Submitted:
02 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Baseline Evaluation
Wireless Motility Capsule
2.3. Intervention
2.4. Final Evaluation
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
3.2. Motility Parameters
| Motility parameters | Celiac disease (n=12) | Non gluten celiac sensitivity (n=12) | ||
| Before | After 4 weeks of GFD | Before | After 4 weeks of GFD | |
| Transit time (minutes) | ||||
|
156 ± 38 | 216 ± 48 | 183.6 ± 60 | 186 ± 54 |
| 252 ± 39 | 196±27 * | 264 ± 41 | 181±18 * | |
| 2150±1020 & | 1450±348 * | 1278 ± 452 | 1139 ± 365 | |
| 2394±960 & | 2104 ± 660 | 1672 ± 429 | 1577 ± 412 | |
| Small bowel motility parameters | ||||
|
109 ± 23 | 198±17 * | 116 ± 28 | 162±28 * |
|
2.4 ± 0.8 | 3.3±0.4 * | 2.8± 0.6 | 3.0 ± 0.7 |
|
1.68 ± 1.4 | 3.74±1.3 * | 3.63± 0.9 | 5.2±2.3 * |
|
136 ± 32 | 206±24 * | 160.5 ± 50 | 222±41 * |
| Colon motility parameters | ||||
|
98 ± 19 | 173±41 * | 107 ± 32 | 132 ± 47 |
|
3.1 ± 1.8 | 4.1 ± 1.9 | 4.2 ± 1.9 | 4.7 ±1.3 |
|
1.9 ± 0.7 | 3.1±0.8 * | 2.1 ± 0.8 | 2.7 ± 0.9 |
|
154 ± 61 | 208 ± 32 * | 197 ± 52 | 224 ± 68 |
| pH median | ||||
|
2.1 ± 0.5 | 1.9 ± 0.8 | 1.9 ± 0.3 | 1.8 ± 0.5 |
|
7.3 ± 0.6 | 6.9 ± 1.1 | 7.2 ± 0.8 | 7.1 ± 0.8 |
|
6.8 ± 0.8 | 6.7 ± 0.9 | 6.9 ±1.1 | 6.9 ± 0.7 |
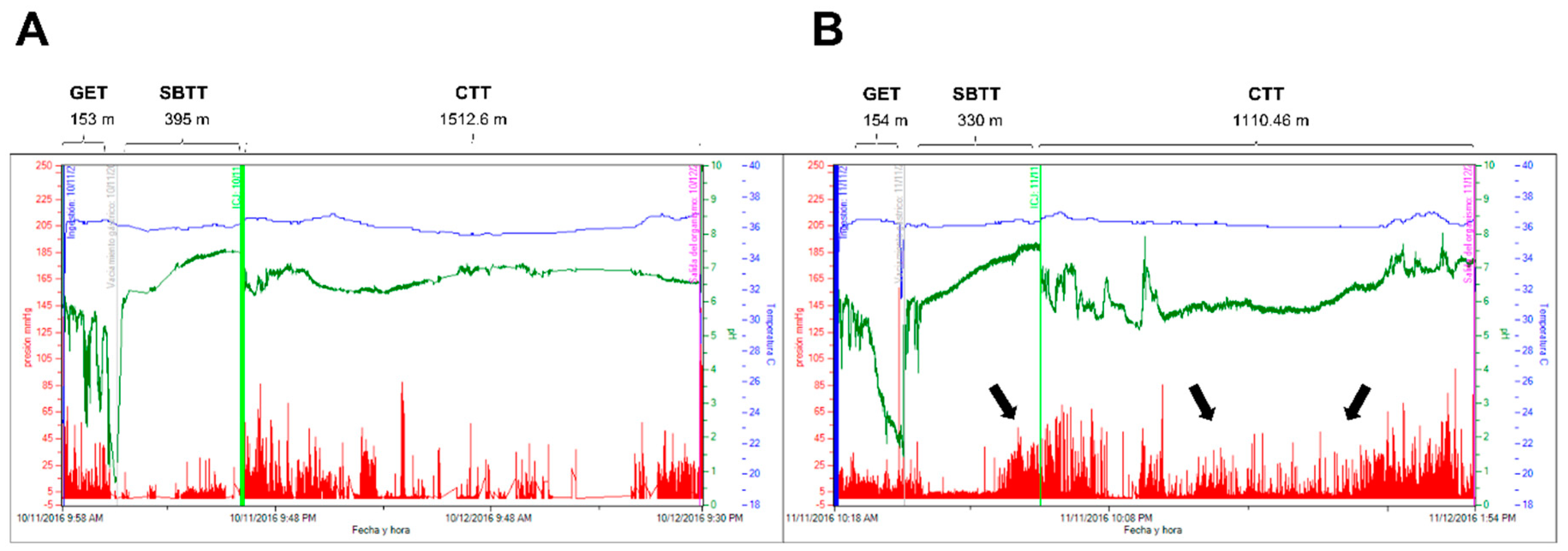
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludvigsson J.F., Leffler D.A., Bai J.C., Biagi F., Fasano A., Green P.H., Hadjivassiliou M., Kaukinen K., Kelly C.P., Leonard J.N., Lundin K.E., Murray J.A., Sanders D.S., Walker M.M., Zingone F., Ciacci C. The Oslo definitions for coeliac disease and related terms. Gut 2013; 62(1):43-52. [CrossRef]
- Singh P., Arora A., Strand T.A., Leffler D.A., Catassi C., Green P.H., Kelly C.P., Ahuja V., Makharia G.K. Global prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018; 16:823-836. [CrossRef]
- Jansson-Knodell C.L., Rubio-Tapia A. Gluten-related disorders from bench to bedside. Clin Gastroenterol Hepatol. 2023: S1542-3565(23)00844-3. [CrossRef]
- Lindfors K., Ciacci C., Kurppa K., Lundin K.E.A., Makharia G.K., Mearin M.L., Murray J.A., Verdu E.F., Kaukinen K. Coeliac disease. Nat Rev Dis Primers. 2019 ;5(1):3. [CrossRef]
- Catassi C., Elli L., Bonaz B., Bouma G., Carroccio A., Castillejo G., Cellier C., Cristofori F., de Magistris L., Dolinsek J., Dieterich W., Francavilla R., Hadjivassiliou M., Holtmeier W., Körner U., Leffler D.A., Lundin K.E., Mazzarella G., Mulder C.J., Pellegrini N., Rostami K., Sanders D., Skodje G.I., Schuppan D., Ullrich R., Volta U., Williams M., Zevallos V.F., Zopf Y., Fasano A. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts' Criteria. Nutrients. 2015 18;7(6):4966-77. [CrossRef]
- Shiha M.G., Raju S.A., Penny H.A., Sanders D.S. Non-coeliac gluten sensitivity: from Salerno to Rome. Lancet Gastroenterol Hepatol. 2024;9(2):94-95. [CrossRef]
- Pinto-Sánchez M.I., Verdú E.F. Non-coeliac gluten sensitivity: are we closer to separating the wheat from the chaff? Gut 2016;65(12):1921-1922. [CrossRef]
- Junker Y., Zeissig S., Kim S.J, Barisani D., Wieser H., Leffler D.A., Zevallos V., Libermann T.A., Dillon S., Freitag T.L., Kelly C.P., Schuppan D. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med 2012; 209:2395–2408. [CrossRef]
