1. Introduction
The prevalence of Urinary incontinence ranges from 10 to 60% of non-pregnant women above 20 years of age and from 50 to 70% of women older than 60 years of age [
1,
2,
3,
4].
In women with stress urinary incontinence (SUI), involuntary pee leakage occurs when intra-abdominal pressure increases (e.g., with exercise, sneezing, coughing, laughing) in the absence of a bladder contraction. [
5].
SUI is hypothesized to be caused by a lack of mechanical support of the urethra and/or poor coaptation of the urethral tissues, resulting in insufficient resistance to urine outflow with elevated abdominal pressures.
The two main mechanisms involved in the physiopathology of SUI are urethral hypermobility, which develops when pelvic floor muscles and vaginal connective tissues provide insufficient urethral support, and Intrinsic Sphincteric Deficiency (ISD), caused by a loss of intrinsic urethral mucosal and muscle tone.
Although the reported cure rates of surgery for stress incontinence suggest a high degree of success in alleviating this symptom, these rates only consider stress incontinence. The usual argument for urethra support playing an important role in stress incontinence is the fact that urethral support operations are able to treat stress incontinence without changing urethral function [
6].
However, contrary to the"pelvic-centric" theory, a new "urethro-centric" hypothesis is emerging, according to which urethral hypermobility is a characteristic that can be both associated or not to the condition of ISD but does not constitute the etiology of SUI.
This idea emphasizes how urethral hypermobility is not the primary cause of SUI and ISD plays a critical part in the progression of this pathologic process.
The debate on the predominance of either of these two causes has dominated the urogynecological scene in terms of the interpretation of SUI physiopathology.
Worldwide, there is a continuous search for increasingly less invasive treatments for urinary incontinence surgery. In England there was even a 30% decrease in the use of mid-urethral slings following the FDA warning.
The use of SIS and UBA has increased as a first-line treatment option to try to reduce complications.
SIS are known to show less post-operative groin pain, less bleeding and shorter surgical times than traditional slings. On the other hand, UBAs are considered less effective in the long term but show fewer total complications.
Urethral bulking agent therapies (UBA) were traditionally used to treat women with painful SUI caused by ISD [
7]. Currently, UBA are seldom used as a first-line therapy for SUI although this procedure might be preferred by women who would rather have fewer postoperative problems in lieu of performing this treatment several times to reduce the likelihood of a SUI recurrence.
The aim of this study is to compare UBA and SIS in terms of efficacy, quality of life and sexual function in women being treated first-line for stress urinary incontinence
2. Materials and Methods
From January 2016 to January 2021, 159 consecutive patients affected by SUI were included in the study. A prospective analysis was performed.
All data were prospectively evaluated from a urogynecological internal database. The Institutional Review Boards (IRB) approved the study. An informed written consent was obtained from all women. The research was conducted according to Good Clinical Practice Guidelines. Sixty-four patients underwent UBA and 75 were treated with SIS. The study was approved by the institutional review board. Physical examinations, voiding diaries, and urodynamic tests were performed at our urogynecology outpatient clinic at the beginning and at the end of treatment.
The present study included only individuals who had symptoms for more than 1 year, had failed conservative treatment, and had incontinence episodes more than once every 24 hours.
The following conditions were used as exclusion criteria: neurogenic bladder, pure UUI and/or exclusive symptoms of OAB, ongoing and/or suspected breast cancer, ongoing and/or suspected hormone-dependent tumors, urological tumors, endometrial hyperplasia and atypical uterine bleeding, ongoing or past venous thromboembolism, clinical evidence of chronic inflammation or urinary tract infection, and treatment history involving pelvic radiation.
Each patient conducted a supine and standing cough stress test at 300-mL bladder filling during the urogynecological examination. Urodynamic examinations were carried out in accordance with the International Continence Society (ICS) guidelines.
The maximum urethral closure pressure of 20cm H2O and the Valsalva leaking point pressure of 60cm H2O were regarded as indicators of intrinsic sphincter deficiency.
