1. Introduction
The life expectancy of PWH has significantly increased in the post-ART era. While advancements in ART have undeniably revolutionized the landscape for PWH, contributing significantly to prolonged lifespans and improved overall health [
1,
2], they have also given rise to a new set of challenges in the form of chronic comorbidities of HIV infection, such as chronic inflammation, dyslipidemia, and insulin resistance, potentially leading to cardiovascular complications and neuronal injuries including peripheral neuropathies [
3,
4]. HIV-associated distal sensory polyneuropathy (HIV DSP) is a common complication of HIV infection, with prevalence rates ranging from 10% to 45% in PWH [
5,
6,
7]. DSP symptoms include pain, decreased sensation, allodynia, paresthesia, and pain in a symmetric stocking-glove distribution. [
8,
9] HIV-1 proteins such as gp120 are implicated in HIV-DSP (e.g., impaired large-diameter fibers) [
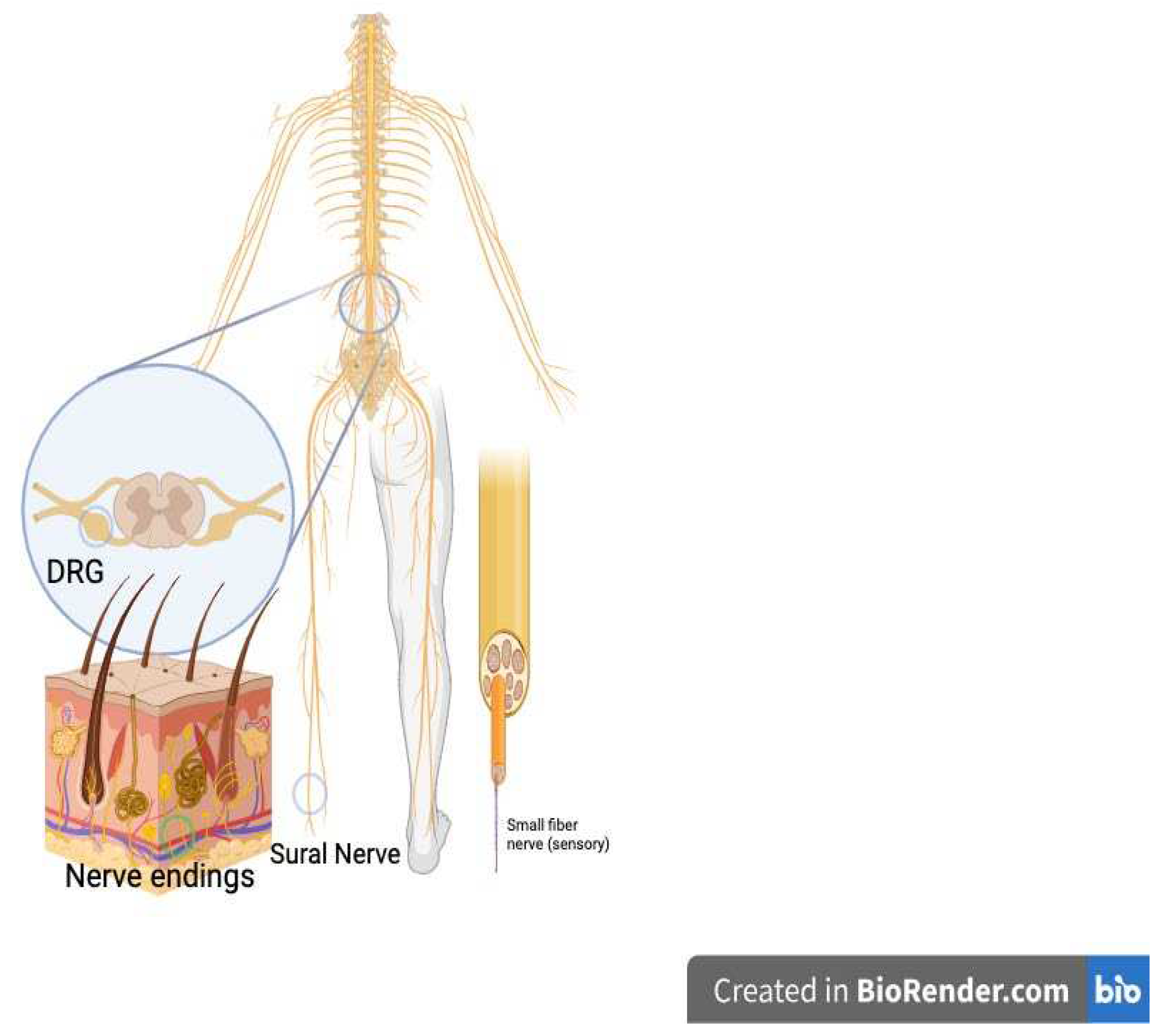
10] [
Figure 1]. In addition, it has been shown that some ART have neurotoxic effects, such as efavirenz (EFV), nonnucleoside reverse transcriptase inhibitors (NNRTI) (“D-drugs,” principally Stavudine (d4T), Didanosine (ddI), and Zalcitabine (ddC)) which cause neuronal mitochondrial toxicity [
11,
12]. Moreover, certain ART has recently been linked to DSP in HIV by impairing thin fiber [
9]. For instance, Zidovudine (AZT) causes myopathy; ddC, ddl, and lamivudine (3TC) cause neuropathy; d4T and fialuridine (FIAU) cause neuropathy or myopathy and lactic acidosis [
13]. The complex interplay between the virus, treatment modalities, and individual factors underscores the importance of a comprehensive approach to healthcare for PWH, addressing not only viral suppression but also monitoring and controlling the long-term conditions that can arise alongside prolonged ART use.
