Submitted:
01 February 2024
Posted:
02 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- (1)
- Systematic testing and treatment of latent TB infection in adult contacts of pulmonary TB cases
- (2)
- Systematic testing and treatment of latent TB infection in immigrants from high TB burden countries.
- (3)
- Detection of latent TB infection based on interferon-gamma release assays (IGRA) or Mantoux tuberculin skin test (TST).
- (4)
- Detection of TB disease in individuals with TB symptoms or radiological abnormalities.
- (5)
- Administration of TB preventive treatments in individuals with latent TB infection.
- (6)
- Clinical monitoring of individuals receiving TB preventive treatment.
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.2. Data Analysis
2.3. Bivariate Correlation among Study Variables
2.4. Multivariate Logistic Regression Analysis
3. Results
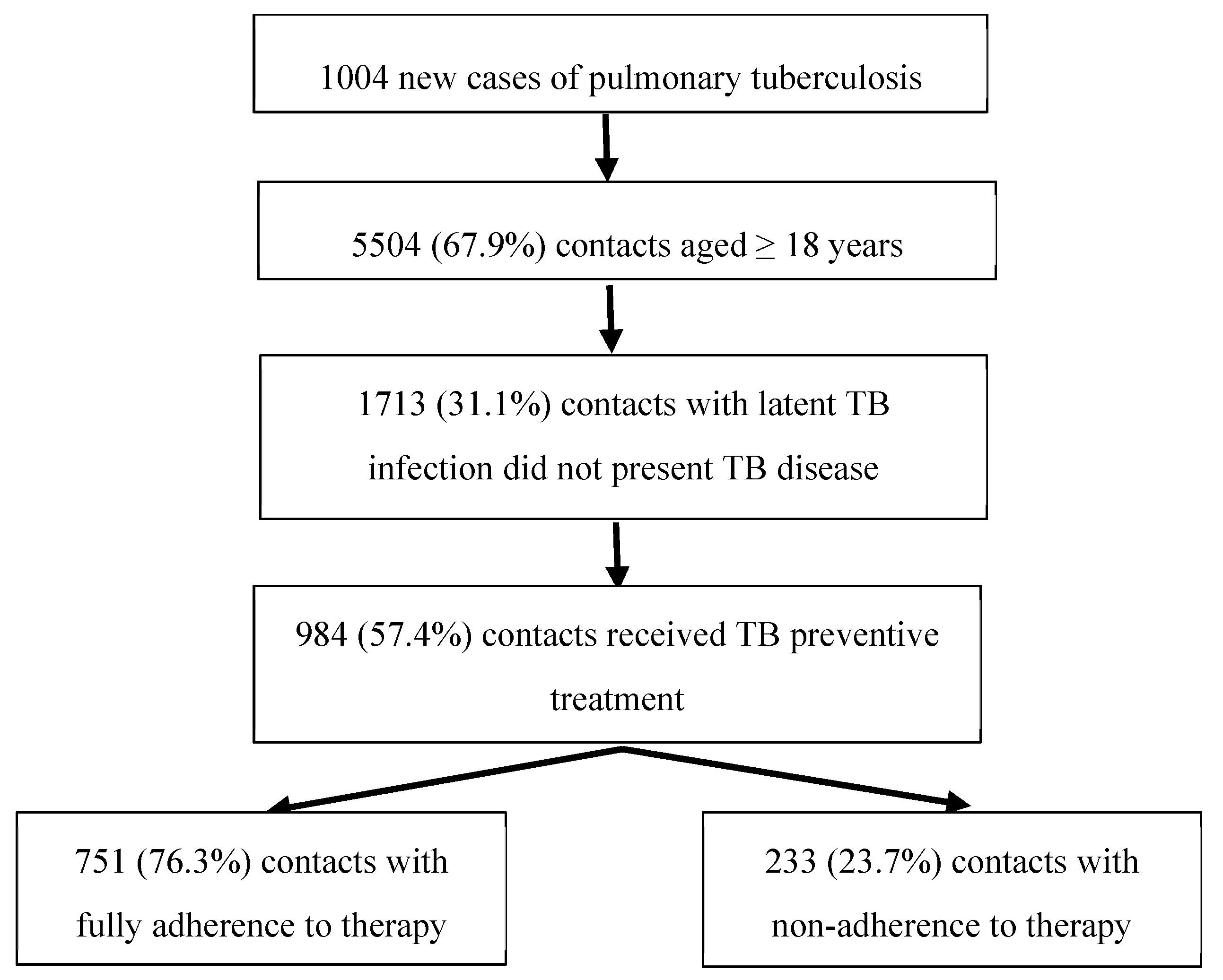
3.1. Population Studied
3.1. Non-adherence to TB Preventive Treatement
3.2. Bivariate Correlation among Study Variables
3.3. Multivariate Logistic Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, M.; Dorman, S.E. Latent Tuberculosis Infection. N. Engl. J. Med 2021, 385, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global tuberculosis report 2023; World Health Organization: Geneva, 2023. [Google Scholar]
- Menzies, N.A.; Wolf, E.; Connors, D.; Bellerose, M.; Sbarra, A.N.; Cohen, T.; Hill, A.N.; Yaesoubi, R.; Galer, K.; White, P.J.; Abubakar, I.; Salomon, J.A. Progression from latent infection to active disease in dynamic tuberculosis transmission models: a systematic review of the validity of modelling assumptions. Lancet Infect. Dis. 2018; 18, e228–e38. [Google Scholar]
- World Health Organization (WHO). The End TB strategy. World Health Organization: Geneve, 2015; Available online: https://iris.who.int/bitstream/handle/10665/331326/WHO-HTM-TB-2015.19-eng.pdf?sequence=1 (accessed on 22 December 2023).
