Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
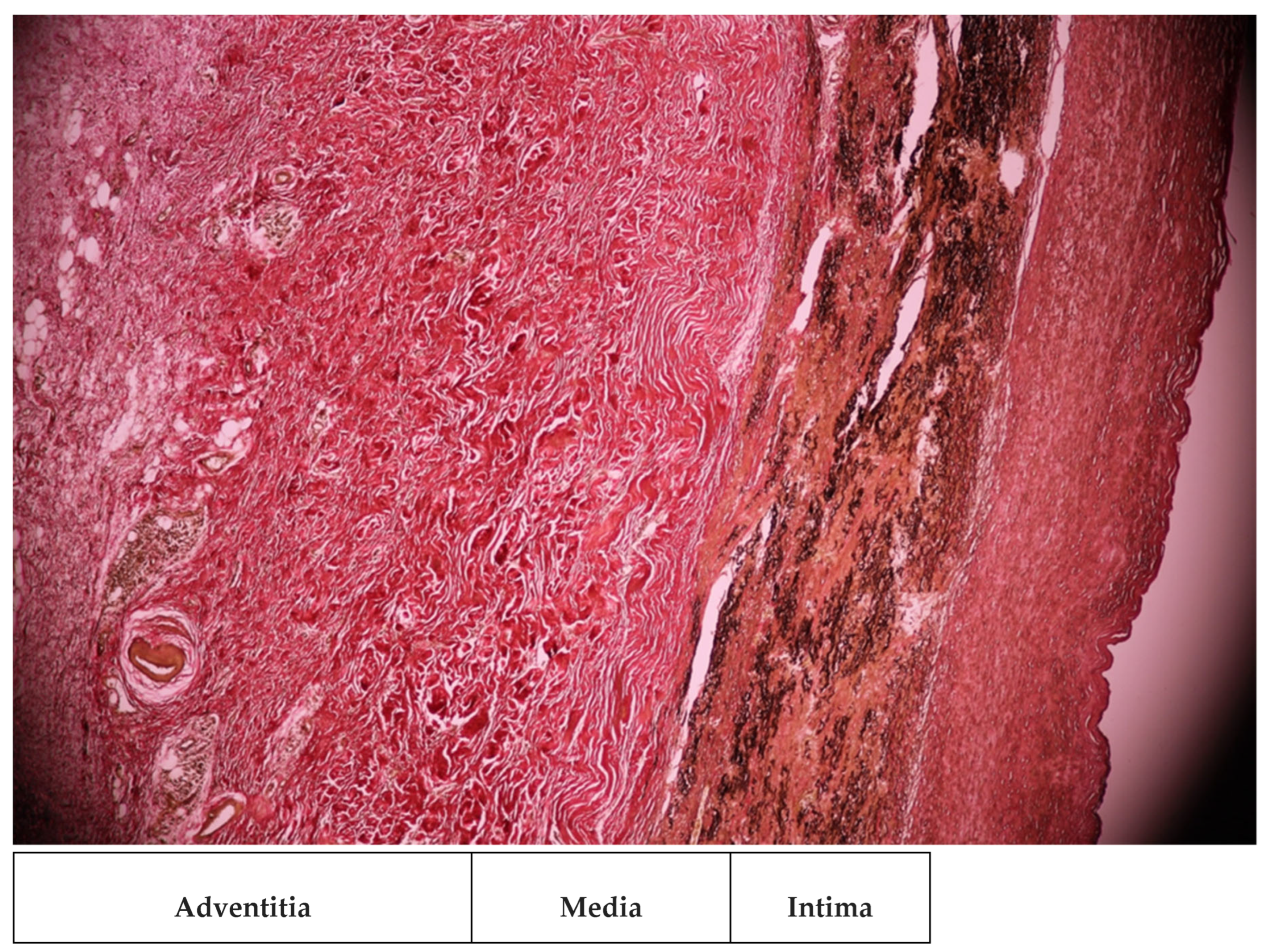
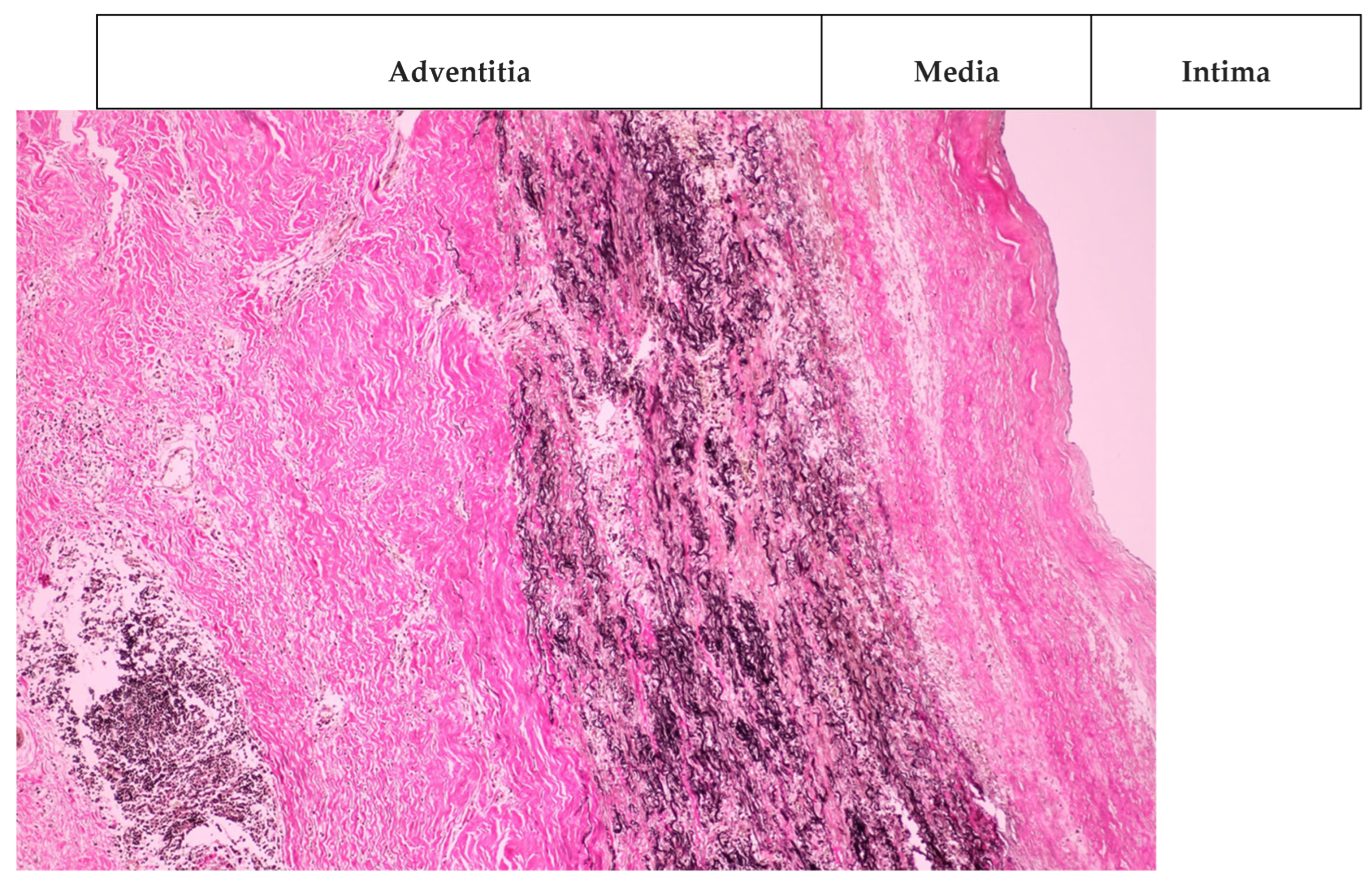
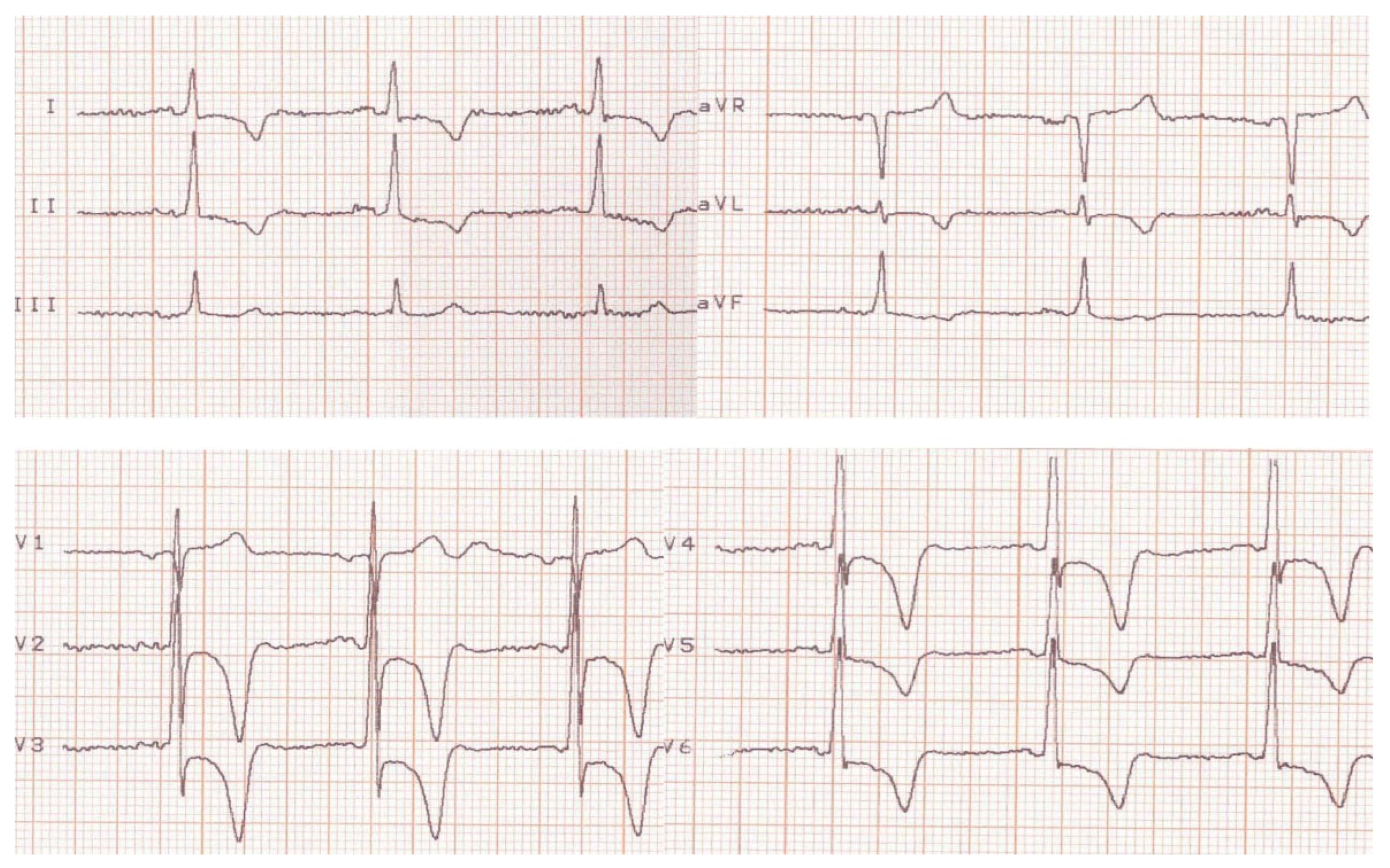
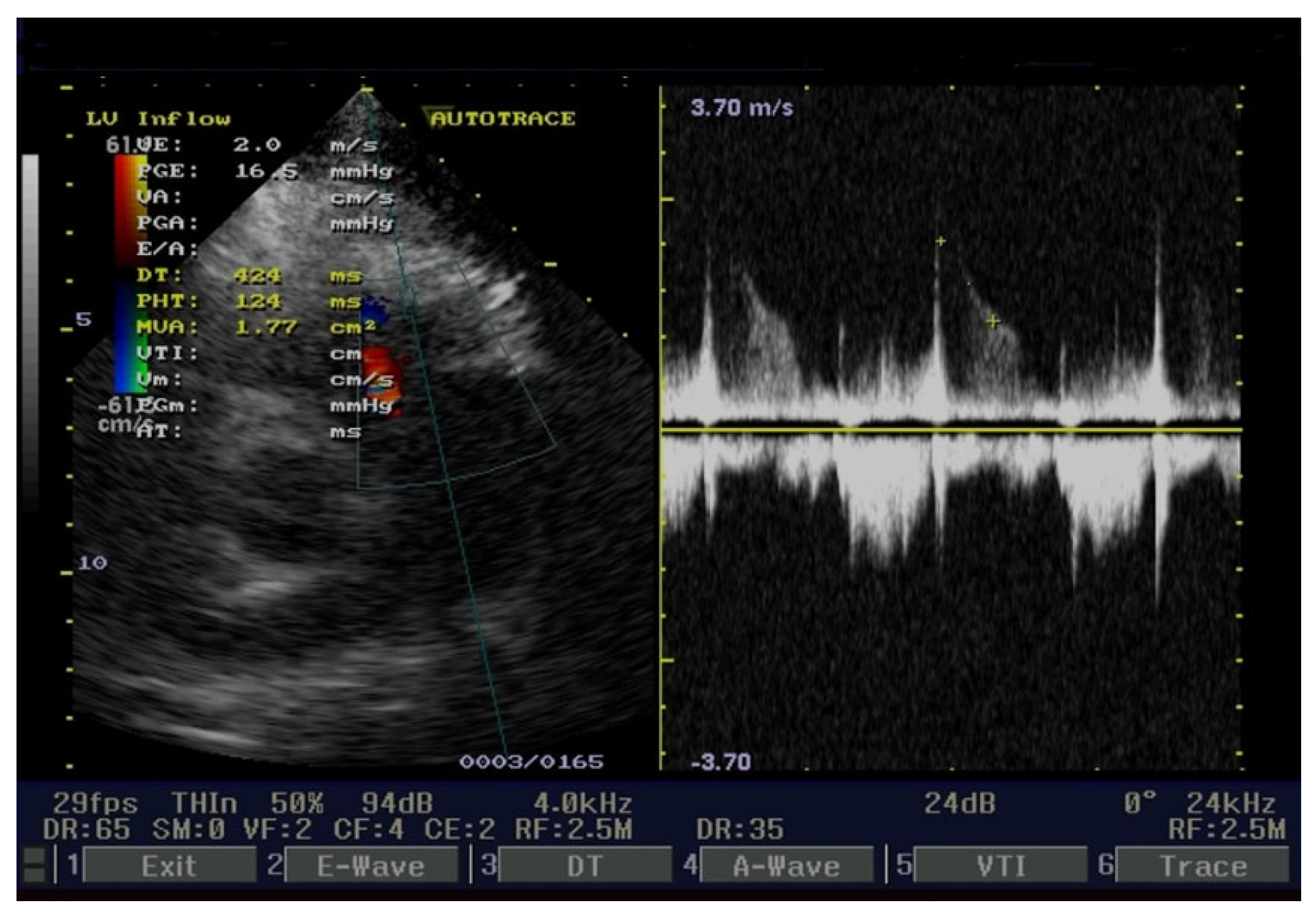
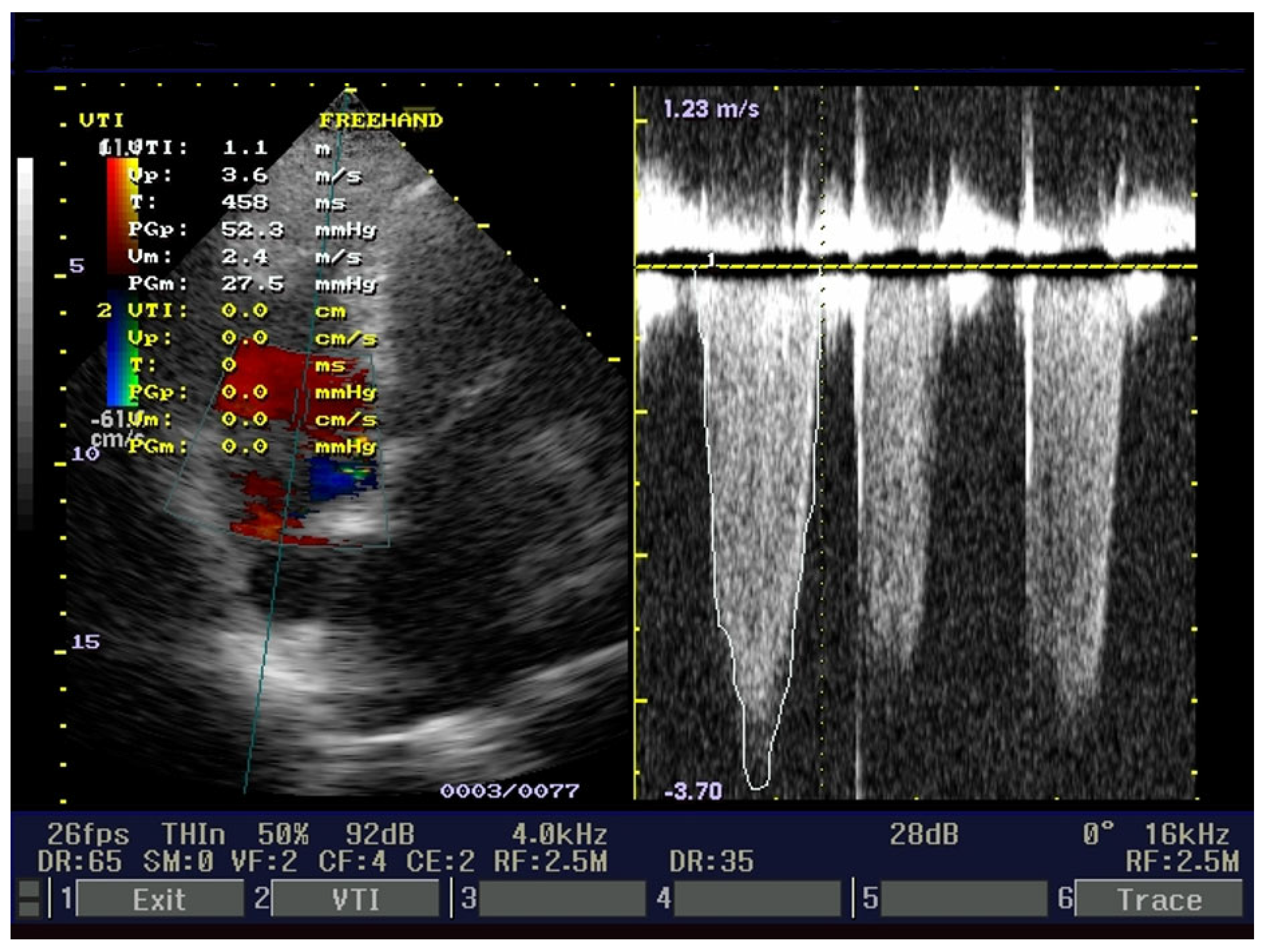
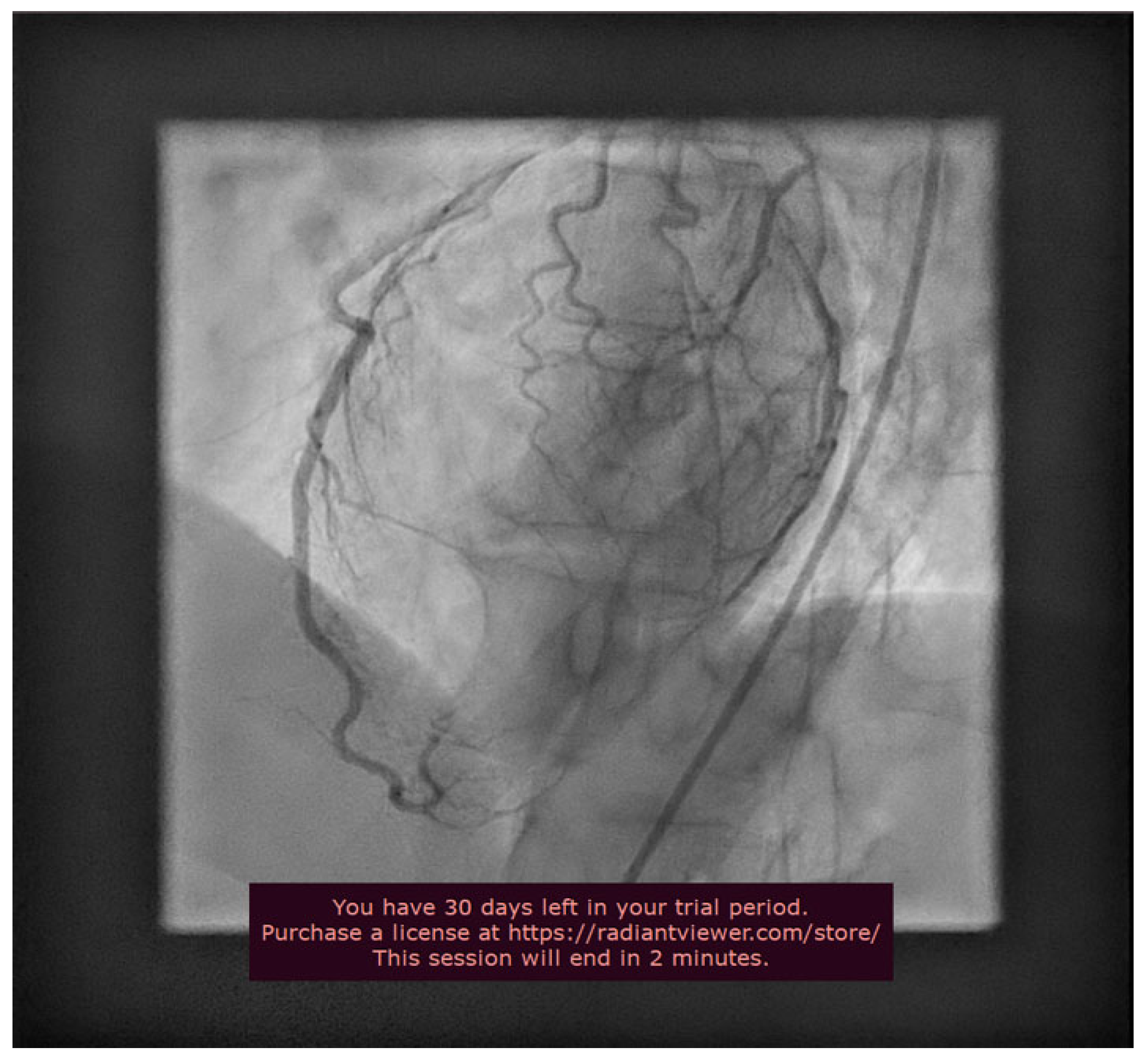
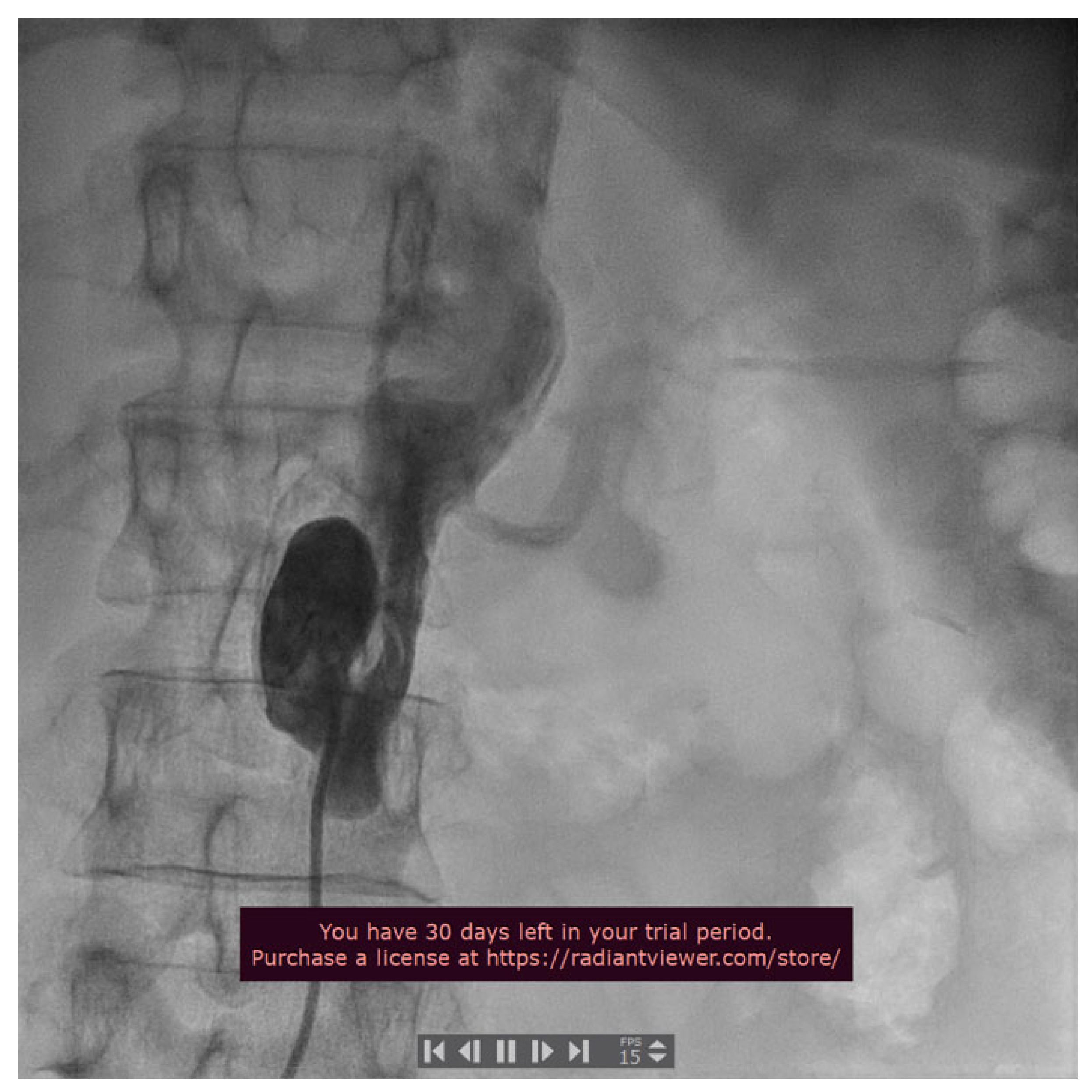
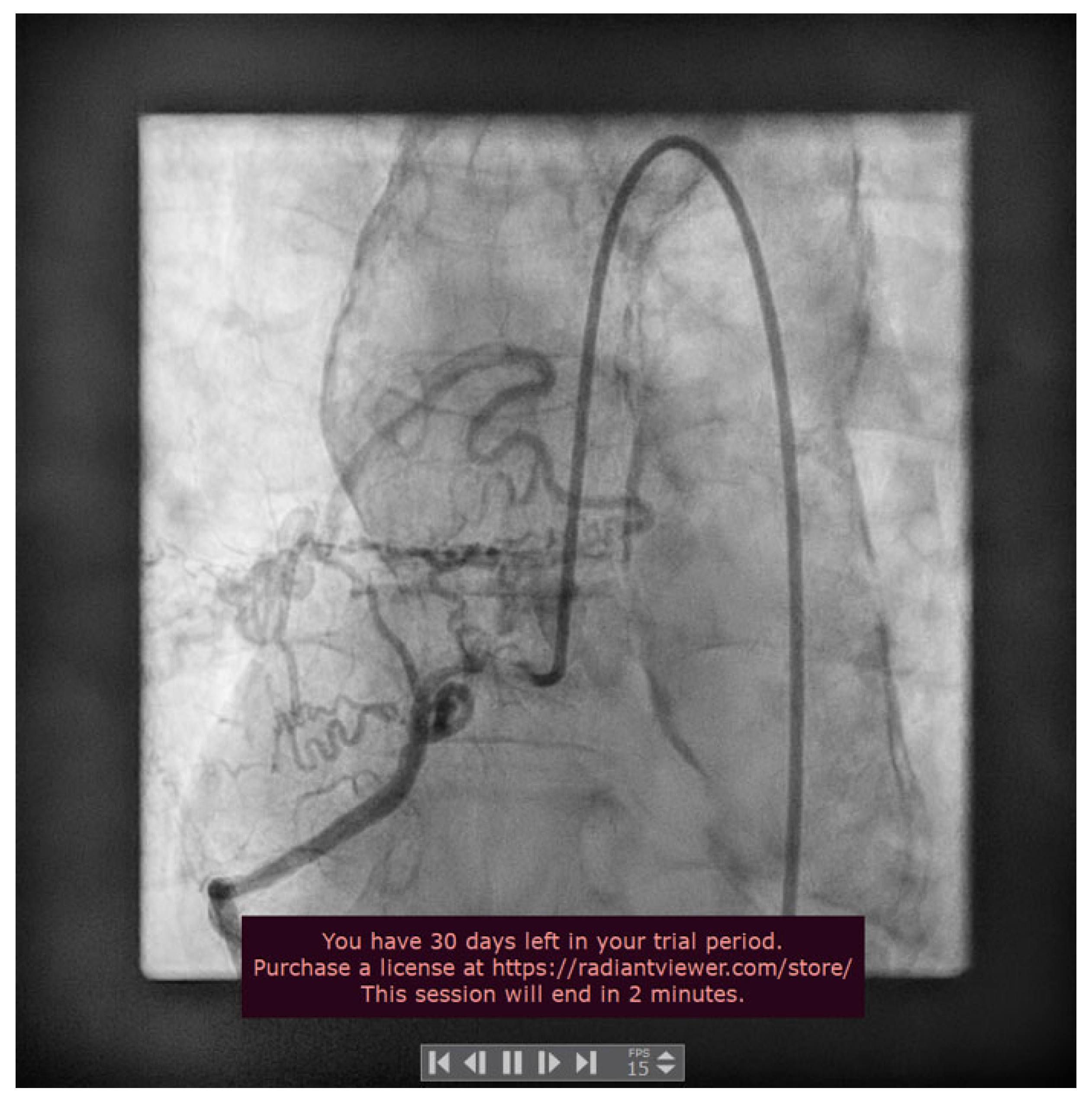
2. Case Presentation
3. Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, D.; Du, X.; Ma, C.S. Takayasu’s arteritis: diagnosis, treatment and prognosis. Int Rev Immunol 2012, 31, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, M. A case with peculiar changes of the retinal central vessels. Acta Soc Ophtal Jpn 1908, 12, 554–555. [Google Scholar]