All patients in this investigation exhibited urodynamically verified urinary stress incontinence, with a median maximum cystometric capacity of 322 mL (ranging 245-498 mL) and a median Valsalva leaking pressure of 59 cm H2O (ranging 40-100 cm H2O), and no indication of outflow obstruction (Qmax 15 mL/sec, Pvesmax >50 cm H2O). Patients with concurrent urinary tract infection, previous surgery for stress incontinence, functional bladder capacity of 200 mL, and stage 2 pelvic organ prolapse were excluded from the study.
Moreover, the patients completed a voiding diary before and after the treatment. Postoperatively, the International Consultation on Incontinence Questionnaire - Urine Incontinence - Short Form (ICIQ-UI-SF) was completed to assess the impact of urine symptoms. To assess sexual function, the standardized Female Sexual Function Index (FSFI), the Female Sexual Distress Scale (FSDS) and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaires short form (PISQ-12) questionnaires were administered on the first visit and again after 3 months. Finally, after treatment, the Patient Global Index of Improvement (PGI-I) was calculated.
Cefazoline 2 g was administered to all patients as a preventative measure 30 minutes before surgery.
The patient was placed on the operation table with her hips slightly flexed.
In UBA group a local anesthetic-containing lubricant was applied within the urethra, followed by a gradual trans-urethral instillation of 2% lidocaine solution. The PAHG injection was performed under endoscopic control with a single use PAHG Bulkamid® cystoscope linked to a 0-degree optic to provide precise and accurate PAHG submucosal injection. The rotating sheath over the cystoscope allows the working channel to revolve 360-degrees, allowing for better access and visual control of the injection sites without having to move the entire cystoscope. Technique points include cautious needle advancement to avoid unintentional urethral mucosa injury and an angulation of fewer than 5-degrees to avoid too deep injections. The best submucosal injection locations are at 2, 5, 7, 10 a.m., and 1 cm within of the bladder neck (proximal urethra). To ensure good urethral wall coaptation, 1-2 mL of Bulkamid® are injected at three sites, with no more than 0.5 mL injected at each site.
In SIS group a spinal anesthesia was performed. The Altis® Single Incision Sling System is a transobturator MUS that is adjustable and authorized for the treatment of stress urine incontinence.Â
Approximately 1 cm proximal to the urethral meatus and extending downward towards the bladder neck, a 1.5 cm midurethral incision was made on the anterior vaginal wall. Then, the scissors are inserted into the vaginal incision and a “push spread” technique (at least 1.5 cm wide) is used to dissect back to the ipislateral ischiopubic ramus. Secondly, the introducer and sling are inserted into the midline vaginal incision using an inside-out approach, with the tip of the introducer targeted via the previously dissected periurethral site towards the obturator membrane landmarks (a "10" and "2" o'clock locations). Finally, the sling is adjusted by dragging the suture loop across the patient's midline until the required support is attained, and it should be positioned tension-free beneath the urethra, allowing a right angle tool to easily slip between the sling and the urethra. This sling employs one static and one dynamic anchor at either end of a pulley suture, allowing for simple intraoperative tension modulation.
Clinical evaluation and exam, uroflowmetry, urodynamic exam and questionnaires were performed at the first appointment and after at least 24 months after surgical intervention.
Using Fisher's exact test, we determined the statistical significance of each event based on its incidence. For each comparison, an odds ratio (OR) and 95% confidence interval (CI) were generated. To evaluate whether data were sampled from a Gaussian distribution, normality tests (D'Agostino and Pearson tests) were used. To compare continuous parametric and non-parametric variables (data that does not fall into a normal distribution), the T-test and Mann-Whitney U test were employed, respectively. The Spearman rank coefficient was used to calculate correlations between numerical parameters. A matched T-test was used to evaluate the change in questionnaire results (ICIQ-UI-SF, PISQ-12, FSFI, FSDS, PGI-I). All analyses were carried out with the Statistical Package for the Social Sciences (SPSS) 22.0 for Mac (SSPS, Chicago, Illinois, USA). A p-value of less than 0.05 was considered significant.
3. Results
The total number of patients was 211. Since 33 patients did not match the inclusion criteria and 39 were lost to follow-up, the sample consisted of 159 patients.
