Submitted:
02 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
Introduction
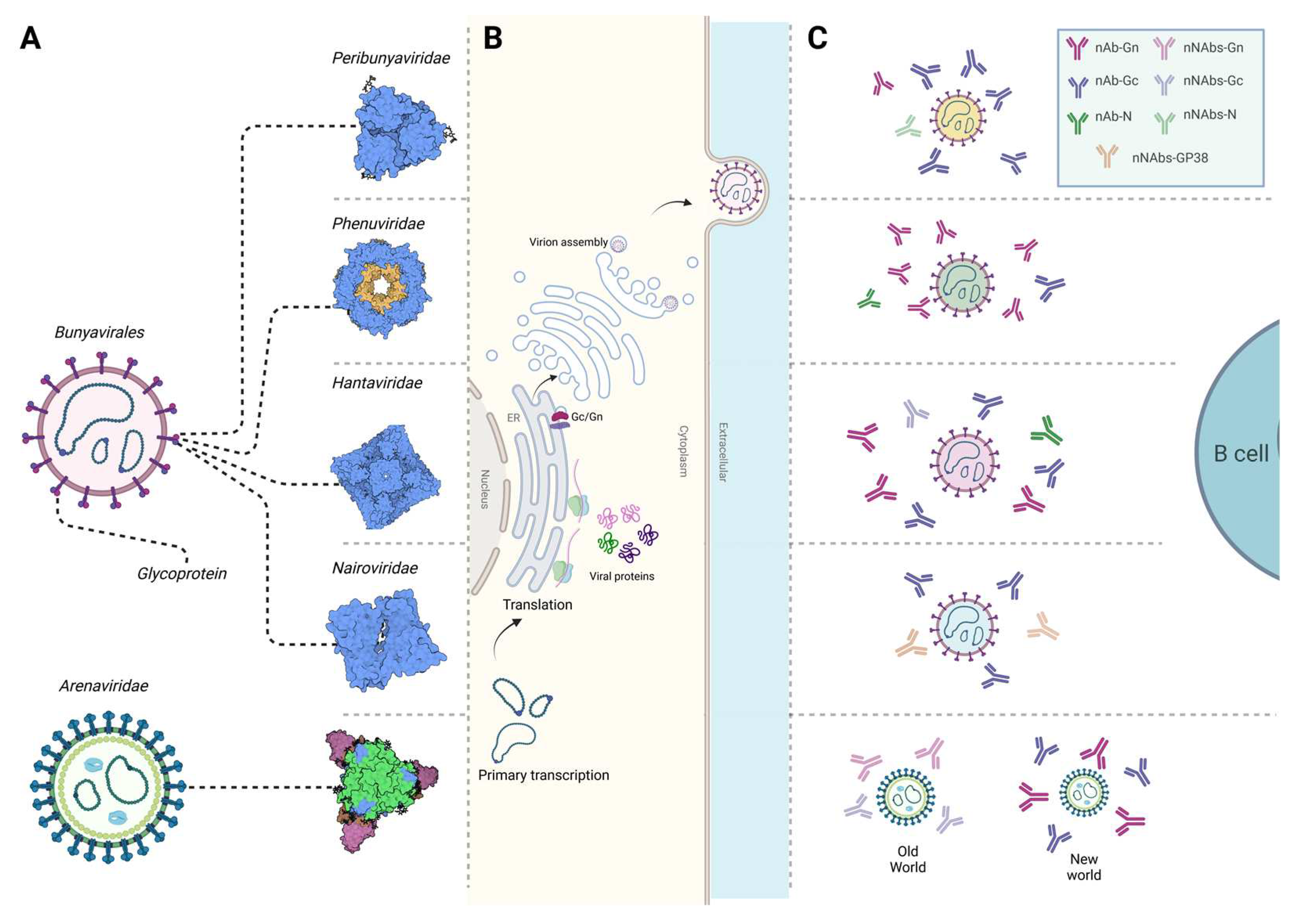
Structure and Life Cycle of Bunyaviruses
T Cell Responses against Bunyaviruses
Peribunyaviridae
Phenuiviridae
Hantaviridae
Nairoviridae
Antibody Responses against Bunyaviruses
Peribunyaviridea
Phenuiviridae
Hantaviridae
Nairoviridea
Bunyavirus Vaccines and Therapeutic Strategies
Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Arch. Virol. 2017, 162, 2505–2538. [Google Scholar] [CrossRef]
- Abudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.D.; et al. Taxonomy of the order Bunyavirales: update 2019. Arch Virol 2019, 164, 1949–1965. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Amarasinghe, G.K.; Anthony, S.J.; Avšič-Županc, T.; Ayllón, M.A.; Bahl, J.; Balkema-Buschmann, A.; et al. 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch Virol 2020, 165, 3023–3072. [Google Scholar] [CrossRef]
- Orba, Y.; Abu, Y.E.; Chambaro, H.M.; Lundu, T.; Muleya, W.; Eshita, Y.; Qiu, Y.; Harima, H.; Kajihara, M.; Mori-Kajihara, A.; et al. Expanding diversity of bunyaviruses identified in mosquitoes. Scientific Reports 2023, 13, 18165. [Google Scholar] [CrossRef] [PubMed]
- Horne, K.M.; Vanlandingham, D.L. Bunyavirus-vector interactions. Viruses 2014, 6, 4373–4397. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M. Bunyaviruses and climate change. Clinical Microbiology and Infection 2009, 15, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Soldan, S.S.; González-Scarano, F. Emerging infectious diseases: The Bunyaviridae. Journal of NeuroVirology 2005, 11, 412–423. [Google Scholar] [CrossRef]
- Elliott, R.M. Emerging Viruses: The Bunyaviridae. Molecular Medicine 1997, 3, 572–577. [Google Scholar] [CrossRef]
- Family - Bunyaviridae. In Virus Taxonomy; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: San Diego, 2012. [Google Scholar] [CrossRef]
- Hastie, K.M.; Melnik, L.I.; Cross, R.W.; Klitting, R.M.; Andersen, K.G.; Saphire, E.O.; Garry, R.F. The Arenaviridae Family: Knowledge Gaps, Animal Models, Countermeasures, and Prototype Pathogens. The Journal of Infectious Diseases 2023, 228, S359–S375. [Google Scholar] [CrossRef]
- Leventhal, S.S.; Wilson, D.; Feldmann, H.; Hawman, D.W. A Look into Bunyavirales Genomes: Functions of Non-Structural (NS) Proteins. Viruses 2021, 13, 314. [Google Scholar] [CrossRef]
- Hulswit, R.J.G.; Paesen, G.C.; Bowden, T.A.; Shi, X. Recent Advances in Bunyavirus Glycoprotein Research: Precursor Processing, Receptor Binding and Structure. Viruses 2021, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.; López-Montero, N.; Elliott, R.M.; Fernández, J.J.; Risco, C. The unique architecture of Bunyamwera virus factories around the Golgi complex. Cellular Microbiology 2008, 10, 2012–2028. [Google Scholar] [CrossRef] [PubMed]
- Won, S.; Ikegami, T.; Peters, C.J.; Makino, S. NSm protein of Rift Valley fever virus suppresses virus-induced apoptosis. J Virol 2007, 81, 13335–13345. [Google Scholar] [CrossRef] [PubMed]
- Ferron, F.; Weber, F.; de la Torre, J.C.; Reguera, J. Transcription and replication mechanisms of Bunyaviridae and Arenaviridae L proteins. Virus Res 2017, 234, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Suda, Y.; Fukushi, S.; Tani, H.; Murakami, S.; Saijo, M.; Horimoto, T.; Shimojima, M. Analysis of the entry mechanism of Crimean-Congo hemorrhagic fever virus, using a vesicular stomatitis virus pseudotyping system. Arch Virol 2016, 161, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Léger, P.; Tetard, M.; Youness, B.; Cordes, N.; Rouxel, R.N.; Flamand, M.; Lozach, P.Y. Differential use of the C-type lectins L-SIGN and DC-SIGN for phlebovirus endocytosis. Traffic 2016, 17, 639–656. [Google Scholar] [CrossRef]
- Shimojima, M.; Kawaoka, Y. Cell surface molecules involved in infection mediated by lymphocytic choriomeningitis virus glycoprotein. J Vet Med Sci 2012, 74, 1363–1366. [Google Scholar] [CrossRef]
- Albornoz, A.; Hoffmann, A.B.; Lozach, P.Y.; Tischler, N.D. Early Bunyavirus-Host Cell Interactions. Viruses 2016, 8. [Google Scholar] [CrossRef]
- Garrison, A.R.; Radoshitzky, S.R.; Kota, K.P.; Pegoraro, G.; Ruthel, G.; Kuhn, J.H.; Altamura, L.A.; Kwilas, S.A.; Bavari, S.; Haucke, V.; et al. Crimean-Congo hemorrhagic fever virus utilizes a clathrin- and early endosome-dependent entry pathway. Virology 2013, 444, 45–54. [Google Scholar] [CrossRef]
- Boshra, H. An Overview of the Infectious Cycle of Bunyaviruses. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; daSilva, L.L.P.; Crump, C.M. Mechanisms of bunyavirus morphogenesis and egress. Journal of General Virology 2023, 104. [Google Scholar] [CrossRef] [PubMed]
- Urata, S.; Yasuda, J. Molecular Mechanism of Arenavirus Assembly and Budding. Viruses 2012, 4, 2049–2079. [Google Scholar] [CrossRef]
- Goldsmith, C.S.; Elliott, L.H.; Peters, C.J.; Zaki, S.R. Ultrastructural characteristics of Sin Nombre virus, causative agent of hantavirus pulmonary syndrome. Arch Virol 1995, 140, 2107–2122. [Google Scholar] [CrossRef]
- Ravkov, E.V.; Nichol, S.T.; Compans, R.W. Polarized entry and release in epithelial cells of Black Creek Canal virus, a New World hantavirus. J Virol 1997, 71, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Dutuze, M.F.; Nzayirambaho, M.; Mores, C.N.; Christofferson, R.C. A Review of Bunyamwera, Batai, and Ngari Viruses: Understudied Orthobunyaviruses With Potential One Health Implications. Frontiers in Veterinary Science 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M. Orthobunyaviruses: recent genetic and structural insights. Nature Reviews Microbiology 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.K.; Tayebi, M.; Rahman, M.M. Immunoinformatics Approach for Epitope-Based Peptide Vaccine Design and Active Site Prediction against Polyprotein of Emerging Oropouche Virus. J Immunol Res 2018, 2018, 6718083. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.; Aiman, S.; Alshammari, A.; Alasmari, A.F.; Alharbi, M.; Khan, A.; Wei, D.Q.; Zheng, G. Immunoinformatics-based potential multi-peptide vaccine designing against Jamestown Canyon Virus (JCV) capable of eliciting cellular and humoral immune responses. Int J Biol Macromol 2023, 253, 126678. [Google Scholar] [CrossRef]
- Nelluri, K.D.D.; Ammulu, M.A.; Durga, M.L.; Sravani, M.; Kumar, V.P.; Poda, S. In silico multi-epitope Bunyumwera virus vaccine to target virus nucleocapsid N protein. Journal of Genetic Engineering and Biotechnology 2022, 20, 89. [Google Scholar] [CrossRef]
- Ghosh, P.; Bhattacharya, M.; Patra, P.; Sharma, G.; Patra, B.C.; Lee, S.S.; Sharma, A.R.; Chakraborty, C. Evaluation and Designing of Epitopic-Peptide Vaccine Against Bunyamwera orthobunyavirus Using M-Polyprotein Target Sequences. Int J Pept Res Ther 2022, 28, 5. [Google Scholar] [CrossRef]
- Boshra, H.Y.; Charro, D.; Lorenzo, G.; Sánchez, I.; Lazaro, B.; Brun, A.; Abrescia, N.G. DNA vaccination regimes against Schmallenberg virus infection in IFNAR(-/-) mice suggest two targets for immunization. Antiviral Res 2017, 141, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Schuh, T.; Schultz, J.; Moelling, K.; Pavlovic, J. DNA-based vaccine against La Crosse virus: protective immune response mediated by neutralizing antibodies and CD4+ T cells. Hum Gene Ther 1999, 10, 1649–1658. [Google Scholar] [CrossRef]
