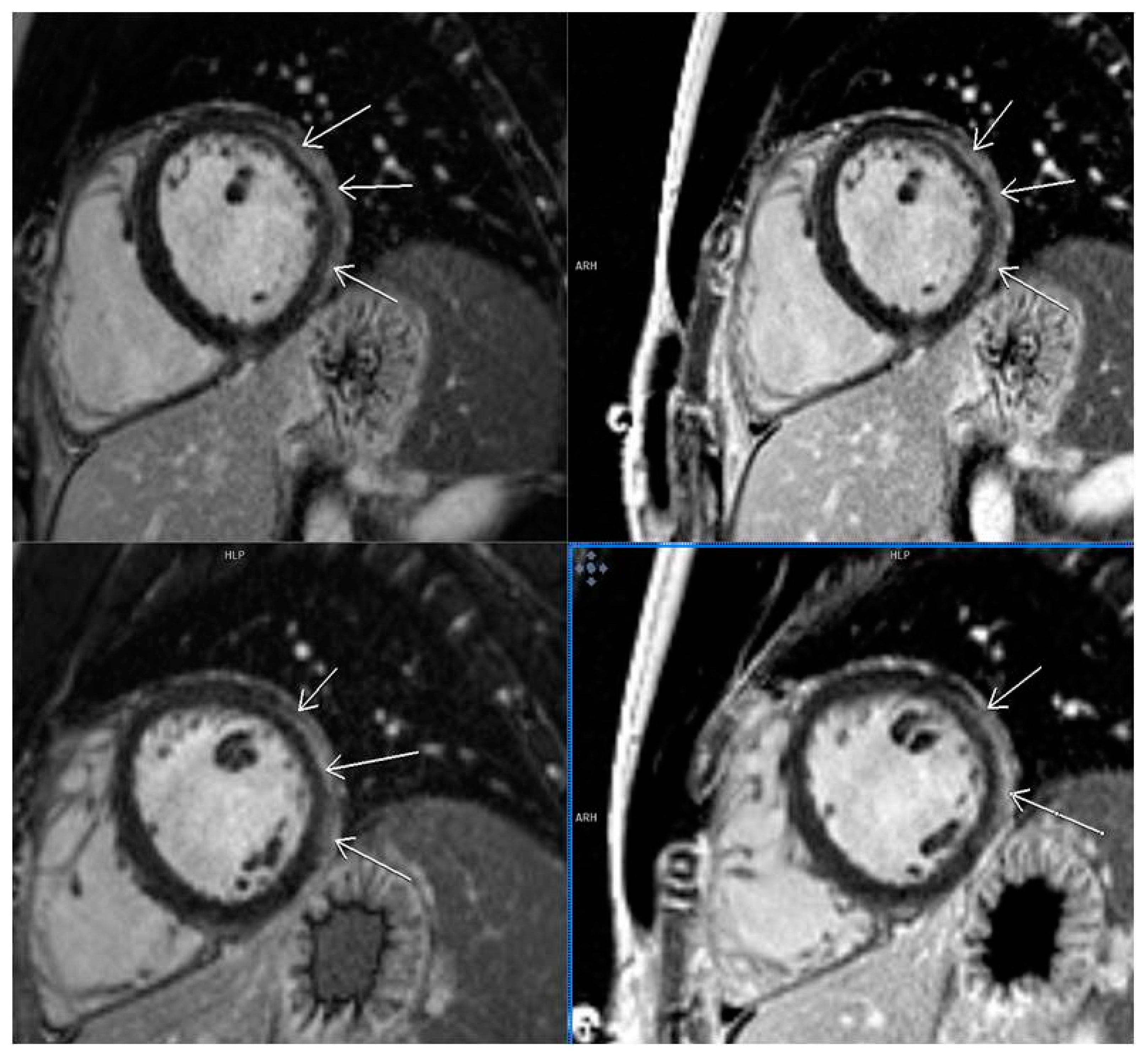
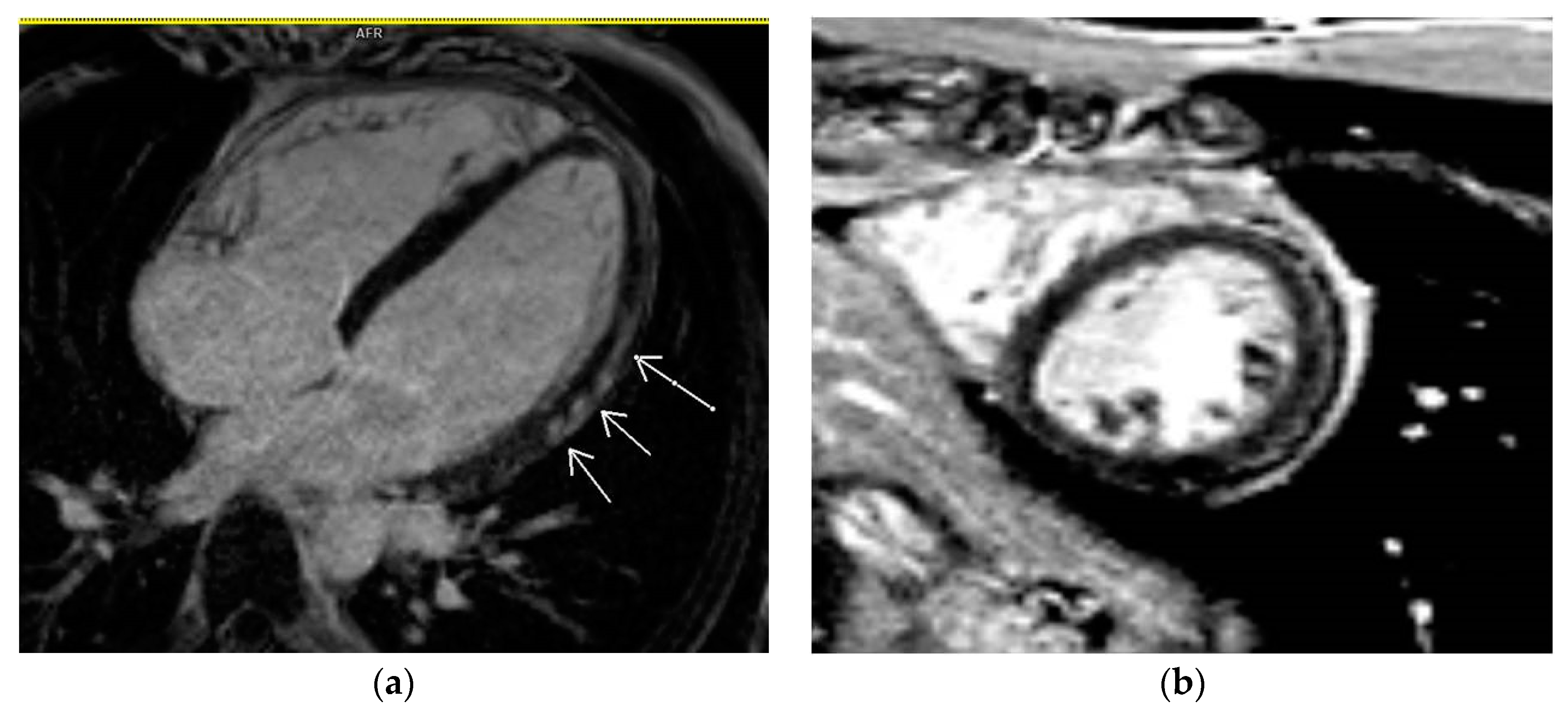
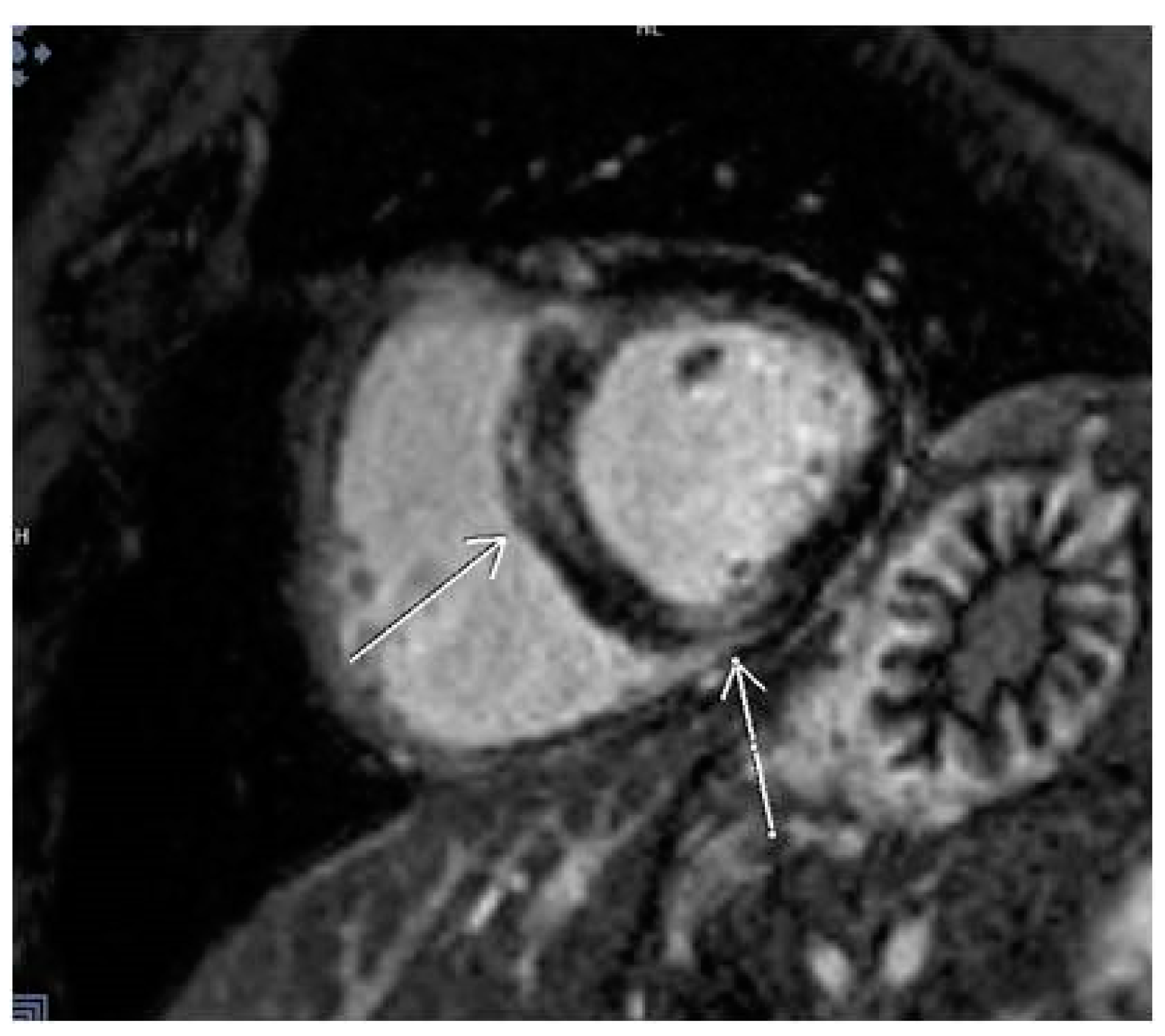
3.5. LGE Localization
4. Discussion
5. Conclusion
Limitation of study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vidal-Perez, R.; Abou Jokh Casas, C.; Agra-Bermejo, R.M.; Alvarez-Alvarez, B.; Grapsa, J.; Fontes-Carvalho, R.; Rigueiro Veloso, P.; Garcia Acuña, J.M.; Gonzalez-Juanatey, J.R. Myocardial infarction with non-obstructive coronary arteries: A comprehensive review and future research directions. World J Cardiol 2019, 11, 305–315. [Google Scholar] [CrossRef]
- Seferović, P.M.; Tsutsui, H.; Mcnamara, D.M.; Ristić, A.D.; Basso, C.; Bozkurt, B.; Cooper, L.T.; Filippatos, G.; Ide, T.; Inomata, T.; Klingel, K.; Linhart, A.; Lyon, A.R.; Mehra, M.R.; Polovina, M.; Milinković, I.; Nakamura, K.; Anker, S.D.; Veljić, I.; Ohtani, T.; Okumura, T.; Thum, T.; Tschöpe, C.; Rosano, G.; Coats, A.J.S.; Starling, R.C. Heart Failure Association, Heart Failure Society of America, and Japanese Heart Failure Society Position Statement on Endomyocardial Biopsy. J Card Fail. 2021, 27, 727–743. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; Huang, H.; Yang, B.; Huang, C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 1–18. [Google Scholar] [CrossRef]
- Klem, I.; Shah, D.J.; White, R.D.; Pennell, D.J.; van Rossum, A.C.; Regenfus, M.; Sechtem, U.; Schvartzman, P.R.; Hunold, P.; Croisille, P.; Parker, M.; Judd, R.M.; Kim, R.J. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: An international, multicenter study. Circ. Cardiovasc. Imaging 2011, 4, 610–619. [Google Scholar] [CrossRef]
- Broncano, J.; Bhalla, S.; Caro, P.; Hidalgo, A.; Vargas, D.; Williamson, E.; Gutiérrez, F.; Luna, A. Cardiac MRI in Patients with Acute Chest Pain. RadioGraphics 2021, 41, 8–31. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; Atar, D.; Kaski, J.C.; Sechtem, U.; Tornvall, P. WG on Cardiovascular Pharmacotherapy. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017, 38, 143–153. [Google Scholar] [CrossRef]