- Numaro, F. Introductory remarks for special issue of Takayasu’ s arteritis. Heart Vess 1992, Suppl. 7, 3–5. [Google Scholar] [CrossRef]
- Chikashi, T. History of Takayasu’s arteritis and Dr. M.Takayasu. IntJ Rheum Dis 2014, 17, 931–935. [Google Scholar] [CrossRef]
- Shimizu, K.; Sano, K. Pulseless disease. J Neuropat Exp Neurol 1951, 1, 37–47. [Google Scholar]
- Kojima,K.; Shimizu, S.; Awaja, S.; Watanabe, I.; Niimi, K.; Yoshida, N. A case of pulseless disease associated with obstruction of the branch of the central retinal artery. Rinsho Ganka 1961, Dec 15, 1246–1249.
- Hirose, K. Entity of Takayasu-Ohnishi’ s syndrome (so called pulseless disease). Rinsho Ganka 1962, Apr16, 441–445. [Google Scholar]
- Hidano, A.; Watanabe, K. Pyoderma gangrenosum and cardiovasculopathies, particularly Takayasu’s arteritis. Review of the Japanese literature. Ann Dermatol Venereol 1981, 108, 13–21. [Google Scholar] [PubMed]
- Arend, W.P.; Michel, B.A.; Bloch, D.A.; Hunder, G.G.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; Lightfoot, W.R. et al. The ACR 1990 criteria for the classification of Takayasu’s arteritis. Arthritis Rheum 1990, 33, 1129-1134. [CrossRef]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, C.J.; Gribbons, K.B.; Judge, A.; Craven, A.; Kalid, S.; Hutchings, A.; Danda, D.; Lukmani, R.A.; Watts, R.A.; Merkel, P.A.; DCVAS Study Group. 2022 ACR/EULAR classification criteria for Takayasu’s arteritis. Ann Rheum Dis 2022, 81, 1654-1660. [CrossRef]
- Marvisi, C.; Bolek, E.C.; Alman, M.A.; Alessi, H.; Redmond, C.; Muratore, F.; Galli, E.; Ricordi, C.; Kaymaz-Tahra, S.; Ozguven, S.; Alibaz-Oner, F.; Direskeneli, H.; Salvarani, C.; Quinn, K.A.; Grayson, P.C. Development