- Usai-Satta P., Oppia F., Lai M., Cabras F. Motility Disorders in Celiac Disease and Non-Celiac Gluten Sensitivity: The Impact of a Gluten-Free Diet. Nutrients. 2018;10(11):1705. [CrossRef]
- Bassotti, G.; Castellucci, G.; Betti, C.; Fusaro, C.; Cavalletti, M.L.; Bertotto, A.; Spinozzi, F.; Morell, A.; Pello, M.A. Abnormal gastrointestinal motility in patients with celiac sprue. Dig. Dis. Sci. 1994, 39, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Cucchiara, S.; Bassotti, G.; Castellucci, G.; Minella, R.; Betti, C.; Fusaro, C.; Morelli, A.; Bertotto, A.; Auricchio, S. Upper gastrointestinal motor abnormalities in children with active celiac disease. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Villanacci, V.; Mazzocchi, A.; Mariano, M.; Incardona, P.; Clerici, C.; Morelli, A. Antroduodenojejunal motor activity in untreated and treated celiac disease patients. J. Gastroenterol. Hepatol. 2008, 23, e23–e28. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez M.I., Bercik P., Verdu E.F. Motility alterations in celiac disease and non-celiac gluten sensitivity. Dig Dis. 2015;33(2):200-207. [CrossRef]
- Benini F., Mora A., Turini D., Bertolazzi S., Lanzarotto F., Ricci C., Villanacci V., Barbara G., Stanghellini V., Lanzini A. Slow gallbladder emptying reverts to normal but small intestinal transit of a physiological meal remains slow in celiac patients during gluten-free diet. Neurogastroenterol Motil. 2012;24(2):100-7, e79-80. [CrossRef]
- Chiarioni G., Bassotti G., Germani U., Battaglia E., Brentegani M.T., Morelli A., Vantini I. Gluten-free diet normalizes mouth-to-cecum transit of a caloric meal in adult patients with celiac disease. Dig Dis Sci. 1997 ;42(10):2100-5. [CrossRef]
- Saad R.J. The Wireless Motility Capsule: a One-Stop Shop for the Evaluation of GI Motility Disorders. Curr Gastroenterol Rep. 2016;18(3):14. [CrossRef]
- Arora Z., Parungao .JM., Lopez R., Heinlein C., Santisi J., Birgisson S. Clinical utility of wireless motility capsule in patients with suspected multiregional gastrointestinal dysmotility. Dig Dis Sci. 2015;60(5):1350-7. [CrossRef]
- Rouphael C., Arora Z., Thota P.N., Lopez R., Santisi J., Funk C., Cline M. Role of wireless motility capsule in the assessment and management of gastrointestinal dysmotility in patients with diabetes mellitus. Neurogastroenterol Motil. 2017 ;29(9). [CrossRef]
- Kuo B., Maneerattanaporn M., Lee A.A., Baker J.R., Wiener S.M., Chey W.D., Wilding G.E., Hasler W.L. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Dig Dis Sci. 2011;56(10):2928-38. [CrossRef]
- Parkman H.P., Sharkey E., McCallum R.W., Hasler W.L., Koch K.L, Sarosiek I., Abell T.L., Kuo B., Shulman R.J., Grover M., Farrugia G., Schey R., Tonascia J., Hamilton F., Pasricha P.J; NIH/NIDDK Gastroparesis Consortium. Constipation in Patients With Symptoms of Gastroparesis: Analysis of Symptoms and Gastrointestinal Transit. Clin Gastroenterol Hepatol. 2022;20(3):546-558.e5. [CrossRef]
- Oberhuber G., Granditsch G., Vogelsang H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol, 1999;11: 1185-1194. [CrossRef]
