1. Introduction
The prevalence of cancer in the general population of the United States is on the rise due to the continued improvement in cancer survivorship [
1]. This improvement has been mainly attributed to advances in surgical adjuncts and techniques, which have allowed for safer and more comprehensive surgery, improvements in conformal radiotherapy (RT) techniques, and the emergence of effective targeted anticancer therapeutic agents for most cancer types [
2,
3,
4]. This improved cancer survivorship has also led to an increased incidence of spinal metastases (SM), which is becoming epidemiologically significant [
1].
The spine is currently the third most common location for metastatic disease after the lungs and liver [
5]. A large autopsy series by Gomez et al. found that up to 80% of patients who died of cancer had evidence of SM, and up to 70% of patients with metastatic cancer were shown to have SM in a more recent review [
6,
7]. Ninety percent of SM originate from primary cancers of the lung, breast, prostate, or kidney [
4,
8]. The thoracic spine segment is most frequently affected (60-80%), followed by the lumbosacral spine (15-30%), and the cervical spine (10-15%) [
9].
SM can become symptomatic from mechanical back or neck pain, compression of the spinal cord and neural elements, or lead to the development of pathologic vertebral compression fractures (VCF) and/or spinal instability [
7,
10]. The latter two often result in debilitating mechanical pain with disabling neurological symptoms and sensorimotor dysfunction. This can lead to worsened overall prognosis and markedly decreased quality of life [
7,
10,
11]. Patchell’s sentinel trial in 2005 established the surgical standard of care for SM as surgical decompression followed by adjuvant RT with the goal of restoring neurological function [
12]. More recently, this treatment paradigm has evolved to circumferential tumor debulking with or without instrumented fusion for the goal of spinal decompression to create a safe distance between the tumor and the spinal cord for subsequent spinal stereotactic RT [
13]. This approach, coined “separation surgery,” was primarily adapted as a palliative modality, allowing for patients to resume systemic treatments earlier. Its minimally invasive footprint reduces intraoperative and postoperative complications in terminally ill and/or frail cancer patients while restoring and/or preserving neurological function [
14,
15,
16,
17]. Posterior decompression is a less invasive, yet time-proven method for decompression of neural elements. However, posterior decompression even with instrumentation can fail to maintain spinal alignment and result in poor functional outcome in a select cohort of SM patients (
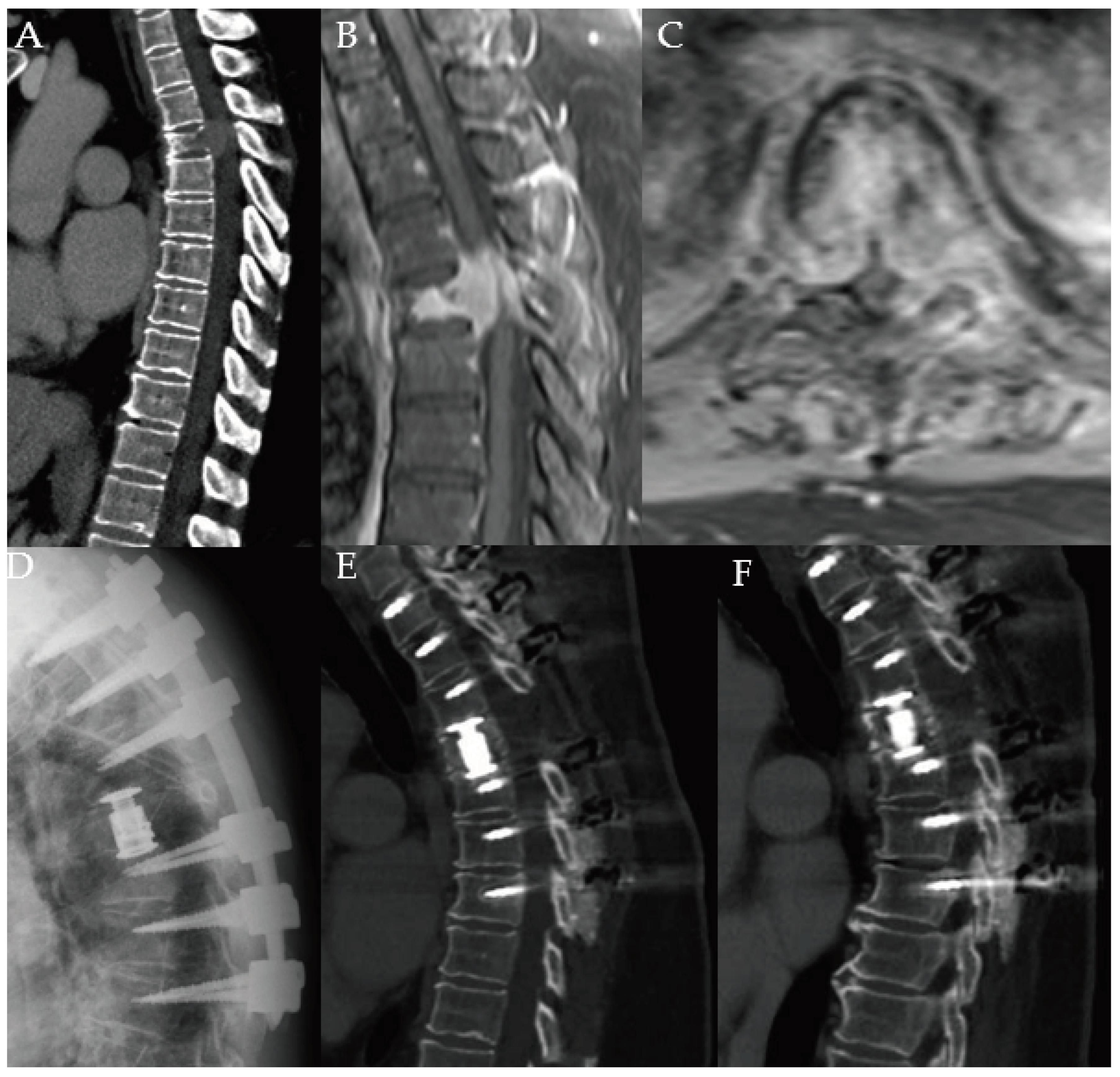
Figure 1).
Given the continued advances in cancer survivorship, this treatment paradigm may need to be revisited. While preserving and restoring neurological function remains a primary goal of spinal oncology surgery, maximal tumor debulking with excision of part or all of the vertebral body (corpectomy) for improved local disease control, restoration of spinal alignment and spinal stabilization with the goal of long-term pain relief, functional independence, and improved disease-free survival may need to be taken into account in a select cohort of patients [
9,
18,
19]. Corpectomy of one or more levels may prove indicated and necessary where other less invasive treatment modalities fail leading to gradually worsening clinical outcome (
Figure 2). In oligometastatic spinal disease (< 5 sites of metastases, patients with good functional status and favorable long-term prognosis, this approach would target long-term spinal stabilization, reduce the risk of local tumor recurrence, and reduce the need for further surgical intervention. However, the evidence concerning this matter is still pending [
12].
Herein, this paper presents a retrospective, single-institutional series of 63 consecutive patients with SM who underwent corpectomy and instrumentation for decompression of neural elements and spinal column reconstruction. Treatment algorithm, patient survival and neurological outcomes, and complications are analyzed.
2. Materials and Methods
2.1. Institutional Setting
This study was approved by the University of Iowa institutional review board (IRB # 201902751). A retrospective review of hospital records was performed for patients who underwent surgical intervention with corpectomy, with or without posterior instrumentation, for the management of spinal metastatic disease from January 2005 to August 2023. The requirement for informed consent was waived by the IRB for all subjects due to the retrospective study design. The study was conducted at the University of Iowa Hospitals and Clinics, an academic tertiary care facility with a level 1 trauma center. Information was obtained from patients seen in the Departments of Neurosurgery and Orthopedics and Rehabilitation. Chart records were obtained from the EPIC (Epic Systems Corporation, Madison, WI) electronic medical record.
2.2. Data Collection
Sixty-three patients were included in this study with no exclusion criteria applied. Data collected included demographics, tumor characteristics, clinical and radiographic features, treatment modalities, and long-term neurological and survival outcomes. Radiological evaluations included plain weight-bearing radiographs, computed tomography (CT), and magnetic resonance imaging (MRI). The evaluated radiographic characteristics included lesion location and appearance, extent of bony destruction/deformity and presence of instability as quantified by the spinal instability neoplastic score (SINS). Prognosis was graded with the Tomita and modified Tokuhashi scoring systems.
Surgical treatment modalities included anterior or posterior corpectomy with or without preoperative tumor embolization, cement augmentation, and/or instrumentation with lateral plating and/or posterior pedicle screws. The decision for cement augmentation was based on the biomechanical integrity of the cancellous bone based on available preoperative CT and bone mineral density scans. Pedicle screw instrumentation used titanium alloy screws with cement augmentation capabilities.
Primary tumor histology, local tumor recurrence, and postoperative complications were recorded along with their corresponding management. Surgical details such as operative time, estimated blood loss (EBL), and the need for transfusion were collected. Neurological status was documented with pre- and postoperative American Spinal Cord Injury Association Impairment Scale (ASIA) scores, and functional status was documented with pre- and postoperative Karnofsky performance scores (KPS) [
20,
21]. Health-related quality of life outcomes was assessed with the visual analogue pain scale (VAS), Oswestry Disability Index (ODI), and RAND SF-36 survey. Information on neoadjuvant and adjuvant therapies, such as chemotherapy, RT, and chemoradiation was collected. Overall survival (OS), progression-free survival (PFS), and follow-up duration was collected.
2.3. Statistical Methods
Descriptive statistics were used to describe patient demographics, tumor characteristics, clinical course, and treatment factors. Continuous variables were expressed with mean, median, standard deviation, and range where applicable. Categorical variables were described as frequencies with percentages as appropriate. GraphPad Prism 9 (Dogmatics LLC, San Diego, CA, USA) was used for quantitative analysis. Categorical variables were compared using the Fisher’s exact and chi-square tests and numerical variables were analyzed using the Mann-Whitney U test. Survival analysis was performed using Kaplan-Meier (KM) estimation and/or Pearson’s correlation coefficient. OS was calculated from the date of initial surgery to the date of death as reported in patient medical records. PFS was calculated from the date of initial surgery to the date of detection of tumor recurrence or progression on surveillance imaging. Patients who were not documented as deceased or had no tumor recurrence or progression were censored from the analysis of OS and PFS, respectively. Results were deemed significant at P-value < 0.05.