- Haussner, W.; DeRosa, A.P.; Haussner, D.; Tran, J.; Torres-Lavoro, J.; Kamler, J.; Shah, K. COVID-19 associated myocarditis: a systematic review. Am J Emerg Med 2022, 51, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Castiello, T.; Georgiopoulos, G.; Finocchiaro, G.; Claudia, M.; Gianatti, A.; Delialis, D.; Aimo, A.; Prasad, S. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022, 27, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Duan, X.; Feng, G.; Sirajuddin, A.; Yin, G.; Zhuang, B.; He, J.; Xu, J.; Yang, W.; Wu, W.; Sun, X.; Zhao, S.; Wang, H.; Teng, Z.; Lu, M. Multiparametric Cardiovascular Magnetic Resonance in Acute Myocarditis: Comparison of 2009 and 2018 Lake Louise Criteria With Endomyocardial Biopsy Confirmation. Front Cardiovasc Med. 2021, 8, 739892. [Google Scholar] [CrossRef] [PubMed]
- Luetkens, J.A.; Faron, A.; Isaak, A.; Dabir, D.; Kuetting, D.; Feisst, A.; Schmeel, F.C.; Sprinkart, A.M.; Thomas, D. Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: results of a validation cohort. Radio Cardiothorac Imaging 2019, 1, e190010. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; Friedrich, M.G. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; Cheng, Z.; Yu, T.; Xia, J.; Wei, Y.; Wu, W.; Xie, X.; Yin, W.; Li, H.; Liu, M.; Xiao, Y.; Gao, H.; Guo, L.; Xie, J.; Wang, G.; Jiang, R.; Gao, Z.; Jin, Q.; Wang, J.; Cao, B. Clinical features of patients infected with 2019. novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, D.; Isaak, A.; Zimmer, S.; Mesropyan, N.; Reinert, M.; Faron, A.; Pieper, C.; Heine, A.; Velten, M.; Nattermann, J.; Kuetting, D.; Duerr, G.; Attenberger, U.; Luetkens, J. Cardiac MRI in Patients with Prolonged Cardiorespiratory Symptoms after Mild to Moderate COVID-19. Radiology 2021, 301, E419–E425. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; Vehreschild, M.; Nagel, E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020, 5, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef]