HIV has been implicated in impairing mitochondrial function [
14]. As previously described, HIV may directly impact mitochondrial respiration and ETC activity, disrupting the delicate balance required for optimal cellular function [
15]. Moreover, the destructive effects on mtDNA replication add another layer of complexity to the interaction between HIV and cellular physiology [
16]. The mitochondrial 𝛾-polymerase, responsible for mtDNA replication, has been identified as a target for the detrimental impact of both HIV and certain ARTs. Consequently, the integrity of the mitochondrial genome is compromised, potentially leading to mitochondrial dysfunction [
17]. Neurons depend on ATP for axonal transport and maintaining ionic gradients, which is crucial for generating action potentials and facilitating synaptic activity [
18]. The consequences of impaired mtDNA replication extend beyond the immediate context of HIV infection, as they may contribute to the development of various metabolic and neurodegenerative disorders associated with mitochondrial dysfunction [
19].
mtDNA damage has emerged as a focal point of investigation in understanding the mechanisms underlying neuropathy in PWH undergoing ART [
20]. The specific metric utilized to gauge mtDNA damage in this context is the CD a molecular marker indicative of genetic alterations within the mitochondrial genome [
21]. The mtDNA CD is a 4977-base pair deletion that has been found in increasing abundance in older age in human tissue obtained from multiple sites, including the CNS, heart, liver, kidney, and skeletal muscles [
22,
23,
24]. In a noteworthy prior study, researchers observed a pronounced increase in the frequency of this CD in HIV-positive individuals undergoing ART [
25].
The connection drawn between elevated mtDNA damage, as reflected by the CD, and the manifestation of neuropathy sheds light on the potential role of mitochondrial dysfunction in the neuropathic processes associated with HIV and its treatment.
While the mechanisms underlying these phenomena are still under investigation, the recognition of HIV-mediated mitochondrial impairment has important implications for both the understanding of HIV pathogenesis and the development of therapeutic strategies. Further elucidating the molecular pathways involved in HIV-induced mitochondrial dysfunction may unveil novel targets for intervention, not only in the context of HIV treatment but also in addressing broader mitochondrial-related diseases.
Considering that axonal mitochondria are assembled in the neuronal cell body and transported down the length of axons, we hypothesize that there are distinct molecular alterations in the mtDNA content and integrity within DRG and the SuN specimens from PWH, particularly in those displaying signs and symptoms of DSP. We expected to observe a reduced quantity of mtDNA per cell among PWH with DSP symptoms compared to those without such symptoms. Additionally, we predicted to see an increased abundance of the CD in mtDNA in the SuN compared to DRGs confirming the cumulative effect of mtDNA CD along nerve fibers.
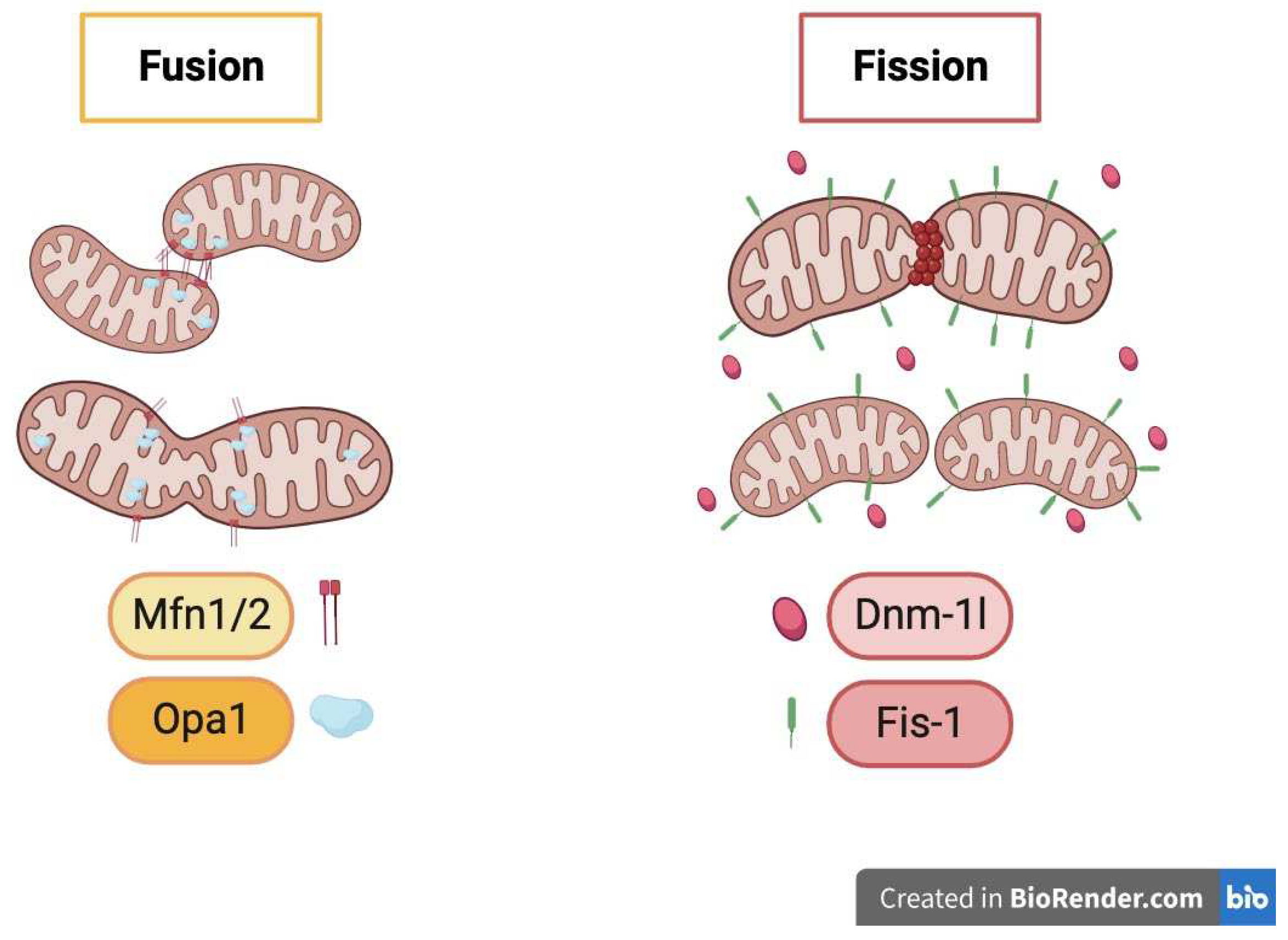
We also anticipated changes in expression levels of ETC complex proteins, mitofusin 1 (MFN1), and dynamin-related protein-1 (DRP1) as revealed by SDS-PAGE and immunoblotting. Expression levels of DRP1, a crucial enzyme that facilitates mitochondrial fission, are altered in conditions marked by chronic neuroinflammation. These alterations impact neuronal apoptosis, synaptic activity, and axonal integrity [
26,
27]. A previous study linked the administration of DRP1 inhibitors to decreased DSP in rats [
28] [
Figure 2]. Considering that axonal mitochondria play a crucial role in neuronal function; we further predict that alterations in mtDNA and mitochondrial protein expression will correlate with the progression of DSP.