3. Results
3.1. Patient Demographics
Sixty-three patients who underwent corpectomy for SM were included (
Table 1). The mean age at presentation was 63.5 ± 9.5 years (range, 37-84 years) and 28 patients (44.4%) were female. Forty-nine patients (77.8%) had an established cancer diagnosis at presentation, and 36 (57.1%) had previously received treatment for their primary cancer.
All patients were symptomatic at presentation, most often with mechanical back or neck pain (n=54, 85.7%), followed by sensorimotor symptoms (n=35, 55.6%). Of the patients presenting with neurological symptoms, thirty-one (49.2%) had epidural spinal cord compression (ESCC). Eleven patients (17.4%) underwent emergent spinal cord decompression surgery, and 3 (4.7%) underwent emergent RT.
3.2. Baseline Oncologic History and Spinal Metastatic Presentations
The most common primary cancers were renal cell carcinoma (RCC), breast cancer, and lung cancer (
Figure S1A). SM most often occurred in the thoracic spine (n=37, 58.7%), followed by the lumbar (n=15, 23.8%) and cervical spine (n=11, 17.5%) (
Figure S1B). Fifty-six patients (88.9%) had SM at one level, five (7.9%) at two levels, and one (1.6%) at three and four levels, each (
Figure S1C). On presentation, 31 patients (49.2%) had extraspinal metastases (ESM) (
Figure S1D), with a median of 2 ESM (range, 1-4) (
Table 1).
3.3. Neoadjuvant and Adjuvant Therapy
Thirty-one patients (49.2%) underwent neoadjuvant therapy for SM. Seven patients (11.1%) underwent chemotherapy, 9 (14.2%) RT, and 15 (23.8%) chemoradiation (
Table 2). Twelve patients (19.0%) did not receive adjunctive therapy. Fourteen (22.2%) underwent chemotherapy, 20 (31.7%) RT, and 17 (27.0%) chemoradiation.
3.4. Surgical Management
All patients underwent surgical treatment with corpectomy for ventral decompression and anterior column reconstruction, with or without posterior instrumentation dependent on the approach. Preoperative tumor embolization was performed for selected patients with hypervascular SM, particularly RCC. Twenty-two patients (34.9%) underwent preoperative embolization of their SM, in a window of 24-36 hours prior to surgery; this did not significantly impact any outcomes in this subgroup (
Table S1).
Thirty-three patients (52.4%) underwent corpectomy with a posterior approach, and 30 (47.6%) with an anterior approach (
Table 2). In the anterior approach, 17 patients (27.0%) received posterior instrumentation and 13 (20.6%) did not. All patients that underwent the posterior approach also received posterior instrumentation. Corpectomy was performed at levels concordant with the location of the SM (
Figure S1C). A median of 5 spinal levels were instrumented (range, 2-13 levels) during surgery. In only 2 patients (3.2%), one cervical and one lumbar, surgery was staged with anterior corpectomy separated by posterior instrumentation by 2 and 5 days, respectively. Intraoperative navigation with C-Arm fluoroscopy was used in all patients. Additional intraoperative navigation with the O-Arm/Stealth system (Medtronic, Minneapolis, MN) was used in 3 cases (4.8%).
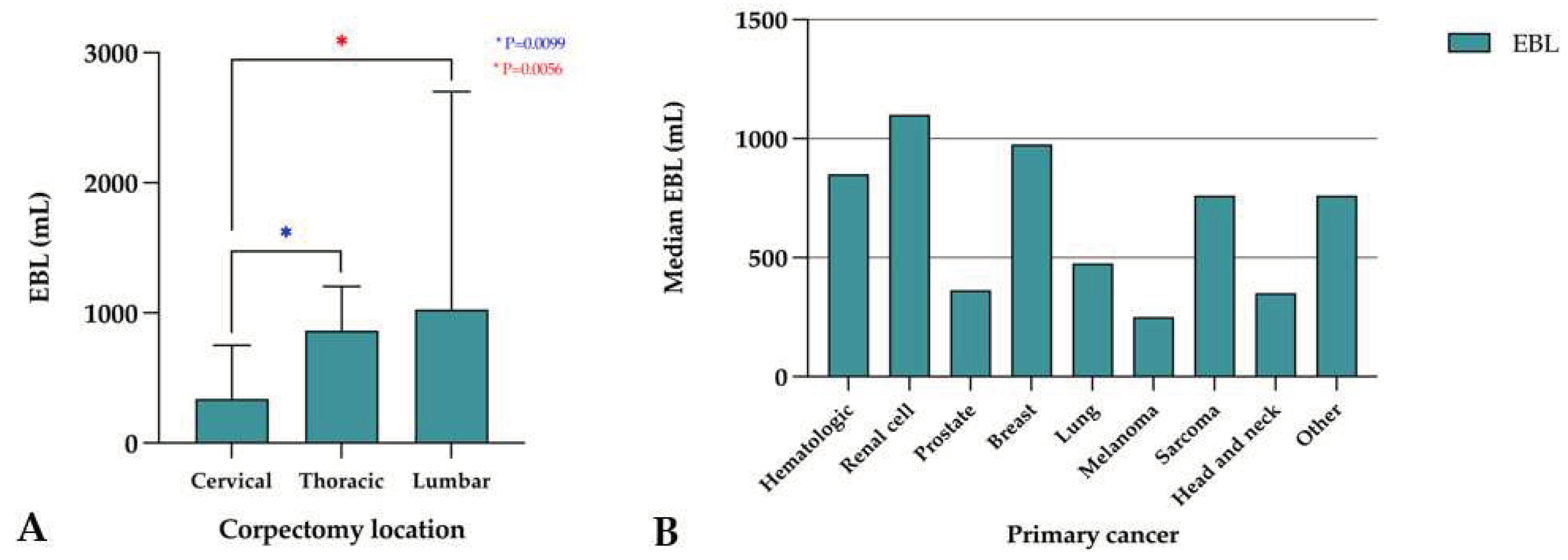
Overall, the median intraoperative EBL was 800 mL (range, 100-35,200 mL). EBL was highest in the lumbar spine (median 1025 mL, range, 250-8800 mL), followed by the thoracic (median 860 mL, range, 100-35,200 mL) and cervical spine (median 337.5 mL, range, 150-1000 mL). There was a significant difference in EBL between the cervical and thoracic spine (p=0.010) and the cervical and lumbar spine (p=0.006), but not the thoracic and lumbar spine (p=0.395) (
Figure 3A). The median EBL stratified by primary cancer is described in
Figure 3B. Twenty-eight patients (45.2%) required blood transfusion, with a median of 650.0 mL (range, 300.0-7975.0 mL) of blood products transfused. The median duration of surgery was 327.0 minutes (range, 111-830 minutes) and the median length of stay (LOS) for postoperative recovery was 6.0 days (range, 1-28 days).
3.5. Outcomes and Survival
3.5.1. Survival Analysis
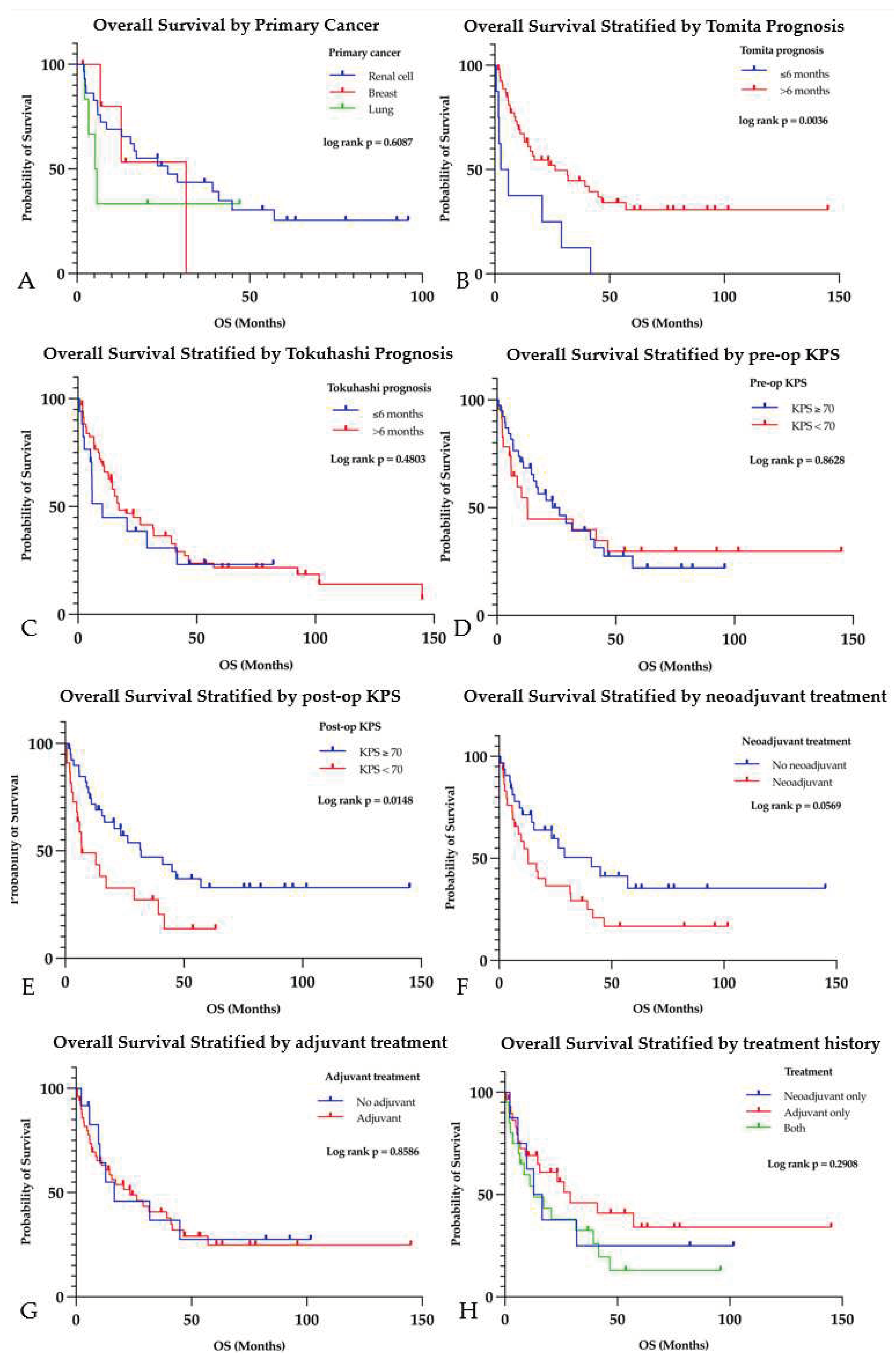
The median OS in this cohort was 14.4 months (range, 0.6-145.0 months) with a median PFS of 268.5 days (range, 10-4351 days) (
Table 3). The median follow-up time was 15.5 months (range, 0.5-145.0 months). As of August 2023, 40 patients (63.5%) had succumbed to complications from cancer. The 3 most common primary cancer pathologies that patients succumbed to were RCC (n=21), lung cancer (n=4), and breast cancer (n=3). The median OS for these cancers, stratified by tumor pathology, was as follows: 26.3 months (range, 1.9-95.9 months) for RCC, 5.5 months (range, 2.2-47.1 months) for lung cancer, and 31.6 months (range, 1.6-31.6 months) for breast cancer (p=0.609) (
Figure 4A). The presence of ESM negatively affected survival; patients with no ESM had a median OS of 46.6 months (range, 1.6-145.0 months) compared to 11.1 months (range, 0.6-57.1 months) for patients with ESM (p<0.001). Having multiple ESM (≥ 2) (median OS 6.7 months, range, 0.6-57.1 months) worsened survival compared to patients with one or no ESM (median OS 31.9 months, range, 1.6-145.0 months) (p=0.002).