- Boshra, H.; Lorenzo, G.; Charro, D.; Moreno, S.; Guerra, G.S.; Sanchez, I.; Garrido, J.M.; Geijo, M.; Brun, A.; Abrescia, N.G.A. A novel Schmallenberg virus subunit vaccine candidate protects IFNAR(-/-) mice against virulent SBV challenge. Sci Rep 2020, 10, 18725. [Google Scholar] [CrossRef]
- Jain, A.; Tripathi, P.; Shrotriya, A.; Chaudhary, R.; Singh, A. In silico analysis and modeling of putative T cell epitopes for vaccine design of Toscana virus. 3 Biotech 2015, 5, 497–503. [Google Scholar] [CrossRef]
- Suleman, M.; Asad, U.; Arshad, S.; Rahman, A.U.; Akbar, F.; Khan, H.; Hussain, Z.; Ali, S.S.; Mohammad, A.; Khan, A.; et al. Screening of immune epitope in the proteome of the Dabie bandavirus, SFTS, to design a protein-specific and proteome-wide vaccine for immune response instigation using an immunoinformatics approaches. Comput Biol Med 2022, 148, 105893. [Google Scholar] [CrossRef]
- Adhikari, U.K.; Rahman, M.M. Overlapping CD8+ and CD4+ T-cell epitopes identification for the progression of epitope-based peptide vaccine from nucleocapsid and glycoprotein of emerging Rift Valley fever virus using immunoinformatics approach. Infection, Genetics and Evolution 2017, 56, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Harmon, J.R.; Barbeau, D.J.; Nichol, S.T.; Spiropoulou, C.F.; McElroy, A.K. Rift Valley fever virus vaccination induces long-lived, antigen-specific human T cell responses. NPJ Vaccines 2020, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Watts, D.M.; Costanzo, M.C.; Tang, X.; Venegas, L.A.; Jiao, F.; Sette, A.; Sidney, J.; Sewell, A.K.; Wooldridge, L.; et al. The nucleocapsid protein of Rift Valley fever virus is a potent human CD8+ T cell antigen and elicits memory responses. PLoS One 2013, 8, e59210. [Google Scholar] [CrossRef]
- Barbeau, D.J.; Cartwright, H.N.; Harmon, J.R.; Spengler, J.R.; Spiropoulou, C.F.; Sidney, J.; Sette, A.; McElroy, A.K. Identification and Characterization of Rift Valley Fever Virus-Specific T Cells Reveals a Dependence on CD40/CD40L Interactions for Prevention of Encephalitis. J Virol 2021, 95, e0150621. [Google Scholar] [CrossRef]
- Abdulla, F.; Nain, Z.; Hossain, M.M.; Syed, S.B.; Ahmed Khan, M.S.; Adhikari, U.K. A comprehensive screening of the whole proteome of hantavirus and designing a multi-epitope subunit vaccine for cross-protection against hantavirus: Structural vaccinology and immunoinformatics study. Microbial Pathogenesis 2021, 150, 104705. [Google Scholar] [CrossRef]
- Van Epps, H.L.; Schmaljohn, C.S.; Ennis, F.A. Human memory cytotoxic T-lymphocyte (CTL) responses to Hantaan virus infection: identification of virus-specific and cross-reactive CD8(+) CTL epitopes on nucleocapsid protein. J Virol 1999, 73, 5301–5308. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, J.; Kang, Z.; Zhao, Q.; Wang, X.; Hui, L. Kinetics and Immunodominance of Virus-Specific T Cell Responses During Hantaan Virus Infection. Viral Immunol 2015, 28, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, Y.; Wang, J.; Lv, T.; Jin, B. Identification of three novel CTL epitopes within nucleocapsid protein of Hantaan virus. Viral Immunol 2011, 24, 449–454. [Google Scholar] [CrossRef]
- Lee, K.Y.; Chun, E.; Kim, N.Y.; Seong, B.L. Characterization of HLA-A2.1-restricted epitopes, conserved in both Hantaan and Sin Nombre viruses, in Hantaan virus-infected patients. J Gen Virol 2002, 83, 1131–1136. [Google Scholar] [CrossRef]
- Ennis, F.A.; Cruz, J.; Spiropoulou, C.F.; Waite, D.; Peters, C.J.; Nichol, S.T.; Kariwa, H.; Koster, F.T. Hantavirus pulmonary syndrome: CD8+ and CD4+ cytotoxic T lymphocytes to epitopes on Sin Nombre virus nucleocapsid protein isolated during acute illness. Virology 1997, 238, 380–390. [Google Scholar] [CrossRef]
- Tang, K.; Cheng, L.; Zhang, C.; Zhang, Y.; Zheng, X.; Zhang, Y.; Zhuang, R.; Jin, B.; Zhang, F.; Ma, Y. Novel Identified HLA-A*0201-Restricted Hantaan Virus Glycoprotein Cytotoxic T-Cell Epitopes Could Effectively Induce Protective Responses in HLA-A2.1/K(b) Transgenic Mice May Associate with the Severity of Hemorrhagic Fever with Renal Syndrome. Front Immunol 2017, 8, 1797. [Google Scholar] [CrossRef]
- Manigold, T.; Mori, A.; Graumann, R.; Llop, E.; Simon, V.; Ferrés, M.; Valdivieso, F.; Castillo, C.; Hjelle, B.; Vial, P. Highly differentiated, resting gn-specific memory CD8+ T cells persist years after infection by andes hantavirus. PLoS Pathog 2010, 6, e1000779. [Google Scholar] [CrossRef] [PubMed]
- Terajima, M.; Van Epps, H.L.; Li, D.; Leporati, A.M.; Juhlin, S.E.; Mustonen, J.; Vaheri, A.; Ennis, F.A. Generation of recombinant vaccinia viruses expressing Puumala virus proteins and use in isolating cytotoxic T cells specific for Puumala virus. Virus Res 2002, 84, 67–77. [Google Scholar] [CrossRef]
- Nosrati, M.; Behbahani, M.; Mohabatkar, H. Towards the first multi-epitope recombinant vaccine against Crimean-Congo hemorrhagic fever virus: A computer-aided vaccine design approach. J Biomed Inform 2019, 93, 103160. [Google Scholar] [CrossRef]
- Shrivastava, N.; Verma, A.; Dash, P.K. Identification of functional epitopes of structural proteins and in-silico designing of dual acting multiepitope anti-tick vaccine against emerging Crimean-Congo hemorrhagic fever virus. Eur J Pharm Sci 2020, 151, 105396. [Google Scholar] [CrossRef]
- Oany, A.R.; Ahmad, S.A.; Hossain, M.U.; Jyoti, T.P. Identification of highly conserved regions in L-segment of Crimean-Congo hemorrhagic fever virus and immunoinformatic prediction about potential novel vaccine. Adv Appl Bioinform Chem 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Maotoana, M.G.; Burt, F.J.; Goedhals, D. Identification of T cell responses to the nonstructural glycoproteins in survivors of Crimean-Congo hemorrhagic fever in South Africa. J Med Virol 2023, 95, e29154. [Google Scholar] [CrossRef] [PubMed]
- Goedhals, D.; Paweska, J.T.; Burt, F.J. Long-lived CD8+ T cell responses following Crimean-Congo haemorrhagic fever virus infection. PLOS Neglected Tropical Diseases 2017, 11, e0006149. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Meade-White, K.; Leventhal, S.; Mihalakakos, E.; Carmody, A.; Feldmann, H.; Hawman, D.W. CD8(+) T-cells target the Crimean-Congo haemorrhagic fever virus Gc protein to control the infection in wild-type mice. EBioMedicine 2023, 97, 104839. [Google Scholar] [CrossRef] [PubMed]
- Abass, O.A.; Timofeev, V.I.; Sarkar, B.; Onobun, D.O.; Ogunsola, S.O.; Aiyenuro, A.E.; Aborode, A.T.; Aigboje, A.E.; Omobolanle, B.N.; Imolele, A.G.; et al. Immunoinformatics analysis to design novel epitope based vaccine candidate targeting the glycoprotein and nucleoprotein of Lassa mammarenavirus (LASMV) using strains from Nigeria. J Biomol Struct Dyn 2022, 40, 7283–7302. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, S.; Hartnett, J.N.; Ngo, N.; Goba, A.; Momoh, M.; Sandi, J.D.; Kanneh, L.; Cubitt, B.; Garcia, S.D.; Ware, B.C.; et al. Identification of Common CD8(+) T Cell Epitopes from Lassa Fever Survivors in Nigeria and Sierra Leone. J Virol 2020, 94. [Google Scholar] [CrossRef]