- United Nations (UN). Resolution adopted by the General Assembly on 5 October 2023. Political declaration of the high-level meeting on the fight against tuberculosis. Advancing science, finance and innovation, and their benefits, to urgently end the global tuberculosis epidemic, in particular by ensuring equitable access to prevention, testing, treatment and care. Available online: https://documents-dds-ny.un.org/doc/UNDOC/GEN/N23/306/91/PDF/N2330691.pdf?OpenElement (accessed on 12 January 2024).
- World Health Organization (WHO). Consolidated guidelines on tuberculosis. Module 1: prevention – tuberculosis preventive treatment; World Health Organization: Geneva, 2020. [Google Scholar]
- World Health Organization (WHO). Guidelines on the management of latent tuberculosis infection; WHO: Genera, 2015. [Google Scholar]
- Getahun, H.; Matteelli, A.; Abubakar, I.; Aziz, M.A.; Baddeley, A.; Barreira, D.; Den Boon, S.; Borroto Gutierrez, S.M.; Bruchfeld, J.; Burhan, E.; Cavalcante, S.; Cedillos, R.; Chaisson, R.; Chee, C.B.; Chesire, L.; Corbett, E.; Dara, M.; Denholm, J.; de Vries, G.; Falzon, D.; Ford, N.; Gale-Rowe, M.; Gilpin, C.; Girardi, E.; Go, U.Y.; Govindasamy, D.; Grant, A.; Grzemska, M.; Harris, R.; Horsburgh, C.R.; Ismayilov, A.; Jaramillo, E.; Kik, S.; Kranzer, K.; Lienhardt, C.; LoBue, P.; Lönnroth, K.; Marks, G.; Menzies, D.; Migliori, G.B.; Mosca, D.; Mukadi, Y.D.; Mwinga, A.; Nelson, L.; Nishikiori, N.; Oordt-Speets, A.; Rangaka, M.X.; Reis, A.; Rotz, L.; Sandgren, A.; Sañé Schepisi, M.; Schünemann, H.J.; Sharma, S.K.; Sotgiu, G.; Stagg, H.R.; Sterling, T.R.; Tayeb, T.; Uplekar, M.; van der Werf, M.J.; Vandevelde, W.; van Kessel, F.; van't Hoog, A.; Varma, J.K.; Vezhnina, N.; Voniatis, C.; Vonk Noordegraaf-Schouten, M.; Weil, D.; Weyer, K.; Wilkinson, R.J.; Yoshiyama, T.; Zellweger, J.P.; Raviglione, M. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur. Respir. J. 2015, 46, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Stagg, H.R.; Zenner, D.; Harris, R.J.; Muñoz, I.; Lipman, M.C.; Abubakar, I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann. Intern. Med. 2014, 161, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, M.; Brugueras, S.; de Andre´s, A.; Simon, P.; Gorrindo, P.; Ros, M.; et al. Tuberculosis incidence among infected contacts detected through contact tracing of smear-positive patients. PLoS ONE 2019, 14, e0215322. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Programmatic management of latent tuberculosis infection in the European Union; ECDC: Stockholm, 2018. [Google Scholar]
- Departament de Salut. Memòria de la Secretaria de Salut Pública; Departament de Salut: Barcelona, 2023; Available online: https://salutpublica.gencat.cat/web/.content/minisite/aspcat/publicacio_formacio_recerca/publicacions/corporatives/memoria-sp-2021.pdf.