Analysis of OS as stratified by the Tomita and modified Tokuhashi prognostication scores was also performed. The median Tomita and Tokuhashi scores were 4 (range, 2-10) and 11 (range, 3-15), respectively. Among patients with a prognosis of ≤ 6 months by Tomita score, the median OS was 4.3 months (n=8, range, 0.6-41.7 months). Patients with a prognosis of > 6 months had a median OS of 26.3 months (n=55, range, 0.6-145.0 months), which was significantly higher (p=0.004) (
Figure 4B). By the Tokuhashi scoring system, patients with a prognosis of ≤ 6 months had a median OS of 10.3 months (n=17, range, 0.6-82.3 months), and patients with a prognosis of > 6 months had a median OS of 17.2 months (n=46, range, 0.6-145.0 months (p=0.480) (
Figure 4C).
While preoperative KPS did not have a significant impact on OS, patients with a postoperative KPS ≥ 70 had a median OS of 31.9 months (n=41, range, 1.6-145.0 months) compared to 6.8 months (n=22, range, 0.6-63.3 months) in those with a postoperative KPS < 70 (p=0.012) (Figure 4D-E). Stratifying survival by factors such as age, sex, ASIA score did not find significant differences (
Figure S2A-D).
3.5.2. Spinal Metastatic Disease Neoadjuvant and Adjuvant Therapy
The median OS for patients who received neoadjuvant therapy was 12.9 months (n=31, range, 0.6-101.6 months), compared to 41.0 months (n=32, range, 0.6-145.0 months) for those who did not (p=0.057) (
Figure 4F). When stratified by type of treatment, patients receiving only chemotherapy had a median OS of 11.1 months (n=7, range, 0.6-82.3 months), patients receiving only RT 31.6 months (n=9, range, 2.2-95.9 months), and patients who received chemoradiation 12.9 months (n=15, range, 1.9-101.6 months); these were not significantly different (p=0.544) (
Figure S3B). Neoadjuvant therapy did not impact PFS regardless of treatment type (
Figure S3A, C).
The median OS for patients who received adjuvant therapy was 23.3 months (n=51, range, 0.6-145.0 months), compared to 16.5 months (n=12, range, 2.2-101.6 months) for those who did not (p=0.859) (
Figure 4G). When stratified by type of adjuvant treatment, patients receiving only chemotherapy had a median OS of 23.3 months (n=14, range, 0.6-145.0 months), patients receiving only RT 29.0 months (n=20, range, 0.6-95.9 months), and patients who received chemoradiation 16.3 months (n=17, range, 2.4-53.7 months); these were not significantly different (p=0.310) (
Figure S3E). Adjuvant therapy did not impact PFS regardless of type (
Figure S3D,F).
Patients who received neoadjuvant therapy but not adjuvant therapy had a median OS of 14.7 months (n=8, range, 2.2-101.6 months), patients who received adjuvant therapy but not neoadjuvant therapy had a median OS of 29.0 months (n=28, range, 0.6-145.0 months), and patients who received both had a median OS of 12.9 months (n=23, range, 0.6-46.6 months) (p=0.291) (
Figure 4H). There was no significant difference in PFS between these groups (p=0.660) (
Figure S3G). Patients who did not receive any neoadjuvant or adjuvant therapies had a median OS of 27.6 months (n=4, range, 5.3-92.6 months).
3.5.3. Neurological, Functional, and Surgical Outcomes
Postoperatively, 47 patients (74.6%) had full recovery of neurological function within 90 days (
Table 3). None worsened postoperatively based on their ASIA score. No patients died within 30 days of surgery from causes related to their spinal surgeries.
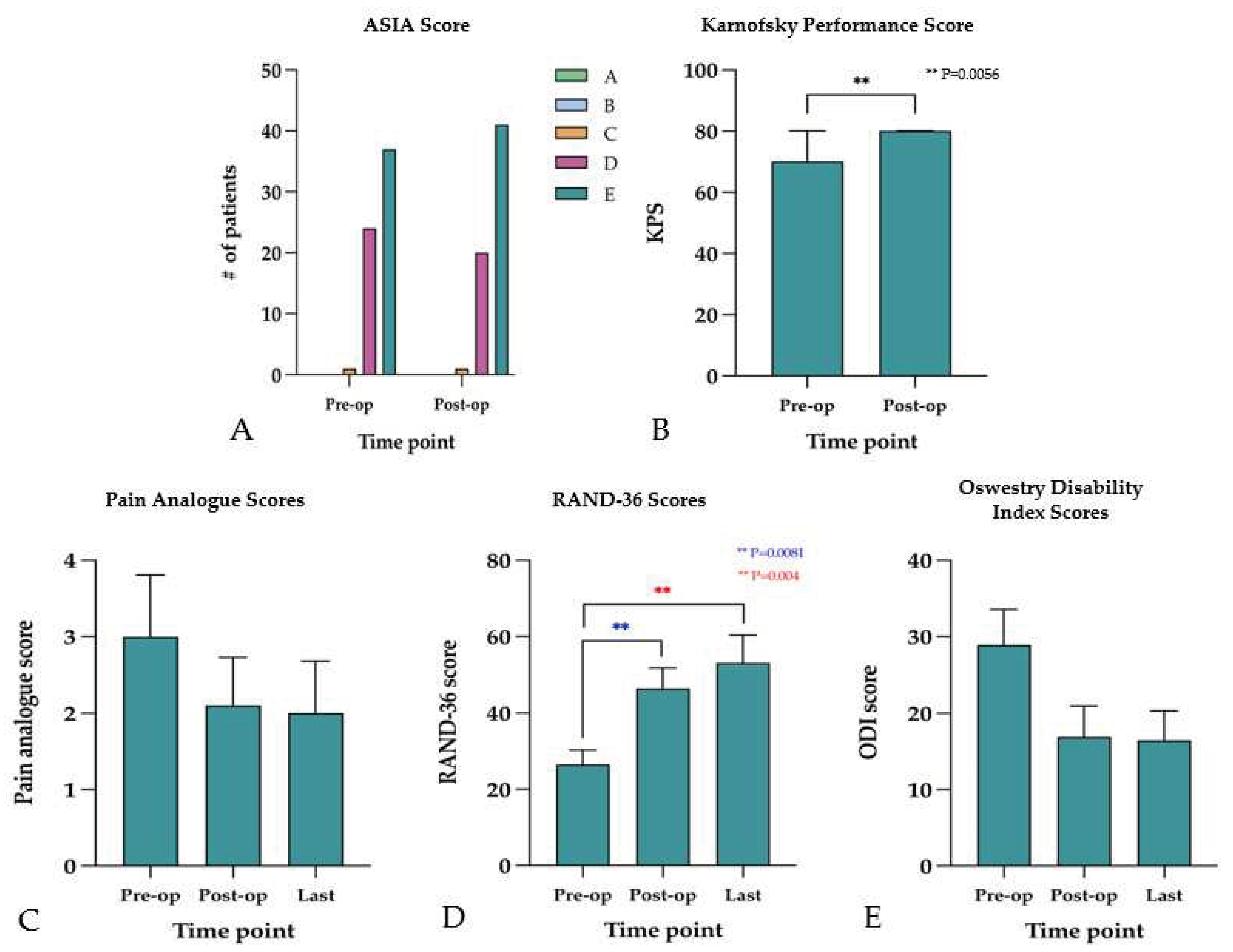
Neurological deficit, measured by ASIA score at presentation found 37 patients (58.7%) with grade E indicating normal neurological function, 25 (39.7%) with grade D, and 1 (1.6%) with grade C scores. Postoperatively, 41 patients (65.1%) had a grade E, 21 (33.3%) had a grade D, and 1 (1.6%) had grade C scores (
Figure 5A). Function, measured by KPS, found a median preoperative KPS of 70 (range, 20-90), which improved significantly (p=0.006) to 80 (range, 40-100) postoperatively (
Figure 5B).
Forty-five patients (71.4%) reported requiring narcotic analgesic use for pain management at their first postoperative follow-up. The mean preoperative score for pain measured with VAS was 3 ± 2.9. Postoperatively, the mean VAS score at first follow up was 2.1 ± 2.8 (p=0.382), and 2.0 ± 2.5 (p=0.350) at the last follow-up. Mean VAS score did not vary significantly between preoperative, postoperative, and last follow-up (p=0.580) (
Figure 5C). However, health-related quality of life, measured by the RAND-36 survey found a mean score of 26.5 ± 14.8 at initial presentation, which significantly improved (p=0.008) to 46.4 ± 24.4 at the first postoperative follow-up. Improvement continued in the follow-up period, with a mean RAND-36 score of 53.1 ± 29.9 at the last follow-up (p=0.004) (
Figure 5D). There was also an improvement in disability, measured by ODI, from presentation (28.9 ± 16.1) to first postoperative follow-up (16.9 ± 15.1) that trended toward significance (p=0.061); however, this improvement was statistically significant (p=0.049) in the long-term as the mean ODI score at last follow-up was 16.9 ± 15.9 (
Figure 5E).