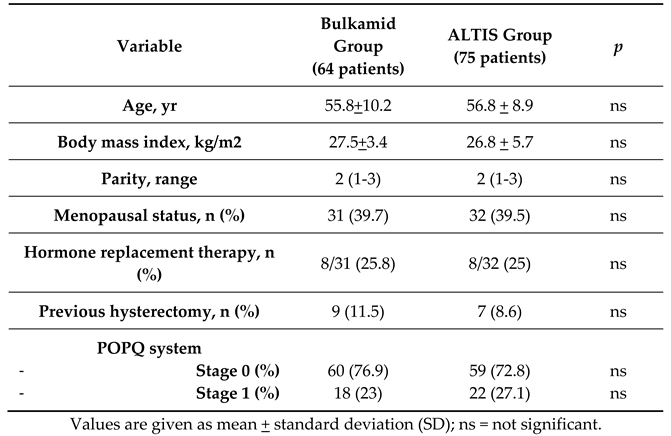
The 159 evaluated patients were divided into 2 groups: 64 patients who underwent UBA and 75 with SIS. Patients’ characteristics as age, BMI, parity, menopausal status, use of HRT, previous hysterectomy and POPQ status are similar between the two groups and shown in
Table 1.
Median follow-up was 29 months (24-37).
The two procedures had an almost overlapping intervention time (22.87+6.32 min. for the Bulking agents injection vs. 23.22+7.44 for ALTIS). We registered the post-operative complications in both groups (
Table 2) but only two of them reached a statistical significance with no patients of the Bulkamid group complaining pain after the procedure unlike the ALTIS group where 9 patients out of 75 (10.8%) experienced post-operative groin pain (p=0.03). Five patients in the ALTIS group developed de novo urgency compared to none in the UBA group (0.04).
The comparison of the voiding diary before and 24 months after treatment also showed interesting results (
Table 3).
In the Bulkamid group only 4 patients (5.1%) had a persistently positive Stress Test (p<0.0001), as well as the ALTIS group in which 5 patients (6.2%) had a positive Stress Test (p<0.0001).
The results of the urodynamic assessment conducted both before and after the therapy are shown in
Table 4. In terms of first voiding desire (from 91 to 138 ml in the Bulkamid group and from 89 to 142 ml in the ALTIS group), maximal cystometric capacity (from 301 to 387 ml in the Bulkamid group and from 298 to 398 ml in the ALTIS group), detrusorial pressure at peak flow (from 18 to 14 mmh20 in the Bulkamid group and from 19 to 13 mmh20 in the ALTIS group) and peak flow (from 20 to 23 ml/s in the Bulkamid group and from 19 to 25 ml/s in the ALTIS group), the table demonstrates the significant outcomes achieved with these treatments and supports the efficacy of both methods without differences between the 2 groups.
In both groups we observed a notable improvement of the QoL with a halving score in ICIQ-UI-SF 24 months after treatment (Bulkamid group from 14.58 ± 5.11 at baseline to 5.67±1.90 after 24 months; p<0.0001 vs. ALTIS group from 13.75 ± 5.89 to 5.83 ± 1.78; p<0.0001).
Likewise, we noted an improvement in sexual function, with a number of sexually active patients increasing from 29 to 44 (56.4%) in Bulkamid group (p=0.041) and from 31 to 51 (61.7%) in ALTIS group (p=0.034). In accordance with the last data, the scores derived from PISQ-12, FSFI and FSDS also showed an improvement in women’s sexual function (
Table 5).
4. Discussion
For the first time in literature, our study compares two treatment options for SUI: the contemporary SIS vs. UBA. The comparison is based on the assessment of treatment effectiveness, safety, and enhancement of sexual function and quality of life.
SUI is a frequent condition among women and has a significant impact on quality of life (QoL). The first line approach should include conservative therapies such as lifestyle advice, physical therapies (PFMT), scheduled voiding regimes, behavioral therapies and medications [
5]. When all of these therapies fail, patients with limited bladder neck mobility may undergo the full range of surgical treatments, such as midurethral sling, gold standard treatment, or when indicated, UBA injection.