- Wang, H.; Li, R.; Zhou, Z.; Jiang, H.; Yan, Z.; Tao, X.; Li, H.; Xu, L. Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2021, 23, 14. [Google Scholar] [CrossRef]
- Kravchenko, D.; Isaak, A.; Zimmer, S.; Mesropyan, N.; Reinert, M.; Faron, A.; Pieper, C.C.; Heine, A.; Velten, M.; Nattermann, J.; Kuetting, D.; Duerr, G.D.; Attenberger, U.I.; Luetkens, J.A. Cardiac MRI in Patients with Prolonged Cardiorespiratory Symptoms after Mild to Moderate COVID-19. Radiology 2021, 301, E419–E425. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Habtemicael, Y.G.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Lanzillo, C.; Scatteia, A.; Di Roma, M.; Pontone, G.; Marra, M.P.; Barison, A.; Di Bella, G. Prognostic Value of Repeating Cardiac Magnetic Resonance in Patients With Acute Myocarditis. J Am Coll Cardiol. 2019, 74, 2439–2448. [Google Scholar] [CrossRef]
- Hijazi, Z.; Oldgren, J.; Andersson, U.; Connolly, S.J.; Ezekowitz, M.D.; Hohnloser, S.H. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation. 2012, 125, 1605–1616. [Google Scholar] [CrossRef]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular Magnetic Resonance in Acute ST-Segment-Elevation Myocardial Infarction: Recent Advances, Controversies, and Future Directions. Circulation 2018, 137, 1949–1964. [Google Scholar] [CrossRef]
- Eitel, I.; de Waha, S.; Wohrle, J.; Fuernau, G.; Lurz, P.; Pauschinger, M.; Desch, S.; Schuler, G.; Thiele, H. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2014, 64, 1217–1226. [Google Scholar] [CrossRef]
