1. Introduction
Cancer, as the second leading cause of death in the world, has seriously hindered the healthy development of human body, and is one of the problems to be solved urgently in the world [
1]. At present, the traditional means of cancer treatment, such as surgery, radiotherapy and chemotherapy, have achieved encouraging clinical results. However, traditional treatment is often accompanied by clinical symptom specificity, low tumor response rate and patient resistance. Therefore, the treatment of cancer still needs new treatment methods to avoid the problems and limitations of traditional treatment methods.
The 2018 Nobel Prize in Physiology or Medicine was awarded to James P. Allison (an United States immunologist, and Tasuku Honjo ((Japan immunologist (for their discovery of cancer therapies that inhibit negative immune regulation, which has successfully promoted the rapid development of tumor immunotherapy [2-4]. In recent year, tumor immunotherapy has gradually overcome various difficulties in that treatment of malignant tumor, among which immune checkpoint blocking (ICB), chimeric antigen receptor (CAR) cell therapy and tumor vaccines have shown excellent therapeutic effects. NK cell-based tumor immunotherapy is also becoming one of the clinically effective treatment methods [
5]. NK cells, as innate immune cells, mainly rely on the balance of cell surface signals to maintain the stability of patients ’tissues, and use their own cytotoxicity to monitor and kill tumor cells. This is different from T cells that need specific antigen stimulation, so the clinical application prospect of anti-tumor based on NK cells is relatively broader [
6].
However, NK cell-based immunotherapy also faces problems such as decreased cell viability, delayed cell homing, tumor immunosuppression and insufficient supply of NK cells. However, nanomaterials have successfully promoted the progress of NK cell immunotherapy by virtue of their own effective load transfer, interaction with immune cells and modification of material structure [7-9]. Moreover, the compatibility of NK cells and the versatility of nanomaterials also show great potential for new immunotherapy.
This review focuses on the research progress of NK cell-based immunotherapy from the perspective of tumor immunotherapy methods and research status, clarifies the application prospect and limitations of NK cell-based immunotherapy, and introduces the research progress and application status of nanomaterials according to its limitations. Finally, the clinical advantages and specific mechanisms of NK cell-based nanomaterials in tumor immunotherapy are summarized to achieve technical progress and improve the efficacy of tumor immunotherapy.
2. Tumor Immunotherapy
The occurrence of malignant tumor has brought great challenge to the development of human health. Currently, the most widely used cancer therapies include surgery, chemoradiotherapy, etc. However, tumor metastasis, patient resistance and clinical heterogeneity still lead to the low efficacy of traditional therapies. In recent years, with the development of science and technology, tumor immunotherapy has emerged as the times require. In 1893, William Coley, an United States surgeon, discovered that bacteria could inhibit the development of sarcoma, which was the first time that immunosuppression was found in the history of human tumor [
10]. In the 1980s and 1990s, the interaction between immune cells and melanoma was discovered, and the concept of tumor immunotherapy was put forward.[
11,
12]. In recent years, clinical studies have proved that tumor immunotherapy can not only prolong the survival time of patients, but also significantly improve the quality of life of patients [
13].
2.1. Tumor Immunotherapy Related Cells
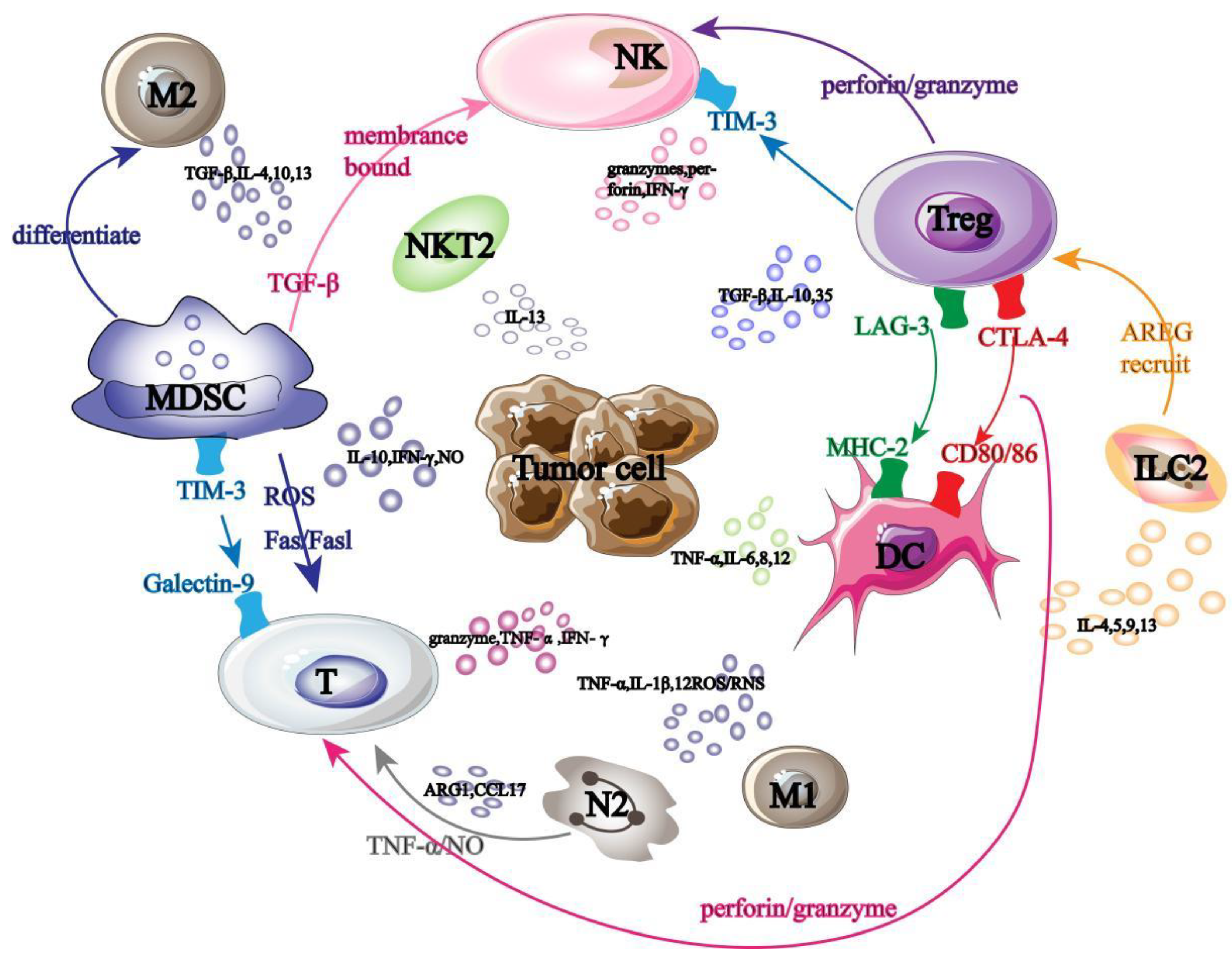
Tumor immunotherapy-related cells fall into two main categories: anti-tumor immune cells and pro-tumor immune cells. The antitumor cells mainly include T lymphocyte cells, NK cells, dendritic cells and M1 macrophages. Tumor promoting cells are composed of Treg (Regulatory Cells, Treg) cells, MDSC (Myeloid-derived Suppressor Cells, MDSC) cells, M2 macrophages, N2 neutrophils, ILC2 (Group 2 innate lymphoid Cells, ILC2) cells and NKT2 (Natural Killer T Cells, NKT2) cells. These immune cells interfere with each other and jointly participate in tumor immunotherapy (
Figure 1).
Among them, T cells, as the primary executor of tumor immunotherapy, mainly use the secretion of granzyme and tumor cell apoptosis induced by death ligand to play a role in killing tumor [
14]; DC cells can initiate adaptive immune response by stimulating T cell activation [
15,
16]; NK cells use the mechanism of "missing self" to prevent the binding of autoinhibitory receptor to MHC-1 on tumor surface, and then activate themselves to transmit anti-tumor immune signals [
17,
18]; M1 macrophages can not only promote Th1 cell recruitment by secreting chemokines, but also release ROS/RNS to directly kill tumor cells [19-21].
However, tumor-promoting cells play an opposite immune role, for example, Treg cells suppress tumor immune response by secreting immunosuppressive cytokines [
22], while MDSC cells damage T cell function by interacting with Treg cells, and can also reduce the number of NK cells and prevent them from killing tumors [
23]; M2 macrophages and N2 neutrophils secrete immunosuppressive factors and chemokines respectively to promote Treg cell recruitment and MDSC cell differentiation and transmit immunosuppressive signals [24-27]; ILC2 promotes tumor cell immune escape by inhibiting NK cell activity and T cell immune response [
28,
29].