- Kulich K. R, Madisch A., Pacini F, Piqué J.M., Regula J., Van Rensburg C.J., Ujszászy L., Carlsson J., Halling K., Wiklund I.K. Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: a six-country study Health Qual Life Outcomes, 2008; 6:12. [CrossRef]
- Heaton K.W., Radvan J., Cripps H., Mountford R.A., Braddon F.E., Hughes A.O. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33(6):818-24. [CrossRef]
- Rao S.S.C., Camilleri M., Hasler W.L., Maurer A.H., Parkman H.P., Saad R., Scott M.S., Simren M., Soffer E., Szarka L.Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23:8-23. Neurogastroenterol Motil. [CrossRef]
- Farmer A.D., Wegeberg A.L., Brock B., Hobson A.R., Mohammed S.D., Scott S.M., Bruckner-Holt C.E., Semler J.R., Hasler W.L., Hellström P.M., Drewes A.M., Brock C. Regional gastrointestinal contractility parameters using the wireless motility capsule: inter-observer reproducibility and influence of age, gender, and study country. Aliment Pharmacol Ther. 2018;47(3):391-400. [CrossRef]
- Bai, J.C.; Mauriño, E.; Martínez, C.; Vázquez, H.; Niveloni, S.; Soifer, G.; Flores, D.; Boerr, L.A. Abnormal colonic transit time in untreated celiac sprue. Acta Gastroenterol Latinoam. 1995, 25, 277–284. [Google Scholar] [PubMed]
- Kelly C.P., Bai J.C., Liu E., Leffler D.A. Advances in diagnosis and management of celiac disease. Gastroenterology. 2015;148(6):1175-86. [CrossRef]
- Sánchez-Vargas L.A., Thomas-Dupont P., Torres-Aguilera M., Azamar-Jacome A.A., Ramírez-Ceervanes K.L., Aedo-Garcés M.R., Meixueiro-Daza A., Roesch-Dietlen F., Grube-Pagola P., Vivanco-Cid H., Remes-Troche J.M. Prevalence of celiac disease and related antibodies in patients diagnosed with irritable bowel syndrome according to the Rome III criteria. A case-control study. Neurogastroenterol Motil. 2016;28(7):994-1000. [CrossRef]
- Benini, L.; Sembenini, C.; Salandini, L.; Dall’O, E.; Bonfante, F.; Vantini, I. Gastric emptying of realistic meals with and without gluten in patients with coeliac disease. Effect of jejunal mucosal recovery. Scand. J. Gastroenterol. 2001, 36, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez M.I., Nardelli A., Borojevic R., De Palma G., Calo N.C., McCarville J., Caminero A., Basra D., Mordhorst A., Ignatova E., Hansen S., Uhde M., Norman G.L., Murray J.A., Smecuol E., Armstrong D., Bai J.C., Schuppan D., Collins S.M., Alaedini A., Moayyedi P., Verdu E.F., Bercik P. Gluten-Free Diet Reduces Symptoms, Particularly Diarrhea, in Patients With Irritable Bowel Syndrome and Antigliadin IgG. Clin Gastroenterol Hepatol. 2021;19(11):2343-2352.e8. [CrossRef]
- Barbaro M.R., Cremon C., Wrona D., Fuschi D., Marasco G., Stanghellini V., Barbara G. Non-Celiac Gluten Sensitivity in the Context of Functional Gastrointestinal Disorders. Nutrients 2020;12(12):3735. [CrossRef]
- Verdu E.F., Huang X., Natividad J., Lu J., Blennerhassett P.A., David C.S., McKay D.M., Murray J.A. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G217-225. [CrossRef]
- Verdu E.F., Armstrong D., Murray J.A. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104(6):1587–94. [CrossRef]
- de Punder, K.; Pruimboom, L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients 2013, 5, 771–787. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