- Ugwu, C.; Olumade, T.; Nwakpakpa, E.; Onyia, V.; Odeh, E.; Duruiheoma, R.O.; Ojide, C.K.; Eke, M.A.; Nwafor, I.E.; Chika-Igwenyi, N.; et al. Humoral and cellular immune responses to Lassa fever virus in Lassa fever survivors and their exposed contacts in Southern Nigeria. Sci Rep 2022, 12, 22330. [Google Scholar] [CrossRef]
- Meulen, J.; Badusche, M.; Satoguina, J.; Strecker, T.; Lenz, O.; Loeliger, C.; Sakho, M.; Koulemou, K.; Koivogui, L.; Hoerauf, A. Old and New World arenaviruses share a highly conserved epitope in the fusion domain of the glycoprotein 2, which is recognized by Lassa virus-specific human CD4+ T-cell clones. Virology 2004, 321, 134–143. [Google Scholar] [CrossRef]
- ter Meulen, J.; Badusche, M.; Kuhnt, K.; Doetze, A.; Satoguina, J.; Marti, T.; Loeliger, C.; Koulemou, K.; Koivogui, L.; Schmitz, H.; et al. Characterization of human CD4(+) T-cell clones recognizing conserved and variable epitopes of the Lassa virus nucleoprotein. J Virol 2000, 74, 2186–2192. [Google Scholar] [CrossRef]
- La Posta, V.J.; Auperin, D.D.; Kamin-Lewis, R.; Cole, G.A. Cross-protection against lymphocytic choriomeningitis virus mediated by a CD4+ T-cell clone specific for an envelope glycoprotein epitope of Lassa virus. J Virol 1993, 67, 3497–3506. [Google Scholar] [CrossRef]
- Vahey, G.M.; Lindsey, N.P.; Staples, J.E.; Hills, S.L. La Crosse Virus Disease in the United States, 2003-2019. Am J Trop Med Hyg 2021, 105, 807–812. [Google Scholar] [CrossRef]
- Winkler, C.W.; Myers, L.M.; Woods, T.A.; Carmody, A.B.; Taylor, K.G.; Peterson, K.E. Lymphocytes have a role in protection, but not in pathogenesis, during La Crosse Virus infection in mice. J Neuroinflammation 2017, 14, 62. [Google Scholar] [CrossRef]
- Sun, M.-H.; Ji, Y.-F.; Li, G.-H.; Shao, J.-W.; Chen, R.-X.; Gong, H.-Y.; Chen, S.-Y.; Chen, J.-M. Highly adaptive Phenuiviridae with biomedical importance in multiple fields. Journal of Medical Virology 2022, 94, 2388–2401. [Google Scholar] [CrossRef]
- Mehand, M.S.; Al-Shorbaji, F.; Millett, P.; Murgue, B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antiviral Res 2018, 159, 63–67. [Google Scholar] [CrossRef]
- Kwaśnik, M.; Rożek, W.; Rola, J. Rift Valley Fever - a Growing Threat To Humans and Animals. J Vet Res 2021, 65, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Huang, H.; Jiang, L.; Li, J. Overview of the immunological mechanism underlying severe fever with thrombocytopenia syndrome (Review). Int J Mol Med 2022, 50. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, N.; Charrel, R.N. An update on Toscana virus distribution, genetics, medical and diagnostic aspects. Clin Microbiol Infect 2020, 26, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Ayari-Fakhfakh, E.; Ghram, A.; Albina, E.; Cêtre-Sossah, C. Expression of cytokines following vaccination of goats with a recombinant capripoxvirus vaccine expressing Rift Valley fever virus proteins. Vet Immunol Immunopathol 2018, 197, 15–20. [Google Scholar] [CrossRef] [PubMed]
- López-Gil, E.; Lorenzo, G.; Hevia, E.; Borrego, B.; Eiden, M.; Groschup, M.; Gilbert, S.C.; Brun, A. A single immunization with MVA expressing GnGc glycoproteins promotes epitope-specific CD8+-T cell activation and protects immune-competent mice against a lethal RVFV infection. PLoS Negl Trop Dis 2013, 7, e2309. [Google Scholar] [CrossRef] [PubMed]
- Pavulraj, S.; Stout, R.W.; Barras, E.D.; Paulsen, D.B.; Chowdhury, S.I. A Novel Quadruple Gene-Deleted BoHV-1-Vectored RVFV Subunit Vaccine Induces Humoral and Cell-Mediated Immune Response against Rift Valley Fever in Calves. Viruses 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.E.; Kim, Y.I.; Park, S.J.; Yu, M.A.; Kwon, H.I.; Eo, S.; Kim, T.S.; Seok, J.; Choi, W.S.; Jeong, J.H.; et al. Development of a SFTSV DNA vaccine that confers complete protection against lethal infection in ferrets. Nat Commun 2019, 10, 3836. [Google Scholar] [CrossRef]
- Kang, J.G.; Jeon, K.; Choi, H.; Kim, Y.; Kim, H.I.; Ro, H.J.; Seo, Y.B.; Shin, J.; Chung, J.; Jeon, Y.K.; et al. Vaccination with single plasmid DNA encoding IL-12 and antigens of severe fever with thrombocytopenia syndrome virus elicits complete protection in IFNAR knockout mice. PLoS Negl Trop Dis 2020, 14, e0007813. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jeon, K.; Hong, J.J.; Park, S.I.; Cho, H.; Park, H.J.; Kwak, H.W.; Park, H.J.; Bang, Y.J.; Lee, Y.S.; et al. Heterologous vaccination utilizing viral vector and protein platforms confers complete protection against SFTSV. Sci Rep 2023, 13, 8189. [Google Scholar] [CrossRef]
- Park, J.Y.; Hewawaduge, C.; Sivasankar, C.; Lloren, K.K.S.; Oh, B.; So, M.Y.; Lee, J.H. An mRNA-Based Multiple Antigenic Gene Expression System Delivered by Engineered Salmonella for Severe Fever with Thrombocytopenia Syndrome and Assessment of Its Immunogenicity and Protection Using a Human DC-SIGN-Transduced Mouse Model. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Taniguchi, S.; Kato, H.; Iwata-Yoshikawa, N.; Tani, H.; Kurosu, T.; Fujii, H.; Omura, N.; Shibamura, M.; Watanabe, S.; et al. A highly attenuated vaccinia virus strain LC16m8-based vaccine for severe fever with thrombocytopenia syndrome. PLoS Pathog 2021, 17, e1008859. [Google Scholar] [CrossRef]
- Gori Savellini, G.; Di Genova, G.; Terrosi, C.; Di Bonito, P.; Giorgi, C.; Valentini, M.; Docquier, J.D.; Cusi, M.G. Immunization with Toscana virus N-Gc proteins protects mice against virus challenge. Virology 2008, 375, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Harmon, J.R.; Spengler, J.R.; Coleman-McCray, J.D.; Nichol, S.T.; Spiropoulou, C.F.; McElroy, A.K. CD4 T Cells, CD8 T Cells, and Monocytes Coordinate To Prevent Rift Valley Fever Virus Encephalitis. J Virol 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.A.; McElroy, A.K.; Jones, M.E.; Nichol, S.T.; Spiropoulou, C.F. Rift Valley fever virus clearance and protection from neurologic disease are dependent on CD4+ T cell and virus-specific antibody responses. J Virol 2013, 87, 6161–6171. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.A.; McElroy, A.K.; Jones, T.L.; Zaki, S.R.; Nichol, S.T.; Spiropoulou, C.F. Rift valley Fever virus encephalitis is associated with an ineffective systemic immune response and activated T cell infiltration into the CNS in an immunocompetent mouse model. PLoS Negl Trop Dis 2014, 8, e2874. [Google Scholar] [CrossRef] [PubMed]
- Michaely, L.M.; Rissmann, M.; Keller, M.; König, R.; von Arnim, F.; Eiden, M.; Rohn, K.; Baumgärtner, W.; Groschup, M.; Ulrich, R. NSG-Mice Reveal the Importance of a Functional Innate and Adaptive Immune Response to Overcome RVFV Infection. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Nair, N.; Osterhaus, A.; Rimmelzwaan, G.F.; Prajeeth, C.K. Rift Valley Fever Virus-Infection, Pathogenesis and Host Immune Responses. Pathogens 2023, 12. [Google Scholar] [CrossRef]