2.2. Tumor Immunotherapy
2.2.1. Immune Checkpoint Blocking (ICB)
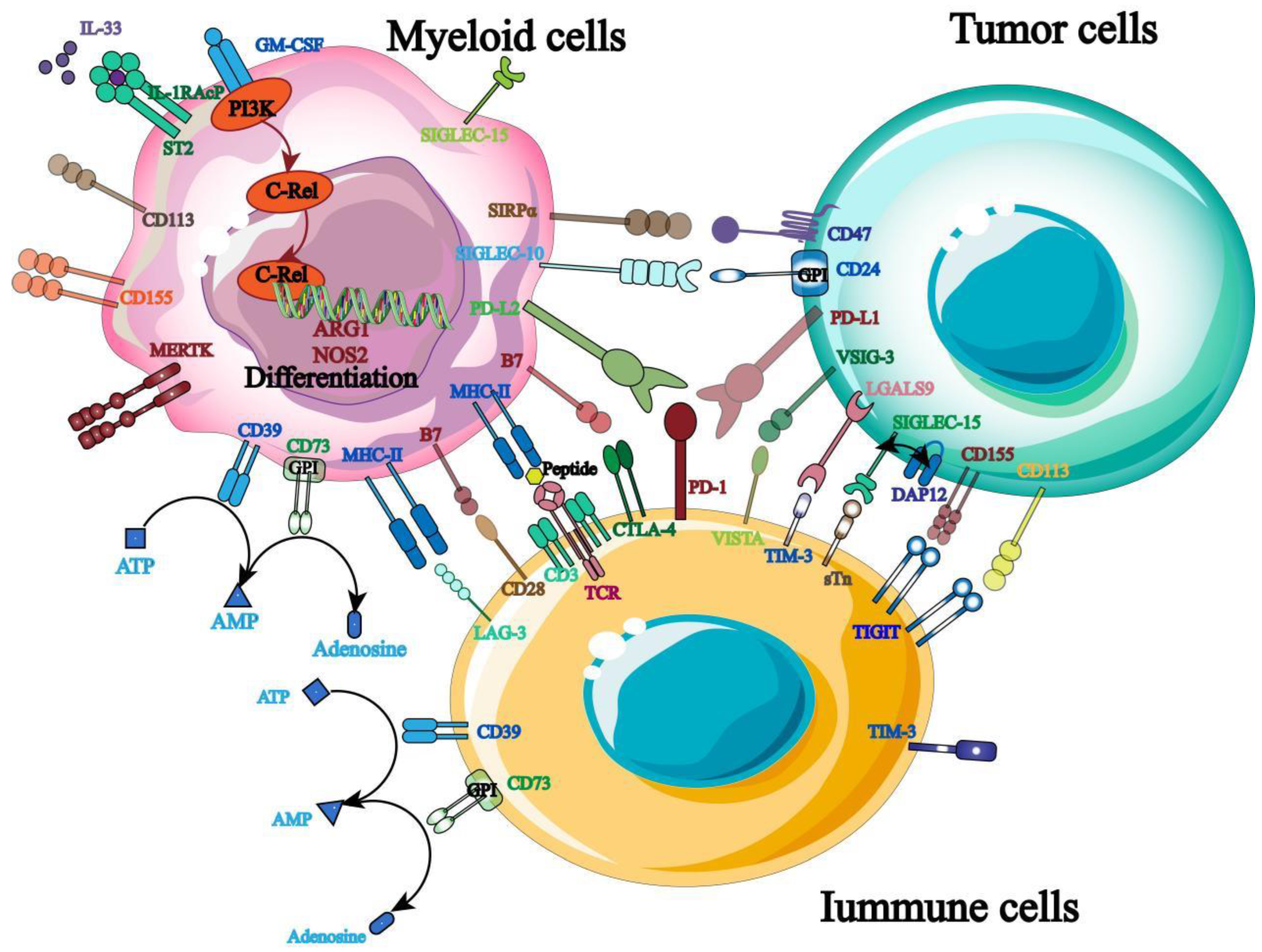
Immune target is one of the key factors to maintain the stability of immune homeostasis and host survival, so keeping the balance of immune signals is the main means to protect host cells from foreign antigen damage. At present, ICB is widely used in solid tumors and hematologic malignancies, and more and more tumor immunotherapy targets have emerged (
Figure 2). Three of the most widely used targets for clinical treatment are described below.
PD-1: As an inhibitory receptor, PD-1 mainly prevents the patient’s own immune response by inhibiting the immune function of T cells [
30], and its binding ligands mainly include PD-L1 and PD-L2 highly expressed on the surface of tumor cells. Its specific mechanism of action is to use ligand binding to conduct negative co-stimulation signal to inhibit T cell activation and mediate tumor cell immune escape [
31,
32]. In addition, studies have shown that PD-1 is not only expressed on the surface of T cells, but also on the surface of NK cells, DC cells and other immune cells, which indicates that PD-1 may control the systemic anti-tumor immune response, so the subsequent tumor immunotherapy can start from blocking this target.[
33].
At present, PD-1 inhibitors have been widely used in clinical practice. Studies have shown that PD-1 inhibitors can not only inhibit tumor cell proliferation, but also directly mediate tumor cell apoptosis by binding with PD-L1 [
34]. For example, the PD-1 blocking antibody Opdivo was approved in 2015 for the treatment of advanced squamous cell lung cancer, marking the first clinical use of anti-PD-1 [
35,
36]. However, in order to promote the wider application of PD-1 inhibitors, it is necessary to solve the problems of patient heterogeneity and personalized formulation to maximize the therapeutic effect.
CTLA-4: CTLA-4 is also highly expressed on the surface of T cells as an inhibitory receptor, and its mechanism of action is to inhibit T cell activation by binding to CD80/86, thus reducing the autoimmune expression of patients [
37]. In addition, it has been clinically demonstrated that the mechanism of action of CTLA-4 inhibitors is to release CD28-mediated positive co-stimulatory signals to reduce Treg cell inhibition and enhance T cell antitumor effect to achieve antitumor signal transmission [38-40]. At present, ipilimumab, a CTLA-4-targeted inhibitor approved by FDA, has been applied in clinical practice, marking the beginning of ICB immunotherapy. However, CTLA-4 blockade also faces the side effect of immuno-overexpression, so there is also a need to optimize the treatment to reduce its side effects.
TIGIT: TIGIT is a type I transmembrane protein of the immunoglobulin superfamily that is highly expressed on T cells, NK cells and Treg cells. Its mechanism of action is mainly to mediate the activation of T cells and NK cells by binding with ligands such as CD155 and CD112 expressed on antigen presenting cells, thus transmitting anti-tumor immune signals [41-43]. It was also found that blocking TIGIT signaling pathway of malignant tumors could significantly increase the expression of IFN-γ and TNF-α in tumor-specific CD8
+ T cells and significantly improve the anti-tumor immune response [
44].
2.2.2. Cell Engineering Therapies
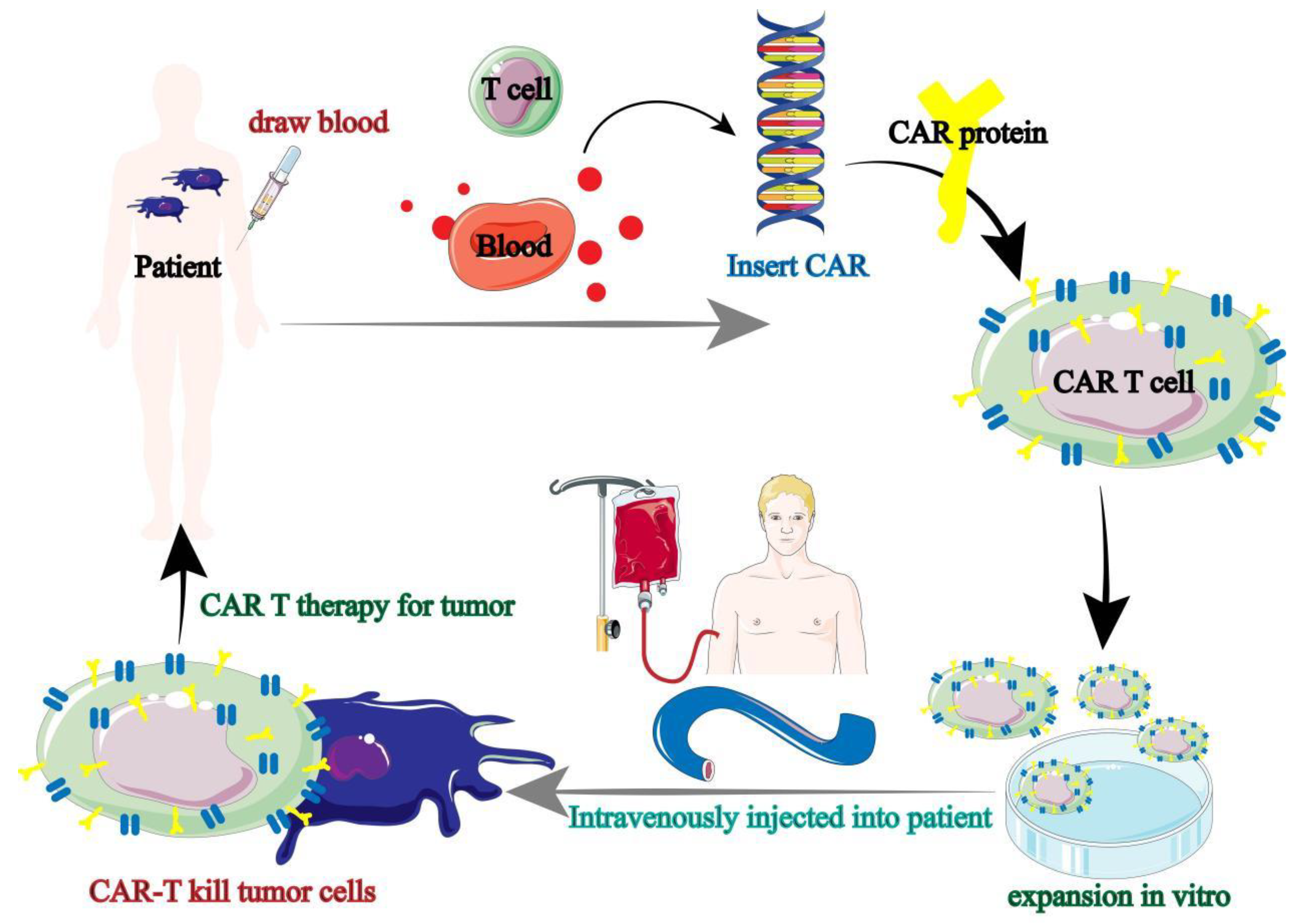
Immune cell-based therapies are mainly called chimeric antigen receptor immunotherapy (CAR), of which CAR-T is the most widely used. During CAR-T treatment, doctors first collect T cells from patients (autologous), but most patients have too few endogenous T cells to support the realization of anti-tumor immune response [
45,
46]. However, the study found that T cells can also be collected from healthy people (allogeneic) for patients ’needs, and then the collected autologous or allogeneic T cells are used for genetic engineering, that is, antigens with specific target tumor cells are presented to T cells, and finally CAR-T cells are injected into patients to cause anti-tumor immune response of patients (
Figure 3) [
47].
At present, CAR-T cell therapy has achieved great clinical success, but the toxic side effects of CAR-T cell therapy are still unavoidable, and this toxic side effect is not specific, in other words, it is very likely to damage normal tissue cells. In addition, the therapeutic effect of CAR-T in solid tumors is not optimistic, so new tumor immunotherapy methods are still needed for clinical treatment of malignant tumors.
2.2.3. Tumor Vaccines
Although ICB and CAR-T have shown good treatment effects, response rates are not high in some tumor types or patient types [
48,
49]. Therefore, the development of new therapeutic regimens is needed to reverse the stagnation of cancer immunotherapy. In recent years, the development of tumor immunotherapy has brought out a new therapeutic method-tumor vaccine.