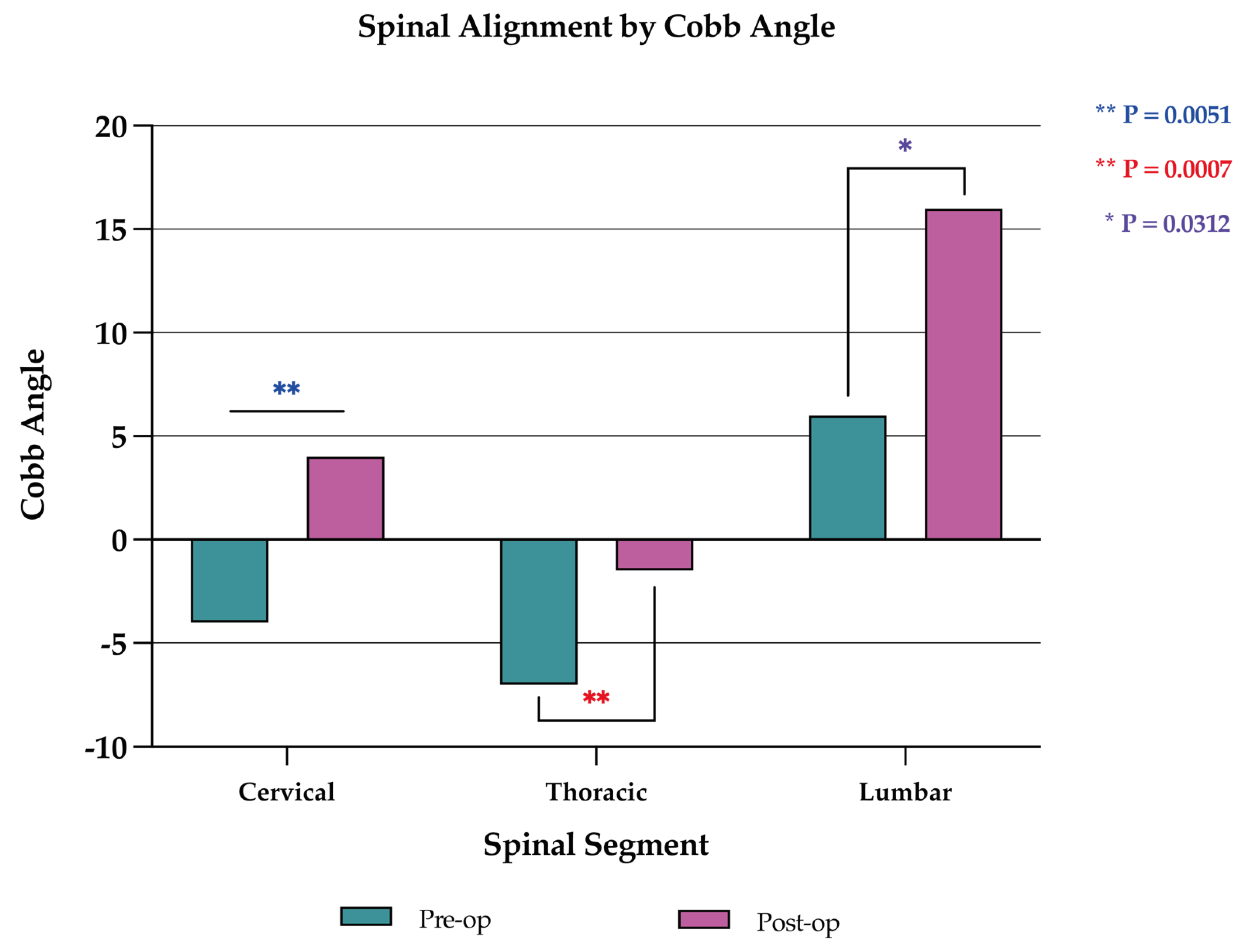
Corpectomy with anterior column reconstruction also improved spinal alignment, as spinal kyphotic deformity significantly improved with surgery. Spinal kyphotic deformity was measured with the Cobb angle. Spinal lordosis was denoted by a positive Cobb angle and kyphosis denoted by a negative Cobb angle. Overall, the median Cobb angle significantly changed from -5.0 (range, -40.0 to +36.0) to 0.0 (range, -24.0 to +38.0) after surgery (p=0.021). Stratification by spinal segment found a significant improvement (p=0.005) in the cervical spine from –4.0 (-25.0 to +17.0) to +4.0 (-10.0 to +18.0), in the thoracic spine (p=0.001) from –7.0 (-49.0 to +20.0) to –1.5 (-24.0 to +26.0), and in the lumbar spine (p=0.031) from +6.0 (-5.0 to +36.0) to +16.0 (-2.0 to +38.0) (
Figure 6).
Nine patients (14.2%) had postoperative complications, including wound dehiscence (n=4, 6.3%), wound infection (n=2, 3.2%), and other complications (n=3, 4.8%). These other complications were cerebrospinal fluid (CSF) leak, hemothorax, and enterospinal fistula (n=1 each, 1.6%). Seven patients (11.1%) required a total of 16 reoperations for surgical complications and/or disease progression. The most common indications for reoperation were wound infection (n=8, 12.7%), followed by wound dehiscence (n=4, 6.3%). Four patients (6.3%) required reoperation for other causes. These include enterospinal fistula, disease progression, hardware failure, and CSF leak (n=1 each, 1.6%).
Nine patients (14.2%) required readmission within 30 days of their initial surgery. Two patients (3.2%) were deceased within 30 days. Neither of these patients experienced postoperative surgical complications and neither died because of their surgery. Twenty-two patients (34.9%) died within a year of surgery.
4. Discussion
4.1. Introduction
The increasing prevalence of cancer has resulted in an increased incidence of SM, with up to 80% of cancer patients now exhibiting evidence of SM at autopsy [
6,
7]. While most SM are asymptomatic, some can present with severe mechanical back pain, compression of the spinal cord and neural elements, or lead to the development of spinal instability, which can significantly affect overall survival and health-related quality of life in cancer patients [
7,
10,
11].
Currently, the standard surgical treatment for symptomatic SM with ESCC is separation surgery. While the minimal footprint of separation surgery allows patients to resume systemic therapy earlier, the continued improvement in cancer survivorship means such an approach could predispose a select cohort of patients to redo surgeries and/or recurrent radiation treatments (due to local tumor recurrence). Furthermore, it can also result in significant long-term disability and loss of functional independence from chronic pain symptoms as it doesn’t restore spinal alignment and does not achieve circumferential stabilization, resulting in worsening kyphotic deformity and hardware failure (
Figure 1) [
9,
18,
19]. In oligometastatic spinal disease patients with good functional status and favorable long-term prognosis, corpectomy with fusion can allow for maximal tumor debulking for improved local disease control, restoration of spinal alignment, and spinal stabilization for long-term pain relief, functional independence, and improved disease-free survival (
Figure 7). This study examines corpectomy in selected SM patients for decompression of neural elements and spinal column reconstruction while focusing on the safety, efficacy, and outcomes.
4.2. Clinical Presentation and Diagnostic Workup
SM often presents in cancer patients in their 6-7th decade of life, as reflected in this cohort’s mean age of 63.5 years [
12,
13,
22]. Males are more frequently affected, with around 60% of patients with SM being male (55.6% in this cohort) [
13,
22]. The thoracic spine (60-80%) is the most commonly affected spinal segment followed by the lumbar (15-30%) and cervical (<10%) segments, which is consistent with this cohort’s tumor location composition [
9]. Rates of predilection for cancers to metastasize to the spine vary by study, especially with regards to rates of prostate and breast cancer as these are inherently affected by sex distribution. However, most literatures cite primaries from lung (21-23%), breast (7-12%), and prostate (8-19%) cancers, as most frequently metastasizing to the spine [
23,
24]. In contrast, this cohort most frequently had SM in RCC, followed by breast and lung cancer. Patients with SM also often develop ESM, which is a marker of poor long-term prognosis, with the prognosis often worsening with an increasing number of ESM [
13,
25]. Half of this cohort (n=31, 49.2%) had ESM, with a median of 2 ESM; patients with increased tumor burden and ESM had worse survival.
Symptomatic SM was historically the first manifestation of cancer in up to 50% of patients, but this number has dwindled significantly over the past decade [
26]. This cohort had 14 patients (22.2%) that received their first cancer diagnosis after presenting with symptomatic SM. Most SM are asymptomatic, but when symptoms do develop, most present with back pain and around half have neurological deficits [
7,
13,
27]. In this cohort, around 85.7% of patients presented with mechanical back or neck pain, and of these, over half had evidence of compression deformity on initial presentation.
Sensorimotor deficits compose most neurological deficits seen in SM, but bowel and bladder dysfunction may rarely occur [
13]. Despite over half of this cohort having some neurological deficits, most patients could ambulate with or without assistance. About one-fifth of SM patients present with sudden onset neurological compromise due to ESCC and/or VCF necessitating emergent surgery and/or RT [
28]. A higher SINS (≥ 7) also can predict an increased likelihood of VCF in patients receiving RT [
30,
31].
The Tomita and Tokuhashi scoring systems have been widely used to assist in estimating overall prognosis in patients with SM based on preoperative factors such as functional status, primary malignancy, and ESM [
32]. A Tomita score between 1-7 indicated a prognosis > 6 months, with higher numbers suggesting a worse prognosis. A Tokuhashi score above 9 indicated a prognosis > 6 months, with higher numbers suggesting a more favorable prognosis. Patients in this study tended to have good preoperative function, with only one patient having an ASIA grade of C, and a median preoperative KPS of 70 (range, 20-90). Selecting for patients with good preoperative function and prognosis to undergo more invasive surgeries, like corpectomy, ensures that risks are minimized and benefits from improved outcomes are appreciated in the long-term.
4.3. Management and Outcomes
Preoperative management varies by the patient’s clinical presentation and goals of treatment. The current standard of care treatment for SM with ESCC is separation surgery for circumferential tumor debulking followed by spinal stereotactic radiosurgery (SRS) to improve local tumor control. As the incidence of SM continues to increase linearly with improving cancer survivorship, however, there is an increasing focus on maximal tumor debulking; particularly in patients with oligometastatic spinal disease and good functional status. Improvements in perioperative care, surgical techniques, and intraoperative navigation have made it safer to perform more invasive surgical interventions like corpectomy with reduced complication risk [
14,
33]. This allows for maximal tumor resection while preserving function, reducing pain, and improving spinal alignment and stability.
4.3.1. Adjuvant Treatment
Traditionally, RT been broadly used as an adjuvant for SM [
28]. RT aims to control pain, decrease tumor burden and spillage, and decompress neural structures [
28]. Conventional external-beam radiotherapy (EBRT) is most often used, and 60-70% of patients have a partial or complete response with pain reduction and functional improvement [
34,
35]. SRS may also be used to deliver high doses of radiation precisely to the SM without injuring important adjacent organs. It allows for greater local disease control than EBRT and has efficacy against EBRT-resistant tumors [
28]. Similarly, systemic medical therapies have also shown promise in treating SM [
36]. Patients that received neoadjuvant therapy in this cohort had a lower OS, likely due to these patients having more advanced disease. However, survival for patients that received adjuvant therapy was higher.