- Patel, A.R.; Campbell, J.R.; Sadatsafavi, M.; Marra, F.; Johnston, J.C.; Smillie, K.; Lester, R.T. Burden of non-adherence to latent tuberculosis infection drug therapy and the potential cost-effectiveness of adherence interventions in Canada: a simulation study. BMJ Open 2017, 7, e015108. [Google Scholar] [CrossRef] [PubMed]
- Stuurman, A.L.; Vonk Noordegraaf-Schouten, M.; van Kessel, F.; Oordt-Speets, A.M.; Sandgren, A.; van der Werf, M.J. Interventions for improving adherence to treatment for latent tuberculosis infection: a systematic review. BMC Infect. Dis. 2016, 16, 257. [Google Scholar] [CrossRef] [PubMed]
- Pina-Gutiérrez, J.M.; Ferrer-Traid, A.; Arias, C.; Sala-Farré, M.R.; López-Sanmartín, J.I. Cumplimiento y efectividad del tratamiento de la infección tuberculosa con isoniazida durante 9 meses en una cohorte de 755 pacientes. Med. Clin. (Barcelona) 2018, 103, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Ambrona de Marcos, V.; Bach Foradada, P.; Alsedà Graells, M.; Duque Jiménez, T.; Delgado Roche, E.; Aguilar Ariza, R.; Bravo Andrés, N.; Godoy, P. Cumplimiento del tratamiento de la infección tuberculosa latente en una cohorte de contactos de enfermos de tuberculosis. Rev. Esp. Salud Pública 2018, 92, e1–e11. [Google Scholar]
- Gallardo, C.R.; Gea Velázquez de Castro, M.T.; Requena Puche, J.; Miralles Bueno, J.J.; Rigo Medrano, M.V.; Aranaz Andrés, J.M. Factores asociados a la adherencia en el tratamiento de la infección tuberculosa. Aten. Primaria. 2014, 46, 6–14. [Google Scholar] [CrossRef]
- Puyana Ortiz, J.D.; Garcés Rodríguez, A.C.; Aznar, M.L.; Espinosa Pereiro, J.; Sánchez-Montalvá, A.; Martínez-Campreciós, J.; Saborit, N.; Rodrigo-Pendás, J.Á.; García Salgado, G.; Broto Cortes, C.; Delcor, N.S.; Oliveira, I.; Treviño Maruri, B.; Ciruelo, D.P.; Salvador, F.; Bosch-Nicolau, P.; Torrecilla-Martínez, I.; Zules-Oña, R.; Tórtola Fernández, M.T.; Molina, I. Adherence and Toxicity during the Treatment of Latent Tuberculous Infection in a Referral Center in Spain. Trop. Med. Infect. Dis. 2023, 8, 373. [Google Scholar] [CrossRef]
- Liu, Y.; Birch, S.; Newbold, K.B.; Essue, B.M. Barriers to treatment adherence for individuals with latent tuberculosis infection: A systematic search and narrative synthesis of the literature. Int. J. Health Plann. Manage. 2018, 33, e416–e433. [Google Scholar] [CrossRef] [PubMed]
- Schein, Y.L; Madebo, T.; Andersen, H.E.; Arnesen, T.M.; Dyrhol-Riise, A.M.; Tveiten, H.; White, R.A.; Winje, B.A. Treatment completion for latent tuberculosis infection in Norway: a prospective cohort study. BMC Infect. Dis. 2018, 18, 587. [Google Scholar] [CrossRef] [PubMed]
- Kan, B.; Kalin, M.; Bruchfeld, J. Completing treatment for latent tuberculosis: patient background matters. Int. J. Tuberc. Lung Dis. 2013, 17, 597–602. [Google Scholar] [CrossRef]
- Hirsch-Moverman, Y.; Daftary, A.; Franks, J.; Colson, P.W. Adherence to treatment for latent tuberculosis infection: systematic review of studies in the US and Canada. Int. J. Tuberc. Lung Dis. 2008, 12, 1235–1254. [Google Scholar] [PubMed]
- Rustage, K.; Lobe, J.; Hayward, S.E.; Kristensen, K.L.; Margineanu, I.; Stienstra, Y.; Goletti, D.; Zenner, D.; Noori, T.; Pareek, M.; Greenaway, C.; Friedland, J.S.; Nellums, L.B.; Hargreaves, S.; ESGITM and ESGMYC study groups. Initiation and completion of treatment for latent tuberculosis infection in migrants globally: a systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 1701–1712. [Google Scholar] [CrossRef]