Tumor vaccine mainly acts on tumor associated antigen (TAA) and tumor specific antigen (TSAT), and DC cells are the main target cells for its anti-tumor effect [
50]. The specific mechanism of tumor vaccine is to regulate the effective presentation of antigen by DC cells, thus improving the efficiency of the whole anti-tumor immune response. For example, in 2017, Nature published two reports of successful treatment of advanced melanoma with tumor vaccines. One clinical trial showed that the vaccine successfully prevented tumor development in more than 60% of patients; Another cancer vaccine that has been used has shown that about 70% of patients do not relapse within 25 months [
51]. At present, tumor vaccines have the advantage of triggering long-term immune memory, although few tumor vaccines have been developed and utilized [
52,
53]. Therefore, researchers are still focusing on the development and utilization of tumor vaccines, which is expected to achieve a lasting anti-tumor immune response in patients.
2.2.4. Soluble Viruses
In recent years, the appearance of tumor vaccine provides a better direction for the development of tumor immunotherapy. Now, researchers are starting to use vaccines against the virus to stop the development of malignant tumors. Oncolytic virus (OV) is the most widely used virus, which can not only infect tumor cells and kill tumor cells directly, but also release TAA and other cytokines to enhance anti-tumor immune response. For example, Talimogene Laherparepvec (T-VEC)-the first oncolytic viral therapy approved by FDA for the treatment of advanced melanoma, can specifically replicate and lyse tumor cells in tumor cells and induce local and systemic anti-tumor immune response [
54]. However, OV therapy is clinically effective for only a few tumor type, so that combination of OV therapy with other immunotherapies will become more and more widespread.
2.3. Tumor Immunotherapy Limitations
Tumor immunotherapy has become the "fourth bullet" of anti-tumor therapy after surgery, radiotherapy and chemotherapy. The clinical use of CAR-T shows that patients ’immune systems can be "reprogrammed" to fight malignant tumors. The use of ICBs, tumor vaccines and oncolytic viruses represent important applications for antigen presentation. However, tumor immunotherapy is not perfect, and there are also problems such as toxicity control and low response rate.
Among them, toxicity control has the greatest impact on patients. It can not only cause clinical symptoms such as anemia (45.4%), fatigue (34.3%) and dysphagia (30%), but also lead to adverse events such as neutropenia (19.6%), hypertension (9.3%) and lymphopenia (10.3%) [
55]. In addition, tumor immunotherapy only accounts for 10-30% of the immune response rate in solid tumors. Therefore, it is urgent to control toxicity and improve the response rate of antitumor immunity.
3. Research Progress of Tumor Immunotherapy based on NK Cells
3.1. NK Cell
3.1.1. Origin of NK Cells
NK cells used for tumor immunotherapy have various sources, mainly including peripheral blood (PB), umbilical cord blood (UCB), bone marrow (BM), human embryonic stem cells (hESC) and derived NK cell lines. NK cells from different sources have different advantages and disadvantages (
Table 1) [
56].
NK cells of either autologous or allogeneic origin have been shown to be clinically safe and tolerable. However, autologous NK cells have low proliferation and antitumor activity, while allogeneic NK cells are difficult to find. In addition, when NK cell are isolated from peripheral blood, that NK cell are easily mixed with monocytes and other blood cells, and thus it is time-consuming and expensive.
3.1.2. NK Cell Classification and Subsets
NK cells can be divided into CD56
bright and CD56
dim according to the expression of CD56 on their surfaces. Among them, CD56
brightNK cells mainly play an anti-tumor immune role by secreting cytokines such as INF-γ and TNF-β [
57]. CD56
dimNK cells use highly expressed CD16 to mediate antibody dependent cell Mediated cytotoxicity (ADCC) to induce phosphorylation of immune receptor tyrosine activation motif (ITAM), and finally immunize and kill tumor cells [
58].
In recent years, Crinier
et. al. revealed the heterogeneity of human and mouse NK cells by using high-dimensional single-cell RNAseq, and confirmed that NK cells can also be divided into several cell subsets such as cytotoxic NK (cNK) cells, antigen-presenting NK (AP-NK) cells, helper NK (NKh) cells and regulatory NK (NKreg) cells according to differentiation degree [
59]. Different subsets of NK cells play different roles in anti-tumor immunity in different fields, and have been widely used in clinic.
3.1.3. Role and Function of NK Cells
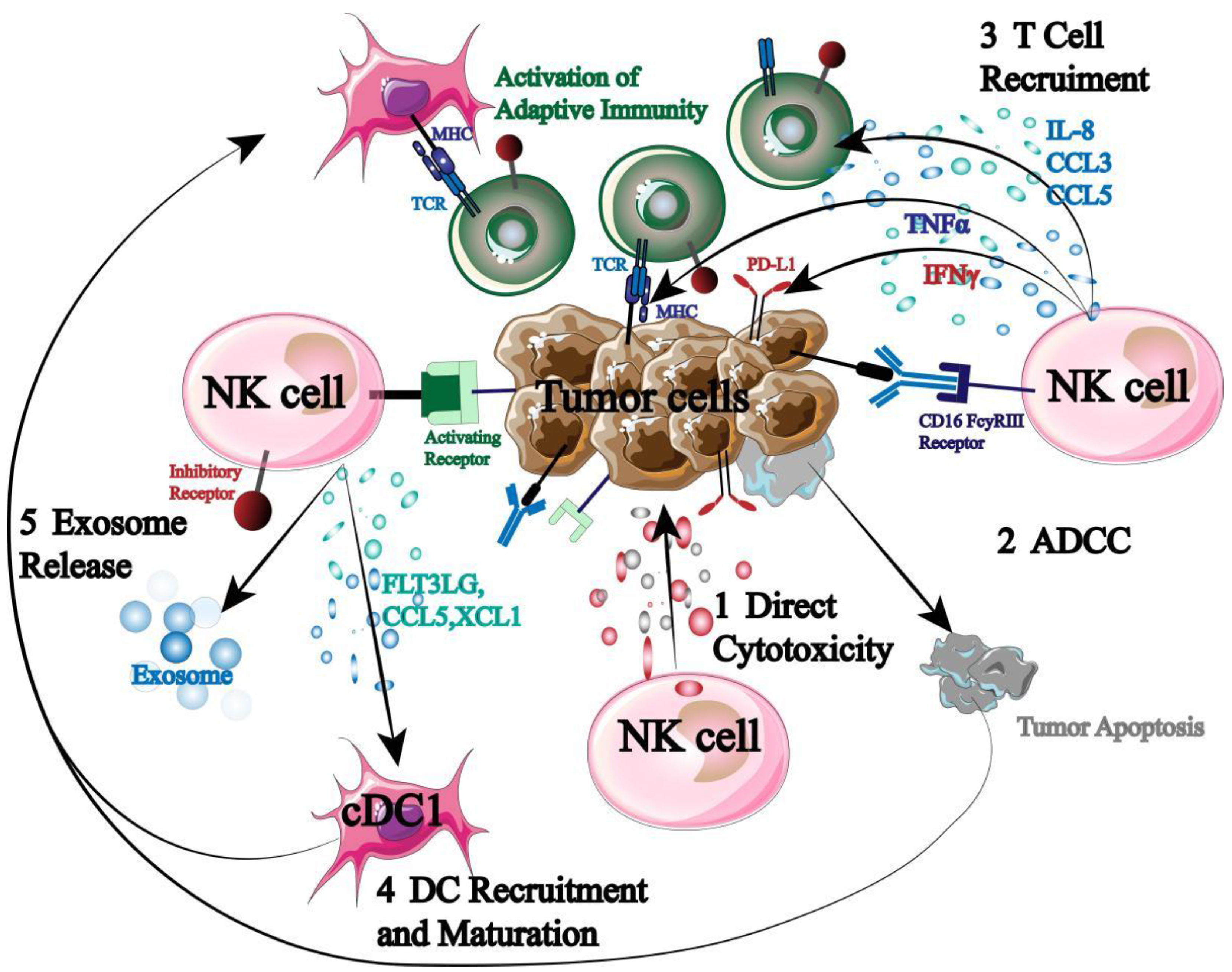
NK cells, as the first responders of the immune system, are key responders for immune surveillance, regulation and anti-tumor (
Figure 4).
First, NK cells can secrete perforin and granzyme to exert autotoxicity directly. Second, when NK cells receive a stronger stimulatory signal than an inhibitory signal, NK cells express high levels of CD16 to trigger ADCC. In addition, NK cells can also secrete chemokines and cytokines to recruit and coordinate other immune cells (T cells, DC cells, etc.), thus starting adaptive immune response to kill tumor cells [60-62]. More importantly, NK cell activation does not require specific antigen presentation and does not require prior sensitization. Therefore, NK cells can not only kill tumor cells preferentially but also induce systemic tumor immune response.
3.2. NK Cell-Based Tumor Immunotherapy
3.2.1. Target Blockade
As mentioned above, the immune function of NK cells is mainly regulated by the balance between its surface inhibitory receptors and activating receptors. When the inhibitory signal is stronger than the activating signal, it will promote the immune escape of tumor cells. Therefore, the anti-tumor immune response of NK cells can be promoted by targeting and blocking the corresponding inhibitory signal. The most widely used inhibitory targets mainly include: PD-I, TIGIT, etc.
PD-1, as described in Chapter 2, is an inhibitory receptor highly expressed on the surface of immune cells and can significantly reduce the immune response of NK cells. In recent years, studies have shown that PD-1 is not only highly expressed in immune cells, but also highly expressed in pleural effusion of patients with primary and metastatic tumors according to clinical data [
63]. In addition, it has been demonstrated that PD-1 not only mediates NK cell inactivation and down-regulates its anti-tumor immune response, but also affects NK cell-dependent immune surveillance and promotes tumor cell immune escape [
64,
65]. Therefore, targeted blockade of PD-1 is of great significance for improving the therapeutic effect of patients.
TIGIT also acts as an inhibitory receptor to transmit immunosuppressive signals. Its binding ligands mainly include CD155 (PVR) and CD112. Among them, CD155 is highly expressed on the surface of tumor cells as the main binding ligand [
66]. When they combine, they undergo autophosphorylation, down-regulating the signal transmission of PI3K/MAPK signaling pathway, and finally reducing the cytotoxicity of NK cells [
67]. Therefore, TIGIT monoclonal antibody can be used to block the transmission of immunosuppressive signals, enhance the anti-tumor activity of NK cells, and then regulate the immune function of patients to achieve the purpose of treatment.