of the Takayasu’s arteritis integrated disease activity index. Arthritis Care Res, 2023, Dec 7. [CrossRef]
- Inder, S.J.; Bobryshev, Y.V.; Cherian, M.S.; Wang, A.Y.; Lord, R. S.; Masuda, K.; Yutani, C. Immunophenothypic analysis of the aortic wall in Takayasu’s arteritis: involvement of lymphocytes, dendritic cells and granulocytes in immunoinflammatory reaction. Cardiovasc Surg, 2000, 8, 141-148. [CrossRef]
- Zhang, Y.; Fan, P.; Luo, F.; Zhang, H.M.; Son, L.; Ma, W.J.; Wu, H.Y.; Cai, J.; Wang, L.P.; Zhou, X.L. Tuberculosis in Takayasu’s arteritis: a retrospective study in 1105 Chinese patients.J Geriatr Cardiol, 2019, 16, 648-655. [CrossRef]
- Moriwaki, R.; Noda, M.; Yajima, M.; Sharma, B.K.; Numano, F. Clinical manifestations of Takayasu’s arteritis in India and Japan-new classification of angiographic findings. Angiol 1997, 48, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.S..; Hollahan, C.W.; Giordano, J.; Leavitt, R.Y.; Fauci, A.S.; Rottem, M.; Hofmann, G.S. Takayasu’s arteritis. Ann Intern Med, 1994, 120, 919-929. [CrossRef]
- Johnston, S.L.; Lock, R.Y..; Gompels, M.M. Takayasu’s arteritis: a review. .J Clin Pathol 2002, 55, 481–486. [CrossRef]
- Schmidt, J.; Kermani, T.; Bacani, A.K.; Crowson, C.S.; Matteson, E.L.; Warrington, K.J. Tumor necrosis factor inhibitors in patients with Takayasu’s arteritis: experience from a referral center with long- term follow up. Arthritis Care Res, 2012, 64, 1079-1083. [CrossRef]