Since retropubic and transobturator mid-urethral slings are associated with severe adverse effects (including bladder rupture, damage to blood vessels and pelvic pain), today single-incision mid-urethral slings (SIS) aim at reducing complications and being less invasive.
Nowadays, literature acknowledges that SIS is an excellent and effective technique despite being minimally invasive, with significantly reduced operating times and pelvic inguinal pain compared to traditional approaches [
8].
Contrary to UBA, SIS is also commonly used as a first-line treatment for SUI. UBA are now largely indicated for IDS and/or urethral hypomobility. However, in both circumstances, they are considered a second-line alternative treatment.
Our initial goal was to compare UBA and single-incision Sling (SIS) in order to establish the efficacy of both despite their minimally invasive nature.
Indeed, much has been published about women with SUI preferring interventions with fewer postoperative complications, although less successful, to more effective procedures with significant side effects [
9,
10].
We assessed the efficacy, safety, and side effects of the two procedures considered herein to show that UBA is also a viable first-line therapy option and that, considering the fewer postoperative complications, women may be tempted to choose a less invasive but equally effective treatment.
Regarding safety, only 8 patients out of 64 from UBA group showed complications. Only one patient experienced acute urinary retention in contrast to Giammò et al. who described 8.2% of this self-limited side effect [
11]. None of our patients reported de novo urgency unlike Itkonen Freitas et al. [
12] who described 9.3% of this complication. On the other hand, 26 patients out of 75 from SIS group showed side effects. The most frequent complication in this group was groin pain, in line with Moran et al. We also registered 6.6% of de novo urgency which is comparable with the 5.3% of Youxiang Han et al. [
13], while Moran et al reported a slightly higher rate (8.1%) as well as of urinary retention cases (7.2%) compared to our single case. Tape extrusion occurred in 2.5% of patients in line with literature [
14,
15].
SIS and UBA demonstrated to be highly effective. This is shown in stress-test data. In fact, at the median follow-up (29 months) the number of patients with positive stress test decreased drastically. Similarly, Q-Tip Swab Test grade almost halfed in both groups. These results appear to be better than the average “objective cure rate” drawn from the studies we analyzed [
10,
12,
14,
16].
We could justify this high cure rate because all procedures had been performed by the same expert surgeon, in the same center with a high volume of patients. Nevertheless, we believe in the need of a standardization of the parameters that define the “objective cure rate”, to align outcomes of these two procedures.
Another fundamental parameter to assess the effectiveness of treatments is the “subjective cure rate”, which could be defined as the personal perception of clinical improvement by the patients.
We obtained this data by submitting to patients questionnaires to evaluate their QoL, such as ICIQ-UI-SF and PGI-I scale. According to Kamarkar et al [
17], the cut-off points in ICIQ to evaluate patients’ satisfaction should be < 6/21. Our data reached these results as the ICIQ-UI-SF decreased by almost three times in both groups. These results are supported by data in literature which show a notable improvement in the ISIQ-UI-SF score after treatment [
17,
10]. The other item we used to evaluate QoL is the PGI-I scale. In our study patients reported to feel “very much better” or “much better" so the “subjective cure rate” of both groups after treatment was approximately in line with literature [
10,
12,
14,
15,
18]. Hence SIS and UBA appeared to be totally comparable in effectiveness and safety at a 24 months follow-up. The only significant difference was the absence of groin pain after UBA treatment.
Another goal of our study was to investigate the changes in sexual function and sexual satisfaction of women treated with bulking agents or SIS. The number of sexually active patients (>2 intercourses/month) increased from 29 (37.2%) to 44 (56.4%) in the UBA group and from 31 (38.2%) to 50 (61.7%) in the SIS group. Similarly, the scores from PISQ-12, FSFI and FSDS showed an improvement in women’s sexual function. There is limited literature available on the evaluation of sexual life after surgical SUI treatment. The two studies we found assess sexual function by using only one questionnaire out of the three we used in our study [
19,
20].
The strength of our study is the mid-term follow-up which enables the evaluation of patients over time, unlike studies with only a short-term follow-up. As mentioned above all patients underwent treatment by a single surgeon in the same high-volume center, minimizing the inter-operator outcome variability. Evaluation of sexual function via more than one questionnaire allows creating a more precise score for sexual activity.