Although ICB is widely used in tumor immunotherapy, the ability of NK cell-mediated immune surveillance is reduced due to the alteration of molecular expression of NK cell-activated receptors due to prolonged exposure to the tumor microenvironment. Therefore, there is a need for new therapies to emerge or combine with them to improve the therapeutic effect of tumor immunotherapy.
3.2.2. CAR-NK
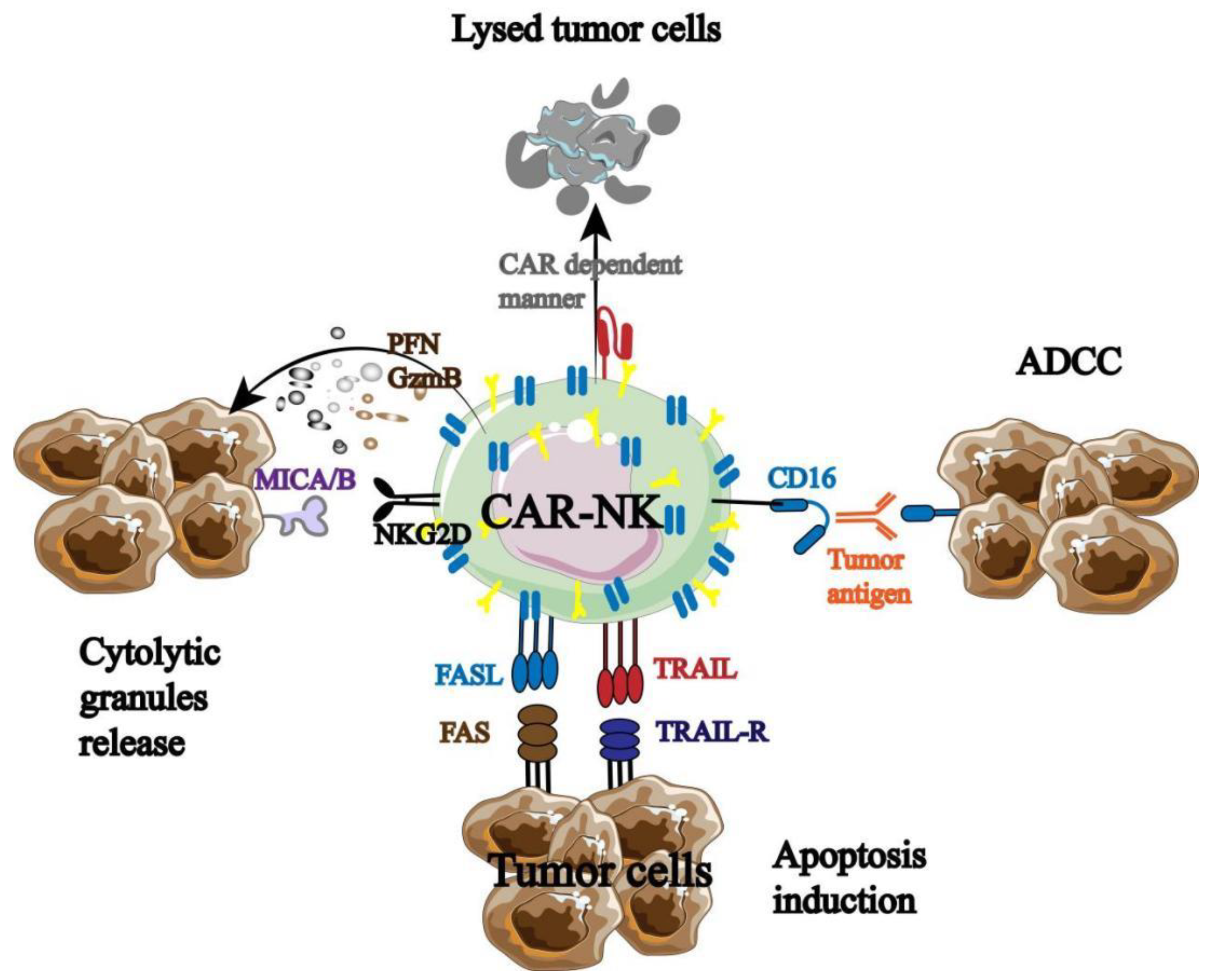
The excellent clinical data of CAR-T promoted the birth of CAR-NK, which has been tested and optimized in animal models and clinical trials. It was found that CAR-NK not only has the inherent anti-tumor properties of NK cells, but also has its own unique anti-tumor immune advantage (
Figure 5) [
68].
CAR-NK therapy has been shown to have an immune effect on a variety of hematologic malignancies, including but not limited to acute myeloid leukemia, lymphoma and multiple myeloma. It has been widely used in the treatment of CD19
+B cell malignancies. Romanski
et. al. [
69] transduced NK-92 cells with CAR against CD19 and demonstrated that CAR-NK-92 can specifically lyse CD19-expressing B cells. In addition, there are data showing that enhanced green fluorescent protein (EGFP)-modified NK cells can significantly improve the lytic capacity of NK cells and show stable antitumor effect in PDX mouse models [
70]. Therefore, EGFP-CAR-NK is often designed to treat triple negative breast cancer [
71].
At present, both ICB and CAR-NK have made encouraging research progress, but as mentioned above, the application of single immunotherapy is inefficient and faces many limitations. In recent years, immune combination therapy is becoming a new therapeutic hotspot, which can not only expand the applicable population and tumor, but also take advantage of each other to improve the efficacy. Therefore, more and more immune combination therapy is applied in clinical practice and becomes a new clinical treatment step by step.
3.2.3. CAR-NK Combined with Gene Modification
Tumor cells can escape from the immune surveillance of NK cells. Therefore, it is necessary to use gene modification to modify NK cells to enhance their anti-tumor immune function, to ensure that NK cells can kill tumor cells directly before tumor cells escape. So far, many genetic modification methods have been applied to NK cell modification. For example, researchers have found that modifying NK cells by inserting suicide genes into CAR can control the potential cytotoxicity of NK cells. Liu
et. al. transduced a retroviral vector incorporating CAR-CD19, IL-15 and caspase-9-based suicide gene into NK cells, and data showed that it had significant killing capacity against CD19 cell line and primary leukemia, and significantly prolonged the survival of Raji lymphoma mice [
72].
3.2.4. Combination of Target Blocking and Monoclonal Antibody
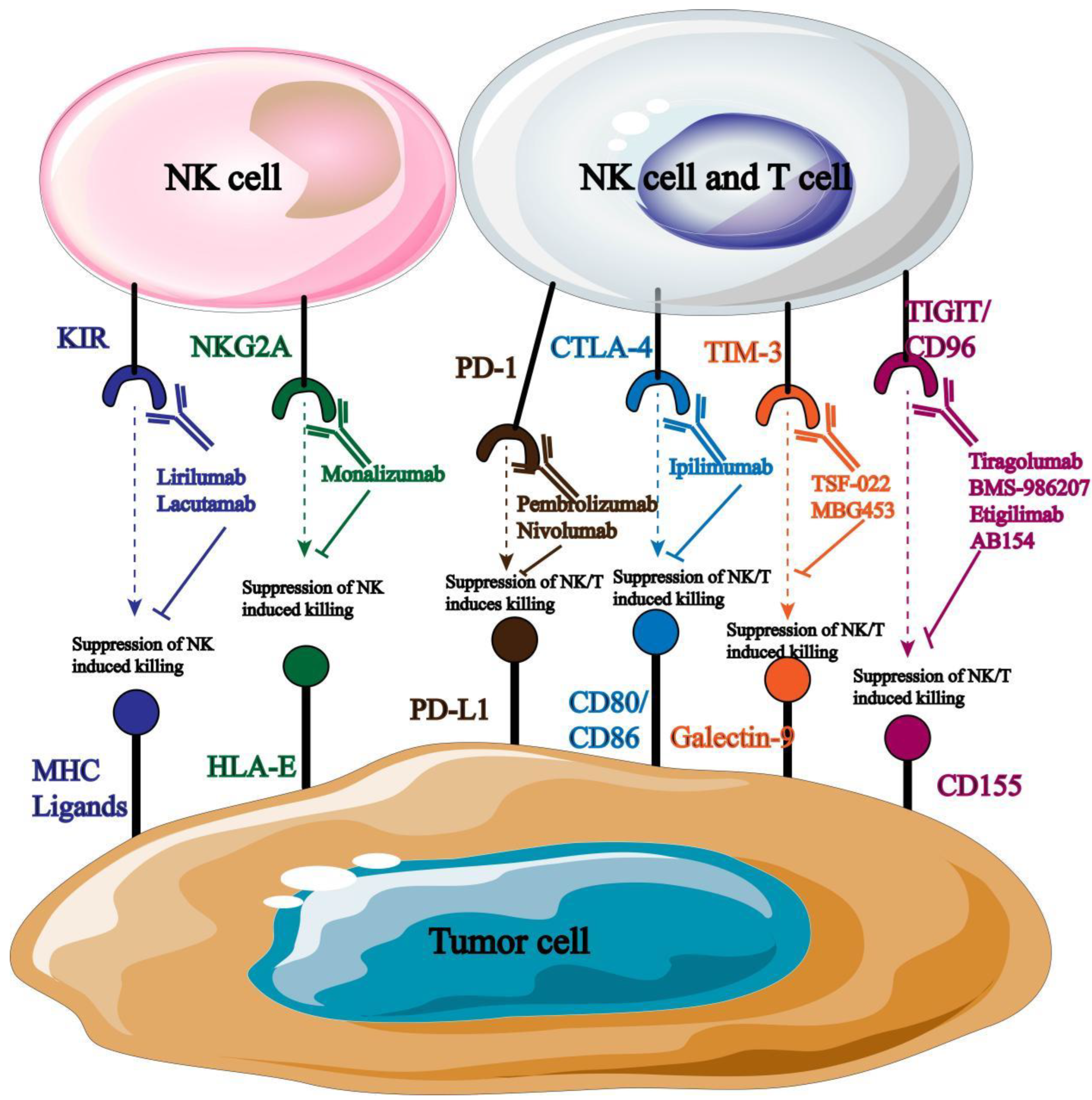
Monoclonal antibody can directly target the target receptor on the surface of NK cells, block its signal transmission and improve the anti-tumor immune activity of NK cells. At present, there are many monoclonal antibodies targeting NK cell surface receptors for clinical treatment (
Figure 6).