- Reindl, M.; Reinstadler, S.J.; Tiller, C.; Kofler, M.; Theurl, M.; Klier, N.; Fleischmann, K.; Mayr, A.; Henninger, B.; Klug, G.; Metzler, B. ACEF score adapted to ST-elevation myocardial infarction patients: The ACEF-STEMI score. Int. J. Cardiol. 2018, 264, 18–24. [Google Scholar] [CrossRef]
- Sangita, S.; Eric, N.; Karl, H.; Scott, M.G.; Geltman, E.M. Use of Biomarkers to Predict Readmission for Congestive Heart Failure. Am. J. Cardiol. 2017, 119, 445–451. [Google Scholar] [CrossRef]
- Costabel, J.P.; Burgos, L.M.; Trivi, M. The Significance Of Troponin Elevation In Atrial Fibrillation. J Atr Fibrillation. 2017, 9, 1530. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; Di Roma, M.; Pontone, G.; Perazzolo, M.M.; Barison, A.; Di Bella, G. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol 2017, 70, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, J.; Li, W.; Xu, Y.; Wan, K.; Zeng, R.; Chen, Y. The prognostic value of late gadolinium enhancement in myocarditis and clinically suspected myocarditis: systematic review and meta-analysis. Eur Radiol 2020, 30, 2616–2626. [Google Scholar] [CrossRef] [PubMed]
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; Jerosch-Herold, M.; Kwong, R.Y. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 2017, 70, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Cadour, F.; Cautela, J.; Rapacchi, S.; Varoquaux, A.; Habert, P.; Arnaud, F.; Jacquier, A.; Meilhac, A.; Paganelli, F.; Lalevée, N.; Scemama, U.; Thuny, F. Cardiac MRI Features and Prognostic Value in Immune Checkpoint Inhibitor-induced Myocarditis. Radiology. 2022, 303, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; Hays, A.G.; Kawel-Boehm, N.; Kramer, C.M.; Nagel, E.; Ntusi, N.A.B.; Ostenfeld, E.; Pennell, D.J.; Raisi-Estabragh, Z.; Reeder, S.B.; Rochitte, C.E.; Starekova, J.; Suchá, D.; Tao, Q.; Schulz-Menger, J.; Bluemke, D.A.S.E.; Friedrich, M.G.; Leiner, T. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc Imaging. 