2. Results
Post-mortem DRG and SuN specimens of 11 PWH were studied in this research. The mean age of the sample at the time of death was 44 years, and 27.2 % were female (
Table 1). Their last median CD4 cell count was 12 (7-50.5) cells/mm
3, and their nadir CD4 cell count was 9.5 (2-23) cells/mm
3. Eight (72.7 %) were on ART at the time of death. Five (45.5%) PWH met the definition of DSP. No significant difference was observed between DSP+ and DSP- in terms of demographic and clinical characteristics, including age, gender, most recent and nadir CD4 cell counts, frequency and duration of ART and regimen, including non-nucleoside analog reverse transcriptase inhibitors (NNRTIs), and highly active ARTs (HAART).
2.2. mtDNA Copy Number and Relative Common Deletion in DSP
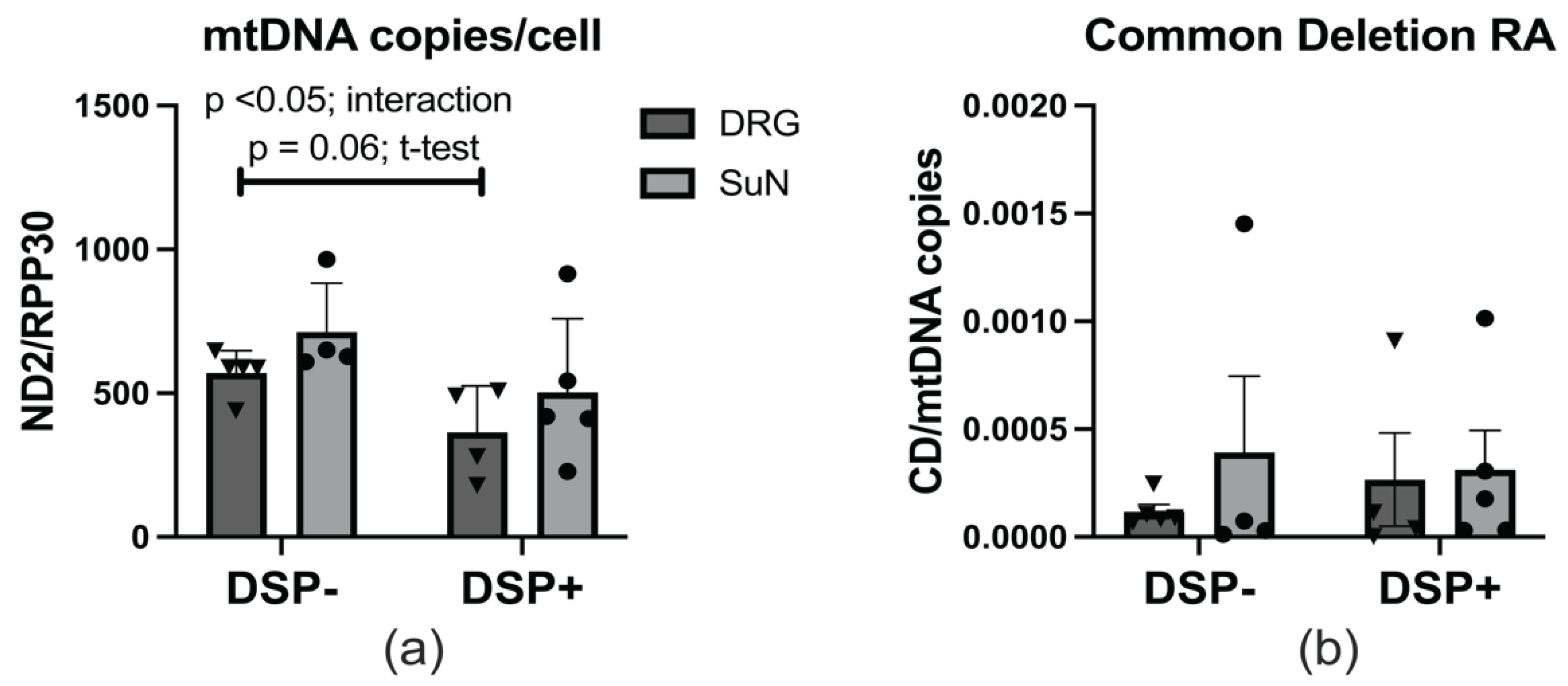
Our investigation into the mtDNA dynamics within the DRG and the SuN of donors with and without DSP revealed a distinct pattern. mtDNA copy number per cell quantified by numbers of ND2/RPP30 using ddPCR. Notably, individuals with DSP exhibited a significant reduction in mtDNA copy number per cell, particularly pronounced in both the DRG and the SuN regions. [
Figure 3a] mtDNA Common deletion (CD) relative abundance (RA) quantified by real-time PCR using primers that span the sequences adjacent to the CD normalized to the total number of mtDNA per cell in each specimen (n= 11). mtDNA CD was lower in the DRG than in the SuN of both groups of PWH with DSP and without this condition. Although mtDNA CD RA was higher in the DRG specimens of PWH with DSP than those without DSP, it was lower in their SuN specimens. [
Figure 3b]. Nevertheless, when considering the impact of a singular data point from one individual in the DSP- group diagnosed with HIV just six months before death, whose SuN specimen showed a markedly higher level of mtDNA CD RA (approximately 100 times more), we observed that mtDNA CD RA was remarkably higher in the DSP+ group compared to the DSP- group (data not shown). This observation highlights a specific alteration in mtDNA content associated with DSP and suggests a potential link between mitochondrial dysfunction and the pathogenesis of sensory neuropathy. Additionally, these alterations in CD mutation RA in mtDNA between distal SuN and dorsal root ganglia suggest the possibility of a cumulative effect of mtDNA CD on the pathogenesis of sensory neuropathy in long nerves.
2.3. Mitochondrial Dynamics and ETC Protein Expression Levels are Altered in DSP.
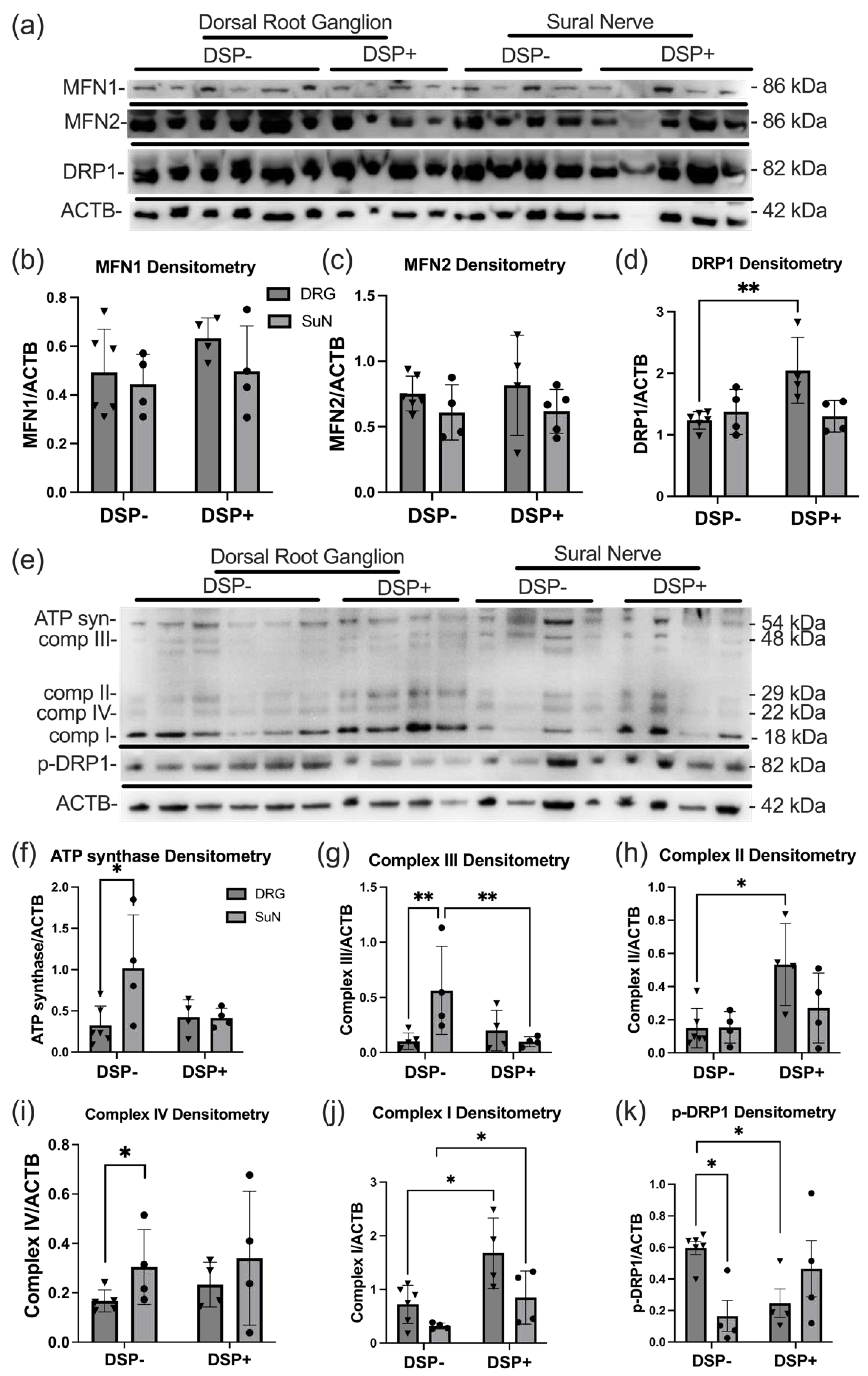
Our investigation extended to exploring ETC proteins, providing insights into the mitochondrial function within the peripheral nervous system in the context of DSP. The immunoblot of DRG and SuN lysates from DSP- and DSP+ decedents revealed bands corresponding to MFN1, MFN2, DRP1, and ACTB (
Figure 4a). Densitometry analyses revealed no significant difference in levels of MFN1 (