Monoclonal antibodies blocked by different targets are directed against different tumor types, such as Anti-PD-1, mainly targeting cervical cancer, breast cancer, liver cancer and lung cancer [
73]; Anti-NKG2A mainly acts on NK cells in hematological malignant tumors [
74]. It has been reported that the application of monoclonal antibodies blocking PD-l/PD-L1 binding can significantly enhance the cytotoxicity of NK cells and the production of related immunosuppressive cytokines, thus inhibiting the growth and development of tumor cells. At present, a variety of monoclonal antibodies targeting PD-1 have been used in clinical practice [
75], and good research results have been achieved.
3.2.5. Molecular Imaging of NK cell
Molecular imaging techniques have also been improving to assess the tracking of NK cells,including quantitative dynamic footprinting (qDF), total internal reflection fluorescence (TIRF) microscopy, optical live-cell imaging(including multiphoton and confocal imaging), light-sheet microscopy and super-resolution microscopy and so on, many of these techniques rely on directly labeling of NK cells surface with fluorophores or contrast agents,cell-permeable fluorophores, radioisotopes to enable real-time visualization of NK cell immunotherapies in tumors. Nanoparticles,such as superparamagnetic iron oxide and ultra-small superparamagnetic iron oxide nanoparticles,have also been used to label NK cells for MRI. Nanoparticles such as superparamagnetic iron oxide and ultra-small superparamagnetic iron oxide nanoparticles have also been used to label NK cells for MRI. Adding iron oxide nanoparticles to NK cells is relatively easy, requiring only a simple incubation electroporation or the use of transfection agents. Iron oxide-labeled NK cells produce a strong low-intensity signal in T2 and T2*-weighted images, and iron oxide labeling can be retained for up to 4 days, that depending on the length of time that the adopted-transferred NK cells survive[
76].
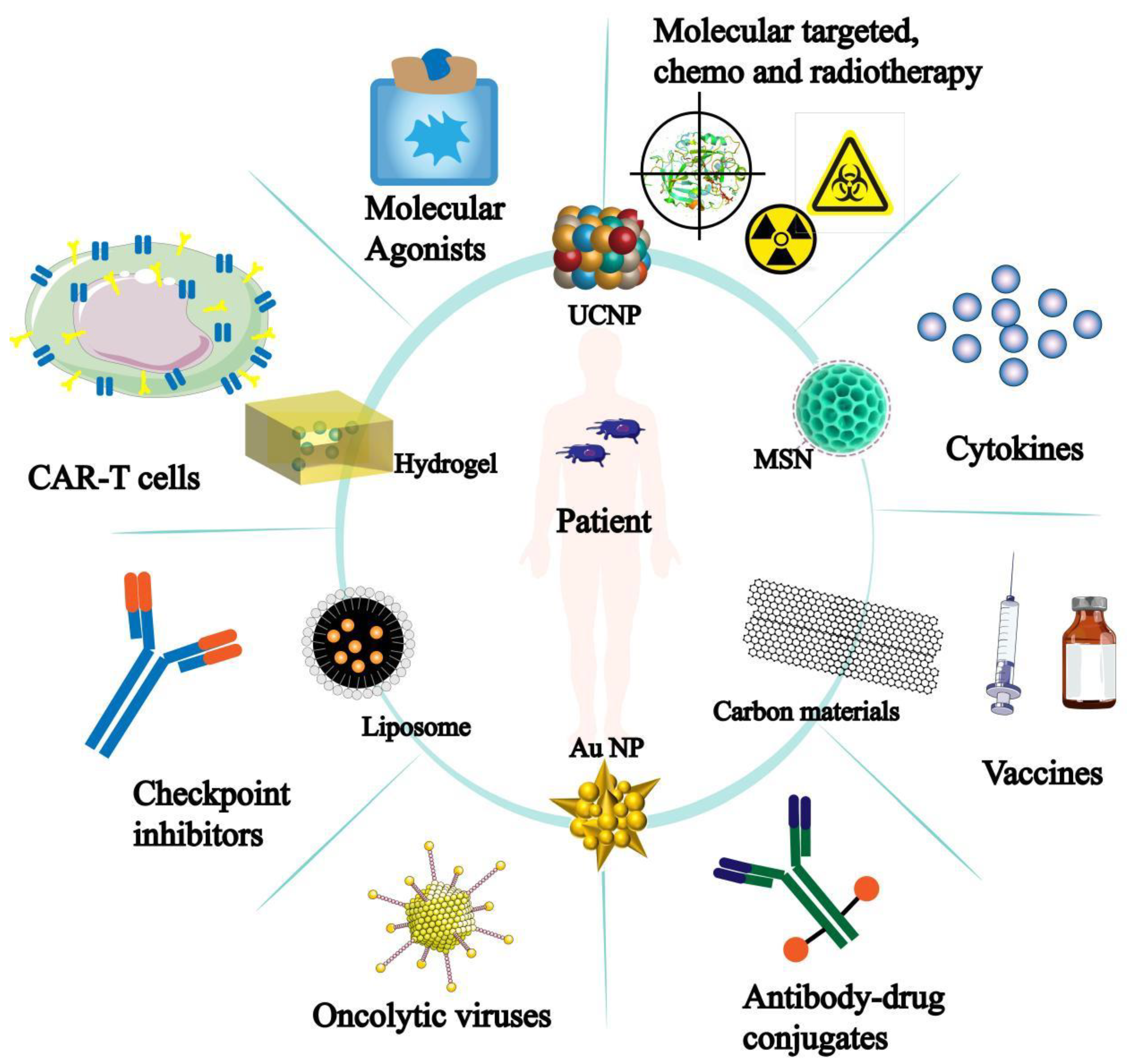
4. Research Progress of Nanomaterials
In recent years, the appearance and clinical application of immunotherapy have promoted the rapid development of tumor treatment. However, the application of immunotherapy is limited, often accompanied by low response rate, patient resistance and adverse reactions. The emergence of nanomaterials has brought new breakthroughs in tumor immunotherapy, and has been gradually applied to various aspects of immunotherapy (
Figure 7).
4.1. Types of Nanomaterials
4.1.1. Metal Nanoparticles
Metal nanoparticles are not only used in the biomedical field [
77], but also can regulate the immune activation of immune cells and host defense [
78,
79]. Among them, gold nanoparticles are widely used in the diagnosis and treatment of tumors by virtue of their light control ability, chemical inertness and low toxicity [80-83]. For example, Ahn used Au-NP to carry endogenous EDB autoantigen as tumor vaccine. Studies showed that Au-NP could present antigen to DC cells and induce T cell immune response, significantly inhibiting the development of tumor cells [
84]. At present, the application of metal nanoparticles in tumor immunotherapy is also increasing. In addition to gold nanoparticles, platinum (Pt), copper (Cu) and iron (Fe) are widely used.
4.1.2. Liposomes
In 1965, Bangham
et. al. proved the existence of liposome and found that liposome is a double-layer spherical vesicle with both hydrophobic and hydrophilic capabilities [
85]. Liposome is one of the most ideal drug delivery systems because of its good drug loading and biocompatibility. Chen
et. al. modified liposomes with anti-PD-1 and mannose, and encapsulated anti-angiogenic drugs and mTOR inhibitors inside liposomes. Studies have shown that this liposome can not only inhibit angiogenesis, glycolysis and tumor volume increase at the same time, but also reprogram immune cells, effectively improving the effect of traditional anti-PD-1 therapy [
86].
At present, although liposome has the advantages of simple preparation and high encapsulation efficiency, it can only be used as a drug delivery system to participate in tumor immunotherapy because it does not have anti-tumor function. However, the development of multi-disciplinary intersection endows liposome with new functions such as targeting and immunotherapy, which makes it better applied in clinical treatment of tumor.
4.1.3. Hydrogels
Hydrogels have excellent biocompatibility and can be efficiently loaded with therapeutic drugs, so they are often designed for tumor immunotherapy. Among them, the most extensive types mainly include hydrogel sprays, hydrogel scaffolds and hydrogel microneedles.
In recent years, routine immunotherapy has been effective in the treatment of primary tumors, but in the treatment of metastatic tumors and recurrent tumors, routine therapy is often inadequate. Therefore, it is urgent to develop a new regimen to control tumor metastasis and reduce tumor recurrence. For example, Chen
et. al. [
87] used PPP (PLGA-PEG-PLGA) and ROCKs inhibitor Y27632 to construct new nanomaterials for tumor immunotherapy. The mixture of the two is a temperature-responsive material, so that the hydrogel state can be maintained during subsequent treatment. It was found that when the hydrogel entered tumor cells, it stimulated tumor cells to form cell debris, and released Y27632 to activate dendritic cells to phagocytize tumor debris and present antigen, thus activating T cells to enhance anti-tumor immune response signal transmission.
4.2. The Role and Application of Nanomaterials in Tumor Immunotherapy
4.2.1. Target Delivery
Nano-materials targeting immune examination sites promote the development of tumor immunotherapy, in which nano-inhibitor conjugates are most widely used. For example, Wang
et. al. [
88] used anti-PD-1 nanoconjugates to treat melanoma tumors in mice, and showed that they not only improved the retention of anti-PD-1 agents in the tumor microenvironment, but also enhanced the therapeutic immune response of immune cells. In addition, another study showed that anti-CTLA-4 nanoconjugates significantly increased the number of tumor-infiltrating immune cells in mouse breast cancer models, better inhibited tumor growth and metastatic spread, and induced a complete anti-tumor immune response. In conclusion, the target nano-inhibitor conjugate is very likely to enhance the reactivity of conventional tumor immunotherapy and reduce its toxic and side effects, thus promoting the development and progress of tumor immunotherapy means.
4.2.2. Tumor Vaccine Nanocarriers
Nanomaterials have greatly improved the success rate of tumor vaccines by virtue of their excellent biological characteristics, and can also have a positive impact on the defense system by activating immunogenic cells to start the body monitoring and protection mechanism [
89]. At present, tumor vaccines based on DC cells are most widely used. The addition of nanomaterials can specifically modify the molecules to be transported to improve the availability of drugs and the uptake rate of DC cells [
90]. It was found that the delivery of tumor vaccine to DC cells by nanomaterials could significantly prolong the antigen/drug action time and improve the therapeutic effect of tumor vaccine. In addition, the researchers also found that the delivery of tumor vaccine by means of nanomaterials can not only directly kill tumor cells [
91], but also indirectly improve the anti-tumor activity of immune cells and realize the transmission of anti-tumor immune signals.