- Wang, D.; Cao, K.; Shen, X.; Zhang, B.; Chen, M.; Yu, W. Clinical Characteristics and Immune Status of Patients with Severe Fever with Thrombocytopenia Syndrome. Viral Immunol 2022. [Google Scholar] [CrossRef]
- Sun, L.; Hu, Y.; Niyonsaba, A.; Tong, Q.; Lu, L.; Li, H.; Jie, S. Detection and evaluation of immunofunction of patients with severe fever with thrombocytopenia syndrome. Clin Exp Med 2014, 14, 389–395. [Google Scholar] [CrossRef]
- Li, M.M.; Zhang, W.J.; Weng, X.F.; Li, M.Y.; Liu, J.; Xiong, Y.; Xiong, S.E.; Zou, C.C.; Wang, H.; Lu, M.J.; et al. CD4 T cell loss and Th2 and Th17 bias are associated with the severity of severe fever with thrombocytopenia syndrome (SFTS). Clin Immunol 2018, 195, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Li, W.; Li, H.; Jie, S. Circulating regulatory T cells in patients with severe fever with thrombocytopenia syndrome. Infect Dis (Lond) 2015, 47, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Heo, S.T.; Seong, G.M.; Lee, K.H.; Yoo, J.R. Severe fever with thrombocytopenia syndrome (SFTS) associated with invasive pulmonary Aspergillosis in a patient with a low CD4+ T-cell count: A case report. Int J Crit Illn Inj Sci 2020, 10, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, Y.; Xing, Y.; Li, S.; Kong, L.; Zhang, Y.; Zhang, L.; Liu, N.; Wang, Q.; Wang, S.; et al. Concurrent measurement of dynamic changes in viral load, serum enzymes, T cell subsets, and cytokines in patients with severe fever with thrombocytopenia syndrome. PLoS One 2014, 9, e91679. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Zhang, W.J.; Liu, J.; Li, M.Y.; Zhang, Y.F.; Xiong, Y.; Xiong, S.E.; Zou, C.C.; Xiong, L.Q.; Liang, B.Y.; et al. Dynamic changes in the immunological characteristics of T lymphocytes in surviving patients with severe fever with thrombocytopenia syndrome (SFTS). Int J Infect Dis 2018, 70, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Yang, F.; Liu, S.; Gao, Y.; Xia, F.; Zheng, M.; Xu, Y. CD8(+) T cells mediate antiviral response in severe fever with thrombocytopenia syndrome. Faseb j 2023, 37, e22722. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, C.B.; Figueiredo, L.T.; Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev 2010, 23, 412–441. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, F.; Díaz, F.E.; Retamal-Díaz, A.; Covián, C.; González, P.A.; Kalergis, A.M. Immune response during hantavirus diseases: implications for immunotherapies and vaccine design. Immunology 2021, 163, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Klingström, J.; Smed-Sörensen, A.; Maleki, K.T.; Solà-Riera, C.; Ahlm, C.; Björkström, N.K.; Ljunggren, H.G. Innate and adaptive immune responses against human Puumala virus infection: immunopathogenesis and suggestions for novel treatment strategies for severe hantavirus-associated syndromes. J Intern Med 2019, 285, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.X.; Cheng, L.F.; Ying, Q.K.; Liu, R.R.; Ma, T.J.; Zhang, X.X.; Liu, Z.Y.; Zhang, L.; Ye, W.; Zhang, F.L.; et al. Screening and Identification of an H-2K(b)-Restricted CTL Epitope within the Glycoprotein of Hantaan Virus. Front Cell Infect Microbiol 2016, 6, 151. [Google Scholar] [CrossRef]
- Wang, M.L.; Zhu, Y.; Wang, J.P.; Liu, J.M.; Fang, L.; Jin, B.Q. [Identification of HTNV-NP-specific T lymphocyte epitopes and analysis of the epitope-specific T cell response]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2005, 21, 704–706. [Google Scholar] [PubMed]
- Maeda, K.; West, K.; Toyosaki-Maeda, T.; Rothman, A.L.; Ennis, F.A.; Terajima, M. Identification and analysis for cross-reactivity among hantaviruses of H-2b-restricted cytotoxic T-lymphocyte epitopes in Sin Nombre virus nucleocapsid protein. J Gen Virol 2004, 85, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Dong, Y.; Zhou, Y.; Ren, H.; Ji, Y.; Lv, S. Levels of HTNV-specific CD8+ T lymphocytes in PBMC from the patients with hemorrhagic fever with renal syndrome. Intern Emerg Med 2013, 8, 503–508. [Google Scholar] [CrossRef]
- Liu, B.; Ma, Y.; Zhang, Y.; Zhang, C.; Yi, J.; Zhuang, R.; Yu, H.; Yang, A.; Zhang, Y.; Jin, B. CD8low CD100- T Cells Identify a Novel CD8 T Cell Subset Associated with Viral Control during Human Hantaan Virus Infection. J Virol 2015, 89, 11834–11844. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhang, Y.; Li, X.; Zhang, C.; Jia, X.; Hu, H.; Chen, L.; Zhuang, R.; Zhang, Y.; Jin, B.; et al. HLA-E-restricted Hantaan virus-specific CD8+ T cell responses enhance the control of infection in hemorrhagic fever with renal syndrome. Biosafety and Health 2023, 5, 289–299. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Yuan, B.; Wang, M.; Zhang, Y.; Xu, Z.; Zhang, C.; Zhang, Y.; Liu, B.; Yi, J.; et al. HLA-A2 and B35 restricted hantaan virus nucleoprotein CD8+ T-cell epitope-specific immune response correlates with milder disease in hemorrhagic fever with renal syndrome. PLoS Negl Trop Dis 2013, 7, e2076. [Google Scholar] [CrossRef]
- Iglesias, A.A.; Períolo, N.; Bellomo, C.M.; Lewis, L.C.; Olivera, C.P.; Anselmo, C.R.; García, M.; Coelho, R.M.; Alonso, D.O.; Dighero-Kemp, B.; et al. Delayed viral clearance despite high number of activated T cells during the acute phase in Argentinean patients with hantavirus pulmonary syndrome. eBioMedicine 2022, 75. [Google Scholar] [CrossRef]
- Liu, R.; Ma, R.; Liu, Z.; Hu, H.; Shu, J.; Hu, P.; Kang, J.; Zhang, Y.; Han, M.; Zhang, X.; et al. HTNV infection of CD8+ T cells is associated with disease progression in HFRS patients. Communications Biology 2021, 4, 652. [Google Scholar] [CrossRef]
- Borges, A.A.; Campos, G.M.; Moreli, M.L.; Moro Souza, R.L.; Saggioro, F.P.; Figueiredo, G.G.; Livonesi, M.C.; Moraes Figueiredo, L.T. Role of mixed Th1 and Th2 serum cytokines on pathogenesis and prognosis of hantavirus pulmonary syndrome. Microbes Infect 2008, 10, 1150–1157. [Google Scholar] [CrossRef]
- Ma, Y.; Yuan, B.; Zhuang, R.; Zhang, Y.; Liu, B.; Zhang, C.; Zhang, Y.; Yu, H.; Yi, J.; Yang, A.; et al. Hantaan virus infection induces both Th1 and ThGranzyme B+ cell immune responses that associated with viral control and clinical outcome in humans. PLoS Pathog 2015, 11, e1004788. [Google Scholar] [CrossRef]