Some limitations include the small number of patients, the need of a longer follow-up (>60 months), the presence of selection bias. Besides, we found in Sekiguchi et al. [
21] a cumulative cure rate of 91% after SIS treatment in a group of patients affected by mixed urinary incontinence, showing SUI together with ISD characteristics. According to these results, various research [
22,
23,
24] showed high rates of success and enhanced quality of life following SIS therapy. This could widen the field of application of SIS treatment but further studies and investigations are needed, including a randomized double-blind design study on a larger cohort of patients.
Overall, to the best of our knowledge this is the first comparative evaluation of these therapies in two groups of patients with comparable features. Furthermore, using a variety of tools for evaluation, our study assesses both the objective and subjective success of the therapies.
Although further research is required, we have demonstrated that UBA are highly successful when compared to traditional surgical methods, and they also have fewer side effects. Our study shows how UBA can be used as a first-line therapy option since it helps reestablish the transient sphincteric mechanism of continence, which is the foundation of incontinence physiology. This gives women the option to choose the therapy that makes them feel more comfortable, and gives them the possibility to favour a less invasive procedure.
Author Contributions
Conceptualization, Schiavi MC, Morciano A, Cervigni M, Palazzetti P, Grossi G, Cignini P, Spina V, calcagno M, Rappa C, Zullo MA, Grilli D and Campanella L.; methodology, Schiavi MC and Campanella L.; software, Schiavi MC.; validation, Schiavi MC. .; formal analysis, Schiavi MC.; investigation, Schiavi MC and Campanella L.; resources, Schiavi MC and Campanella L.; data curation, Schiavi MC and Campanella L.; writing—original draft preparation, Schiavi MC, Campanella L, Gabrielli G, Chiodo E, Stefanachi V.; writing—review and editing, Schiavi MC, Campanella L, Gabrielli G, Chiodo E, Stefanachi V, Pennacchini E; visualization, Schiavi MC.; supervision, Schiavi MC.; project administration, Schiavi MC. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Conflicts of Interest
The authors declare no conflicts of interest.The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A; Standardisation Sub-committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167-78. PMID: 11857671. [CrossRef]
- Mardon RE, Halim S, Pawlson LG, Haffer SC. Management of urinary incontinence in Medicare managed care beneficiaries: results from the 2004 Medicare Health Outcomes Survey. Arch Intern Med. 2006 May 22;166(10):1128-33. PMID: 16717176. [CrossRef]
- Griffiths AN, Makam A, Edwards GJ. Should we actively screen for urinary and anal incontinence in the general gynaecology outpatients setting?--A prospective observational study. J Obstet Gynaecol. 2006 Jul;26(5):442-4. PMID: 16846873. [CrossRef]
- Minassian VA, Yan X, Lichtenfeld MJ, Sun H, Stewart WF. The iceberg of health care utilization in women with urinary incontinence. Int Urogynecol J. 2012 Aug;23(8):1087-93. Epub 2012 Apr 12. PMID: 22527544; PMCID: PMC3905313. [CrossRef]
- Abrams P, Andersson KE, Birder L, Brubaker L, Cardozo L, Chapple C, Cottenden A, Davila W, de Ridder D, Dmochowski R, Drake M, Dubeau C, Fry C, Hanno P, Smith JH, Herschorn S, Hosker G, Kelleher C, Koelbl H, Khoury S, Madoff R, Milsom I, Moore K, Newman D, Nitti V, Norton C, Nygaard I, Payne C, Smith A, Staskin D, Tekgul S, Thuroff J, Tubaro A, Vodusek D, Wein A, Wyndaele JJ; Members of Committees; Fourth International Consultation on Incontinence. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn. 2010;29(1):213-40. PMID: 20025020. [CrossRef]