4.2.3. Engineered T-Cell-Based
CAR-T cell therapy mainly has the disadvantages of low survival rate of transplanted cells and low efficacy, while CAR-T often requires the addition of adjuvant drugs, which leads to more serious toxic and side effects [
92]. However, the emergence of nanoparticle-functionalized T cells has solved these problems well. It has been found that it can not only improve the delivery of chemotherapeutic drugs without pharmacokinetics, but also improve the drug penetration of tumor environment by utilizing the ability of T cells to migrate to tumor cells to reduce the toxic and side effects of drug administration.
In addition, it was found that nanomaterials can also promote the expansion of T cells in vivo through antigen presentation, which significantly improves the low survival rate of T cells. Currently, ipilimumab is the clinically used CTLA antibody, which can promote T cell initiation and expansion [
93]. The application of CTLA4 antibody coated with nanomaterials in T cell engineering therapy can not only directly stimulate naive T cells to differentiate into effector or memory T cells, but also expand mature T cells to overcome the difficulties of adoptive T cell therapy.
4.3. Limitations of Nanomaterials
Although the use of nanomaterials has brought exciting prospects for the development of tumor immunotherapy, there are still some challenges to be overcome to better meet the needs of clinical applications. First of all, the toxicity of materials can not be ignored, which is one of the problems of nanomaterials. Most materials have too few toxic side effects due to the way they are transported in cells, tissues, and systems [
94], and if not degraded in time, can lead to accumulation of materials that can be more toxic [
95]. In addition, tumor heterogeneity and patient specificity also hinder the development of clinical applications of nanomaterials.Moreover, single treatment methods do not achieve a satisfactory therapeutic effect. Therefore, how to combine different therapeutic methods and different nanomaterials into the same nanosystem in a reasonable, compatible and synergistic way to realize the efficient development of combined immunotherapy is also a huge challenge.
5. Research Progress of Nanomaterials Based on NK Cells
Immunosuppression of tumor microenvironment (TME) can down-regulate the expression of NK cell surface activation receptor, and inhibit NK cell activation and expansion [
96]. In addition, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) secreted by tumor cells can inhibit the expression of adhesion factors and affect NK cell infiltration and homing [
97]. Therefore, NK cell-based tumor immunotherapy often suffers from low NK cell activity, insufficient homing infiltration and limited contact frequency with tumor cells [
98]. Moreover, the immune escape of tumor cells itself is also urgent to be solved, so the immunotherapy based on NK cells needs the assistance of nanomaterials. The appearance and reasonable design of nanomaterials provide a broader application prospect for tumor immunotherapy. In recent years, nanomaterials have played an increasingly important clinical role in NK cell-based immunotherapy.
5.1. Nano-Materials Enhance the Anti-Tumor Activity of NK Cells
The immune activity of NK cells is mainly affected by the negative regulatory factors secreted by tumor cells, such as TGF-β and IFN-γ. They inhibit activation signaling by down-regulating the expression of NK cell surface activating receptors, which leads to the reduction of NK cell cytotoxicity and antitumor immune response. Therefore, if we want to improve the anti-tumor activity of NK cells, we can start from blocking TGF-β signal transduction. For example, Liu
et. al. developed nanoemulsions containing both selenocysteine and a TGF-β inhibitor. Studies showed that the nanoemulsion significantly inhibited TGF-β/TGF-βR1/Smad2/3 signaling, and up-regulated the expression of NKG2DL receptor, effectively enhancing the antitumor activity of NK cells [
99]. In addition, nanomaterials can overcome the problems of low response rate, drug resistance and patient heterogeneity of conventional therapies to improve efficacy [
100]. For example, selenium-based nanoparticles can not only enhance NK cell antitumor activity, but also induce non-specific immune response and thus improve the efficacy of overall immunotherapy [
101,
102].
5.2. Immune Modification of NK Cells by Nanomaterials
NK cells are the first effector to recognize and monitor tumor cells, and CAR-NK is gradually becoming the next generation of emerging tools for immunotherapy. At the same time, the development of CAR-NK immunotherapy has promoted the delivery of chimeric antigen receptor genes based on nanomaterials. Studies have found that chitosan nanoparticles loaded with IL-2 and NKG2D genes can effectively activate NK cells in vitro, and can show better anti-tumor effect by using their own permeability, retention effect and aggregation energy [
103]. Therefore, nanomaterials can significantly inhibit tumor cell growth by modifying NK cells with relevant cytokines.
In addition, the engineered NK cells modified by nanomaterials can also directly destroy tumor cells. For example, a novel nanomaterial composed of human ferritin heavy chain (hFTH1) gene-transfected NK cells embedded with gold nanoparticles was found to be able to guide NK cells into TME and provide high-quality imaging of transfected NK cells [
104]. Therefore, the biocompatible multifunctional nanomaterials can realize the real-time monitoring of NK cells in patients by utilizing the immune modification of NK cells [
105].
5.3. Nano-Materials Enhance NK Cells Homing and Infiltration
The homing behavior of NK cells is mainly dependent on the signal transduction between the homing receptor on the surface and the ligand, while the infiltration of NK cells is influenced by the interaction with TME [
106,
107]. Studies have found that once NK cells infiltrate tumor cells and homing receptors bind to ligands, immune cell activation signals will be transmitted and secrete perforin, granzyme and apoptosis-inducing factors [
108] to play an anti-tumor immune role of NK cells. Therefore, it is necessary to use nanomaterials to enhance the homing and infiltration of NK cells to inhibit tumor development. For example, iron oxide nanoparticles conjugated to primary NK cells significantly enhanced their homing ability and significantly increased the secretion of perforin and granzyme [
109]. In addition, some studies have found that the homing and infiltration of NK cells are also affected by external magnetic guidance. For example, Wu
et. al. implanted polydopamine-coated magnetic iron oxide nanoparticles into mice subcutaneously. The results showed that the magnetic particles could not only improve the homing and infiltration of NK cells, but also effectively activate NK cells to directly kill tumor cells, showing more significant anti-tumor effect [
110].
5.4. NK-Cell-Associated RNAi Loaded on Nanomaterials
RNA effectors such as siRNA, miRNA and shRNA can silence specific genes of immune cells, thus changing their genome function and enhancing antitumor activity [
111]. Nanomaterials can enhance the delivery of these RNA effectors and become an excellent nano-delivery system for these effectors. For example, cationic liposomes loaded with epithelial cell adhesion molecules containing siCD47 and siPD-1 were able to significantly knock down CD47 and PD-1 expression. Moreover, the liposome can not only effectively inhibit tumor growth and lung metastasis, but also increase NK cell amplification and accelerate anti-tumor immune response signal transmission [
112].
6. Conclusion and Future Directions
The immune system of human body can protect human body from many diseases including cancer, so the appearance of tumor immunotherapy brings new hope for the prevention and treatment of malignant tumor. however, that clinical result of immunotherapy to date have not been satisfactory, and when tumors are treat with monotherapy, only a small proportion of patients are clinically effective. Moreover, monotherapy is often associated with immune cell depletion, indiscriminate cytotoxicity, and suppression of the tumor microenvironment. Therefore, in recent years, the combination of different immunotherapies has been widely used, and the combination of immunotherapies has achieved unprecedented clinical success.
Among them, tumor immunotherapy based on NK cells has attracted great attention. The anti-tumor immune response of NK cells has tumor-specific cytotoxicity. Moreover, NK cells do not need the specific process of antigen presentation and recognition when they play an anti-tumor immune role. Therefore, the combination of NK cell-based immunotherapy is a promising development direction, such as the combination of target blocking and antibody, CAR-NK and gene-modified therapy, etc.
However, even the combination of NK cell-based immunotherapy also has limitations that cannot be ignored, and the addition of nanomaterials can complement each other and enhance the anti-tumor effect of immune cells. And the size, surface charge and shape of nanomaterials can be changed by design. Different types of nanomaterials can exert different anti-tumor functions. Therefore, nanomaterials will become a key tool to enhance the efficacy of NK cell tumor immunotherapy. Such as nano material for inducing immune cell to home and infiltrate tumor cells; There are also nanomaterials that themselves can reduce the inflammatory response of the tumor microenvironment; In addition, that nano material also has a medical image contrast effect, and can guide NK cells to kill tumor cell and monitor the treatment effect in real time. Therefore, nanomaterials play a variety of functions in the interaction between tumor cells and immune cells, significantly enhance the anti-tumor ability of NK cell immunotherapy, and provide a broader application prospect for tumor immunotherapy.
At the same time, the clinical application of nanomaterials also has some challenges, the most important of which is the toxicity and safety of nanomaterials. Metal nanoparticles have been reported to cause hepatotoxicity [
113]. Studies have shown that chemotherapy drugs rely mainly on kidney decomposition, while nanomaterials rely on the liver. During liver detoxification, nanoparticles accumulate in tissues near the liver and disrupt the degradation process by other enzymes [
114]. In addition, Fe nanoparticles can also cause oxidative stress and ferritin precipitation, affecting immune cell function and clinical therapeutic effect [
115]. Therefore, when designing nanomaterials to assist NK cells in tumor immune response, it is necessary to consider the toxicity of nanomaterials themselves and evaluate their potential cytotoxicity. Using the intersection of multiple disciplines, different design methods are used to develop and design nanoparticle-based immunomodulatory drugs to further improve the effectiveness of immunotherapy. Thus, the versatility of the nanoparticles in the interaction between the immune system and the tumor will allow synergistic combination of anti-cancer effects, broaden the capabilities of NK cell cancer immunotherapy, and contribute to the development of safe and controllable NK cell cancer immunotherapy, providing a more effective approach to the clinical treatment of patients ’tumors.
Author Contributions
All authors contributed to the study’s conception and design. Yachan Feng to draft the article, conduct literature retrieval and provide the overall structure of the article. Haojie Zhang, Jiangtao Shao, Chao Du and Xiaolei Zhou modified the article and added some content. Yingze Wang and Xueling Guo revised the arrangement of the article to help improve the accuracy of the language. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant no.81760750), Natural Science Foundation of Hebei Province (grant no.H2020208018,C2020208023), the Graduate Student Innovation Ability Training project of Hebei University of Science and Technology (grant no.XJCXZZSS202307) and Doctoral research fund project of Hebei university of science and technology (no.QD2023004).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- June, C. H. & Sadelain, M, Chimeric antigen receptor therapy, N Engl J Med. 379 (2018) 64-73.
