Submitted:
01 February 2024
Posted:
02 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
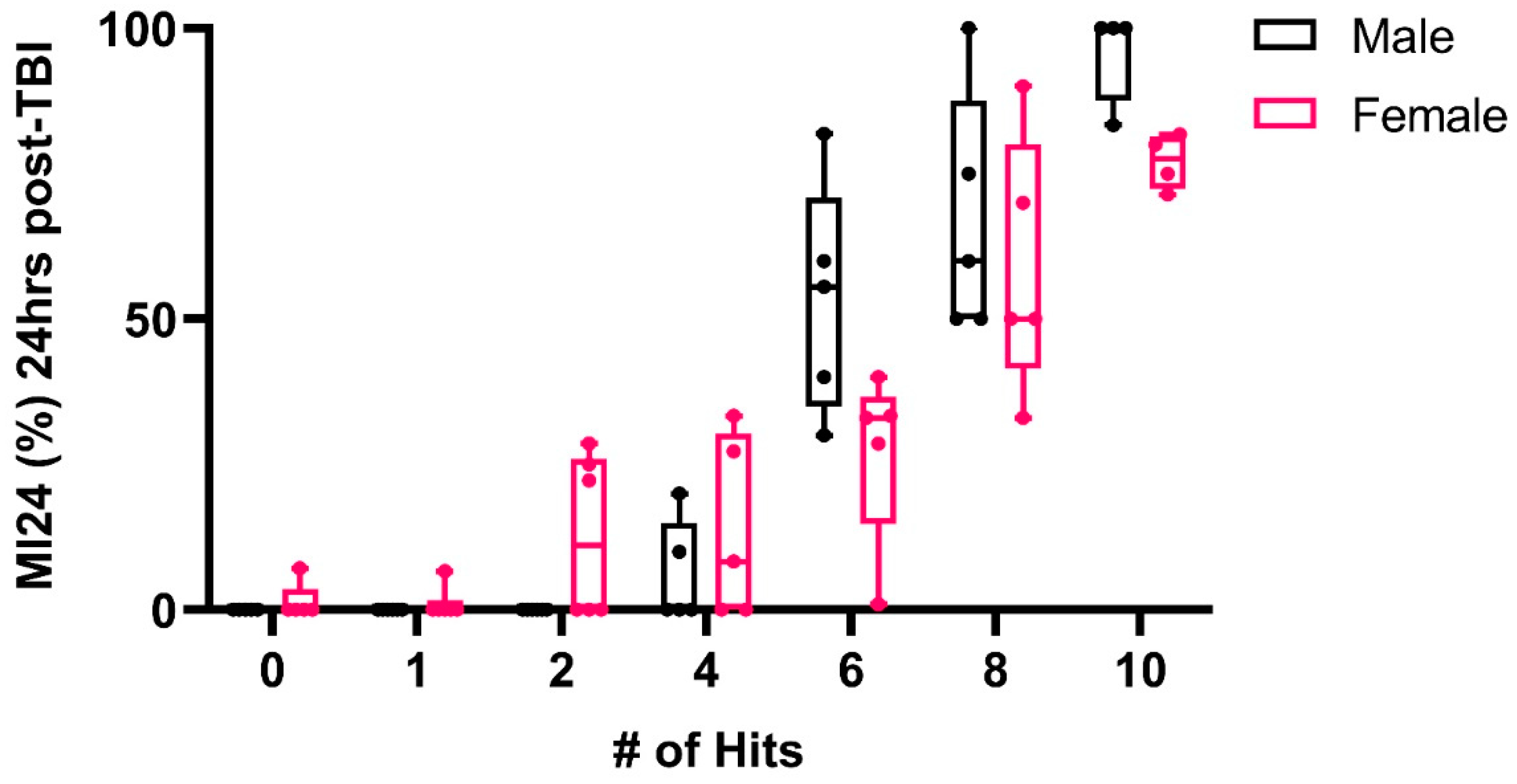
2.1. TBI Paradigm
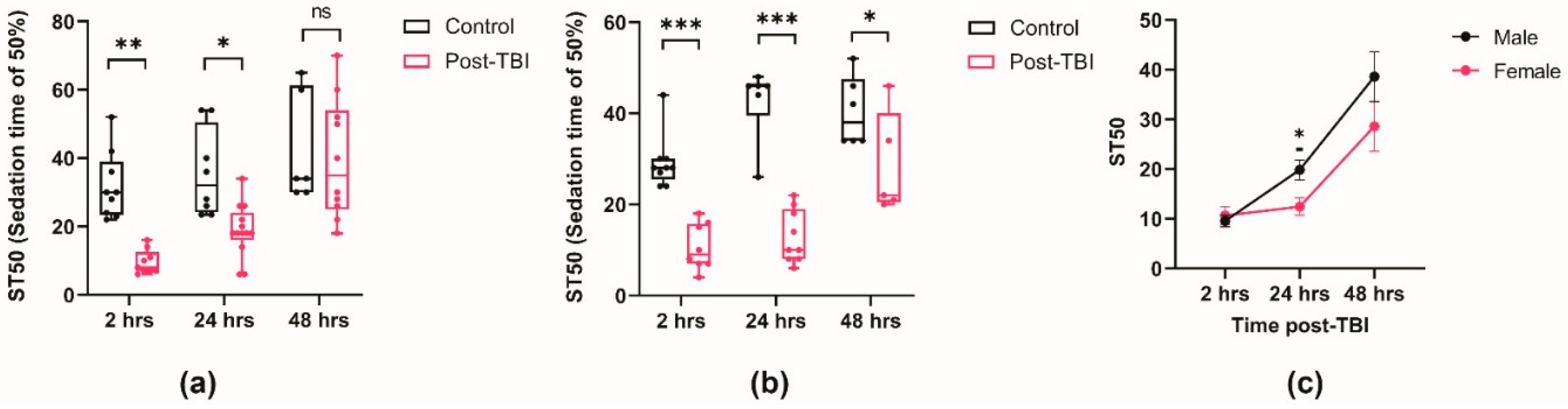
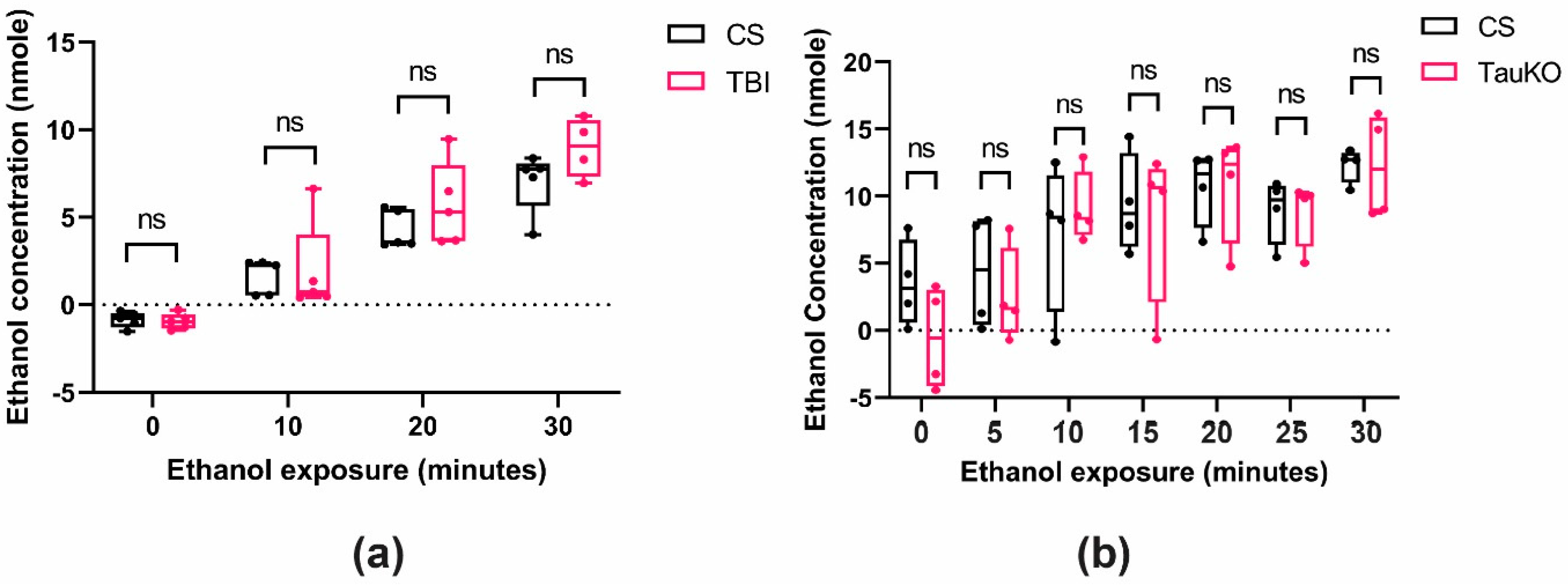
2.2. TBI Alters Ethanol Sensitivity
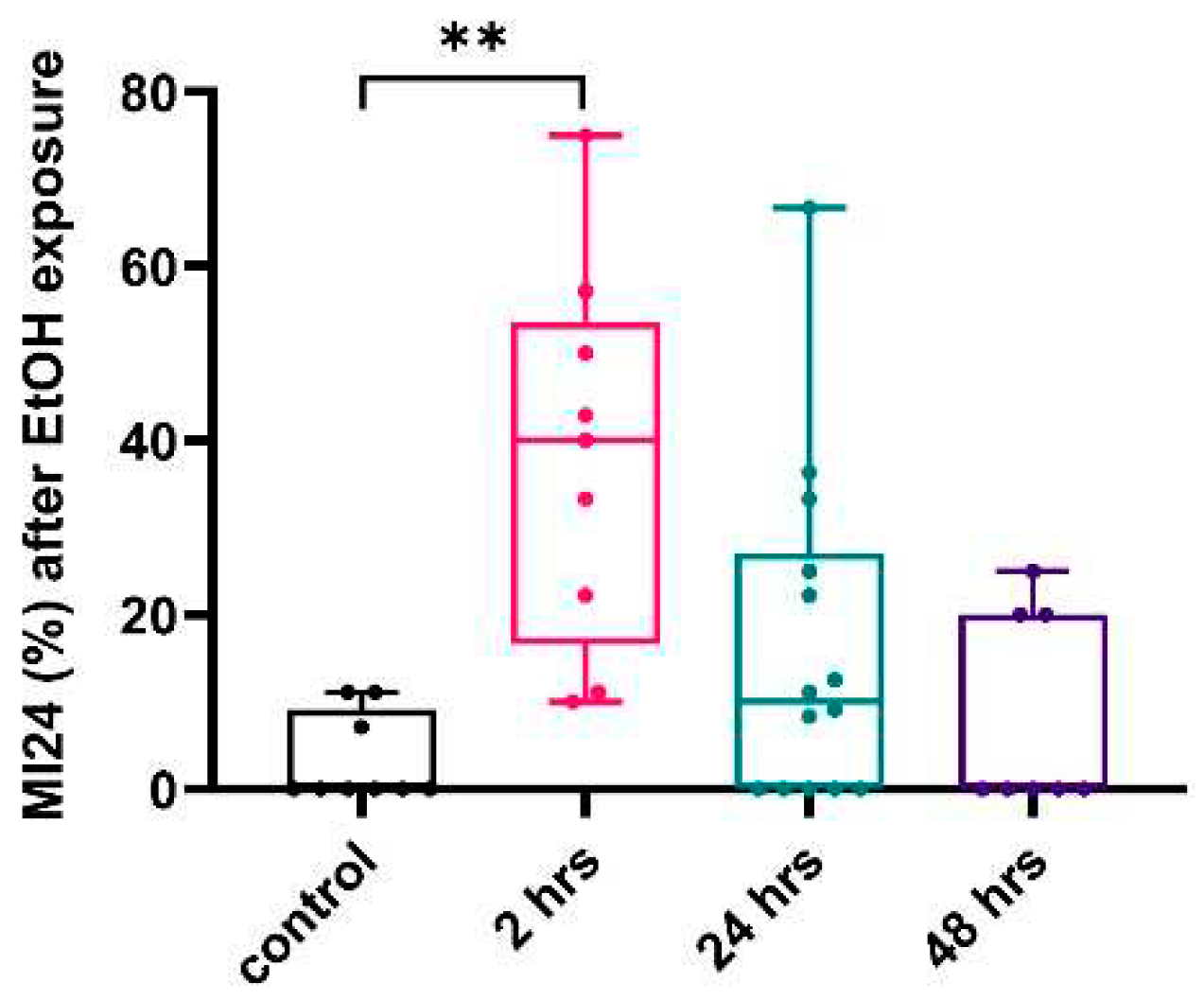
2.3. Ethanol Exposure Post-TBI Increases the Mortality Rate
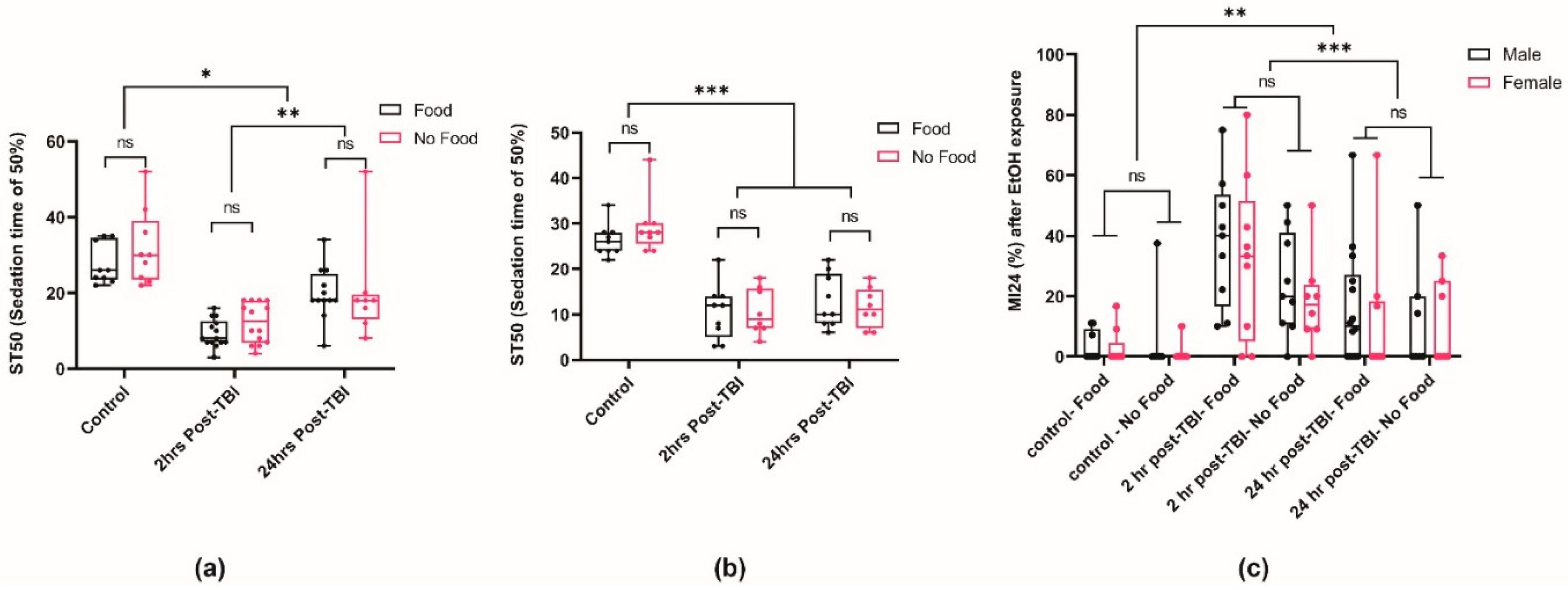
2.4. Post-TBI Diet Does Not Affect Ethanol Sensitivity and Mortality
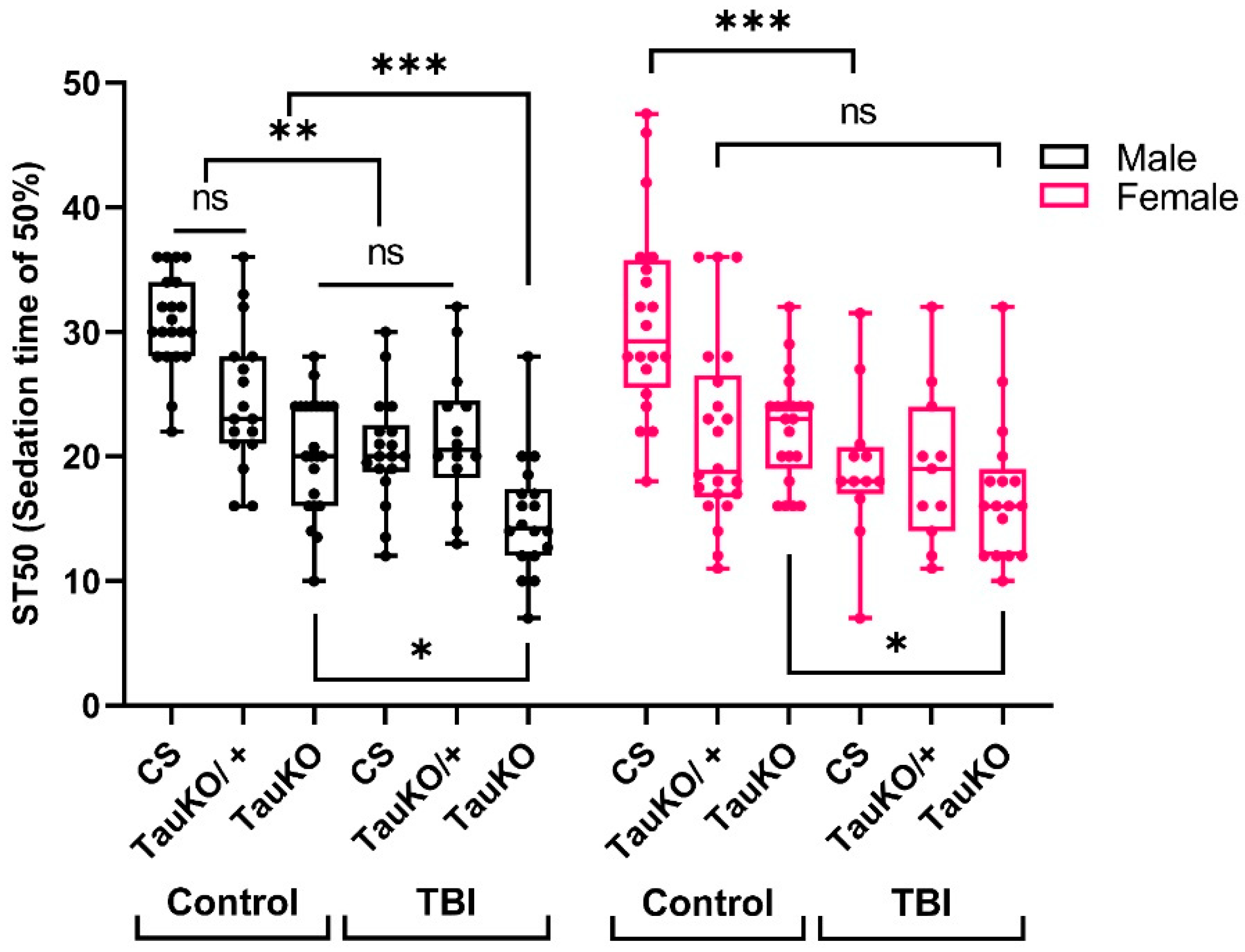
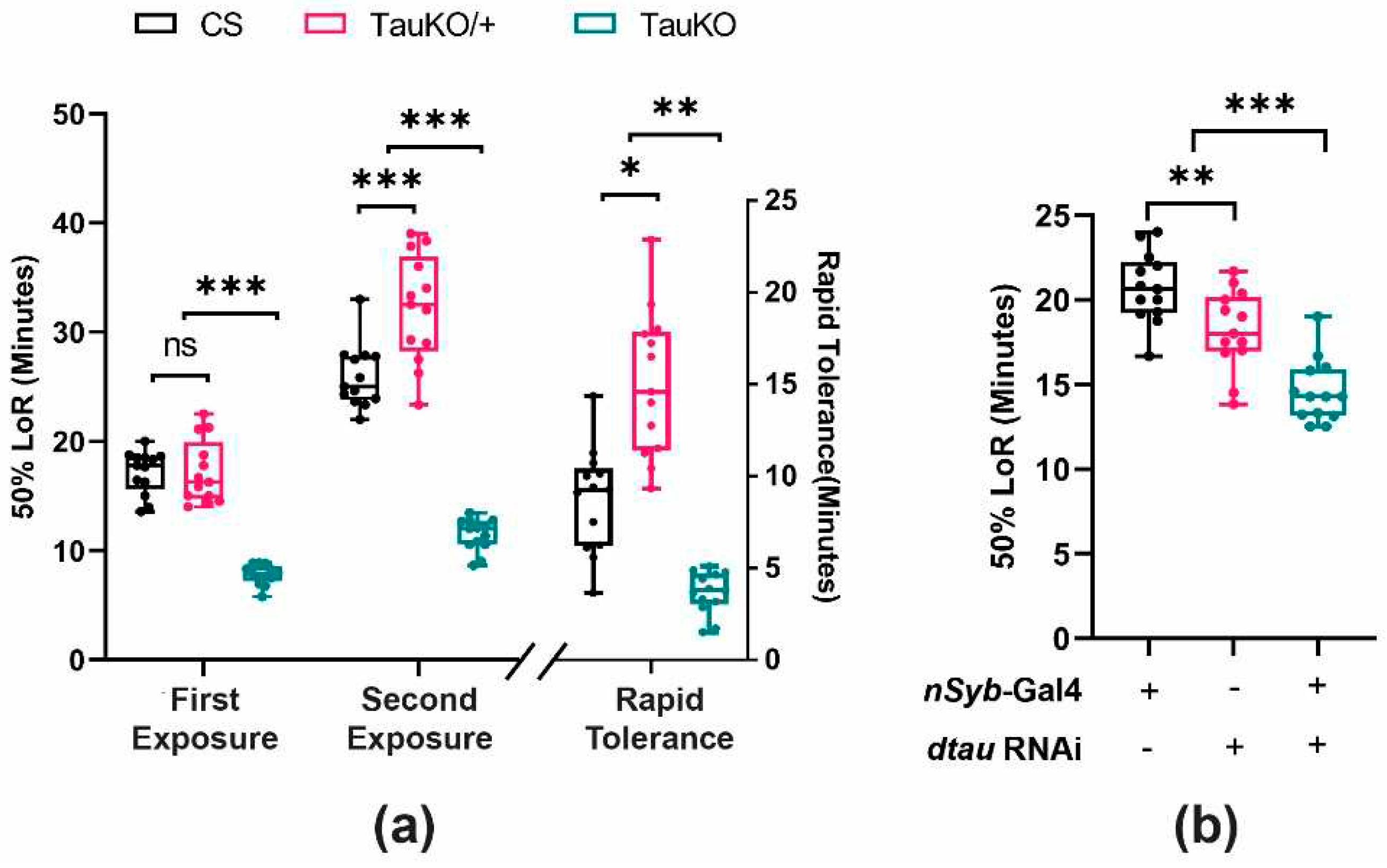
2.5. Loss of dTau Exacerbates Acute Ethanol Sensitivity
3. Discussion
3.1. TBI and Ethanol Sensitivity
3.2. Tau and Ethanol Sensitivity
4. Materials and Methods
4.1. Fly Stocks and Maintenance
4.2. Traumatic Brain Injury (TBI) Paradigm
4.3. Ethanol Sensitivity Assay and Mortality Rate
4.4. Diet Studies
4.5. Statistical Analyses
References
- Zeiler, F.A.; Iturria-Medina, Y.; Thelin, E.P.; Gomez, A.; Shankar, J.J.; Ko, J.H.; Figley, C.R.; Wright, G.E.B.; Anderson, C.M. Integrative Neuroinformatics for Precision Prognostication and Personalized Therapeutics in Moderate and Severe Traumatic Brain Injury. Front. Neurol. 2021, 12, 729184. [Google Scholar] [CrossRef] [PubMed]
- Bigler, E.D. Neurobiology and neuropathology underlie the neuropsychological deficits associated with traumatic brain injury. Archives of Clinical Neuropsychology 2003, 18, 595–621. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Hardy, J.; Zetterberg, H. The Neuropathology and Neurobiology of Traumatic Brain Injury. Neuron 2012, 76, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Baratz, R.; Rubovitch, V.; Frenk, H.; Pick, C.G. The Influence of Alcohol on Behavioral Recovery after mTBI in Mice. J. Neurotrauma 2010, 27, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Masel, B.E.; DeWitt, D.S. Traumatic Brain Injury: A Disease Process, Not an Event. J. Neurotrauma 2010, 27, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.M.; Donders, J. Correlates of verbal learning and memory after pediatric traumatic brain injury. Appl. Neuropsychol. Child 2017, 7, 298–305. [Google Scholar] [CrossRef]
- Alcock, B.; Gallant, C.; Good, D. The relationship between concussion and alcohol consumption among university athletes. Addict. Behav. Rep. 2018, 7, 58–64. [Google Scholar] [CrossRef]
- McCrory, P.; Meeuwisse, W.; Dvorak, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. British journal of sports medicine 2017, 51, 838–847. [Google Scholar] [CrossRef]
- Zuckerman, S.L.; Kerr, Z.Y.; Yengo-Kahn, A.; Wasserman, E.; Covassin, T.; Solomon, G.S. Epidemiology of sports-related concussion in NCAA athletes from 2009-2010 to 2013-2014: incidence, recurrence, and mechanisms. The American journal of sports medicine 2015, 43, 2654–2662. [Google Scholar] [CrossRef]