- Spyridis, N.P.; Spyridis, P.G.; Gelesme, A.; Sypsa, V.; Valianatou, M.; Metsou, F.; Gourgiotis, D.; Tsolia, M.N. The effectiveness of a 9-month regimen of isoniazid alone versus 3-and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin. Infect. Dis. 2007, 45, 715–722. [Google Scholar] [CrossRef]
- Sterling, T.R.; Njie, G.; Zenner, D.; Cohn, D.L.; Reves, R.; Ahmed, A.; Menzies, D.; Horsburgh, C.R.; Crane, C.M.; Burgos, M.; LoBue, P.; Winston, C.A.; Belknap, R. Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm. Rep. 2020, 69, 1–11. [Google Scholar] [CrossRef]
- Chee, C.B.E.; Reves, R.; Zhang, Y.; Belknap, R. Latent tuberculosis infection: Opportunities and challenges. Respirology 2018, 10, 893–900. [Google Scholar] [CrossRef]
- Shah, R.; Khakhkhar, T.; Modi, B. Efficacy and Safety of Different Drug Regimens for Tuberculosis Preventive Treatment: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e38182. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Implementing the end TB strategy: essentials; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization (WHO). Latent TB Infection: Updated and consolidated guidelines for programmatic management. World Health Organization: Geneva, 2018; Available online: http://apps.who.int/iris/bitstream/handle/10665/260233/9789241550239-eng.pdf (accessed on 7 January 2023).
- Centers for Disease Control and Prevention CDC. Reported Tuberculosis in the United States:, 2016; US Department of Health and Human Services; CDC: Atlanta, GA, 2017. Available online: https://www.cdc.gov/tb/statistics/reports/2016/pdfs/2016_Surveillance_FullReport.pdf (accessed on 12 January 2024).
- Taylor, Z.; Marks, S.M.; Ríos Burrows, N.M.; Weis, S.E.; Stricof, R.L.; Miller, B. Causes and costs of hospitalization of tuberculosis patients in the United States. Int. J. Tuberc. Lung Dis. 2000, 4, 931−939. [Google Scholar]
- Vrijens, B.; De, G.S.; Hughes, D.A.; Przemys law, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; Matyjaszczyk, M.; Mshelia, C.; Clyne, W.; Aronson, J.K.; Urquhart, J.; ABC Project Team. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Stagg, H.R.; Flo Stagg, H.R.; Flook, M.; Martinecz, A.; Kielmann, K.; Abel Zur Wiesch, P.; Karat, A.S.; Lipman, M.C.I.; Sloan, D.J.; Walker, E.F.; Fielding, K.L. All nonadherence is equal but is some more equal than others? Tuberculosis in the digital era. ERJ Open Res. 2020, 6, 00315–2020. [Google Scholar] [CrossRef] [PubMed]
- Menzies, D.; Dion, M.J.; Rabinovitch, B.; Mannix, S.; Brassard, P.; Schwartzman, K. Treatment completion and costs of a randomized trial of rifampin for 4 months versus isoniazid for 9 months. Am. J. Respir. Crit. Care Med. 2004, 170, 445–449. [Google Scholar] [CrossRef] [PubMed]
|
Variable |
Values |
Non-adherence to TB preventive treatment among contacts of pulmonary TB cases | |||
| No. | % (95% CI) | OR (95% CI) | n | ||
| Age (years) | 18 – 29 30 – 44 45 – 64 ≥64 |
47 75 99 12 |
20.9 (15.3−26.4) 23.4 (16.6−28.2) 25.7 (21.2−30.2) 22.2 (10.2−34.2) |
Reference 1.15 (0.77−1.75) 1.31 (0.88−1.94) 1.08 (0.53−2.21) |
225 320 385 54 |
| Total | 233 | 23.7 (21.0−26.4) | − | 984 | |
| Age (years) | 18 – 29 ≥ 30 years |
47 186 |
20.9 (15.3−26.4) 24.5 (21.4−27.6) |
Reference 1.23 (0.86−1.74) |
225 759 |
| Gender |
Male Female |
142 91 |