- Ashton-Miller JA, Howard D, DeLancey JO. The functional anatomy of the female pelvic floor and stress continence control system. Scand J Urol Nephrol Suppl. 2001;(207):1-7; discussion 106-25. PMID: 11409608; PMCID: PMC1192576. [CrossRef]
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A; Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003 Jan;61(1):37-49. [CrossRef]
- Kasi AD, Pergialiotis V, Perrea DN, Khunda A, Doumouchtsis SK. Polyacrylamide hydrogel (Bulkamid®) for stress urinary incontinence in women: a systematic review of the literature. Int Urogynecol J. 2016 Mar;27(3):367-75. Epub 2015 Jul 26. PMID: 26209952. [CrossRef]
- Patel T, Sugandh F, Bai S, Varrassi G, Devi A, Khatri M, Kumar S, Dembra D, Dahri S. Single Incision Mini-Sling Versus Mid-Urethral Sling (Transobturator/Retropubic) in Females With Stress Urinary Incontinence: A Systematic Review and Meta-Analysis. Cureus. 2023 Apr 18;15(4):e37773. PMID: 37214065; PMCID: PMC10194431. [CrossRef]
- Brosche T, Kuhn A, Lobodasch K, Sokol ER. Seven-year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourology and Urodynamics. 2021;40:502−508. [CrossRef]
- Giammò A, Geretto P, Ammirati E, et al. Urethral bulking with Bulkamid: An analysis of efficacy, safety profile, and predictors of functional outcomes in a single-center cohort. Neurourology and Urodynamics. 2020;1–6. [CrossRef]
- Itkonen Freitas Anna-Maija, Maarit Mentula, Päivi Rahkola-Soisalo, Sari Tulokas and Tomi S. Mikkola. Tension-Free Vaginal Tape Surgery versus Polyacrylamide Hydrogel Injection for Primary Stress Urinary Incontinence: A Randomized Clinical Trial. THE JOURNAL OF UROLOGY® Vol. 203, 372-378, February 2020. [CrossRef]
- Jonathan Youxiang Han, Eugene Youjin Huang, Jiayi Liu, Rehena Sultana, How Chuan Han. Short-medium term outcomes of Altis® single-incision sling for stress urinary incontinence in an Asian single-centre. Continence Volume 3, September 2022, 100498. [CrossRef]
- Kocjancic E, Erickson T, Tu LM, Gheiler E, Van Drie D. Two-year outcomes for the Altis® adjustable single incision sling system for treatment of SUI.. Neurourol Urodyn. 2017 Aug;36:1582-1587. [CrossRef]
- Dias J, Xambre L, Costa L, Costa P, Ferraz L. Short-term outcomes of Altis single-incision sling procedure for SUI: a prospective single-center study. Int Urogynecol J. 2014 Aug;25:1089-95. [CrossRef]
- Morán E, Pérez-Ardavín J, Sánchez JV, Bonillo MA, Martínez-Cuenca E, Arlandis S, Broseta E, Boronat F. Mid-term safety and efficacy of the ALTIS® single-incision sling for female stress urinary incontinence: less mesh, same results. BJU Int. 2019 May;123(5A):E51-E56. Epub 2018 Oct 26. PMID: 30267560. [CrossRef]
- Karmakar D, Mostafa A, Abdel-Fattah M.A new validated score for detecting patient-reported success on postoperative ICIQ-SF: a novel two-stage analysis from two large RCT cohorts. Int Urogynecol J. 2017 Jan;28:95-100. [CrossRef]
- 18- Sokol ER, Karram MM and Dmochowski R: Efficacy and safety of polyacrylamide hydrogel for the treatment of female stress incontinence: a randomized, prospective, multicenter North American study. J Urol 2014; 192: 843. [CrossRef]
- Leone Roberti Maggiore U, Alessandri F, Medica M, Gabelli M, Venturini PL, Ferrero S. Periurethral injection of polyacrylamide hydrogel for the treatment of stress urinary incontinence: the impact on female sexual function. J Sex Med. 2012 Dec;9(12):3255-63. PMID: 23206347. [CrossRef]
- Gert Naumann, Joscha Steetskamp, Mira Meyer, Rosa Laterza, Christine Skala, Stefan Albrich, Heinz Koelbl. Sexual function and quality of life following retropubic TVT and single-incision sling in women with stress urinary incontinence: results of a prospective study. Arch Gynecol Obstet (2013) 287:959–966. [CrossRef]
- Sekiguchi Y, Kinjyo M, Inoue H, Sakata H, Kubota Y. Outpatient mid urethral tissue fixation system sling for urodynamic SUI. J Urol 2009;182:2810–3.