4.3.2. Surgical Management
Preoperative embolization of spinal tumors, particularly hypervascular tumors, is commonly done to reduce intraoperative bleeding [
37]. RCC was the most frequently embolized SM in this cohort, which in consistent with current practices [
38]. The literature on preoperative embolization for SM shows mixed findings on whether it can decrease intraoperative bleeding. In our subgroup of RCC patients, patients treated with preoperative embolization had a lower mean EBL (1835.8 ± 2119.4 mL) compared to RCC patients without preoperative embolization (4902.5 ± 10,786.4 mL), though this was not found to be significant (p=0.9369), likely due to the cohort being underpowered. Moreover, preoperative embolization reduced average operative time by 40 minutes, and there was a near significant reduction in length of stay in these patients (5 days, range, 3-13 days) compared to patients that did not receive embolization (8 days, range, 4-14 days) (p=.0591)
All patients in this cohort underwent corpectomy with a posterior or anterior approach. Given the goal of attaining circumferential spinal stabilization and improving spinal alignment in addition to tumor resection, all but 13 patients also underwent posterior instrumentation. The standard approach to supplementing corpectomy with posterior pedicle screws was to place them 2 level(s) above and below the level of the corpectomy, with further instrumentation at the discretion of the surgeon [
39].
4.3.3. Outcomes
Most patients in this study had partial or full neurological recovery after surgery, and all patients either maintained or improved on their ASIA scores. Functional and health-related quality of life metrics, quantified by KPS and RAND-36, improved significantly postoperatively. The VAS and ODI quality of life metrics also improved postoperatively, though the p-value did not reach significance. Additionally, the improvement in postoperative KPS (with scores of ≥ 70) correlated with improved OS, as has been previously reported in other series [
13]. Interestingly, age, sex, preoperative KPS, and pre- and postoperative ASIA score did not correlate with OS. Of note, this is one of the first studies examining both neurological and quality of life outcomes in patients undergoing corpectomy for SM. These results are reassuring that it is a safe and viable alternative to separation surgery for SM in select group of patients [
7,
13,
14,
21,
22,
27,
33,
42,
44,
45,
46,
47,
48,
49].
A goal of this study was to determine if corpectomy improved spinal alignment and instability in the immediate and long term. This study revealed that corpectomy for SM significantly improves spinal kyphotic deformity in all spinal segments and allows for long-term correction as measured by Cobb angle (
Figure 7). While there is literature in deformity spine surgery that supports the use of corpectomy for improving spinal kyphotic deformity, this study serves to further corroborate that these previously reported findings also apply to SM patients [
50,
51].
The disease course of SM stemming from a variety of cancers presents a challenge in comparing survival across different primary pathologies. The one-year mortality rate in this study (34.9%) is lower than what is previously reported in other series, although the OS is comparable [
7,
13]. In terms of primary tumor histology, this cohort showed a significant difference in survival across the three most common cancers treated (RCC, breast cancer, and lung cancer). Lung cancer patients had a much lower OS than other cancers, concordant with the literature [
52,
53].
4.3.5. Complications
As corpectomy surgery for the treatment of SM has more risks compared to a less invasive separation surgery, this study sought to establish the safety of corpectomy surgery to justify its advantages. In this cohort, the mean length of stay (LOS) was 6 days (range, 1-28 days). This is comparable to reported postoperative admission times for SM patients undergoing simple decompression or separation surgery as reported by Azad et al. (6.9 days) and slightly lower than the median LOS reported by Patchell et al. [
12,
14]. Traditionally, complication rates are higher in patients undergoing corpectomy compared to separation surgery, but both surgeries have wide ranges reported in the literature. The complication rate in this study (n=9, 14.2%) was lower than the corpectomy complication rates reported by Gokaslan et al. (29.2%) and Azad et al. (45.6%), as well as the separation surgery/decompression complication rates reported by Azad et al. (29.0%) and Patchell et al. (40.0%) [
7,
12,
14]. It was, however, slightly higher than the complication rates reported for separation surgery by Silva et al. (13.9%) and Xu et al. (3.5%) [
40,
41]. No patients had long-term surgical complications nor did any patients die within 30 days of surgery for reasons related to their surgery.
Examining the literature of surgical tumor excision and instrumented stabilization for SM, the most common surgical complications were wound infection/dehiscence (8%), pulmonary complications (4%), instrument failure (4%), deep vein thrombosis/pulmonary embolism (2%), and CSF leak (2%) [
42]. These complications were similarly represented in this cohort, with most complications stemming from surgical site infection (SSI) or wound dehiscence (9.5%). Reoperations for complications were similarly most frequent for wound infection (6.3%) and wound dehiscence (6.3%). One patient each also had reoperation for enterospinal fistula, disease progression, hardware failure, and CSF leak in comparable proportions to the literature [
42]. A retrospective review by Sebaaly et al. focused on postoperative SSI for patients with operatively treated SM and found an incidence of 5.1% [
43]. Risk factors for developing SSI included smoking, higher body mass index, higher number of SM, higher number of fused vertebrae, intraoperative bleeding ≥ 2000 mL, and neurological deterioration [
43]. These factors should be considered during patient selection and postoperative management.
The 30-day readmission (14.2%) and mortality (3.2%) rates in this cohort is lower than what has been previously reported in the literature [
7,
14]. Notably, two patients died within one month of their spinal surgery. A 78-year-old male with marginal zone lymphoma diagnosed 3 years prior and treated with chemotherapy developed arm and shoulder pain from SM. This was treated with an uncomplicated C5 corpectomy with a carbon-fiber reinforced polymer graft and anterior plating, and histopathological diagnosis yielded squamous cell carcinoma from a lung primary. Further workup found multiple metastases to the brain, liver, and skeleton. He started palliative RT to the whole brain and lung but died 2 weeks after surgery while receiving treatment due to unknown causes. The second patient, a 69-year-old female with a 50-year history of smoking presented with 6 months of back pain. The patient underwent an uncomplicated T9 corpectomy with T7-T11 posterior instrumented fusion, and histopathological diagnosis yielded metastatic large cell neuroendocrine carcinoma from a lung primary. Further workup found multiple lesions to the skeleton and liver. Owing to her poor prognosis, the patient opted for hospice care and did not receive additional treatment. She survived for 2 weeks postoperatively before succumbing to her primary cancer. It is important to acknowledge that these patients were poor surgical candidates and should not have undergone surgery.
This study is not without limitations. The retrospective nature of this study, done at a single-center academic institution, limits the generalizability of its findings. Surgical techniques varied be-tween patients based on tumor location, characteristics, and preoperative imaging. Most study patients had metastatic spread from renal cell carcinoma, so the results and implications of the study may best apply to patients with this specific malignancy. Similarly, nearly all patients in the study were of non-Hispanic white ethnicity. Future research should focus on larger, multicenter studies with long-term follow-up to further elucidate the benefits and drawbacks of corpectomy for patients with SM.
5. Conclusions
In conclusion, this study highlights the evolution of SM management in the setting of improved cancer survivorship. Tumor resection, spinal cord decompression, and circumferential stabilization with corpectomy is viable option for patients with SM, particularly in those with oligometastatic disease, kyphotic deformity, good functional status, and favorable prognosis. Moreover, this study suggests that corpectomy for decompression of neural elements and spinal column reconstruction is a relatively safe intervention with minimal morbidity and favorable neurological and health-related quality of life outcomes in a select cohort of cancer patients with SM.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
Conceptualization, P.H., N.T., and A.C.; methodology, P.H., N.T., and A.C.; data curation, A.C., J.V.; investigation, A.C., N.T., S.L., J.V., and D.C.; project administration, N.T. and A.C.; supervision, P.H. and N.T.; validation, N.T., J.V., and P.H.; visualization, A.C., S.L., and M.P.; writing – original draft, A.C., N.T., S.L., J.V., D.C., C.L.; writing – review & editing, A.C., N.T., M.C., M.P., H.O., and P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
IRB # 201902751. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of The University of Iowa for studies involving humans.
Informed Consent Statement
Patient consent was waived by the Institutional Review Board for all patients.