Figure 4b) or MFN2 (
Figure 4c) between any of the groups. Densitometry analyses of the band corresponding to DRP1 revealed a ~40% increase in DRP1 levels in the DRG of DSP+ decedents compared to DSP- decedents (
Figure 4d; **p < 0.01). Noteworthy, another immunoblot of DRG and SuN lysates from DSP- and DSP+ decedents revealed bands corresponding to ATP synthase, Complex III, Complex II, Complex IV, Complex I, p-DRP-1, and ACTB (
Figure 4e). Densitometry analyses of the band corresponding to ATP synthase revealed a significant increase in its levels in the SuN of DSP- decedents compared to the ATP synthase levels in their DRG (
Figure 4f; *p < 0.05). Densitometry analyses of the band corresponding to Complex III revealed a significant increase in the SuN of DSP- decedents compared to their DRG and SuN of DSP+ decedents (
Figure 4g; **p < 0.03). Densitometry analyses of the band corresponding to Complex II revealed a significant elevation in its levels in DGR of DSP+ decedents compared to DSP- decedents (
Figure 4h; *p < 0.05). Densitometry analyses of the band corresponding to Complex IV revealed a significant increase in the SuN of DSP- decedents compared to their DRG (
Figure 4i; *p < 0.05). we observed a significant decrease in the densitometry of Complex I within the SuN of DSP+ and DSP- decedents compared to their DRG, respectively (
Figure 4j; *p < 0.03). In addition, p-DRP-1 densitometry exhibited a remarkable increase in the DRG of DSP- decedents compared to their SuN and the DRG of DSP+ decedents (
Figure 4k; *p < 0.01).
3. Discussion
This investigation utilized a combination of molecular and histopathological methods to analyze mtDNA dynamics and ETC protein expression in post-mortem DRG and SuN specimens from individuals with and without DSP. These methods allowed for a comprehensive exploration of mitochondrial dynamics and function in the context of sensory neuropathy in PWH. We found a significant reduction in mtDNA copy number per cell in both DRG and the SuN of individuals with DSP. This observation suggests a link between HIV infection, ART, mitochondrial dysfunction, and the pathogenesis of sensory neuropathy. The reduction in mtDNA copy number suggests that mitochondrial dysfunction may result from or cause neurodegenerative processes in the context of DSP. The alteration in mtDNA dynamics, particularly in the SuN, emphasizes the cumulative effect of mtDNA changes along the course of long nerves, potentially contributing to the specific manifestations of peripheral neuropathy. In addition, a primate model of SIV-associated peripheral neuropathy has affirmed the primary role of mitochondrial dysfunction in this process [
16].
Previous studies link HIV infection to DSP resulting from the virus itself or to a toxic neuropathy associated with certain dideoxynucleoside analog reverse transcriptase inhibitors, specifically d4T, ddI, and ddC [