- Morissette, S.B.; Woodward, M.; Kimbrel, N.A.; Meyer, E.C.; Kruse, M.I.; Dolan, S.; Gulliver, S.B. Deployment-related TBI, persistent postconcussive symptoms, PTSD, and depression in OEF/OIF veterans. Rehabilitation psychology 2011, 56, 340. [Google Scholar] [CrossRef] [PubMed]
- Warden, D. Military TBI During the Iraq and Afghanistan Wars. J. Head Trauma Rehabilitation 2006, 21, 398–402. [Google Scholar] [CrossRef]
- Katzenberger, R.J.; Chtarbanova, S.; Rimkus, S.A.; Fischer, J.A.; Kaur, G.; Seppala, J.M.; Swanson, L.C.; Zajac, J.E.; Ganetzky, B.; Wassarman, D.A. Death following traumatic brain injury in Drosophila is associated with intestinal barrier dysfunction. eLife 2015, 4, e04790. [Google Scholar] [CrossRef]
- Gadani, S.P.; Walsh, J.T.; Lukens, J.R.; Kipnis, J. Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron 2015, 87, 47–62. [Google Scholar] [CrossRef]
- Clark, R.S.B.; Kochanek, P.M.; Watkins, S.C.; Chen, M.; Dixon, C.E.; Seidberg, N.A.; Melick, J.; Loeffert, J.E.; Nathaniel, P.D.; Jin, K.L.; et al. Caspase-3 Mediated Neuronal Death After Traumatic Brain Injury in Rats. J. Neurochem. 2000, 74, 740–753. [Google Scholar] [CrossRef]
- Uzan, M.; Erman, H.; Tanriverdi, T.; Sanus, G.Z.; Kafadar, A.; Uzun, H. Evaluation of apoptosis in cerebrospinal fluid of patients with severe head injury. Acta Neurochir. 2006, 148, 1157–1164. [Google Scholar] [CrossRef]
- Schindler, A.G.; Baskin, B.; Juarez, B.; Lee, S.J.; Hendrickson, R.; Pagulayan, K.; Zweifel, L.S.; Raskind, M.A.; Phillips, P.E.; Peskind, E.R.; et al. Repetitive blast mild traumatic brain injury increases ethanol sensitivity in male mice and risky drinking behavior in male combat veterans. Alcohol. Clin. Exp. Res. 2021, 45, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.W.; Foster, E.; Comper, P.; Langer, L.; Hutchison, M.G.; Chandra, T.; Bayley, M. Cannabis, alcohol and cigarette use during the acute post-concussion period. Brain Inj. 2020, 34, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Lowing, J.L.; Susick, L.L.; Caruso, J.P.; Provenzano, A.M.; Raghupathi, R.; Conti, A.C. Experimental Traumatic Brain Injury Alters Ethanol Consumption and Sensitivity. J. Neurotrauma 2014, 31, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Ramchand, R.; Miles, J.; Schell, T.; Jaycox, L.; Marshall, G.N.; Tanielian, T. Prevalence and Correlates of Drinking Behaviors Among Previously Deployed Military and Matched Civilian Populations. Mil. Psychol. 2011, 23, 6–21. [Google Scholar] [CrossRef]