Data Availability Statement
The data presented in this study are available within this article and its supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Jellinger, K.; Kothbauer, P.; Sunder-Plassmann, E.; Weiss, R. Intramedullary spinal cord metastases. J Neurol 1979, 220, 31–41. [Google Scholar] [CrossRef]
- Dam-Hieu, P.; Seizeur, R.; Mineo, J.F.; Metges, J.P.; Meriot, P.; Simon, H. Retrospective study of 19 patients with intramedullary spinal cord metastasis. Clin Neurol Neurosurg 2009, 111, 10–17. [Google Scholar] [CrossRef]
- Choi, D.; Crockard, A.; Bunger, C.; Harms, J.; Kawahara, N.; Mazel, C.; Melcher, R.; Tomita, K. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J 2010, 19, 215–222. [Google Scholar] [CrossRef]
- Witham, T.F.; Khavkin, Y.A.; Gallia, G.L.; Wolinsky, J.P.; Gokaslan, Z.L. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol 2006, 2, 87–94, quiz 116. [Google Scholar] [CrossRef]
- Ortiz Gómez, J.A. The incidence of vertebral body metastases. Int Orthop 1995, 19, 309–311. [Google Scholar] [CrossRef]
- Gokaslan, Z.L.; York, J.E.; Walsh, G.L.; McCutcheon, I.E.; Lang, F.F.; Putnam, J.B.; Wildrick, D.M.; Swisher, S.G.; Abi-Said, D.; Sawaya, R. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg 1998, 89, 599–609. [Google Scholar] [CrossRef]
- Ciftdemir, M.; Kaya, M.; Selcuk, E.; Yalniz, E. Tumors of the spine. World J Orthop 2016, 7, 109–116. [Google Scholar] [CrossRef]
- Furlan, J.C.; Wilson, J.R.; Massicotte, E.M.; Sahgal, A.; Fehlings, M.G. Recent advances and new discoveries in the pipeline of the treatment of primary spinal tumors and spinal metastases: a scoping review of registered clinical studies from 2000 to 2020. Neuro Oncol 2022, 24, 1–13. [Google Scholar] [CrossRef]
- Pinter, N.K.; Pfiffner, T.J.; Mechtler, L.L. Neuroimaging of spine tumors. Handb Clin Neurol 2016, 136, 689–706. [Google Scholar] [CrossRef]
- Wewel, J.T.; O’Toole, J.E. Epidemiology of spinal cord and column tumors. Neurooncol Pract 2020, 7, i5–i9. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Haag, E.; Joerger, A.K.; Jost, P.; Combs, S.E.; Wostrack, M.; Gempt, J.; Meyer, B. Comprehensive surgical treatment strategy for spinal metastases. Sci Rep 2021, 11, 7988. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.D.; Varshneya, K.; Ho, A.L.; Veeravagu, A.; Sciubba, D.M.; Ratliff, J.K. Laminectomy Versus Corpectomy for Spinal Metastatic Disease-Complications, Costs, and Quality Outcomes. World Neurosurg 2019, 131, e468–e473. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Garcés-Ambrossi, G.L.; McGirt, M.J.; Witham, T.F.; Wolinsky, J.P.; Bydon, A.; Gokaslan, Z.L.; Sciubba, D.M. Thoracic vertebrectomy and spinal reconstruction via anterior, posterior, or combined approaches: clinical outcomes in 91 consecutive patients with metastatic spinal tumors. J Neurosurg Spine 2009, 11, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.G.; Lad, S.P.; Santarelli, J.; Boakye, M. National inpatient complications and outcomes after surgery for spinal metastasis from 1993-2002. Cancer 2007, 110, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, H.; Yoneoka, D. Trends in the surgical treatment for spinal metastasis and the in-hospital patient outcomes in the United States from 2000 to 2009. Spine J 2014, 14, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.Q.; Zhang, H.R.; Ma, R.X.; Li, R.F.; Hu, Y.C. Prognostic Factors for Bone Survival and Functional Outcomes in Patients With Breast Cancer Spine Metastases. Technol Cancer Res Treat 2022, 21, 15330338221122642. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, D.M.; Gokaslan, Z.L.; Suk, I.; Suki, D.; Maldaun, M.V.; McCutcheon, I.E.; Nader, R.; Theriault, R.; Rhines, L.D.; Shehadi, J.A. Positive and negative prognostic variables for patients undergoing spine surgery for metastatic breast disease. Eur Spine J 2007, 16, 1659–1667. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). The Journal of Spinal Cord Medicine 2011, 34, 535–546. [Google Scholar] [CrossRef]
- Karnofsky, D.A. The clinical evaluation of chemotherapeutic agents in cancer. Evaluation of chemotherapeutic agents 1949, 191–205. [Google Scholar]
- Okai, B.K.; Lipinski, L.J.; Ghannam, M.M.; Fabiano, A.J. Expected motor function change following decompressive surgery for spinal metastatic disease. N Am Spine Soc J 2023, 15, 100240. [Google Scholar] [CrossRef]
- Onken, J.; Fekonja, L.S.; Wehowsky, R.; Hubertus, V.; Vajkoczy, P. Metastatic dissemination patterns of different primary tumors to the spine and other bones. Clinical & Experimental Metastasis 2019, 36, 493–498. [Google Scholar] [CrossRef]
- Paholpak, P.; Sirichativapee, W.; Wisanuyotin, T.; Kosuwon, W.; Jeeravipoolvarn, P. Prevalence of Known and Unknown Primary Tumor Sites in Spinal Metastasis Patients. Open Orthopaedics Journal 2012, 6, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, D.M.; Pennington, Z.; Colman, M.W.; Goodwin, C.R.; Laufer, I.; Patt, J.C.; Redmond, K.J.; Saylor, P.; Shin, J.H.; Schwab, J.H.; Schoenfeld, A.J. Spinal metastases 2021: a review of the current state of the art and future directions. The Spine Journal 2021, 21, 1414–1429. [Google Scholar] [CrossRef] [PubMed]
- STARK, R.J.; HENSON, R.A.; EVANS, S.J.W. SPINAL METASTASES: A RETROSPECTIVE SURVEY FROM A GENERAL HOSPITAL. Brain 1982, 105, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.; Larsen, B.H.; Rohde, K.; Børgesen, S.E.; Gjerris, F.; Bøge-Rasmussen, T.; Agerlin, N.; Rasmusson, B.; Stjernholm, P.; Sørensen, P.S. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien) 1990, 107, 37–43. [Google Scholar] [CrossRef]
- Spratt, D.E.; Beeler, W.H.; de Moraes, F.Y.; Rhines, L.D.; Gemmete, J.J.; Chaudhary, N.; Shultz, D.B.; Smith, S.R.; Berlin, A.; Dahele, M.; et al. An integrated multidisciplinary algorithm for the management of spinal metastases: an International Spine Oncology Consortium report. The Lancet Oncology 2017, 18, e720–e730. [Google Scholar] [CrossRef]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010, 35, E1221–1229. [Google Scholar] [CrossRef]
- Lee, S.-H.; Tatsui, C.E.; Ghia, A.J.; Amini, B.; Li, J.; Zavarella, S.M.; Tannir, N.M.; Brown, P.D.; Rhines, L.D. Can the spinal instability neoplastic score prior to spinal radiosurgery predict compression fractures following stereotactic spinal radiosurgery for metastatic spinal tumor?: a post hoc analysis of prospective phase II single-institution trials. Journal of Neuro-Oncology 2016, 126, 509–517. [Google Scholar] [CrossRef]
- Lee, J.; Rhee, W.J.; Chang, J.S.; Chang, S.K.; Koom, W.S. Evaluation of predictive factors of vertebral compression fracture after conventional palliative radiotherapy for spinal metastasis from colorectal cancer. J Neurosurg Spine 2018, 28, 333–340. [Google Scholar] [CrossRef]
- Papastefanou, S.L.; Alpantaki, K.; Akra, G.A.; Katonis, P. Predictive value of Tokuhashi and Tomita scores in patients with metastatic spine disease. Acta orthopaedica et traumatologica turcica 2012, 46 1, 50–56. [Google Scholar] [CrossRef]
- Zhou, R.P.; Mummaneni, P.V.; Chen, K.-Y.; Lau, D.; Cao, K.; Amara, D.; Zhang, C.; Dhall, S.; Chou, D. Outcomes of Posterior Thoracic Corpectomies for Metastatic Spine Tumors: An Analysis of 90 Patients. World Neurosurgery 2019, 123, e371–e378. [Google Scholar] [CrossRef]