- de Carvalho Nicacio, C.; Sällberg, M.; Hultgren, C.; Lundkvist, Å. T-helper and humoral responses to Puumala hantavirus nucleocapsid protein: identification of T-helper epitopes in a mouse model. J Gen Virol 2001, 82, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.-x.; Cheng, L.-f.; Ying, Q.-k.; Liu, R.-r.; Ma, T.-j.; Zhang, X.-x.; Liu, Z.-y.; Zhang, L.; Ye, W.; Zhang, F.-l.; et al. Screening and Identification of an H-2Kb-Restricted CTL Epitope within the Glycoprotein of Hantaan Virus. Frontiers in Cellular and Infection Microbiology 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cheng, L.; Yuan, B.; Zhang, Y.; Zhang, C.; Zhang, Y.; Tang, K.; Zhuang, R.; Chen, L.; Yang, K.; et al. Structure and Function of HLA-A*02-Restricted Hantaan Virus Cytotoxic T-Cell Epitope That Mediates Effective Protective Responses in HLA-A2.1/K(b) Transgenic Mice. Front Immunol 2016, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tang, K.; Zhang, Y.; Zhang, C.; Zhang, Y.; Jin, B.; Ma, Y. Design and synthesis of HLA-A*02-restricted Hantaan virus multiple-antigenic peptide for CD8(+) T cells. Virol J 2020, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tang, K.; Zhang, Y.; Zhang, C.; Cheng, L.; Zhang, F.; Zhuang, R.; Jin, B.; Zhang, Y. Protective CD8(+) T-cell response against Hantaan virus infection induced by immunization with designed linear multi-epitope peptides in HLA-A2.1/K(b) transgenic mice. Virol J 2020, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Kallio-Kokko, H.; Leveelahti, R.; Brummer-Korvenkontio, M.; Lundkvist, A.; Vaheri, A.; Vapalahti, O. Human immune response to Puumala virus glycoproteins and nucleocapsid protein expressed in mammalian cells. J Med Virol 2001, 65, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Lindkvist, M.; Lahti, K.; Lilliehöök, B.; Holmström, A.; Ahlm, C.; Bucht, G. Cross-reactive immune responses in mice after genetic vaccination with cDNA encoding hantavirus nucleocapsid proteins. Vaccine 2007, 25, 1690–1699. [Google Scholar] [CrossRef]
- Lundkvist, A.; Meisel, H.; Koletzki, D.; Lankinen, H.; Cifire, F.; Geldmacher, A.; Sibold, C.; Gött, P.; Vaheri, A.; Krüger, D.H.; et al. Mapping of B-cell epitopes in the nucleocapsid protein of Puumala hantavirus. Viral Immunol 2002, 15, 177–192. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Nicacio, C.; Gonzalez Della Valle, M.; Padula, P.; Björling, E.; Plyusnin, A.; Lundkvist, A. Cross-protection against challenge with Puumala virus after immunization with nucleocapsid proteins from different hantaviruses. J Virol 2002, 76, 6669–6677. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.R.; Alkhovsky Альхoвский Сергей Владимирoвич, S.V.; Avšič-Županc, T.; Bente, D.A.; Bergeron, É.; Burt, F.; Di Paola, N.; Ergünay, K.; Hewson, R.; Kuhn, J.H.; et al. ICTV Virus Taxonomy Profile: Nairoviridae. J Gen Virol 2020, 101, 798–799. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, S.; Jara, M.; Frias-De-Diego, A.; Machado, G. Nairobi Sheep Disease Virus: A Historical and Epidemiological Perspective. Front Vet Sci 2020, 7, 419. [Google Scholar] [CrossRef]
- Rodriguez, S.E.; Hawman, D.W.; Sorvillo, T.E.; O'Neal, T.J.; Bird, B.H.; Rodriguez, L.L.; Bergeron, É.; Nichol, S.T.; Montgomery, J.M.; Spiropoulou, C.F.; et al. Immunobiology of Crimean-Congo hemorrhagic fever. Antiviral Research 2022, 199, 105244. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Nain, Z.; Syed, S.B.; Abdulla, F.; Moni, M.A.; Sheam, M.M.; Karim, M.M.; Adhikari, U.K. Computational formulation and immune dynamics of a multi-peptide vaccine candidate against Crimean-Congo hemorrhagic fever virus. Mol Cell Probes 2021, 55, 101693. [Google Scholar] [CrossRef]
- Golden, J.W.; Fitzpatrick, C.J.; Suschak, J.J.; Clements, T.L.; Ricks, K.M.; Sanchez-Lockhart, M.; Garrison, A.R. Induced protection from a CCHFV-M DNA vaccine requires CD8(+) T cells. Virus Res 2023, 334, 199173. [Google Scholar] [CrossRef]
- Appelberg, S.; John, L.; Pardi, N.; Végvári, Á.; Bereczky, S.; Ahlén, G.; Monteil, V.; Abdurahman, S.; Mikaeloff, F.; Beattie, M.; et al. Nucleoside-Modified mRNA Vaccines Protect IFNAR(-/-) Mice against Crimean-Congo Hemorrhagic Fever Virus Infection. J Virol 2022, 96, e0156821. [Google Scholar] [CrossRef]
- Crimean-Congo Hemorrhagic Fever Virus Subunit Vaccines Induce High Levels of Neutralizing Antibodies But No Protection in STAT1 Knockout Mice. Vector-Borne and Zoonotic Diseases 2015, 15, 759–764. [CrossRef] [PubMed]
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Hunter, L.; Watson, R.; Taylor, I.; Rule, A.; Carroll, M.W.; Hewson, R. Protective effects of a Modified Vaccinia Ankara-based vaccine candidate against Crimean-Congo Haemorrhagic Fever virus require both cellular and humoral responses. PLoS One 2016, 11, e0156637. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Meade-White, K.; Leventhal, S.; Feldmann, F.; Okumura, A.; Smith, B.; Scott, D.; Feldmann, H. Immunocompetent mouse model for Crimean-Congo hemorrhagic fever virus. eLife 2021, 10, e63906. [Google Scholar] [CrossRef]
- Akinci, E.; Yilmaz, M.; Bodur, H.; Ongürü, P.; Bayazit, F.N.; Erbay, A.; Ozet, G. Analysis of lymphocyte subgroups in Crimean-Congo hemorrhagic fever. Int J Infect Dis 2009, 13, 560–563. [Google Scholar] [CrossRef]
- Salvati, M.V.; Salaris, C.; Monteil, V.; Del Vecchio, C.; Palù, G.; Parolin, C.; Calistri, A.; Bell-Sakyi, L.; Mirazimi, A.; Salata, C. Virus-Derived DNA Forms Mediate the Persistent Infection of Tick Cells by Hazara Virus and Crimean-Congo Hemorrhagic Fever Virus. J Virol 2021, 95, e0163821. [Google Scholar] [CrossRef]
- Ohta, K.; Saka, N.; Nishio, M. Hazara Orthonairovirus Nucleoprotein Antagonizes Type I Interferon Production by Inhibition of RIG-I Ubiquitination. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Tapia-Ramírez, G.; Lorenzo, C.; Navarrete, D.; Carrillo-Reyes, A.; Retana, Ó.; Carrasco-Hernández, R. A Review of Mammarenaviruses and Rodent Reservoirs in the Americas. Ecohealth 2022, 19, 22–39. [Google Scholar] [CrossRef]
- Briese, T.; Paweska, J.T.; McMullan, L.K.; Hutchison, S.K.; Street, C.; Palacios, G.; Khristova, M.L.; Weyer, J.; Swanepoel, R.; Egholm, M.; et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog 2009, 5, e1000455. [Google Scholar] [CrossRef]
- Abdel-Hakeem, M.S. Viruses Teaching Immunology: Role of LCMV Model and Human Viral Infections in Immunological Discoveries. Viruses 2019, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Oldstone, M.B.; Lewicki, H.; Homann, D.; Nguyen, C.; Julien, S.; Gairin, J.E. Common antiviral cytotoxic t-lymphocyte epitope for diverse arenaviruses. J Virol 2001, 75, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Muzaffar, A.; Shoaib, R.M.; Khan, A.; Waheed, Y.; Wei, D.Q. Towards specie-specific ensemble vaccine candidates against mammarenaviruses using optimized structural vaccinology pipeline and molecular modelling approaches. Microb Pathog 2022, 172, 105793. [Google Scholar] [CrossRef]
- Azim, K.F.; Lasker, T.; Akter, R.; Hia, M.M.; Bhuiyan, O.F.; Hasan, M.; Hossain, M.N. Combination of highly antigenic nucleoproteins to inaugurate a cross-reactive next generation vaccine candidate against Arenaviridae family. Heliyon 2021, 7, e07022. [Google Scholar] [CrossRef] [PubMed]
- Botten, J.; Alexander, J.; Pasquetto, V.; Sidney, J.; Barrowman, P.; Ting, J.; Peters, B.; Southwood, S.; Stewart, B.; Rodriguez-Carreno, M.P.; et al. Identification of protective Lassa virus epitopes that are restricted by HLA-A2. J Virol 2006, 80, 8351–8361. [Google Scholar] [CrossRef]
- Boesen, A.; Sundar, K.; Coico, R. Lassa fever virus peptides predicted by computational analysis induce epitope-specific cytotoxic-T-lymphocyte responses in HLA-A2.1 transgenic mice. Clin Diagn Lab Immunol 2005, 12, 1223–1230. [Google Scholar] [CrossRef]