- Zullo MA, Schiavi MC, Luffarelli P, Bracco G, Iuliano A, Grilli D, Esperto F, Cervigni M. Efficacy and safety of anterior vaginal prolapse treatment using single incision repair system: Multicentric study. Taiwan J Obstet Gynecol. 2022 Jul;61(4):646-651.PMID: 35779915. [CrossRef]
- Schiavi MC, Carletti V, Yacoub V, Cardella G, Luffarelli P, Valensise HCC, Palazzetti P, Spina V, Zullo MA. Evaluation of the efficacy and safety of single incision sling vs TVT-O in obese patients with stress urinary incontinence: Quality of life and sexual function analysis. Taiwan J Obstet Gynecol. 2023 Jan;62(1):89-93. PMID: 36720557. [CrossRef]
- Zullo MA, Schiavi MC, Luffarelli P, Prata G, Di Pinto A, Oliva C. TVT-O vs. TVT-Abbrevo for stress urinary incontinence treatment in women: a randomized trial. Int Urogynecol J. 2020 Apr;31(4):703-710. Epub 2019 Aug 13. PMID: 31410518. [CrossRef]
Table 1.
Patient characteristics.
Table 1.
Patient characteristics.
Table 2.
Postoperative complications in 159 patients.
Table 2.
Postoperative complications in 159 patients.
| Variable |
Bulkamid Group
(64 patients) |
|
ALTIS Group
(75 patients) |
p |
| Operative time, min |
22.87+6.32
|
|
23.22+7.44
|
ns |
|
Fever, n(%)
|
1 (1.2) |
|
0 (0) |
ns |
|
Groin pain, n(%)
|
0 (0) |
|
9 (10.8) |
0.03 |
|
Urinary tract infection, n(%)
|
2 (2.6) |
|
3 (3.6) |
ns |
|
Deep vein thrombosis, n(%)
|
0 (0) |
|
0 (0) |
ns |
| Urinary retention for up to 7 days, n (%) |
1 (1.2) |
|
1 (1.3) |
ns |
|
Tape extrusion, n(%)
|
1 (1.2) |
|
2 (2.5) |
ns |
|
Severe pain, n(%)
|
0 |
|
0 (0) |
ns |
|
Dyspareunia, n(%)†
|
0 |
|
2 (2.5) |
ns |
|
De novo urgency(%)
|
0 |
|
5 (6.6) |
0.04 |
|
De novo SUI(%)
|
4 (5.1) |
|
4 (5) |
ns |
Table 3.
Comparison of Voiding Diary before and after treatment (29 months Follow-Up).
Table 3.
Comparison of Voiding Diary before and after treatment (29 months Follow-Up).
| Variables |
Bulkamid Group
(64 patients) |
ALTIS Group
(75 patients) |
|
| Follow Up |
Baseline |
Median FU |
p |
Baseline |
Median FU |
p |
p |
| Positive Stress Test (%) |
78 (100) |
4 (5.1) |
<0.0001 |
81 (100) |
5 (6.2) |
<0.0001 |
ns |
| Q-Tip swab test (grade) |
12.10 |
10.41 |
<0.0001 |
11.11 |
8.56 |
<0.0001 |
ns |
| Mean number of voids (24 h) |
1.65 |
2.22 |
0.04 |
2.12 |
1.98 |
ns |
0.03 |
| Mean number of nocturia events |
0.43 |
0.45 |
ns |
0.88 |
0.95 |
ns |
ns |
Table 4.
Pre and Post urodynamic evaluation.
Table 4.