11], or, most likely, both together. Ten participants had prior exposure to the D-drugs. mtDNA CD RA increased in the SuN compared to DRGs in both DSP+ and DSP- groups, suggesting a potential cumulative effect of mtDNA CD on the pathogenesis of DSP, which predominantly affects the distal parts of the nerve fibers compared to DRG. This finding supports the notion that mitochondrial genome damage, as reflected by the CD, may play a role in the development and progression of DSP in long nerves. The specificity of this alteration in distal nerves highlights the importance of investigating regional differences in mitochondrial dynamics within the peripheral nervous system.
Our study extended to an analysis of ETC proteins, which demonstrated a significant elevation in the levels of these proteins within the DRG of DSP+ donors, particularly in complex I and DRP-1. NRTIs cause a notable decrease in the activity of complex IV and a targeted blockage of complex I [
30]. In this study, 91.9% (10/11) of the donors had a history of NRTIs, specifically d-drugs. No differences were observed in the expression of ETC complex-IV between DSP+ and DSP- samples. However, there was an upregulation of complex IV in the SuN compared to DRG in DSP- donors. Lehmann et al. reported a decrease in the expression of subunits of complex IV in SuN samples from DSP+ PWH compared to both DSP- individuals and controls [
16]. This regional disparity in ETC protein expression suggests a compensatory mechanism in response to mitochondrial dysfunction. The upregulation of ETC proteins may indicate an attempt to overcome impaired mitochondrial function by enhancing the production of key components involved in energy production. However, the lack of a similar upregulation in the SuN suggests potential limitations in the transport of mitochondria to the distal portions of sensory neurons, possibly contributing to the observed neuropathic symptoms. [
29]
Several studies have highlighted the involvement of DRP-1 in neuroinflammation and DSP through multiple pathways [
29,
31,
32]. Research has demonstrated that the spinal intrathecal administration of ODN antisense to Drp1 reduces the expression level of Drp-1 in primary afferent fibers, reducing ddC-induced mechanical hyperalgesia in male Sprague Dawley rats. Furthermore, this study revealed that the intradermal injection of Mitochondrial Division Inhibitor 1 (mdivi-1), a selective inhibitor of Drp1-dependent mitochondrial fission, significantly alleviated mechanical hyperalgesia induced by ROS and ddC [
32]. The current study found that DRP-1 expression within the DRG was markedly higher in donors with DSP+ than DSP- donors. ATP synthase is the complex assembled in the mitochondria and transported to the cell surface by DRP-1 [
33]. We found that ATP synthase exhibited a remarkable upregulation in the SuN compared to the DRG in donors without DSP.