- Clegg, J.C.S. Current progress towards vaccines for arenavirus-caused diseases. Vaccine 1992, 10, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Hoch, S.P.; McCormick, J.B. Towards a human Lassa fever vaccine. Rev Med Virol 2001, 11, 331–341. [Google Scholar] [CrossRef]
- Jahrling, P.B.; Frame, J.D.; Rhoderick, J.B.; Monson, M.H. Endemic Lassa fever in Liberia. IV. Selection of optimally effective plasma for treatment by passive immunization. Trans R Soc Trop Med Hyg 1985, 79, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Port, J.R.; Wozniak, D.M.; Oestereich, L.; Pallasch, E.; Becker-Ziaja, B.; Müller, J.; Rottstegge, M.; Olal, C.; Gómez-Medina, S.; Oyakhliome, J.; et al. Severe Human Lassa Fever Is Characterized by Nonspecific T-Cell Activation and Lymphocyte Homing to Inflamed Tissues. J Virol 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Flatz, L.; Rieger, T.; Merkler, D.; Bergthaler, A.; Regen, T.; Schedensack, M.; Bestmann, L.; Verschoor, A.; Kreutzfeldt, M.; Brück, W.; et al. T cell-dependence of Lassa fever pathogenesis. PLoS Pathog 2010, 6, e1000836. [Google Scholar] [CrossRef]
- Carballal, G.; Oubiña, J.R.; Rondinone, S.N.; Elsner, B.; Frigerio, M.J. Cell-mediated immunity and lymphocyte populations in experimental Argentine hemorrhagic fever (Junín Virus). Infect Immun 1981, 34, 323–327. [Google Scholar] [CrossRef]
- Barrios, H.A.; Rondinone, S.N.; Blejer, J.L.; Giovanniello, O.A.; Nota, N.R. Development of specific immune response in mice infected with Junin virus. Acta Virol 1982, 26, 156–164. [Google Scholar]
- Evans, A.B.; Peterson, K.E. Cross reactivity of neutralizing antibodies to the encephalitic California Serogroup orthobunyaviruses varies by virus and genetic relatedness. Sci Rep 2021, 11, 16424. [Google Scholar] [CrossRef]
- Wernike, K.; Aebischer, A.; Sick, F.; Szillat, K.P.; Beer, M. Differentiation of Antibodies against Selected Simbu Serogroup Viruses by a Glycoprotein Gc-Based Triplex ELISA. Veterinary Sciences 2021, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Skinner, B.; Mikula, S.; Davis, B.S.; Powers, J.A.; Hughes, H.R.; Calvert, A.E. Monoclonal antibodies to Cache Valley virus for serological diagnosis. PLoS Negl Trop Dis 2022, 16, e0010156. [Google Scholar] [CrossRef] [PubMed]
- Srihongse, S.; Grayson, M.A.; Deibel, R. California serogroup viruses in New York State: the role of subtypes in human infections. Am J Trop Med Hyg 1984, 33, 1218–1227. [Google Scholar] [CrossRef]
- Heinz, F.; Asera, J. Presence of viruse-neutralizing antibodies ot the Tahyna virus in the inhabitants of North Moravia. Folia Parasitol (Praha) 1972, 19, 315–320. [Google Scholar] [PubMed]
- Blitvich, B.J.; Saiyasombat, R.; Talavera-Aguilar, L.G.; Garcia-Rejon, J.E.; Farfan-Ale, J.A.; Machain-Williams, C.; Loroño-Pino, M.A. Orthobunyavirus Antibodies in Humans, Yucatan Peninsula, Mexico. Emerg Infect Dis. 18(10):1629-1632. 2012, 18. [Google Scholar] [CrossRef]
- Grimstad, P.R.; Schmitt, S.M.; Williams, D.G. Prevalence of neutralizing antibody to Jamestown Canyon virus (California group) in populations of elk and moose in northern Michigan and Ontario, Canada. J Wildl Dis 1986, 22, 453–458. [Google Scholar] [CrossRef]
- Putkuri, N.; Vaheri, A.; Vapalahti, O. Prevalence and protein specificity of human antibodies to Inkoo virus infection. Clin Vaccine Immunol 2007, 14, 1555–1562. [Google Scholar] [CrossRef]
- Gonzalez-Scarano, F.; Shope, R.E.; Calisher, C.E.; Nathanson, N. Characterization of monoclonal antibodies against the G1 and N proteins of LaCrosse and Tahyna, two California serogroup bunyaviruses. Virology 1982, 120, 42–53. [Google Scholar] [CrossRef]
- Wernike, K.; Brocchi, E.; Cordioli, P.; Sénéchal, Y.; Schelp, C.; Wegelt, A.; Aebischer, A.; Roman-Sosa, G.; Reimann, I.; Beer, M. A novel panel of monoclonal antibodies against Schmallenberg virus nucleoprotein and glycoprotein Gc allows specific orthobunyavirus detection and reveals antigenic differences. Veterinary Research 2015, 46, 27. [Google Scholar] [CrossRef]
- Kingsford, L.; Hill, D.W. The effect of proteolytic cleavage of La Crosse virus G1 glycoprotein on antibody neutralization. J Gen Virol 1983, 64 (Pt 10) Pt 10, 2147–2156. [Google Scholar] [CrossRef]
- Powers, J.A.; Boroughs, K.L.; Mikula, S.; Goodman, C.H.; Davis, E.H.; Thrasher, E.M.; Hughes, H.R.; Biggerstaff, B.J.; Calvert, A.E. Characterization of a monoclonal antibody specific to California serogroup orthobunyaviruses and development as a chimeric immunoglobulin M-positive control in human diagnostics. Microbiology Spectrum 2023, 11, e01966–01923. [Google Scholar] [CrossRef]
- Hellert, J.; Aebischer, A.; Wernike, K.; Haouz, A.; Brocchi, E.; Reiche, S.; Guardado-Calvo, P.; Beer, M.; Rey, F.A. Orthobunyavirus spike architecture and recognition by neutralizing antibodies. Nature Communications 2019, 10, 879. [Google Scholar] [CrossRef]
- Roman-Sosa, G.; Brocchi, E.; Schirrmeier, H.; Wernike, K.; Schelp, C.; Beer, M. Analysis of the humoral immune response against the envelope glycoprotein Gc of Schmallenberg virus reveals a domain located at the amino terminus targeted by mAbs with neutralizing activity. J Gen Virol 2016, 97, 571–580. [Google Scholar] [CrossRef]
- Kingsford, L. Enhanced neutralization of La Crosse virus by the binding of specific pairs of monoclonal antibodies to the G1 glycoprotein. Virology 1984, 136, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Eguchi, M.; Shimoji, Y. Two Akabane virus glycoprotein Gc domains induce neutralizing antibodies in mice. J Vet Med Sci 2022, 84, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Kingsford, L.; Boucquey, K.H. Monoclonal antibodies specific for the G1 glycoprotein of La Crosse virus that react with other California serogroup viruses. J Gen Virol 1990, 71 ( Pt 3) Pt 3, 523–530. [Google Scholar] [CrossRef]
- Bréard, E.; Lara, E.; Comtet, L.; Viarouge, C.; Doceul, V.; Desprat, A.; Vitour, D.; Pozzi, N.; Cay, A.B.; De Regge, N.; et al. Validation of a commercially available indirect ELISA using a nucleocapside recombinant protein for detection of Schmallenberg virus antibodies. PLoS One 2013, 8, e53446. [Google Scholar] [CrossRef] [PubMed]
- Roman-Sosa, G.; Karger, A.; Kraatz, F.; Aebischer, A.; Wernike, K.; Maksimov, P.; Lillig, C.H.; Reimann, I.; Brocchi, E.; Keller, M.; et al. The amino terminal subdomain of glycoprotein Gc of Schmallenberg virus: disulfide bonding and structural determinants of neutralization. J Gen Virol 2017, 98, 1259–1273. [Google Scholar] [CrossRef]
- Bennett, R.S.; Gresko, A.K.; Nelson, J.T.; Murphy, B.R.; Whitehead, S.S. A recombinant chimeric La Crosse virus expressing the surface glycoproteins of Jamestown Canyon virus is immunogenic and protective against challenge with either parental virus in mice or monkeys. J Virol 2012, 86, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Operschall, E.; Schuh, T.; Heinzerling, L.; Pavlovic, J.; Moelling, K. Enhanced protection against viral infection by co-administration of plasmid DNA coding for viral antigen and cytokines in mice. J Clin Virol 1999, 13, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Pekosz, A.; Griot, C.; Stillmock, K.; Nathanson, N.; Gonzalez-Scarano, F. Protection from La Crosse virus encephalitis with recombinant glycoproteins: role of neutralizing anti-G1 antibodies. J Virol 1995, 69, 3475–3481. [Google Scholar] [CrossRef]