- Adams, R.S.; Larson, M.J.; Corrigan, J.D.; Horgan, C.M.; Williams, T.V. Frequent Binge Drinking After Combat-Acquired Traumatic Brain Injury Among Active Duty Military Personnel With a Past Year Combat Deployment. J. Head Trauma Rehabilitation 2012, 27, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Faul, M.; Coronado, V. Epidemiology of traumatic brain injury. Handbook of clinical neurology 2015, 127, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Coronado, V.G.; McGuire, L.C.; Sarmiento, K.; Bell, J.; Lionbarger, M.R.; Jones, C.D.; Geller, A.I.; Khoury, N.; Xu, L. Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995–2009. J. Saf. Res. 2012, 43, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.K.; Liu, Y.; Esser, M.B.; Mesnick, J.B.; Lu, H.; Pan, Y.; Greenlund, K.J. Binge drinking among adults, by select characteristics and state—United States, 2018. American journal of transplantation 2021, 21, 4084–4091. [Google Scholar] [CrossRef]
- Gupte, R.P.; Brooks, W.M.; Vukas, R.R.; Pierce, J.D.; Harris, J.L. Sex Differences in Traumatic Brain Injury: What We Know and What We Should Know. J. Neurotrauma 2019, 36, 3063–3091. [Google Scholar] [CrossRef]
- Roof, R.L.; Duvdevani, R.; Stein, D.G. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993, 607, 333–336. [Google Scholar] [CrossRef]
- Farin, A.; Deutsch, R.; Biegon, A.; Marshall, L.F. Sex-related differences in patients with severe head injury: greater susceptibility to brain swelling in female patients 50 years of age and younger. J. Neurosurg. 2003, 98, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.K.; Bayir, H.; Ren, D.; Puccio, A.; Zafonte, R.D.; Kochanek, P.M. Relationships between Cerebrospinal Fluid Markers of Excitotoxicity, Ischemia, and Oxidative Damage after Severe TBI: The Impact of Gender, Age, and Hypothermia. J. Neurotrauma 2004, 21, 125–136. [Google Scholar] [CrossRef]
- Becker, J.B.; Koob, G.F. Sex Differences in Animal Models: Focus on Addiction. Pharmacol. Rev. 2016, 68, 242–263. [Google Scholar] [CrossRef] [PubMed]
- Radke, A.K.; Sneddon, E.A.; Frasier, R.M.; Hopf, F.W. Recent Perspectives on Sex Differences in Compulsion-Like and Binge Alcohol Drinking. Int. J. Mol. Sci. 2021, 22, 3788. [Google Scholar] [CrossRef]
- Ceylan-Isik, A.F.; McBride, S.M.; Ren, J. Sex difference in alcoholism: who is at a greater risk for development of alcoholic complication? Life sciences 2010, 87, 133–138. [Google Scholar] [CrossRef]
- Lenz, B.; Müller, C.P.; Stoessel, C.; Sperling, W.; Biermann, T.; Hillemacher, T.; Bleich, S.; Kornhuber, J. Sex hormone activity in alcohol addiction: Integrating organizational and activational effects. Prog. Neurobiol. 2012, 96, 136–163. [Google Scholar] [CrossRef] [PubMed]
- Erol, A.; Karpyak, V.M. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015, 156, 1–13. [Google Scholar] [CrossRef]
- Kaun, K.R.; Devineni, A.V.; Heberlein, U. Drosophila melanogaster as a model to study drug addiction. Hum. Genet. 2012, 131, 959–975. [Google Scholar] [CrossRef]