- Waldmann, T.A. Cytokines in Cancer Immunotherapy. Cold Spring Harb. Perspect. Biol. 2017, 10, a028472. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK cell–cancer cycle: advances and new challenges in NK cell–based immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Huntington, N.D.; Cursons, J.; Rautela, J. The cancer–natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Dane, E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Choi, H.; Yu, B.; Kim, D.-H. Synergistic Local Combination of Radiation and Anti-Programmed Death Ligand 1 Immunotherapy Using Radiation-Responsive Splintery Metallic Nanocarriers. ACS Nano 2020, 14, 13115–13126. [Google Scholar] [CrossRef]
- Yu, B.; Choi, B.; Li, W.; Kim, D.-H. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coley, W.B. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc. R. Soc. Med. 1910, 3, 1–48. [Google Scholar] [CrossRef]
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS et al., Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2, J Natl Cancer Inst. 86 (1994) 1159-66.
- Van Der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van Den Eynde, B.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef]
- Kruger, S.; Ilmer, M.; Kobold, S.; Cadilha, B.L.; Endres, S.; Ormanns, S.; Schuebbe, G.; Renz, B.W.; D’haese, J.G.; Schloesser, H.; et al. Advances in cancer immunotherapy 2019 – latest trends. J. Exp. Clin. Cancer Res. 2019, 38, 1–11. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef]
- Böttcher JP, Reis e Sousa C, The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity, Trends Cancer. 4 (2018) 784-92.
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2020, 18, 85–100. [Google Scholar] [CrossRef]
- Cózar, B.; Greppi, M.; Carpentier, S.; Narni-Mancinelli, E.; Chiossone, L.; Vivier, E. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2021, 11, 34–44. [Google Scholar] [CrossRef]
- Van Dalen, F.J.; Van Stevendaal, M.H.M.E.; Fennemann, F.L.; Verdoes, M.; Ilina, O. Molecular Repolarisation of Tumour-Associated Macrophages. Molecules 2018, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Goswami, K.K.; Bose, A.; Baral, R. Macrophages in tumor: An inflammatory perspective. Clin. Immunol. 2021, 232, 108875. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, J.; Gu, Q.; Huang, M.; Zhang, W.; Guo, J.; Zhou, X. Reciprocal Expression of IL-35 and IL-10 Defines Two Distinct Effector Treg Subsets that Are Required for Maintenance of Immune Tolerance. Cell Rep. 2017, 21, 1853–1869. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Gerhardt, L.; Vareki, S.M. Immunosuppressive Effects of Myeloid-Derived Suppressor Cells in Cancer and Immunotherapy. Cells 2021, 10, 1170. [Google Scholar] [CrossRef]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, Z.; Li, X.; Han, X.; Shi, S.; Zhang, T. Tumor-associated macrophages: role in tumorigenesis and immunotherapy implications. J. Cancer 2021, 12, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Griess, B.; Mir, S.; Datta, K.; Teoh-Fitzgerald, M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free. Radic. Biol. Med. 2019, 147, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Maggi, E.; Veneziani, I.; Moretta, L.; Cosmi, L.; Annunziato, F. Group 2 Innate Lymphoid Cells: A Double-Edged Sword in Cancer? Cancers 2020, 12, 3452. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, D.C.; Shevde, L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Steinberger, P.; Drinić, M.; Wiedermann, U. Emerging targets for anticancer vaccination: PD-1. ESMO Open 2021, 6, 100278. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-H.; Chan, L.-C.; Li, C.-W.; Hsu, J.L.; Hung, M.-C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.C.; Sen, D.R.; Al Abosy, R.; Bi, K.; Virkud, Y.V.; LaFleur, M.W.; Yates, K.B.; Lako, A.; Felt, K.; Naik, G.S.; et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019, 20, 326–336. [Google Scholar] [CrossRef]
- Hill, M.; Segovia, M.; Russo, S.; Girotti, M.R.; Rabinovich, G.A. The Paradoxical Roles of Inflammation during PD-1 Blockade in Cancer. Trends Immunol. 2020, 41, 982–993. [Google Scholar] [CrossRef]
- Heine, A.; Kristiansen, G.; Schild, H.H.; Brossart, P. Successful treatment of refractory leiomyosarcoma with the PD-1 inhibitor nivolumab. Ann. Oncol. 2016, 27, 1813–1814. [Google Scholar] [CrossRef]
- Wang X, Wang F, Zhong M, Yarden Y, Fu L, The biomarkers of hyperprogressive disease in PD-1/PD-L1 blockage therapy, Mol Cancer. 19 (2020) 81.
- Kim CG, Kim C, Yoon SE, Kim KH, Choi SJ, Kang B et al., Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma, J Hepatol. 74 (2021) 350-359.
- Cascone, T.; Weissferdt, A.; Godoy, M.C.B.; William, W.N.; Leung, C.H.; Lin, H.Y.; Basu, S.; Yadav, S.S.; Pataer, A.; Mitchell, K.G.; et al. Nodal immune flare mimics nodal disease progression following neoadjuvant immune checkpoint inhibitors in non-small cell lung cancer. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu Y, Zheng P, How Does an Anti-CTLA-4 Antibody Promote Cancer Immunity? Trends Immunol. 39 (2018) 953-956.
- Bengsch F, Knoblock DM, Liu A, McAllister F, Beatty GL, CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer, Cancer Immunol Immunother. 66 (2017) 1609–1617.
- Pol, J.; Kroemer, G. Anti-CTLA-4 immunotherapy: uncoupling toxicity and efficacy. Cell Res. 2018, 28, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2019, 200, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.-M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Immuno-oncology target TIGIT attracts a new contender. Nat. Rev. Drug Discov. 2021, 20, 576–576. [Google Scholar] [CrossRef] [PubMed]
- Banta, K.L.; Xu, X.; Chitre, A.S.; Au-Yeung, A.; Takahashi, C.; O’gorman, W.E.; Wu, T.D.; Mittman, S.; Cubas, R.; Comps-Agrar, L.; et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses. Immunity 2022, 55, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Bai, M.; Li, X.; Wei, J.; Chen, D.; Wu, Z.; Wang, Y.; Han, W. Adaptive T cell immunotherapy in cancer. Sci. China Life Sci. 2020, 64, 363–371. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2020, 18, 842–859. [Google Scholar] [CrossRef]
- Adeel, K.; Fergusson, N.J.; Shorr, R.; Atkins, H.; Hay, K.A. Efficacy and safety of CD22 chimeric antigen receptor (CAR) T cell therapy in patients with B cell malignancies: a protocol for a systematic review and meta-analysis. Syst. Rev. 2021, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gounant, V.; Duruisseaux, M.; Soussi, G.; Van Hulst, S.; Bylicki, O.; Cadranel, J.; Wislez, M.; Trédaniel, J.; Spano, J.-P.; Helissey, C.; et al. Does Very Poor Performance Status Systematically Preclude Single Agent Anti-PD-1 Immunotherapy? A Multicenter Study of 35 Consecutive Patients. Cancers 2021, 13, 1040. [Google Scholar] [CrossRef]
- Khaki, A.R.; Glisch, C.; Petrillo, L.A. Immunotherapy in Patients With Poor Performance Status: The Jury Is Still Out on This Special Population. JCO Oncol. Pr. 2021, 17, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Sasada, T. Cancer Vaccines: Toward the Next Breakthrough in Cancer Immunotherapy. J. Immunol. Res. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Huang, C.; Zhu, Q.; Ferguson, A.K.; Durham, J.N.; Anders, R.A.; Thompson, E.D.; Rozich, N.S.; Thomas, D.L.; Nauroth, J.M.; et al. A phase 2 study of GVAX colon vaccine with cyclophosphamide and pembrolizumab in patients with mismatch repair proficient advanced colorectal cancer. Cancer Med. 2019, 9, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu-Lieskovan, S.; Chmielowski, B.; Govindan, R.; Naing, A.; Bhardwaj, N.; Margolin, K.; Awad, M.M.; Hellmann, M.D.; Lin, J.J.; et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer. Cell 2020, 183, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.; Freeman, D.J.; Kelly, B.; Harper, J.; Soria, J.-C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 2019, 18, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yao, Z.; Bai, H.; Duan, J.; Wang, Z.; Wang, X.; Zhang, X.; Xu, J.; Fei, K.; Zhang, Z.; et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol. 2021, 22, 1265–1274. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, W.; Tian, Z. Challenges of NK cell-based immunotherapy in the new era. Front. Med. 2018, 12, 440–450. [Google Scholar] [CrossRef]
- Wagner, J.A.; Rosario, M.; Romee, R.; Berrien-Elliott, M.M.; Schneider, S.E.; Leong, J.W.; Sullivan, R.P.; Jewell, B.A.; Becker-Hapak, M.; Schappe, T.; et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Investig. 2017, 127, 4042–4058. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Wang, T.T.; Dahan, R.; Maamary, J.; Ravetch, J.V. Signaling by Antibodies: Recent Progress. Annu. Rev. Immunol. 2017, 35, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Crinier, A.; Milpied, P.; Escalière, B.; Piperoglou, C.; Galluso, J.; Balsamo, A.; Spinelli, L.; Cervera-Marzal, I.; Ebbo, M.; Girard-Madoux, M.; et al. High-Dimensional Single-Cell Analysis Identifies Organ-Specific Signatures and Conserved NK Cell Subsets in Humans and Mice. Immunity 2018, 49, 971–986. [Google Scholar] [CrossRef]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; e Sousa, C.R. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037. [Google Scholar] [CrossRef]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Cichocki F, Bjordahl R, Gaidarova S, Mahmood S, Abujarour R, Wang H et al., iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy, Sci Transl Med. 12 (2020) eaaz5618.
- Tumino, N.; Martini, S.; Munari, E.; Scordamaglia, F.; Besi, F.; Mariotti, F.R.; Bogina, G.; Mingari, M.C.; Vacca, P.; Moretta, L. Presence of innate lymphoid cells in pleural effusions of primary and metastatic tumors: Functional analysis and expression of PD-1 receptor. Int. J. Cancer 2019, 145, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Togashi, Y.; Tay, C.; Ha, D.; Sasaki, A.; Nakamura, Y.; Sato, E.; Fukuoka, S.; Tada, Y.; Tanaka, A.; et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 9999–10008. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, J.; Chen, Y.; Cui, J.; Lei, Y.; Cui, Y.; Jiang, N.; Jiang, W.; Chen, L.; Chen, Y.; et al. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma). Int. Immunopharmacol. 2020, 80, 106198. [Google Scholar] [CrossRef]
- Kim, N.; Lee, H.H.; Lee, H.-J.; Choi, W.S.; Lee, J.; Kim, H.S. Natural killer cells as a promising therapeutic target for cancer immunotherapy. Arch. Pharmacal Res. 2019, 42, 591–606. [Google Scholar] [CrossRef]
- Kumar, S. Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology 2018, 154, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS et al., CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies, J Cell Mol Med. 20 (2016) 1287-94.