- Stubbs, S.H.; Cornejo Pontelli, M.; Mishra, N.; Zhou, C.; de Paula Souza, J.; Mendes Viana, R.M.; Lipkin, W.I.; Knipe, D.M.; Arruda, E.; Whelan, S.P.J. Vesicular Stomatitis Virus Chimeras Expressing the Oropouche Virus Glycoproteins Elicit Protective Immune Responses in Mice. mBio 2021, 12, e0046321. [Google Scholar] [CrossRef]
- Hertz, T.; Beatty, P.R.; MacMillen, Z.; Killingbeck, S.S.; Wang, C.; Harris, E. Antibody Epitopes Identified in Critical Regions of Dengue Virus Nonstructural 1 Protein in Mouse Vaccination and Natural Human Infections. J Immunol 2017, 198, 4025–4035. [Google Scholar] [CrossRef]
- Sootichote, R.; Puangmanee, W.; Benjathummarak, S.; Kowaboot, S.; Yamanaka, A.; Boonnak, K.; Ampawong, S.; Chatchen, S.; Ramasoota, P.; Pitaksajjakul, P. Potential Protective Effect of Dengue NS1 Human Monoclonal Antibodies against Dengue and Zika Virus Infections. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-C.; Chuang, Y.-C.; Liu, C.-C.; Ho, T.-S.; Lin, Y.-S.; Anderson, R.; Yeh, T.-M. Antibodies Against Modified NS1 Wing Domain Peptide Protect Against Dengue Virus Infection. Scientific Reports 2017, 7, 6975. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Allen, E.R.; Clark, M.H.A.; Gitonga, J.N.; Karanja, H.K.; Hulswit, R.J.G.; Taylor, I.; Biswas, S.; Marshall, J.; Mwololo, D.; et al. Naturally Acquired Rift Valley Fever Virus Neutralizing Antibodies Predominantly Target the Gn Glycoprotein. iScience 2020, 23, 101669. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.S.; Zhao, H.; Kose, N.; Westover, J.B.; Kalveram, B.; Bombardi, R.; Rodriguez, J.; Sutton, R.; Genualdi, J.; LaBeaud, A.D.; et al. Potent neutralization of Rift Valley fever virus by human monoclonal antibodies through fusion inhibition. Proceedings of the National Academy of Sciences 2021, 118, e2025642118. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, L.; Qian, J.; Wu, X.; Wang, Z.; Wang, H.; Liu, D.; Deng, F.; Shen, S. The Neutralizing Monoclonal Antibodies against SFTS Group Bandaviruses Suggest New Targets of Specific or Broad-Spectrum Antivirals. Am J Trop Med Hyg 2023, 109, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.R.; Krumm, S.A.; Raghwani, J.; Halldorsson, S.; Elliott, A.; Graham, V.A.; Koudriakova, E.; Harlos, K.; Wright, D.; Warimwe, G.M.; et al. A Protective Monoclonal Antibody Targets a Site of Vulnerability on the Surface of Rift Valley Fever Virus. Cell Rep 2018, 25, 3750–3758. [Google Scholar] [CrossRef] [PubMed]
- Besselaar, T.G.; Blackburn, N.K. Topological mapping of antigenic sites on the Rift Valley fever virus envelope glycoproteins using monoclonal antibodies. Arch Virol 1991, 121, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, Y.; Gao, F.; Jiao, Y.; Oladejo, B.O.; Chai, Y.; Bi, Y.; Lu, S.; Dong, M.; Zhang, C.; et al. Structures of phlebovirus glycoprotein Gn and identification of a neutralizing antibody epitope. Proc Natl Acad Sci U S A 2017, 114, E7564–e7573. [Google Scholar] [CrossRef]
- Hao, M.; Zhang, G.; Zhang, S.; Chen, Z.; Chi, X.; Dong, Y.; Fan, P.; Liu, Y.; Chen, Y.; Song, X.; et al. Characterization of Two Neutralizing Antibodies against Rift Valley Fever Virus Gn Protein. Viruses 2020, 12, 259. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Zhang, W.; Chi, Y.; Zeng, X.; Li, X.; Qi, X.; Jin, Q.; Zhang, X.; Huang, M.; et al. Human antibody neutralizes severe Fever with thrombocytopenia syndrome virus, an emerging hemorrhagic Fever virus. Clin Vaccine Immunol 2013, 20, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, C.; Prathyumn, S.; Terrosi, C.; Anichini, G.; Gori Savellini, G.; Corti, D.; Bracci, L.; Lanzavecchia, A.; Roman-Sosa, G.; Cusi, M.G. Identification of a Neutralizing Epitope on TOSV Gn Glycoprotein. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Magurano, F.; Nicoletti, L. Humoral response in Toscana virus acute neurologic disease investigated by viral-protein-specific immunoassays. Clin Diagn Lab Immunol 1999, 6, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Bosco, S.; Mochi, S.; Accardi, L.; Ciufolini, M.G.; Nicoletti, L.; Giorgi, C. Human antibody response to Toscana virus glycoproteins expressed by recombinant baculovirus. J Med Virol 2002, 68, 615–619. [Google Scholar] [CrossRef]
- Fernandez, J.C.; Billecocq, A.; Durand, J.P.; Cêtre-Sossah, C.; Cardinale, E.; Marianneau, P.; Pépin, M.; Tordo, N.; Bouloy, M. The nonstructural protein NSs induces a variable antibody response in domestic ruminants naturally infected with Rift Valley fever virus. Clin Vaccine Immunol 2012, 19, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Findlay, G.M. The Mechanism of Immunity in Rift Valley Fever. Br J Exp Pathol. 1936, 17, 89–104. [Google Scholar]
- Pierro, A.; Ficarelli, S.; Ayhan, N.; Morini, S.; Raumer, L.; Bartoletti, M.; Mastroianni, A.; Prati, F.; Schivazappa, S.; Cenni, P.; et al. Characterization of antibody response in neuroinvasive infection caused by Toscana virus. Clin Microbiol Infect 2017, 23, 868–873. [Google Scholar] [CrossRef]
- Hu, L.; Kong, Q.; Liu, Y.; Li, J.; Bian, T.; Ma, X.; Ye, Y.; Li, J. Time Course of Severe Fever With Thrombocytopenia Syndrome Virus and Antibodies in Patients by Long-Term Follow-Up Study, China. Front Microbiol 2021, 12, 744037. [Google Scholar] [CrossRef]
- Mhamadi, M.; Badji, A.; Barry, M.A.; Ndiaye, E.H.; Gaye, A.; Ndiaye, M.; Mhamadi, M.; Touré, C.T.; Ndiaye, O.; Faye, B.; et al. Human and Livestock Surveillance Revealed the Circulation of Rift Valley Fever Virus in Agnam, Northern Senegal, 2021. Trop Med Infect Dis 2023, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Huang, B.; Ma, X.; Zhu, L.; Zheng, N.; Xu, S.; Nawaz, W.; Xu, C.; Wu, Z. A single-domain antibody inhibits SFTSV and mitigates virus-induced pathogenesis in vivo. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Li, J.C.; Ding, H.; Wang, G.; Zhang, S.; Yang, X.; Wu, Y.X.; Peng, X.F.; Zhang, X.A.; Yang, Z.D.; Cui, N.; et al. Dynamics of neutralizing antibodies against severe fever with thrombocytopenia syndrome virus. Int J Infect Dis 2023, 134, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chang, H.; Jia, B.; Liu, Y.; Huang, R.; Wu, W.; Hao, Y.; Yan, X.; Xia, J.; Chen, Y.; et al. Nucleocapsid protein-specific IgM antibody responses in the disease progression of severe fever with thrombocytopenia syndrome. Ticks Tick Borne Dis 2019, 10, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Salekwa, L.P.; Wambura, P.N.; Matiko, M.K.; Watts, D.M. Circulation of Rift Valley Fever Virus Antibody in Cattle during Inter-Epizootic/Epidemic Periods in Selected Regions of Tanzania. The American Journal of Tropical Medicine and Hygiene 2019, 101, 459–466. [Google Scholar] [CrossRef]
- Nfon, C.K.; Marszal, P.; Zhang, S.; Weingartl, H.M. Innate Immune Response to Rift Valley Fever Virus in Goats. PLOS Neglected Tropical Diseases 2012, 6, e1623. [Google Scholar] [CrossRef] [PubMed]