- Katzenberger, R.J.; Loewen, C.A.; Wassarman, D.R.; Petersen, A.J.; Ganetzky, B.; Wassarman, D.A. A Drosophila Model of Closed Head Traumatic Brain Injury. Proc. Natl. Acad. Sci. USA 2013, 110, E4152–E4159. [Google Scholar] [CrossRef]
- Katzenberger, R.J.; Ganetzky, B.; A Wassarman, D. Age and Diet Affect Genetically Separable Secondary Injuries that Cause Acute Mortality Following Traumatic Brain Injury in Drosophila. G3 Genes|Genomes|Genetics 2016, 6, 4151–4166. [Google Scholar] [CrossRef]
- Barekat, A.; Gonzalez, A.; Mauntz, R.E.; Kotzebue, R.W.; Molina, B.; El-Mecharrafie, N.; Conner, C.J.; Garza, S.; Melkani, G.C.; Joiner, W.J. Using Drosophila as an integrated model to study mild repetitive traumatic brain injury. Sci. Rep. 2016, 6, 25252–25252. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Zhao, J.; Dash, P.K.; Soto, C.; Moreno-Gonzalez, I. Traumatic Brain Injury Induces Tau Aggregation and Spreading. J. Neurotrauma 2020, 37, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, A.; Takeuchi, H.; Tanaka, F. Tau Pathology in Chronic Traumatic Encephalopathy and Alzheimer’s Disease: Similarities and Differences. Front. Neurol. 2019, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Cantu, R.C.; Nowinski, C.J.; Hedley-Whyte, E.T.; Gavett, B.E.; Budson, A.E.; Santini, V.E.; Lee, H.-S.; Kubilus, C.A.; Stern, R.A. Chronic Traumatic Encephalopathy in Athletes: Progressive Tauopathy After Repetitive Head Injury. J. Neuropathol. Exp. Neurol. 2009, 68, 709–735. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Perry, G. Tau biology, tauopathy, traumatic brain injury, and diagnostic challenges. Journal of Alzheimer’s Disease 2019, 67, 447–467. [Google Scholar] [CrossRef]
- Avila, J.; Lucas, J.J.; Pérez, M.; Hernández, F. Role of Tau Protein in Both Physiological and Pathological Conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Trinczek, B.; Ebneth, A.; Mandelkow, E.M.; Mandelkow, E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J. Cell Sci. 1999, 112, 2355–2367. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Ross, J.L.; Goldman, Y.E.; Holzbaur, E.L.F. Differential Regulation of Dynein and Kinesin Motor Proteins by Tau. Science 2008, 319, 1086–1089. [Google Scholar] [CrossRef]
- Moraga, D.M.; Nuñez, P.; Garrido, J.; Maccioni, R.B. A τ Fragment Containing a Repetitive Sequence Induces Bundling of Actin Filaments. J. Neurochem. 1993, 61, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Widespread Tau and Amyloid-Beta Pathology Many Years After a Single Traumatic Brain Injury in Humans. Brain Pathol. 2011, 22, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Heidary, G.; E Fortini, M. Identification and characterization of the Drosophila tau homolog. Mech. Dev. 2001, 108, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Papanikolopoulou, K.; Roussou, I.G.; Gouzi, J.Y.; Samiotaki, M.; Panayotou, G.; Turin, L.; Skoulakis, E.M. Drosophila tau negatively regulates translation and olfactory long-term memory, but facilitates footshock habituation and cytoskeletal homeostasis. Journal of Neuroscience 2019, 39, 8315–8329. [Google Scholar] [CrossRef]