- Liu, Y.; Zhou, Y.; Huang, K.; Fang, X.; Li, Y.; Wang, F.; An, L.; Chen, Q.; Zhang, Y.; Shi, A.; et al. Targeting epidermal growth factor-overexpressing triple-negative breast cancer by natural killer cells expressing a specific chimeric antigen receptor. Cell Prolif. 2020, 53, e12858. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chu, J.; Chan, W.K.; Zhang, J.; Wang, Y.; Cohen, J.B.; Victor, A.; Meisen, W.H.; Kim, S.-H.; Grandi, P.; et al. CAR-Engineered NK Cells Targeting Wild-Type EGFR and EGFRvIII Enhance Killing of Glioblastoma and Patient-Derived Glioblastoma Stem Cells. Sci. Rep. 2015, 5, 11483. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2017, 32, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xu, J.; Huang, Q.; Huang, M.; Wen, H.; Zhang, C.; Wang, J.; Song, J.; Zheng, M.; Sun, H.; et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. OncoImmunology 2016, 6, e1264562. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Greppi, M.; Grossi, F.; Del Zotto, G.; Moretta, L.; Sivori, S.; Genova, C.; Marcenaro, E. PD/1-PD-Ls Checkpoint: Insight on the Potential Role of NK Cells. Front. Immunol. 2019, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Rajendran, R.L.; Ahn, B.-C. Application of In Vivo Imaging Techniques for Monitoring Natural Killer Cell Migration and Tumor Infiltration. Cancers 2020, 12, 1318. [Google Scholar] [CrossRef]
- Vimbela GV, Ngo SM, Fraze C, Yang L, Stout DA, Antibacterial properties and toxicity from metallic nanomaterials, Int J Nanomedicine. 12 (2017) 3941–3965.
- Wang C, Zhang R, Wei X, Lv M, Jiang Z, Metalloimmunology: The metal ion-controlled immunity, Adv Immunol. 145 (2020) 187-241.
- Li, J.; Ren, H.; Zhang, Y. Metal-based nano-vaccines for cancer immunotherapy. Co-ord. Chem. Rev. 2021, 455, 214345. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, M.; Luo, T.; Qu, J.; Chen, W.R. Photo-activated chemo-immunotherapy for metastatic cancer using a synergistic graphene nanosystem. Biomaterials 2020, 265, 120421–120421. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Sheng, J.; Zheng, P.; Li, C.; Li, D.; Cheng, Z.; Ma, P.; Lin, J. Biodegradable Upconversion Nanoparticles Induce Pyroptosis for Cancer Immunotherapy. Nano Lett. 2021, 21, 8281–8289. [Google Scholar] [CrossRef] [PubMed]
- Guinart, A.; Perry, H.L.; Wilton-Ely, J.D.E.T.; Tetley, T.D. Gold nanomaterials in the management of lung cancer. Emerg. Top. Life Sci. 2020, 4, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Essawy, M.M.; El-Sheikh, S.M.; Raslan, H.S.; Ramadan, H.S.; Kang, B.; Talaat, I.M.; Afifi, M.M. Function of gold nanoparticles in oral cancer beyond drug delivery: Implications in cell apoptosis. Oral Dis. 2020, 27, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, I.-H.; Kang, S.; Kim, D.; Choi, M.; Saw, P.E.; Shin, E.-C.; Jon, S. Gold Nanoparticles Displaying Tumor-Associated Self-Antigens as a Potential Vaccine for Cancer Immunotherapy. Adv. Healthc. Mater. 2014, 3, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Gao, A.; Tu, B.; Wang, Y.; Yu, X.; Wang, Y.; Xiu, Y.; Wang, B.; Wan, Y.; Huang, Y. Metabolic modulation via mTOR pathway and anti-angiogenesis remodels tumor microenvironment using PD-L1-targeting codelivery. Biomaterials 2020, 255, 120187. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tan, Y.; Hu, J.; Jiang, Y.; Wang, Z.; Liu, Z.; Chen, Q. Injectable Immunotherapeutic Thermogel for Enhanced Immunotherapy Post Tumor Radiofrequency Ablation. Small 2021, 17, 2104773. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef]
- Shi, G.-N.; Zhang, C.-N.; Xu, R.; Niu, J.-F.; Song, H.-J.; Zhang, X.-Y.; Wang, W.-W.; Wang, Y.-M.; Li, C.; Wei, X.-Q.; et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Thapa, R.K.; Jiang, L.; Soe, Z.C.; Gautam, M.; Chang, J.-H.; Jeong, J.-H.; Ku, S.K.; Choi, H.-G.; Yong, C.S.; et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J. Control. Release 2018, 281, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, W.; Guo, S.; Wang, Y.; Miao, L.; Xiong, Y.; Huang, L. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat. Commun. 2016, 7, 11822. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef]
- Buss, C.G.; Bhatia, S.N. Nanoparticle delivery of immunostimulatory oligonucleotides enhances response to checkpoint inhibitor therapeutics. Proc. Natl. Acad. Sci. 2020, 117, 13428–13436. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front. Immunol. 2020, 10, 3038. [Google Scholar] [CrossRef] [PubMed]
- Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK, During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium, Nat Med. 2 (1996) 992-7.
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lai, H.; Chen, T. Boosting Natural Killer Cell-Based Cancer Immunotherapy with Selenocystine/Transforming Growth Factor-Beta Inhibitor-Encapsulated Nanoemulsion. ACS Nano 2020, 14, 11067–11082. [Google Scholar] [CrossRef]
- Jindal, A.; Sarkar, S.; Alam, A. Nanomaterials-Mediated Immunomodulation for Cancer Therapeutics. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Gao, S.; Li, T.; Guo, Y.; Sun, C.; Xianyu, B.; Xu, H. Selenium-Containing Nanoparticles Combine the NK Cells Mediated Immunotherapy with Radiotherapy and Chemotherapy. Adv. Mater. 2020, 32, e1907568. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium Nanoparticles for Biomedical Applications: From Development and Characterization to Therapeutics. Adv. Heal. Mater. 2021, 10, 2100598. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Han, S.; Ding, S.; Xiao, W.; Ding, Y.; Qian, L.; Wang, C.; Gong, W. Chitosan nanoparticle-based delivery of fused NKG2D–IL-21 gene suppresses colon cancer growth in mice. Int. J. Nanomed. 2017, ume 12, 3095–3107. [Google Scholar] [CrossRef]
- Zhuo, Y.; Chen, F.; Kong, L.; Li, T.; Lu, L.; Yang, J.; Yu, T.; Shi, X.; Li, K. Magnetic Resonance Imaging of the Human Ferritin Heavy Chain Reporter Gene Carried by Dendrimer-Entrapped Gold Nanoparticles. J. Biomed. Nanotechnol. 2019, 15, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Han, J.-H.; Park, J.-H.; Kim, H.-K.; Choi, S.H.; Kim, G.R.; Song, H.; An, H.J.; Han, D.K.; Park, W.; et al. Multifunctional nanoparticles for genetic engineering and bioimaging of natural killer (NK) cell therapeutics. Biomaterials 2019, 221, 119418. [Google Scholar] [CrossRef] [PubMed]
- Kwak M, Erdag G, Leick KM, Bekiranov S, Engelhard VH, Slingluff CL, Associations of immune cell homing gene signatures and infiltrates of lymphocyte subsets in human melanomas: discordance with CD163+ myeloid cell infiltrates, J Transl Med. 19 (2021) 371.
- Zhang, S.-C.; Hu, Z.-Q.; Long, J.-H.; Zhu, G.-M.; Wang, Y.; Jia, Y.; Zhou, J.; Ouyang, Y.; Zeng, Z. Clinical Implications of Tumor-Infiltrating Immune Cells in Breast Cancer. J. Cancer 2019, 10, 6175–6184. [Google Scholar] [CrossRef] [PubMed]
- Ran, G.H.; Lin, Y.Q.; Tian, L.; Zhang, T.; Yan, D.M.; Yu, J.H.; Deng, Y.C. Natural killer cell homing and trafficking in tissues and tumors: from biology to application. Signal Transduct. Target. Ther. 2022, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Cifaldi L, Doria M, Cotugno N, Zicari S, Cancrini C, Palma P et al. Int J Mol Sci. 20 (2019) 3715.
- Wu, L.; Zhang, F.; Wei, Z.; Li, X.; Zhao, H.; Lv, H.; Ge, R.; Ma, H.; Zhang, H.; Yang, B.; et al. Magnetic delivery of Fe3O4@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater. Sci. 2018, 6, 2714–2725. [Google Scholar] [CrossRef]
- Monty, M.A.; Islam, A.; Nan, X.; Tan, J.; Tuhin, I.J.; Tang, X.; Miao, M.; Wu, D.; Yu, L. Emerging role of RNA interference in immune cells engineering and its therapeutic synergism in immunotherapy. Br. J. Pharmacol. 2021, 178, 1741–1755. [Google Scholar] [CrossRef]
- Lian, S.; Xie, R.; Ye, Y.; Xie, X.; Li, S.; Lu, Y.; Li, B.; Cheng, Y.; Katanaev, V.L.; Jia, L. Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release. EBioMedicine 2019, 42, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, M.; Luo, J.; Zhao, L.; Ye, G.; Shi, F.; Lv, C.; Chen, H.; Wang, Y.; Li, Y. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ. Sci. Eur. 2019, 31, 30. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Powell, L.G.; Stone, V. A review of hepatic nanotoxicology – summation of recent findings and considerations for the next generation of study designs. J. Toxicol. Environ. Heal. Part B 2020, 23, 137–176. [Google Scholar] [CrossRef]
- Wang, P.; Lu, Y.-Q. Ferroptosis: A Critical Moderator in the Life Cycle of Immune Cells. Front. Immunol. 2022, 13, 877634. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).