- Katagiri, H.; Takahashi, M.; Inagaki, J.; Kobayashi, H.; Sugiura, H.; Yamamura, S.; Iwata, H. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys 1998, 42, 1127–1132. [Google Scholar] [CrossRef]
- Chow, E.; Zeng, L.; Salvo, N.; Dennis, K.; Tsao, M.; Lutz, S. Update on the Systematic Review of Palliative Radiotherapy Trials for Bone Metastases. Clinical Oncology 2012, 24, 112–124. [Google Scholar] [CrossRef]
- Fomchenko, E.I.; Bayley, J.C.; Alvarez-Breckenridge, C.; Rhines, L.D.; Tatsui, C.E. Spinal Metastases and the Evolving Role of Molecular Targeted Therapy, Chemotherapy, and Immunotherapy. Neurospine 2022, 19, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Onishi, E.; Hashimura, T.; Ota, S.; Fujita, S.; Tsukamoto, Y.; Matsunaga, K.; Yasuda, T. The Efficacy and Complications of Preoperative Embolization of Metastatic Spinal Tumors: Risk of Paralysis after Embolization. Spine Surg Relat Res 2022, 6, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Pikis, S.; Itshayek, E.; Barzilay, Y.; Hasharoni, A.; Kaplan, L.; Gomori, M.; Cohen, J.E. Preoperative embolization of hypervascular spinal tumors: current practice and center experience. Neurol Res 2014, 36, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Cady-McCrea, C.I.; Gilbert, J.C.; Galgano, M.A. Cement-Augmented and Dual-Headed Posterior Screw Reconstruction After Corpectomy for Metastatic Tumor Resection. World Neurosurg 2021, 152, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Yurac, R.; Guiroy, A.; Bravo, O.; Morales Ciancio, A.; Landriel, F.; Hem, S. Low Implant Failure Rate of Percutaneous Fixation for Spinal Metastases: A Multicenter Retrospective Study. World Neurosurg 2021, 148, e627–e634. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, W.; Cai, W.; Sun, Z.; Fang, M.; Ji, Y.; Wang, S.; Zhang, J.; Hu, T.; Cheng, M.; Yan, W. Comparison of Surgical Outcomes Between Separation Surgery and Piecemeal Spondylectomy for Spinal Metastasis: A Retrospective Analysis. Front Surg 2021, 8, 686930. [Google Scholar] [CrossRef]
- Kim, J.M.; Losina, E.; Bono, C.M.; Schoenfeld, A.J.; Collins, J.E.; Katz, J.N.; Harris, M.B. Clinical outcome of metastatic spinal cord compression treated with surgical excision ± radiation versus radiation therapy alone: a systematic review of literature. Spine 2012, 37, 78–84. [Google Scholar] [CrossRef]
- Sebaaly, A.; Shedid, D.; Boubez, G.; Zairi, F.; Kanhonou, M.; Yuh, S.J.; Wang, Z. Surgical site infection in spinal metastasis: incidence and risk factors. Spine J 2018, 18, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Falicov, A.; Fisher, C.G.; Sparkes, J.; Boyd, M.C.; Wing, P.C.; Dvorak, M.F. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine (Phila Pa 1976) 2006, 31, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.; Rory Goodwin, C.; Abu-Bonsrah, N.; Elder, B.D.; De la Garza Ramos, R.; Sciubba, D.M. Posterior approaches for symptomatic metastatic spinal cord compression. Neurosurgical Focus FOC 2016, 41, E11. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M. Experimental development of the graphic rating method. Psychological Bulletin 1921, 18, 98–99. [Google Scholar]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry disability index. Spine 2000, 25, 2940–2953. [Google Scholar] [CrossRef]
- Hays, R.D.; Sherbourne, C.D.; Mazel, R.M. The rand 36-item health survey 1.0. Health economics 1993, 2, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Younsi, A.; Riemann, L.; Scherer, M.; Unterberg, A.; Zweckberger, K. Impact of decompressive laminectomy on the functional outcome of patients with metastatic spinal cord compression and neurological impairment. Clin Exp Metastasis 2020, 37, 377–390. [Google Scholar] [CrossRef]
- Oni, P.; Schultheiß, R.; Scheufler, K.M.; Roberg, J.; Harati, A. Radiological and Clinical Outcome after Multilevel Anterior Cervical Discectomy and/or Corpectomy and Fixation. J Clin Med 2018, 7. [Google Scholar] [CrossRef]
- Wong, M.L.; Lau, H.C.; Kaye, A.H. The management of malignant spinal cord compression: a modified technique of spinal reconstruction. Neurol Res 2014, 36, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Wibmer, C.; Leithner, A.; Hofmann, G.; Clar, H.; Kapitan, M.; Berghold, A.; Windhager, R. Survival analysis of 254 patients after manifestation of spinal metastases: evaluation of seven preoperative scoring systems. Spine (Phila Pa 1976) 2011, 36, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.B.; Cho, W.; Chang, U.K. Analysis of prognostic factors relating to postoperative survival in spinal metastases. J Korean Neurosurg Soc 2012, 51, 127–134. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
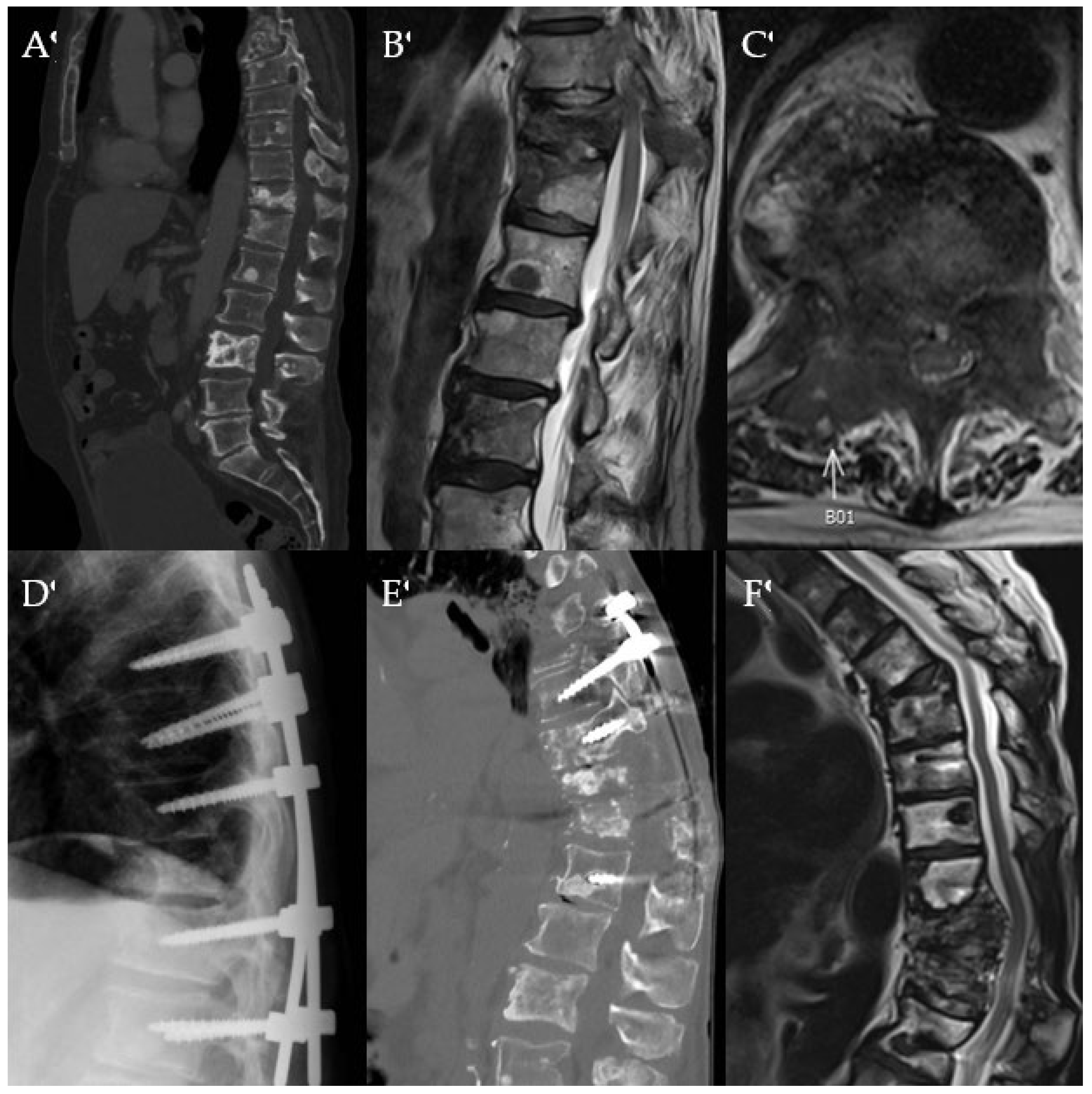
Images of a 69-year-old male with a history of metastatic prostate cancer. He was neurologically intact but was beset with severe back pain 6 years after initial diagnosis. He had evidence of extensive metastatic disease to the entire spine, particularly to T11, as demonstrated by CT (A) and T2-weighted MRI in the sagittal (B), and axial (C) views with concerns for cord compression. He underwent separation surgery with posterior instrumentation. Lateral plain radiograph (D) obtained one day after surgery demonstrates instrumentation spanning T8 to L1. Imaging 3 years later obtained for progressively worsening mechanical back pain shows worsening kyphosis on CT (E) and MRI (F). Patient was scheduled for revision surgery but passed away over 3 years after his index operation.
Figure 1.
Images of a 69-year-old male with a history of metastatic prostate cancer. He was neurologically intact but was beset with severe back pain 6 years after initial diagnosis. He had evidence of extensive metastatic disease to the entire spine, particularly to T11, as demonstrated by CT (A) and T2-weighted MRI in the sagittal (B), and axial (C) views with concerns for cord compression. He underwent separation surgery with posterior instrumentation. Lateral plain radiograph (D) obtained one day after surgery demonstrates instrumentation spanning T8 to L1. Imaging 3 years later obtained for progressively worsening mechanical back pain shows worsening kyphosis on CT (E) and MRI (F). Patient was scheduled for revision surgery but passed away over 3 years after his index operation.
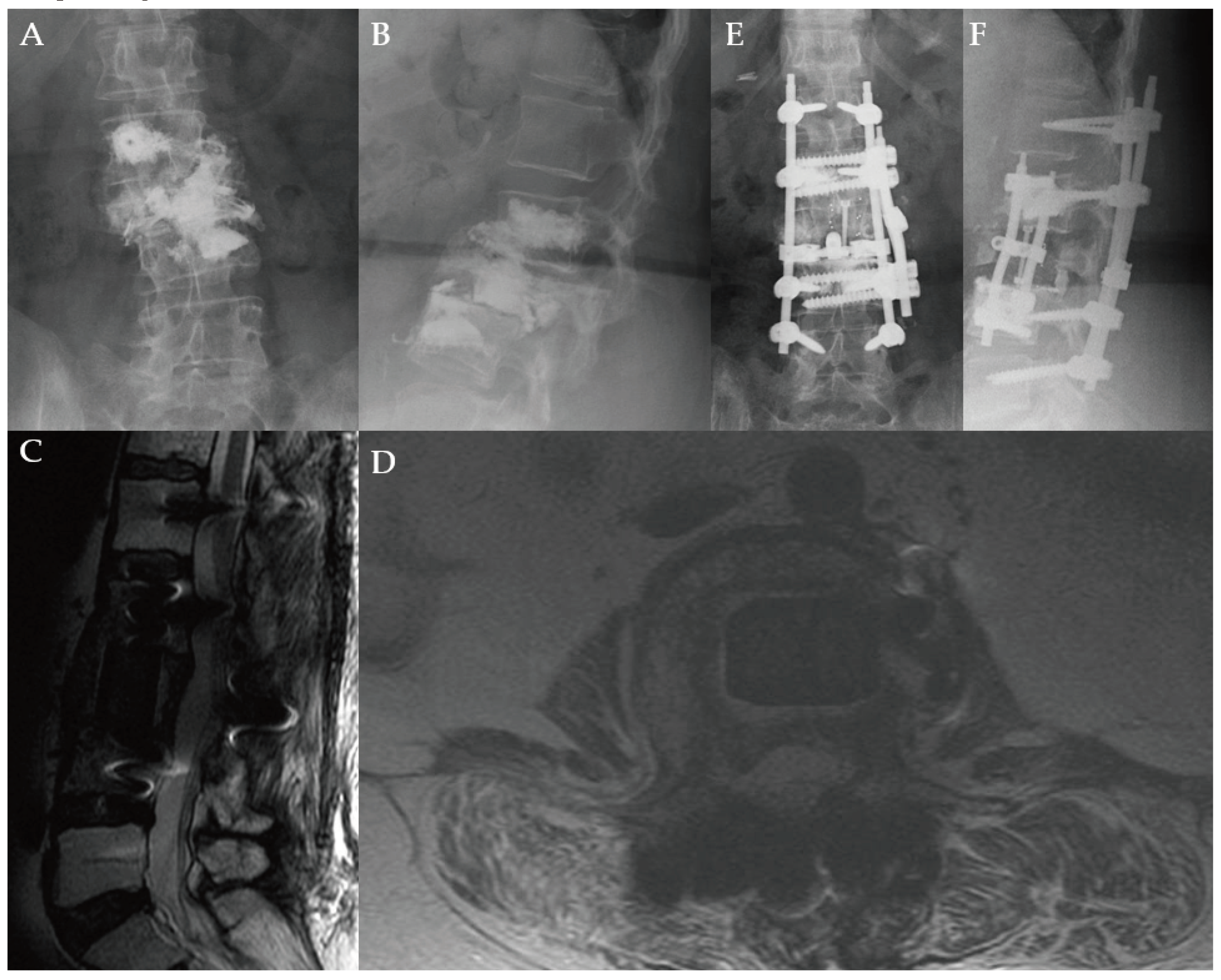
Figure 2.
A 66-year-old female with a history of metastatic breast cancer. She developed low back and right leg pain and underwent vertebroplasty as depicted on plain anteroposterior (A) and lateral (B) radiographs. Eight months later, she began feeling pain and weakness in the right leg requiring significant opioid pain medication usage. Patient underwent L3 corpectomy through a lateral approach with carbon fiber prosthesis and posterior instrumentation spanning T12-L5. Postoperative T2-weighted MRI in the lateral (C) and axial (D) views show decompression of the canal, and restoration of height and spinal alignment. Anteroposterior (E) and lateral (F) plain radiographs obtained one year after surgery show alignment and height maintained without kyphosis. She was ambulatory with a cane and was very pleased with her outcome. She survived over 2.5 years after her surgery.
Figure 2.