- Bolkan, B.J.; Kretzschmar, D. Loss of tau results in defects in photoreceptor development and progressive neuronal degeneration in Drosophila. Dev. Neurobiol. 2014, 74, 1210–1225. [Google Scholar] [CrossRef] [PubMed]
- Weil, Z.M.; Corrigan, J.D.; Karelina, K. Alcohol use disorder and traumatic brain injury. Alcohol research: current reviews 2018, 39, 171. [Google Scholar]
- Biegon, A. Considering Biological Sex in Traumatic Brain Injury. Front. Neurol. 2021, 12, 576366. [Google Scholar] [CrossRef]
- Gilthorpe, M.; Wilson, R.; Moles, D.; Bedi, R. Variations in admissions to hospital for head injury and assault to the head Part 1: age and gender. Br. J. Oral Maxillofac. Surg. 1999, 37, 294–300. [Google Scholar] [CrossRef]
- Devineni, A.V.; Heberlein, U. Acute ethanol responses in Drosophila are sexually dimorphic. Proc. Natl. Acad. Sci. 2012, 109, 21087–21092. [Google Scholar] [CrossRef]
- McDougall, A.; Bayley, M.; Munce, S.E. The ketogenic diet as a treatment for traumatic brain injury: a scoping review. Brain Inj. 2018, 32, 416–422. [Google Scholar] [CrossRef]
- Greco, T.; Glenn, T.C.; A Hovda, D.; Prins, M.L. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J. Cereb. Blood Flow Metab. 2016, 36, 1603–1613. [Google Scholar] [CrossRef]
- Delventhal, R.; Wooder, E.R.; Basturk, M.; Sattar, M.; Lai, J.; Bolton, D.; Muthukumar, G.; Ulgherait, M.; Shirasu-Hiza, M.M. Dietary restriction ameliorates TBI-induced phenotypes in Drosophila melanogaster. Sci. Rep. 2022, 12, 1–16. [Google Scholar] [CrossRef]
- van der Linde, K.; Fumagalli, E.; Roman, G.; Lyons, L.C. The FlyBar: Administering Alcohol to Flies. Journal of visualized experiments: JoVE 2014. [CrossRef]
- Buhlman, L.M.; Krishna, G.; Jones, T.B.; Thomas, T.C. Drosophila as a model to explore secondary injury cascades after traumatic brain injury. Biomed. Pharm. 2021, 142, 112079. [Google Scholar] [CrossRef]
- Shah, E.J.; Gurdziel, K.; Ruden, D.M. Mammalian Models of Traumatic Brain Injury and a Place for Drosophila in TBI Research. Front. Neurosci. 2019, 13, 409. [Google Scholar] [CrossRef]
- Poznanski, P.; Lesniak, A.; Korostynski, M.; Sacharczuk, M. Ethanol consumption following mild traumatic brain injury is related to blood-brain barrier permeability. Addict. Biol. 2020, 25, e12683. [Google Scholar] [CrossRef]