A prior study showed macrophage activation and infiltration in the DRG of PWH with DSP. [
34] Macrophage activation contributes to mitochondrial dysfunction by altering the electron transport chain (ETC) activity and the TCA cycle. [
35] Activated macrophages also induce an upregulation of glucose and glutamine utilization and a shift toward anabolic pathways. These interactions between mA and mitochondrial function are bidirectional. Thus, itaconate, produced in the mitochondrial matrix from the TCA cycle metabolite cis-aconitate, regulates multiple aspects of macrophage functions.
The strength of this work lies in its comprehensive investigation of mitochondrial dynamics and ETC protein expression in the context of distal sensory polyneuropathy (DSP) in people living with HIV. Prior research in this area has often focused on individual aspects of mitochondrial function or neuropathy, but this study integrates multiple facets to provide a better understanding of the relationship between mitochondrial dysfunction and DSP. Additionally, the inclusion of post-mortem DGR and SuN specimens from a well-characterized population of people living with HIV enhances the clinical relevance and applicability of the findings.
Our study has limitations. The relatively small sample size due to limited access to autopsied DRG and the SuN specimens may affect the generalizability of our findings. Future research with larger cohorts is warranted to validate and expand upon our results. Additionally, it is not completely known to which extent the virus itself or ART contributes to the pathogenesis of DSP separately. Therefore, investigating the functional consequences of the observed molecular alterations and the direct impact of specific ART on mitochondrial function could provide a more comprehensive understanding of the underlying mechanisms. Future studies could also explore the potential use of mitochondrial protective agents or interventions to enhance mitochondrial transport as therapeutic strategies for DSP. Longitudinal studies tracking mitochondrial dynamics in PWH over time and in response to different antiretroviral regimens could further elucidate the progressive nature of mitochondrial dysfunction in the context of HIV. Finally, as the risk of mitochondrial toxicity from newer ART regimens decreases, the more significant mitochondrial impacts in PWH moving forward may be direct viral toxicity in combination with metabolic disease.
Future studies could also explore the potential use of mitochondrial protective agents or interventions to enhance mitochondrial transport as therapeutic strategies for DSP. Targeting pathways that facilitate the transport of mitochondria to distal nerve endings may represent a therapeutic approach to address the specific neuropathic symptoms observed in DSP. Longitudinal studies tracking mitochondrial dynamics in PWH over time and in response to different antiretroviral regimens could further elucidate the progressive nature of mitochondrial dysfunction in the context of HIV. The regional differences in ETC protein expression identified here point to new avenues for research into the specific mechanisms by which mitochondrial dysfunction contributes to sensory neuropathy and may lead to identifying novel drug targets for intervention. Additionally, research into the potential interplay between mitochondrial health, immune function, and neuroinflammation in the context of DSP could provide valuable insights into the multiple causes of this condition and inform the development of comprehensive treatment strategies.
4. Materials and Methods
Autopsied specimens of DRG and SuN were obtained from a characterized cohort of PWH. Within this cohort, 6 individuals exhibited no signs or symptoms of DSP, while 5 displayed two or more distinct signs indicative of DSP. Notably, all participants in the study were either currently undergoing cART or had a history of previous exposure to antiretroviral medications. Following the acquisition of DRG and the SuN specimens, total DNA was isolated from these tissues. The investigation focused on quantifying the amount of mtDNA per cell and determining the proportion of mtDNA carrying the CD. This quantitative analysis was conducted using ddPCR, an exact method well-suited for measuring rare events in genetic material. The specimens underwent additional analyses to gain further insights into the molecular landscape. Specifically, they were subjected to homogenization and subsequently resolved using SDS-PAGE. The resolved proteins were then probed using immunoblotting techniques to assess the expression of mitochondrial ETC complex proteins and the levels of MFN1 and DRP1.
4.1. Study Population
PWH were evaluated at the University of California, San Diego, in the HIV Neurobehavioral Research Center and the California NeuroAIDS Tissue Network. An IRB approved this research, and each participant gave informed consent. Data were collected according to a protocol of comprehensive neuromedical, neurobehavioral, and laboratory assessments that were standardized across sites.
4.2. Phenotype Definitions
Clinicians trained in HIV neurological disorders performed a standardized, targeted neurological examination to evaluate DSP signs, including diminished ability to recognize vibration and reduced sharp-dull discrimination in the feet and toes or reduced ankle reflexes. The presence of at least 1 sign bilaterally was considered to be evidence of DSP. Neuropathy symptoms were also assessed in the legs, feet, and toes, including bilateral neuropathic pain and dysesthesias (burning, aching, or shooting), paresthesia, and loss of sensation. Using a standardized form and a structured interview, clinicians classified neuropathic pain into the following 5 severity levels: none, slight (occasional, fleeting), mild (frequent), moderate (frequent, disabling), and severe (constant, daily, disabling, requiring analgesic medication or other treatment). A trained clinician conducted a comprehensive evaluation, assessing limb strength, and sensory and motor symptoms. Based on this evaluation, the cohort was categorized into two groups: individuals without neuropathy (DSP-) and those with neuropathy (DSP+).
4.3. Quantification of mtDNA and the Proportion of Mitochondria Carrying the CD
For each specimen, cell type, and treatment, we quantified total mtDNA and the RA of mitochondria carrying the CD in relationship to a cellular control by ddPCR (BioRadQX100TM). A total of 50 pg and 50 ng of DNA was used to quantify mtDNA and the CD respectively. mtDNA copy number was quantified by targeting the NADH dehydrogenase 2 (ND2) gene in relation to the Ribonuclease P protein subunit 30 (RPP30) gene, which appears in 2 copies per cell.