2022, 15, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Sanguineti, F.; Garot, P.; Mana, M.; O'h-Ici, D.; Hovasse, T.; Unterseeh, T.; Louvard, Y.; Troussier, X.; Morice, M.C.; Garot, J. Cardio-vascular magnetic resonance predictors of clinical outcome in patients with suspected acute myocarditis. J Cardiovasc Magn Reson 2015, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Leite, W.F.; Dos Santos Povoa, R.M.; Caixeta, A.M.; Amodeo, C.; Szarf, G.; Bombig, M.T.N.; Izar, M.C.O.; Gioia, L.N.; Ribeiro, W.N.; Fonseca, F.A.H. Chest Pain in Acute Myocardial Infarction and Its Association With the Culprit Artery and Fibrotic Segment Identified by Cardiac Magnetic Resonance. Cardiol Res. 2023, 14, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.K.; Oraii, A.; Soleimani, A.; Hadadi, A.; Shajari, Z.; Montazeri, M.; Moradi, H.; Talebpour, M.; Naseri, A.S.; Balali, P.; Akhbari, M.; Ashraf, H. The association between cardiac injury and outcomes in hospitalized patients with COVID-19. Internal and Emergency Medicine 2020, 15, 1415–1424. [Google Scholar] [CrossRef] [PubMed]

| Demographic characteristics | Result |
|---|---|
| Age | 46.76 ± 15.251 |
| Sex (male/female) | 68/71 (48.9%/51.1%) |
| Days from virus detection to CMR | 134.67±80.740 |
| Symptoms of COVID-19 | |
| Chest pain | 22 (15.8%) |
| Fatigue | 66 (47.5%) |
| Arrhythmias | 19 (13.7%) |
| Dyspnea | 40 (28.8%) |
| Lost of taste and smell | 24 (17.3%) |
| Digestive symptoms | 31 (22.3%) |
| Headache | 36 (25.9%) |
| Pneumonia | 66 (46.5%) |
| Co-morbidities | |
| Dyslipidemia | 6 (4.3%) |
| Hypertension | 24 (17.3%) |
| Diabetes mellitus type II | 10 (7.2%) |
| Smoking | 10 (7.2%) |
| Former smokers | 6 (4.3%) |
| Symptoms after COVID-19 | |
| Chest pain | 48 (34.5%) |
| Fatigue | 82 (58.9%) |
| Arrhythmias | 54 (38.8%) |
| Dyspnoea | 58 (41.7%) |
| ECG changes after COVID-19 | |
| Ischaemic changes | 18 (12.9%) |