- Saikumar, J.; Byrns, C.N.; Hemphill, M.; Meaney, D.F.; Bonini, N.M. Dynamic neural and glial responses of a head-specific model for traumatic brain injury inDrosophila. Proc. Natl. Acad. Sci. 2020, 117, 17269–17277. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Gurdziel, K.; Liu, J.; Qu, W.; Nuga, O.O.; Burl, R.B.; Hüttemann, M.; Pique-Regi, R.; Ruden, D.M. Smooth, an hnRNP-L Homolog, Might Decrease Mitochondrial Metabolism by Post-Transcriptional Regulation of Isocitrate Dehydrogenase (Idh) and Other Metabolic Genes in the Sub-Acute Phase of Traumatic Brain Injury. Front. Genet. 2017, 8, 175–175. [Google Scholar] [CrossRef]
- Shah, E.J.; Gurdziel, K.; Ruden, D.M. Drosophila Exhibit Divergent Sex-Based Responses in Transcription and Motor Function After Traumatic Brain Injury. Front. Neurol. 2020, 11, 511. [Google Scholar] [CrossRef]
- Parkhurst, S.J.; Adhikari, P.; Navarrete, J.S.; Legendre, A.; Manansala, M.; Wolf, F.W. Perineurial Barrier Glia Physically Respond to Alcohol in an Akap200-Dependent Manner to Promote Tolerance. Cell Rep. 2018, 22, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Bainton, R.J.; Tsai, L.T.-Y.; Schwabe, T.; DeSalvo, M.; Gaul, U.; Heberlein, U. moody Encodes Two GPCRs that Regulate Cocaine Behaviors and Blood-Brain Barrier Permeability in Drosophila. Cell 2005, 123, 145–156. [Google Scholar] [CrossRef]
- Contreras, E.G.; Glavic, Á.; Brand, A.H.; Sierralta, J.A. The serine protease homolog, Scarface, is sensitive to nutrient availability and modulates the development of the Drosophila blood–brain barrier. Journal of Neuroscience 2021, 41, 6430–6448. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.; Kaun, K.R. Alcohol, neuronal plasticity, and mitochondrial trafficking. Proceedings of the National Academy of Sciences 2022, 119, e2208744119. [Google Scholar] [CrossRef]
- Knabbe, J.; Protzmann, J.; Schneider, N.; Berger, M.; Dannehl, D.; Wei, S.; Strahle, C.; Tegtmeier, M.; Jaiswal, A.; Zheng, H.; et al. Single-dose ethanol intoxication causes acute and lasting neuronal changes in the brain. Proc. Natl. Acad. Sci. 2022, 119, e2122477119. [Google Scholar] [CrossRef]
- Tapia-Rojas, C.; Carvajal, F.J.; Mira, R.G.; Arce, C.; Lerma-Cabrera, J.M.; Orellana, J.A.; Cerpa, W.; Quintanilla, R.A. Adolescent binge alcohol exposure affects the brain function through mitochondrial impairment. Molecular neurobiology 2018, 55, 4473–4491. [Google Scholar] [CrossRef]
- Oh, K.H.; Sheoran, S.; Richmond, J.E.; Kim, H. Alcohol induces mitochondrial fragmentation and stress responses to maintain normal muscle function in Caenorhabditis elegans. FASEB J. 2020, 34, 8204–8216. [Google Scholar] [CrossRef]
- Müller, T.E.; Nunes, M.E.; Rodrigues, N.R.; Fontana, B.D.; Hartmann, D.D.; Franco, J.L.; Rosemberg, D.B. Neurochemical mechanisms underlying acute and chronic ethanol-mediated responses in zebrafish: The role of mitochondrial bioenergetics. Neurochem. Int. 2019, 131, 104584. [Google Scholar] [CrossRef]