- Comarmond, C.; Biard, L.; Lambert, M.; Mekinian, A.; Ferfar, Y.; Kahn, J.E.; Benhamou, Y; Chiche, L.; Koskas, K.; Cluzel, P.; Hachulla, E.; Messas, E.; Resche-Rigon, M.; Cacoub, P.; Miraud, T.; Saadoun, D.; French Takayasu Network. Long-term outcomes and prognostic factors of complications Takayasu’s arteritis. Circ 2017, 136, 1114-1122. [CrossRef]
- Tombetti, E.; Mason, J.C. Takayasu arteritis: advanced understanding is leading to new horizons. Rheumatol Oxf Engl, 2019, 58, 206–219. [Google Scholar] [CrossRef]
- Arnaud, L.; Haroche, J.; Mathian, A.; Gorochov, G.; Amoura, Z. Pathogenesis of Takayasu’s arteritis: a 2011 update. Autoimmun Rev, 2011, 11, 61–67. [Google Scholar] [CrossRef]
- Yoshifuji, H. Pathophysiology of large vessel vasculitis and utility of interleukin-6 inhibition therapy. Mod Rheumatol, 2019, 29, 287–293. [Google Scholar] [CrossRef]
- Goel, R.; Kabeerdoss, J.; Ram, B.; Prakash, J.A.J.; Babji, S.; Nair, A.; Jeyaseelan, V.; Matthew, J.; Balaji, V.; Joseph, G.; Danda,D. Serum cytokine profile in Asian Indian patients with Takayasu arteritis and its association with disease activity. Open Rheumatol J, 2017, 11, 23–29. [CrossRef] [PubMed]
- Parakh, R.; Yadav, A. Takayasu’s arteritis: an Indian perspective. Eur J Vasc Endovasc Surg, 2007, 33, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, L.; Cambau, E.; Brocheriou, I.; Koskas, F.; Kieffer, E.; Piette, J.C.; Amoura, Z. Absence of mycobacterium tuberculosis in arterial lesions from patients with Takayasu’s arteritis. J Rheumatol, 2009, 36, 1682–1685. [Google Scholar] [CrossRef] [PubMed]
- Chaubal, N.; Dighe, M.; Shah, M. Sonographic and color doppler findings in Aortoarteritis (Takayasu Arteritis). J Ultrasound Med, 2004, 23, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Hayashi, K.; Sakamoto, I.; Ogawa, Y.; Matsumoto, T. Takayasu arteritis: protean radiologic manifestations and diagnosis. RadioGraphics 1997, 17, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Ferfar, Y.; Mirault, T.; Desbois, A.C.; Comarmond, C.; Messas, E.; Savey, L.; Domot, F.; Cacoub, P.; Saadoun, D. Biotherapies in large vessel vasculitis. Autoimmun Rev, 2016, 15, 544–551. [Google Scholar] [CrossRef] [PubMed]
| Laboratory finding | 1st day | 2nd day | 3rd day |
|---|---|---|---|
| AST (IU/L)1 | 32 | 27 | 31 |
| ALT (IU/L)2 | 25 | 22 | 29 |
| CPK ( IU/L)3 | 48 | 52 | 58 |
| CPK-MB (IU/L)4 | 2 | 1 | 2 |
| Troponin T(ng/L) | 3.1 | 4.3 | 1.5 |
| Troponin I (ng/L) | 2.6 | 3.7 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