| Arrhythmias | 14 (10.1%) |
| Right heart overload | 14 (10.1%) |
| CMR parameters | |
| LVEF | 62.37% ± 4.88 % |
| Patients with LGE | 54 (38.8%) |
| Patients without LGE | 85 (61.2%) |
| Localization of LGE | |
| Lateral | 11 (7.9%) |
| Septal | 9 (6.5%) |
| Multiple segments (more than 3) | 13 (9.4%) |
| Other localizations | 21 (15.1%) |
| Abbreviations: CMR: cardiac magnetic resonance, LVEF: left ventricular ejection fraction, LGE: late gadolinium enhancement. | |
| Laboratory parameters | LGE negative patients (mean; SD) | LGE positive patients (mean; SD) | p values | Test values | Independent predictors for LGE |
|---|---|---|---|---|---|
| Troponin values | 4.9±0.3 ng/L | 13.1±0.4 ng/L | p<0.001 | Z=-9.5 | Yes |
| D-dimer levels | 0.8±0.1 mg/L FEU | 1.8±0.3 mg/L FEU | p=0.005 | Z -3,9 | No |
| Fibrinogen levels | 3.2±0.1 g/L | 4.3±0.23 g/L | p<0.001 | t=-4 | No |
| CK values | 143.9±12.4 u/L | 112.8±6.9 u/L | p=0.042 | Z=-1.6 | No |
| Clinical symptoms | |||||
| Chest pain | 43.7% | 56.3% | p=0.002 | Chi2 9.3 | Yes |
| Fatique | 39% | 61% | p<0.001 | Chi241.2 | Yes |
| Dyspnea | 67.2% | 32.8% | p=0.213 | Chi2 1.5 | No |
| CMR findings | |||||
| T1 mapping values | 1063.5±9.7 ms | 1103.9±17.2 ms | p=0.033 | t=-2.2 | Yes |
| Abbreviations: SD: standard deviation, LGE: late gadolinium enhancement, CMR: cardiac magnetic resonance, CK: creatine kinase. | |||||
| Laboratory parameters | Patients with arrhythmias | Patients without arrhythmias | P values | Test values | Arrhythmias predictor |
|---|---|---|---|---|---|
| Troponin values | 10.4±0.8 ng/L | 7.4±0.5 ng/L | p<0.001 | Z=-3.3 | Yes |
| CMR findings | |||||
| Septal fibrosis | 32% | 3.4% | p=0.005 | Chi2 7.9 | Yes |
| T1 mapping | 1100.8±21.4ms | 1071.6±9.5ms | p=0.362 | Z=-0.912 | No |
| Abbreviations: CMR: cardiac magnetic resonance | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