- Petruccelli, E.; Kaun, K.R. Insights from intoxicated Drosophila. Alcohol 2019, 74, 21–27. [Google Scholar] [CrossRef]
- Troutwine, B.R.; Ghezzi, A.; Pietrzykowski, A.Z.; Atkinson, N.S. Alcohol resistance in Drosophila is modulated by the Toll innate immune pathway. Genes, Brain Behav. 2016, 15, 382–394. [Google Scholar] [CrossRef]
- Kong, E.C.; Allouche, L.; Chapot, P.A.; Vranizan, K.; Moore, M.S.; Heberlein, U.; Wolf, F.W. Ethanol-Regulated Genes That Contribute to Ethanol Sensitivity and Rapid Tolerance in Drosophila. Alcohol. Clin. Exp. Res. 2010, 34, 302–316. [Google Scholar] [CrossRef]
- Morozova, T.V.; Anholt, R.R.; Mackay, T.F. Phenotypic and transcriptional response to selection for alcohol sensitivity in Drosophila melanogaster. Genome Biol. 2007, 8, R231–R231. [Google Scholar] [CrossRef]
- van Alphen, B.; Stewart, S.; Iwanaszko, M.; Xu, F.; Li, K.; Rozenfeld, S.; Ramakrishnan, A.; Itoh, T.Q.; Sisobhan, S.; Qin, Z.; et al. Glial immune-related pathways mediate effects of closed head traumatic brain injury on behavior and lethality in Drosophila. PLOS Biol. 2022, 20, e3001456. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, D.; Leyssen, M.; Koch, M.; Yan, J.; Srahna, M.; Sheeba, V.; Fogle, K.J.; Holmes, T.C.; Hassan, B.A. Axonal Injury and Regeneration in the Adult Brain of Drosophila. J. Neurosci. 2008, 28, 6010–6021. [Google Scholar] [CrossRef]
- Soares, L.; Parisi, M.; Bonini, N.M. Axon Injury and Regeneration in the Adult Drosophila. Sci. Rep. 2014, 4, srep06199. [Google Scholar] [CrossRef]
- Wilson, A.; Periandri, E.M.; Sievers, M.; Petruccelli, E. Drosophila Stat92E Signaling Following Pre-exposure to Ethanol. Neurosci. Insights 2023, 18, 26331055221146755. [Google Scholar] [CrossRef]
- Weber, J.T. Altered Calcium Signaling Following Traumatic Brain Injury. Front. Pharmacol. 2012, 3, 60. [Google Scholar] [CrossRef]
- He, H.J.; Wang, X.S.; Pan, R.; Wang, D.L.; Liu, M.N.; He, R.Q. The proline-rich domain of tau plays a role in interactions with actin. BMC Cell Biol. 2009, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sealey, M.A.; Vourkou, E.; Cowan, C.M.; Bossing, T.; Quraishe, S.; Grammenoudi, S.; Skoulakis, E.M.; Mudher, A. Distinct phenotypes of three-repeat and four-repeat human tau in a transgenic model of tauopathy. Neurobiol. Dis. 2017, 105, 74–83. [Google Scholar] [CrossRef]
- Walker, A.; Chapin, B.; Abisambra, J.; DeKosky, S.T. Association between single moderate to severe traumatic brain injury and long-term tauopathy in humans and preclinical animal models: a systematic narrative review of the literature. Acta Neuropathol. Commun. 2022, 10, 1–20. [Google Scholar] [CrossRef]
- Medina, M.; Hernández, F.; Avila, J. New Features about Tau Function and Dysfunction. Biomolecules 2016, 6, 21. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nature reviews neuroscience 2016, 17, 22–35. [Google Scholar] [CrossRef]