A 66-year-old female with a history of metastatic breast cancer. She developed low back and right leg pain and underwent vertebroplasty as depicted on plain anteroposterior (A) and lateral (B) radiographs. Eight months later, she began feeling pain and weakness in the right leg requiring significant opioid pain medication usage. Patient underwent L3 corpectomy through a lateral approach with carbon fiber prosthesis and posterior instrumentation spanning T12-L5. Postoperative T2-weighted MRI in the lateral (C) and axial (D) views show decompression of the canal, and restoration of height and spinal alignment. Anteroposterior (E) and lateral (F) plain radiographs obtained one year after surgery show alignment and height maintained without kyphosis. She was ambulatory with a cane and was very pleased with her outcome. She survived over 2.5 years after her surgery.
Figure 3.
Estimated blood loss (EBL) associated with corpectomy surgery by (A) spinal segment and (B) primary cancer type.
Figure 3.
Estimated blood loss (EBL) associated with corpectomy surgery by (A) spinal segment and (B) primary cancer type.
Figure 4.
Kaplan-Meier survival analysis showing survival as stratified by (A) tumor histology, preoperative (B) Tomita and (C) Tokuhashi scores, (D) pre- and (E) postoperative KPS, (F) neoadjuvant treatment, (G) adjuvant treatment, and (H) type of adjunctive therapy given.
Figure 4.
Kaplan-Meier survival analysis showing survival as stratified by (A) tumor histology, preoperative (B) Tomita and (C) Tokuhashi scores, (D) pre- and (E) postoperative KPS, (F) neoadjuvant treatment, (G) adjuvant treatment, and (H) type of adjunctive therapy given.
Figure 5.
Neurological grades and health-related quality of life metrics. (A) Pre- and postoperative Frankel Grade. (B) Pre- and postoperative KPS. (C) Pre-, postoperative, and last follow-up pain analogue scores. (D) Pre, postoperative, and last RAND-36 scores. (E) Pre-, postoperative, and last follow-up ODI scores.
Figure 5.
Neurological grades and health-related quality of life metrics. (A) Pre- and postoperative Frankel Grade. (B) Pre- and postoperative KPS. (C) Pre-, postoperative, and last follow-up pain analogue scores. (D) Pre, postoperative, and last RAND-36 scores. (E) Pre-, postoperative, and last follow-up ODI scores.
Figure 6.
Change in spinal alignment as measured by Cobb angle in the cervical, thoracic, and lumbar spine. Positive (+) Cobb angle indicates lordosis, and negative (-) Cobb angle indicates kyphosis. There were improvements in spinal kyphotic deformity in all segments.
Figure 6.
Change in spinal alignment as measured by Cobb angle in the cervical, thoracic, and lumbar spine. Positive (+) Cobb angle indicates lordosis, and negative (-) Cobb angle indicates kyphosis. There were improvements in spinal kyphotic deformity in all segments.
Figure 7.
Images of a 68-year-old female with a history of metastatic RCC. Sagittal CT (A) shows destructive changes of the T5 vertebral body. T1 contrast-enhanced MRI sagittal (B) and axial (C) views of the spine show RCC metastases to T5 with canal compromise and circumferential cord compression. Patient underwent preoperative embolization to reduce intraoperative blood loss, followed by T5 corpectomy and posterior instrumentation from T2 to T5 as demonstrated on the immediate postoperative lateral plain radiograph (D) and the 4-month postoperative CT (E). Two-year follow-up CT (F) shows hardware in place without significant change in kyphosis. The patient survived for nearly 2.5 years after surgery.
Figure 7.
Images of a 68-year-old female with a history of metastatic RCC. Sagittal CT (A) shows destructive changes of the T5 vertebral body. T1 contrast-enhanced MRI sagittal (B) and axial (C) views of the spine show RCC metastases to T5 with canal compromise and circumferential cord compression. Patient underwent preoperative embolization to reduce intraoperative blood loss, followed by T5 corpectomy and posterior instrumentation from T2 to T5 as demonstrated on the immediate postoperative lateral plain radiograph (D) and the 4-month postoperative CT (E). Two-year follow-up CT (F) shows hardware in place without significant change in kyphosis. The patient survived for nearly 2.5 years after surgery.
Table 1.
Baseline demographics, clinical, and imaging characteristics.
Table 1.
Baseline demographics, clinical, and imaging characteristics.
| Characteristic |
Value |
| Age in years, mean ± standard deviation (range) |
63.5 ± 9.5 (37-84) |
| Female sex, n (%) |
28 (44.4) |
| Male sex, n (%) |
35 (55.6) |
| Prior cancer diagnosis, n (%) |
49 (77.8) |
| Prior cancer treatment, n (%) |
36 (57.1) |
| Chemotherapy |
5 (7.9) |
| Radiation therapy |
15 (23.8) |
| Chemoradiation |
16 (25.4) |
| Systemic extraspinal metastases, n (%) |
31 (49.2) |
| Number of extraspinal metastases, median (range) |
2 (1-4) |
| Pain on presentation, n (%) |
54 (85.7) |
| Neurologic deficit on presentation, n (%) |
35 (55.6) |
| Radiculopathy |
16 (25.4) |
| Weakness |
14 (22.2) |
| Paresthesia |
8 (12.7) |
| Spinal cord compression, n (%) |
31 (49.2) |
| Emergent management, n (%) |
14 (22.2) |
| Surgery |
11 (17.4) |
| Radiation |
3 (4.7) |
| Preoperative Tomita score, median (range) |
4 (2-10) |
| Preoperative Tokuhasi score, median (range) |
11 (3-15) |
| Preoperative SINS, median (range) |
10 (7-17) |
| Radiographic characteristics, n (%) |
|
| Evidence of deformity |
|
| None |
40 (63.5) |
| Kyphosis/scoliosis |
2 (3.2) |
| Subluxation/translation |
15 (23.8) |
| Kyphosis/scoliosis + subluxation/translation |
7 (11.1) |
| Compression fractures, n (%) |
37 (58.7) |
| Burst fracture, n (%) |
6 (9.5) |
Table 2.
Treatment characteristics.
Table 2.
Treatment characteristics.
| Characteristic |
Value |
| Neoadjuvant therapy, n (%) |
31 (49.2) |
| Chemotherapy |
7 (11.1) |
| Radiation therapy |
9 (14.2) |
| Chemoradiation |
15 (23.8) |
| Adjuvant therapy, n (%) |
51 (80.9) |
| Chemotherapy |
14 (22.2) |
| Radiation therapy |
20 (31.7) |
| Chemoradiation |
17 (27.0) |
| Surgical approach, n (%) |
|
| Posterior |
33 (52.4) |
| Anterior |
30 (47.6) |
| Anterior corpectomy without posterior instrumentation, n (%) |
13 (20.6) |
| Cervical |
9 (14.2) |
| Thoracic |
3 (4.7) |
| Lumbar |
1 (1.6) |
| Anterior corpectomy with posterior instrumentation, n (%) |
17 (27.0) |
| Cervical |
6 (9.5) |
| Thoracic |
6 (9.5) |
| Lumbar |
5 (7.9) |
| Posterior corpectomy with posterior instrumentation, n (%) |
33 (52.4) |
| Thoracic |
24 (38.1) |
| Lumbar |
9 (14.2) |
| Estimated blood loss in mL, median (range) |
800.0 (100.0-35,200.0) |
| Cervical |
337.5 (150.0-1000.0) |
| Thoracic |
860.0 (100.0-35,200.0) |
| Lumbar |
1025.0 (250.0-8800.0) |
| Intraoperative blood transfusion, n (%) |
28 (45.2) |
| Blood products transfused in mL, median (range) |
650.0 (0.0-7975.0) |
| Cervical |
0 (0-900) |
| Thoracic |
0 (0-2600) |
| Lumbar |
310 (0-3850) |
| Surgery duration in minutes, median (range) |
327 (111-830) |
| Length of postoperative admission in days, median (range) |
6 (1-28) |
Table 3.
Outcomes.
| Characteristic |
Preoperative |
Postoperative |
Last follow-up |
P-value |
| Overall survival in months, median (range) |
|
14.4 (0.6-145.0) |
|
|
| Progression-free survival in days, median (range) |
|
268.5 (10.0-4351.0) |
|
|
| Follow-up times in months, median (range) |
|
15.5 (0.5-145.0) |
|
|
| Mortality, n (%) |
|
40 (63.5) |
|
|
| Neurological recovery, n (%) |
|
47 (74.6) |
|
|
| ASIA Score, n (%) |
|
|
|
|
| C |
1 (1.6) |
1 (1.6) |
|
|
| D |
25 (39.7) |
21 (33.3) |
|
|
| E |
37 (58.7) |
41 (65.1) |
|
|
| KPS, median (range) |
70 (20-90) |
80 (40-100) |
|
0.0056+ |
| Pain analogue score, mean ± SD |
3 ± 2.9 |
2.1 ± 2.8 |
2.0 ± 2.5 |
0.3822, 0.3503 |
| RAND-36 score, mean ± SD |
26.5 ± 14.8 |
46.4 ± 24.4 |
53.1 ± 29.9 |
0.0081+, 0.004+ |
| ODI score, mean ± SD |
28.9 ± 16.1 |
16.9 ± 15.1 |
16.9 ± 15.9 |
0.0613, 0.0485+
|
| Spinal kyphotic (-)/lordotic (+) deformity in degrees, median (range) |
-5.0 (-40.0 to +36.0) |
0.0 (-24.0 to +38.0) |
|
0.021+ |
| Cervical spine (including T1), |
-4 (-25 to +17) |
+4 (-10 to +18.0) |
|
0.0051+ |
| Thoracic spine, |
-7 (-49 to +20) |
-1.5 (-24.0 to +26.0) |
|
0.0007+ |
| Lumbar spine, |
+6.0 (-5.0 to +36.0) |
+16.0 (-2.0 to +38.0) |
|
0.0312+ |
| Complications, n (%) |
|
9 (14.2) |
|
|
| Wound dehiscence |
|
4 (6.3) |
|
|
| Wound infection |
|
2 (3.2) |
|
|
| Other* |
|
3 (4.8) |
|
|
| Reoperations, n |
|
16 |
|
|
| Patients requiring reoperation, n (%) |
|
7 (11.1) |
|
|
| Reoperation indications, n (%) |
|
|
|
|
| Wound infection |
|
8 (12.7) |
|
|
| Wound dehiscence |
|
4 (6.3) |
|
|
| Other** |
|
4 (6.3) |
|
|
| 30-day readmission rate, n (%) |
|
9 (14.2) |
|
|
| 30-day mortality rate, n (%) |
|
2 (3.2)+
|
|
|
| 1-year mortality rate, n (%) |
|
22 (34.9) |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).