26.2 (22.4−30.0)+ 20.6 (16.7−24.5) |
1.37 (1.01−1.85) Reference |
542 442 |
| TB preventive treatment regimena |
Short-term Long-term Undefined |
51 68 114 |
14.6 (10.7−18.4) 26.3 (20.7−31.8)* 30.4 (25.1−32.3)* |
Reference 2.09 (1.39−3.13) 2.54 (1.76−3.68) |
350 259 375 |
| TB preventive treatment regimena | Short-term Long-term or undefined |
51 182 |
14.6 (10.7−18.4) 28.7 (25.1−32.3)* |
Reference 2.36 (1.67−3.32) |
350 634 |
| Exposure duration |
≥6 hours per day <6 h/day and ≥ 6 h/week <6 hours per week Sporadic exposure |
119 35 49 23 |
21.9 (14.8−21.0) 18.1 (30.3−41.6) 37.8 (25.0−26.1)** 28.0 (16.1−55.2) |
1.26 (0.83−1.92) Reference 2.30 (1.39−3.80) 1.76 (0.96−3.22) |
544 193 145 82 |
| Exposure duration |
≥ 6 hours per week <6 hours per week or Sporadic exposure |
154 72 |
20.9 (17.9−23.9)* 31.7 (25.4−38.0) |
Reference 1.76 (1.26−2.45) |
737 227 |
| Exposure type |
Cohabiting household Workplace Recreational School |
110 106 6 10 |
17.9 (14.8−21-0)* 35.9 (30.3−41.6) 14.3 (2.5−26.1) 35.7 (16.1−55.2) |
2.57 (1.88−3.53) 3.36 (1.37−8.25) Reference 1.01 (0.44−2.27) |
615 295 42 28 |
| Exposure type |
Cohabiting household or recreational Workplace or school |
116 116 |
17.7 (14.7−20.6)* 35.9 (30.5−41.3) |
Reference 2.61 (1.93−3.53) |
657 323 |
| Immigrant contact |
Yes No |
115 118 |
27.3 (22.9−31.7)+ 20.9 (17.5−24.4) |
1.42 (1.05−1.90) Reference |
421 563 |
| Smoking habit |
Daily smoker Occasional smoker Ex-smoker Never smoker |
51 49 3 67 |
28.5 (21.6−35.4) 24.9 (18.6−31.2) 14.5 (3.0−36.3) 19.8 (15.4−24.2)+ |
2.39 (0.67−8.47) 1.99 (0.56−7.03) Reference 1.48 (0.42−5.18) |
179 197 21 338 |
| Smoking habit |
Yes No |
100 70 |
26.6 (22.0−31.2)+ 19.5 (15.3−23.7) |
1.50 (1.06−2.11) Reference |
376 359 |
| BCG vaccination |
Yes No |
74 71 |
24.9 (19.8−30.0) 19.2 (15.0−23.3) |
1.37 (0.95−1.99) Reference |
297 370 |
| High-risk alcohol consumption | Yes No |
19 147 |
35.2 (21.5−48.8)+ 21.8 (18.6−25.0) |
1.94 (1.08−3.51) Reference |
54 674 |
| Exposure to an ndex case without laboratory confirmation of TB | Yes No |
34 192 |
38.2 (27.5−48.9)* 22.2 (19.6−25.3) |
2.16 (1.37−3.42) Reference |
89 854 |
| Exposure to an index case with pulmonary TB anomalies detected by computed tomography | Yes No |
49 124 |
25.8 (19.3−32.4) 24.7 (20.8−28.6) |
1.06 (0.72−1.55) Reference |
190 502 |
|
Variable Values compared |
Full regression model | Reduced regression model | |||
| aOR (95% CI) | p | aOR (95% CI) | p | ||
| Sex | men vs. women | 1.56 (0.94−2.57) | 0.012 | 1.75 (0.97−2.63) | 0.007 |
| Exposure duration |
<6 hours per week or sporadic vs. ≥6 hours per week | 1.54 (0.91−2.47) | 0.072 | 1.60 (0.93−2.55) | 0.049 |
| Exposure type |
workplace or school vs. cohabiting household or recreational |
3.19 (1.57−4.81) | <0.001 | 3.34 (1.61−5.00) | <0.001 |
| Smoking habit | yes vs. no | 1.41 (0.81−2.25) | 0.148 | − | − |
| High-risk alcohol consumption | yes vs. no | 0.93 (0.65−1.92) | 0.839 | − | − |
| Immigrant contact | yes vs. no | 2.00 (1.13−3.11) | 0.002 | 1.81 (1.01−2.72) | 0.004 |
| TB preventive treatment regimen | short-term vs. long-term or undefined | 0.44 (−0.34−0.71) | 0.001 | 0.38 (−0.49−0.61) | <0.001 |
| Exposure to an index case without laboratory TB confirmation | yes vs. no | 2.15 (1.38−3.98) | 0.014 | 2.07 (1.33−3.76) | <0.001 |
| Constant | 0.0.6 (−2.05−0.13) | <0.001 | 0.14 (−1.08−0.34) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