- Burnouf, S.; Grönke, S.; Augustin, H.; Dols, J.; Gorsky, M.K.; Werner, J.; Kerr, F.; Alic, N.; Martinez, P.; Partridge, L. Deletion of endogenous Tau proteins is not detrimental in Drosophila. Sci. Rep. 2016, 6, 23102. [Google Scholar] [CrossRef]
- Harada, A.; Oguchi, K.; Okabe, S.; Kuno, J.; Terada, S.; Ohshima, T.; Sato-Yoshitake, R.; Takei, Y.; Noda, T.; Hirokawa, N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 1994, 369, 488–491. [Google Scholar] [CrossRef]
- Shah, E.J.; Gurdziel, K.; Ruden, D.M. Sex-Differences in Traumatic Brain Injury in the Absence of Tau in Drosophila. Genes 2021, 12, 917. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.S.; DeZazzo, J.; Luk, A.Y.; Tully, T.; Singh, C.M.; Heberlein, U. Ethanol Intoxication in Drosophila: Genetic and Pharmacological Evidence for Regulation by the cAMP Signaling Pathway. Cell 1998, 93, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.H.; Kong, E.C.; Dubnau, J.; Tully, T.; Moore, M.S.; Heberlein, U. Ethanol Sensitivity and Tolerance in Long-Term Memory Mutants of Drosophila melanogaster. Alcohol. Clin. Exp. Res. 2008, 32, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Endo, K.; Wu, K.; Rodan, A.R.; Heberlein, U.; Davis, R.L. Drosophila fasciclinII Is Required for the Formation of Odor Memories and for Normal Sensitivity to Alcohol. Cell 2001, 105, 757–768. [Google Scholar] [CrossRef]
- Xu, S.; Pany, S.; Benny, K.; Tarique, K.; Al-Hatem, O.; Gajewski, K.; Leasure, J.L.; Das, J.; Roman, G. Ethanol Regulates Presynaptic Activity and Sedation through Presynaptic Unc13 Proteins inDrosophila. eneuro 2018, 5. [Google Scholar] [CrossRef]
- Myers, J.L.; Porter, M.; Narwold, N.; Bhat, K.; Dauwalder, B.; Roman, G. Mutants of the white ABCG Transporter in Drosophila melanogaster Have Deficient Olfactory Learning and Cholesterol Homeostasis. Int. J. Mol. Sci. 2021, 22, 12967. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Kollah, A.P.; Lewellyn, L.; Chan, R.F.; Grotewiel, M. An inexpensive, scalable behavioral assay for measuring ethanol sedation sensitivity and rapid tolerance in Drosophila. JoVE (Journal of Visualized Experiments) 2015, e52676. [CrossRef]
- van der Linde, K.; Fumagalli, E.; Roman, G.; Lyons, L.C. The FlyBar: Administering Alcohol to Flies. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.Y.; Wachi, Y.; Engdorf, E.; Fumagalli, E.; Wang, Y.; Myers, J.; Massey, S.; Greiss, A.; Xu, S.; Roman, G. Normal Ethanol Sensitivity and Rapid Tolerance Require the G Protein Receptor Kinase 2 in Ellipsoid Body Neurons in Drosophila. Alcohol. Clin. Exp. Res. 2020, 44, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J Am Stat Assoc 1952, 47, 583–621. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