The following ZENTM Double-Quenched Probes (P1) were used:
RPP30 - P1 ATCCTCCCGCTTTGGCCTCC;
MT-ND2–P1 CCACATCATCGAAACCGCAAACA;
CD - TAAACACAAACTACCACCACCTCCCTCACCAT.
4.4. Immunoblot Analysis
DRG and the SuN from human donors were homogenized and fractionated using a buffer to separate the membrane and cytosolic fractions. Tissues (0.1 g) were homogenized in 0.7 mL of fractionation buffer containing phosphatase and protease inhibitor cocktails. After determination of the protein content of all samples by BCA Protein assay (Thermo Fisher Scientific, Rockford, IL), homogenates were loaded (20 mg total protein/lane), separated on 4-12 % Bis-Tris gels, and electrophoresed in 5 % HEPES running buffer, and blotted onto Immobilon-P 0.45 mm membrane using NuPage transfer buffer. The membranes were blocked and then incubated overnight at 4 ºC with primary antibodies.
4.5. Statistical Analysis
For statistical analysis, the number of mitochondria per compartment was averaged for each specimen. For statistical analysis, the Kruskal-Wallis test with Dunn’s post-test was used to compare group data. P<0.05 was considered statistically significant.
5. Conclusions
In conclusion, our investigation into mtDNA dynamics in peripheral nerve tissues from PWH tissue donors with DSP found a reduction in mtDNA copy number in both DRG and the SuN of individuals with DSP, suggesting a potential bidirectional relationship between mitochondrial dysfunction and sensory neuropathy, indicating that impaired mitochondrial function may contribute to, or result from, neurodegenerative processes in the context of DSP. The increased mtDNA RA carrying the CD mutation in the SuN compared to DRGs, particularly in individuals with DSP, highlights the cumulative effect of mtDNA alterations along the course of long nerves. This finding underscores the potential role of mitochondrial genome damage in the development and progression of DSP, with a specific impact on distal nerves. The regional disparity in the expression of mitochondrial ETC complex proteins further emphasizes the complexity of mitochondrial involvement in the pathophysiology of DSP, suggesting a compensatory mechanism within the DRG in response to mitochondrial dysfunction.
The clinical implications of our study suggest that preserving mitochondrial integrity may represent a potential therapeutic target for managing DSP. Strategies aimed at protecting mitochondrial function and preventing mtDNA damage could be explored to mitigate the development and progression of neuropathic symptoms. Furthermore, understanding regional differences in ETC protein expression provides insights into challenges associated with mitochondrial transport within neurons, highlighting potential therapeutic approaches to address specific neuropathic symptoms in DSP.
Exploring the functional consequences of molecular alterations and investigating the direct impact of specific antiretroviral drugs on mitochondrial function could provide a more comprehensive understanding of the underlying mechanisms. Ultimately, our study contributes to the growing body of knowledge aiming to unravel the intricate relationship between HIV, mitochondrial dynamics, and the development of sensory neuropathy, paving the way for potential targeted therapeutic interventions in the future.
Author Contributions
J.A.F. JPS, SM, and R.J.E. conceived the hypothesis, methods, and analyses, contributed to the statistical analysis, and obtained funding. JAF and JPS performed tissue processing and analyses. A.B. and M.S. assisted in statistical analysis, and visualization, drafted and finalized the manuscript. All authors edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Mental Health, grant number MH128108 to JAF.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the National NeuroAIDS Tissue Consortium (Institutional Review Board #080323) for studies involving humans.
Informed Consent Statement
All subjects provided written consent to participate in the study. No individual-protected health information is included in this analysis.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the HIV Neurobehavioral Research Center (HNRC) repository upon reasonable request.
Acknowledgments
We are immensely appreciative of the study subjects and the clinical staff that made this work possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Collaboration ATC. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293-9. [CrossRef]
- Autenrieth CS, Beck EJ, Stelzle D, Mallouris C, Mahy M, Ghys P. Global and regional trends of people living with HIV aged 50 and over: Estimates and projections for 2000-2020. PLoS ONE. 2018;13(11):e0207005. [CrossRef]
- Tavasoli A, Gelman BB, Marra CM, Clifford DB, Iudicello JE, Rubin LH; et al. Increasing Neuroinflammation Relates to Increasing Neurodegeneration in People with HIV. Viruses. 2023;15(9). [CrossRef]
- Lucas S, Nelson AM. HIV and the spectrum of human disease. J Pathol. 2015;235(2):229-41. [CrossRef]
- Keswani SC, Pardo CA, Cherry CL, Hoke A, McArthur JC. HIV-associated sensory neuropathies. AIDS. 2002;16(16):2105-17. [CrossRef]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4(9):543-55. [CrossRef]