Pre and Post urodynamic evaluation.
| Urodynamic data |
Bulkamid Group
(64 patients) |
ALTIS Group
(75 patients) |
Bulkamid
vs
Altis |
| |
Baseline |
12 weeks |
p |
Baseline |
12 weeks |
p |
p |
| Peak flow (ml/s) |
20.71 + 3.60 |
23.23 + 4.23 |
0.01 |
19.65 + 4.23 |
24.81 + 5.88 |
<0.0001 |
0.07 |
| Flow time (ml/s) |
26.22 + 5.11 |
27.67 + 5.18 |
0.11 |
25.68 + 5.51 |
27.77 + 5.11 |
0.09 |
0.81 |
| Post-void residual (ml) |
20.55 + 6.28 |
19.54 + 6.12 |
0.49 |
21.11 + 7.09 |
20.13 + 7.11 |
0.54 |
0.72 |
| First voiding desire (ml) |
91.76 + 20.13 |
138.72 + 19.24 |
0.004 |
89.23 + 21.47 |
142.43 + 19.98 |
<0.0001 |
0.32 |
| Maximum cystometric capacity (ml) |
301.31 +73.56 |
387.76 + 82.44 |
0.002 |
298.65 +77.28 |
398.26 + 91.21 |
0.0031 |
0.55 |
| Detrusor pressure at peak flow (cmH2O) |
18.78 + 5.63 |
14.45 + 6.10 |
0.0012 |
19.11 + 6.12 |
13.89 + 4.89 |
<0.0001 |
0.21 |
| Maximum Urethral Closure Pressure (cmH2O) |
69.87 + 9.11 |
70.32 + 8.34 |
0.69 |
68.91 + 9.71 |
71.09 + 7.91 |
0.51 |
0.72 |
| Urethral Functional Length (mm) |
28.10 + 2.22 |
28.21 + 2.33 |
0.41 |
28.43 + 3.01 |
28.67 + 2.93 |
0.65 |
0.81 |
| Patients with detrusor overactive (%) |
36 (60) |
23 (38.3) |
0.13 |
30 (57.7) |
9 (17.3) |
0.02 |
0.08 |
Table 5.
Quality of Life and Sexual Function at 29 months follow up.
Table 5.
Quality of Life and Sexual Function at 29 months follow up.
| Variables |
Bulkamid Group
(64 patients) |
ALTIS Group
(75 patients) |
|
| |
Preoperative |
Median FU |
p value |
Preoperative |
Median FU |
p value |
p value |
| Quality of Life |
| ICIQ-UI-SF |
14.58 ± 5.11 |
5.67±1.90 |
<0.001 |
13.75 ± 5.89 |
5.83 ± 1.78 |
< 0.001 |
ns |
| Sexual Function |
|
Sexual Activity † (%)
|
29 (37.2) |
44 (56.4) |
0.041 |
31 (38.2) |
50 (61.7) |
0.034 |
ns |
|
PISQ-12‡ |
30.44 ± 7.23 |
36.54 ± 6.98 |
< 0.001 |
31.22 ± 5.65 |
38.33 ± 6.24 |
< 0.001 |
ns |
|
FSFI‡ |
20.43 ± 2.22 |
29.77 ± 1.89 |
< 0.001 |
21.21 ± 1.43 |
29.34 ± 2.11 |
< 0.001 |
ns |
|
FSDS‡ |
21.65 ± 4.76 |
8.32 ± 3.56 |
< 0.001 |
20.98 ± 5.43 |
7.86 ± 4.78 |
< 0.001 |
ns |
Table 6.
Patient impression of global improvement (PGI-I) after 29 months of treatment.
Table 6.
Patient impression of global improvement (PGI-I) after 29 months of treatment.
| Variables |
Bulkamid Group
(64 patients) |
ALTIS Group
(75 patients) |
p |
| 1: very much better (%) |
60 (77) |
60 (74) |
ns |
| 2: much better (%) |
10 (12.8) |
12 (14.8) |
ns |
| 3: a little better (%) |
5 (6.4) |
6 (7.4) |
ns |
| 4: no improvement (%) |
3 (3.8) |
3 (3.7) |
ns |
| 5: a little worse (%) |
0 (0) |
0 (0) |
/ |
| 6: much worse (%) |
0 (0) |
0 (0) |
/ |
| 7: very much worse (%) |
0 (0) |
0 (0) |
/ |
| Success (%) |
70 (89.7) |
72 (88.8) |
ns |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).