- Cornblath DR, Hoke A. Recent advances in HIV neuropathy. Curr Opin Neurol. 2006;19(5):446-50. [CrossRef]
- Vafaei AA, Safakhah HA, Jafari S, Tavasoli A, Rashidy-Pour A, Ghanbari A; et al. Role of Cannabinoid Receptors in Crocin-Induced Hypoalgesia in Neuropathic Pain in Rats. J Exp Pharmacol. 2020;12:97-106. [CrossRef]
- Jazebi N, Evans C, Kadaru HS, Kompella D, Raji M, Fang F; et al. HIV-related Neuropathy: Pathophysiology, Treatment and Challenges. J Neurol Exp Neurosci. 2021;7(1):15-24. [CrossRef]
- Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP; et al. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21(8):2808-19. [CrossRef]
- Holzinger ER, Hulgan T, Ellis RJ, Samuels DC, Ritchie MD, Haas DW; et al. Mitochondrial DNA variation and HIV-associated sensory neuropathy in CHARTER. J Neurovirol. 2012;18(6):511-20. [CrossRef]
- Rozzi SJ, Avdoshina V, Fields JA, Trejo M, Ton HT, Ahern GP; et al. Human Immunodeficiency Virus Promotes Mitochondrial Toxicity. Neurotox Res. 2017;32(4):723-33. [CrossRef]
- Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J Peripher Nerv Syst. 2001;6(1):14-20. [CrossRef]
- Ganta KK, Chaubey B. Mitochondrial dysfunctions in HIV infection and antiviral drug treatment. Expert Opin Drug Metab Toxicol. 2019;15(12):1043-52. [CrossRef]
- Fields JA, Ellis RJ. HIV in the cART era and the mitochondrial: Immune interface in the CNS. Int Rev Neurobiol. 2019;145:29-65. [CrossRef]
- Lehmann HC, Chen W, Borzan J, Mankowski JL, Höke A. Mitochondrial dysfunction in distal axons contributes to human immunodeficiency virus sensory neuropathy. Ann Neurol. 2011;69(1):100-10. [CrossRef]
- Selvaraj S, Ghebremichael M, Li M, Foli Y, Langs-Barlow A, Ogbuagu A; et al. Antiretroviral therapy-induced mitochondrial toxicity: Potential mechanisms beyond polymerase-γ inhibition. Clin Pharmacol Ther. 2014;96(1):110-20. [CrossRef]
- Kwong JQ, Beal MF, Manfredi G. The role of mitochondria in inherited neurodegenerative diseases. J Neurochem. 2006;97(6):1659-75. [CrossRef]
- Nadalutti CA, Ayala-Peña S, Santos JH. Mitochondrial DNA damage as driver of cellular outcomes. Am J Physiol Cell Physiol. 2022;322(2):C136-C50. [CrossRef]
- Roca-Bayerri C, Robertson F, Pyle A, Hudson G, Payne BAI. Mitochondrial DNA Damage and Brain Aging in Human Immunodeficiency Virus. Clin Infect Dis. 2021;73(2):e466-e73. [CrossRef]
- Phillips AF, Millet AR, Tigano M, Dubois SM, Crimmins H, Babin L; et al. Single-Molecule Analysis of mtDNA Replication Uncovers the Basis of the Common Deletion. Mol Cell. 2017;65(3):527-38.e6. [CrossRef]
- Cortopassi GA, Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990;18(23):6927-33. [CrossRef]
- Linnane AW, Baumer A, Maxwell RJ, Preston H, Zhang CF, Marzuki S. Mitochondrial gene mutation: The ageing process and degenerative diseases. Biochem Int. 1990;22(6):1067-76. [PubMed]
- Yen TC, Su JH, King KL, Wei YH. Ageing-associated 5 kb deletion in human liver mitochondrial DNA. Biochem Biophys Res Commun. 1991;178(1):124-31. [CrossRef]
- Roda RH, Bargiela D, Chen W, Perry K, Ellis RJ, Clifford DB; et al. Large Mitochondrial DNA Deletions in HIV Sensory Neuropathy. Neurology. 2021;97(2):e156-e65. [CrossRef]
- Yang Y, Liu Y, Zhu J, Song S, Huang Y, Zhang W; et al. Neuroinflammation-mediated mitochondrial dysregulation involved in postoperative cognitive dysfunction. Free Radic Biol Med. 2022;178:134-46. [CrossRef]
- Ho DH, Je AR, Lee H, Son I, Kweon HS, Kim HG; et al. LRRK2 Kinase Activity Induces Mitochondrial Fission in Microglia via Drp1 and Modulates Neuroinflammation. Exp Neurobiol. 2018;27(3):171-80. [CrossRef]
- Hao S. The Molecular and Pharmacological Mechanisms of HIV-Related Neuropathic Pain. Curr Neuropharmacol. 2013;11(5):499-512. [CrossRef]
- Roda RH, Hoke A. Chapter Four - Mitochondrial dysfunction in HIV-induced peripheral neuropathy. In: Fernyhough P, Calcutt NA, editors. International Review of Neurobiology. 145: Academic Press; 2019. p. 67-82. [CrossRef]
- Pérez-Matute P, Pérez-Martínez L, Blanco JR, Oteo JA. Role of Mitochondria in HIV Infection and Associated Metabolic Disorders: Focus on Nonalcoholic Fatty Liver Disease and Lipodystrophy Syndrome. Oxidative Medicine and Cellular Longevity. 2013;2013:493413. [CrossRef]
- Fields JA, Serger E, Campos S, Divakaruni AS, Kim C, Smith K; et al. HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiology of Disease. 2016;86:154-69. [CrossRef]
- Ferrari LF, Chum A, Bogen O, Reichling DB, Levine JD. Role of Drp1, a Key Mitochondrial Fission Protein, in Neuropathic Pain. The Journal of Neuroscience. 2011;31(31):11404-10. [CrossRef]
- Chang Y-W, Tony Yang T, Chen M-C, Liaw Yg, Yin C-F, Lin-Yan X-Q; et al. Spatial and temporal dynamics of ATP synthase from mitochondria toward the cell surface. Communications Biology. 2023;6(1):427. [CrossRef]
- Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: Insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst. 2001;6(1):21-7. [CrossRef]
- Wang Y, Li N, Zhang X, Horng T. Mitochondrial metabolism regulates macrophage biology. J Biol Chem. 2021;297(1):100904. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).