- Selina, O.; Imatdinov, I.; Balysheva, V.; Akasov, R.; Kryukov, A.; Balyshev, V.; Markvicheva, E. Microencapsulated plasmids expressing Gn and Gc glycoproteins of Rift Valley Fever virus enhance humoral immune response in mice. Biotechnol Lett 2020, 42, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Faburay, B.; Lebedev, M.; McVey, D.S.; Wilson, W.; Morozov, I.; Young, A.; Richt, J.A. A glycoprotein subunit vaccine elicits a strong Rift Valley fever virus neutralizing antibody response in sheep. Vector Borne Zoonotic Dis 2014, 14, 746–756. [Google Scholar] [CrossRef]
- Chrun, T.; Lacôte, S.; Urien, C.; Richard, C.A.; Tenbusch, M.; Aubrey, N.; Pulido, C.; Lakhdar, L.; Marianneau, P.; Schwartz-Cornil, I. A DNA Vaccine Encoding the Gn Ectodomain of Rift Valley Fever Virus Protects Mice via a Humoral Response Decreased by DEC205 Targeting. Front Immunol 2019, 10, 860. [Google Scholar] [CrossRef]
- Kim, D.; Lai, C.J.; Cha, I.; Kang, S.; Yang, W.S.; Choi, Y.; Jung, J.U. SFTSV Gn-Head mRNA vaccine confers efficient protection against lethal viral challenge. J Med Virol 2023, 95, e29203. [Google Scholar] [CrossRef]
- Vapalahti, O.; Kallio-Kokko, H.; Närvänen, A.; Julkunen, I.; Lundkvist, A.; Plyusnin, A.; Lehväslaiho, H.; Brummer-Korvenkontio, M.; Vaheri, A.; Lankinen, H. Human B-cell epitopes of Puumala virus nucleocapsid protein, the major antigen in early serological response. J Med Virol 1995, 46, 293–303. [Google Scholar] [CrossRef]
- Engdahl, T.B.; Crowe, J.E., Jr. Humoral Immunity to Hantavirus Infection. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Hepojoki, J.; Strandin, T.; Vaheri, A.; Lankinen, H. Interactions and oligomerization of hantavirus glycoproteins. J Virol 2010, 84, 227–242. [Google Scholar] [CrossRef]
- Battisti, A.J.; Chu, Y.K.; Chipman, P.R.; Kaufmann, B.; Jonsson, C.B.; Rossmann, M.G. Structural studies of Hantaan virus. J Virol 2011, 85, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Rissanen, I.; Zeltina, A.; Hepojoki, J.; Raghwani, J.; Harlos, K.; Pybus, O.G.; Huiskonen, J.T.; Bowden, T.A. A Molecular-Level Account of the Antigenic Hantaviral Surface. Cell Rep 2016, 15, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, T.B.; Binshtein, E.; Brocato, R.L.; Kuzmina, N.A.; Principe, L.M.; Kwilas, S.A.; Kim, R.K.; Chapman, N.S.; Porter, M.S.; Guardado-Calvo, P.; et al. Antigenic mapping and functional characterization of human New World hantavirus neutralizing antibodies. Elife 2023, 12. [Google Scholar] [CrossRef]
- Stass, R.; Engdahl, T.B.; Chapman, N.S.; Wolters, R.M.; Handal, L.S.; Diaz, S.M.; Crowe, J.E., Jr.; Bowden, T.A. Mechanistic basis for potent neutralization of Sin Nombre hantavirus by a human monoclonal antibody. Nat Microbiol 2023, 8, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Levanov, L.; Iheozor-Ejiofor, R.P.; Lundkvist, Å.; Vapalahti, O.; Plyusnin, A. Defining of MAbs-neutralizing sites on the surface glycoproteins Gn and Gc of a hantavirus using vesicular stomatitis virus pseudotypes and site-directed mutagenesis. J Gen Virol 2019, 100, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Plyusnin, A.; Kedari, A.; Rissanen, I.; Iheozor-Ejiofor, R.P.; Lundkvist, Å.; Vapalahti, O.; Levanov, L. Validation of an antigenic site targeted by monoclonal antibodies against Puumala virus. J Gen Virol 2023, 104. [Google Scholar] [CrossRef] [PubMed]
- Mittler, E.; Serris, A.; Esterman, E.S.; Florez, C.; Polanco, L.C.; O'Brien, C.M.; Slough, M.M.; Tynell, J.; Gröning, R.; Sun, Y.; et al. Structural and mechanistic basis of neutralization by a pan-hantavirus protective antibody. Sci Transl Med 2023, 15, eadg1855. [Google Scholar] [CrossRef]
- Mittler, E.; Wec, A.Z.; Tynell, J.; Guardado-Calvo, P.; Wigren-Byström, J.; Polanco, L.C.; O'Brien, C.M.; Slough, M.M.; Abelson, D.M.; Serris, A.; et al. Human antibody recognizing a quaternary epitope in the Puumala virus glycoprotein provides broad protection against orthohantaviruses. Sci Transl Med 2022, 14, eabl5399. [Google Scholar] [CrossRef]
- Rissanen, I.; Krumm, S.A.; Stass, R.; Whitaker, A.; Voss, J.E.; Bruce, E.A.; Rothenberger, S.; Kunz, S.; Burton, D.R.; Huiskonen, J.T.; et al. Structural Basis for a Neutralizing Antibody Response Elicited by a Recombinant Hantaan Virus Gn Immunogen. mBio 2021, 12, e0253120. [Google Scholar] [CrossRef]
- Lundkvist, A.; Kallio-Kokko, H.; Sjölander, K.B.; Lankinen, H.; Niklasson, B.; Vaheri, A.; Vapalahti, O. Characterization of Puumala virus nucleocapsid protein: identification of B-cell epitopes and domains involved in protective immunity. Virology 1996, 216, 397–406. [Google Scholar] [CrossRef]
- Kalaiselvan, S.; Sankar, S.; Ramamurthy, M.; Ghosh, A.R.; Nandagopal, B.; Sridharan, G. Prediction of Pan-Specific B-Cell Epitopes From Nucleocapsid Protein of Hantaviruses Causing Hantavirus Cardiopulmonary Syndrome. J Cell Biochem 2017, 118, 2320–2324. [Google Scholar] [CrossRef]
- Duehr, J.; McMahon, M.; Williamson, B.; Amanat, F.; Durbin, A.; Hawman, D.W.; Noack, D.; Uhl, S.; Tan, G.S.; Feldmann, H.; et al. Neutralizing Monoclonal Antibodies against the Gn and the Gc of the Andes Virus Glycoprotein Spike Complex Protect from Virus Challenge in a Preclinical Hamster Model. mBio 2020, 11. [Google Scholar] [CrossRef]
- Garrido, J.L.; Prescott, J.; Calvo, M.; Bravo, F.; Alvarez, R.; Salas, A.; Riquelme, R.; Rioseco, M.L.; Williamson, B.N.; Haddock, E.; et al. Two recombinant human monoclonal antibodies that protect against lethal Andes hantavirus infection in vivo. Sci Transl Med 2018, 10. [Google Scholar] [CrossRef]
- Schmaljohn, C.S.; Chu, Y.K.; Schmaljohn, A.L.; Dalrymple, J.M. Antigenic subunits of Hantaan virus expressed by baculovirus and vaccinia virus recombinants. J Virol 1990, 64, 3162–3170. [Google Scholar] [CrossRef]
- Liang, M.; Chu, Y.K.; Schmaljohn, C. Bacterial expression of neutralizing mouse monoclonal antibody Fab fragments to Hantaan virus. Virology 1996, 217, 262–271. [Google Scholar] [CrossRef]
- Arikawa, J.; Yao, J.S.; Yoshimatsu, K.; Takashima, I.; Hashimoto, N. Protective role of antigenic sites on the envelope protein of Hantaan virus defined by monoclonal antibodies. Arch Virol 1992, 126, 271–281. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, L.; Wang, L.; Wang, H.; Jiang, S. The in vitro and in vivo protective activity of monoclonal antibodies directed against Hantaan virus: potential application for immunotherapy and passive immunization. Biochem Biophys Res Commun 2002, 298, 552–558. [Google Scholar] [CrossRef]
- Vial, P.A.; Valdivieso, F.; Calvo, M.; Rioseco, M.L.; Riquelme, R.; Araneda, A.; Tomicic, V.; Graf, J.; Paredes, L.; Florenzano, M.; et al. A non-randomized multicentre trial of human immune plasma for treatment of hantavirus cardiopulmonary syndrome caused by Andes virus. Antivir Ther 2015, 20, 377–386. [Google Scholar] [CrossRef]
- Engdahl, T.B.; Kuzmina, N.A.; Ronk, A.J.; Mire, C.E.; Hyde, M.A.; Kose, N.; Josleyn, M.D.; Sutton, R.E.; Mehta, A.; Wolters, R.M.; et al. Broad and potently neutralizing monoclonal antibodies isolated from human survivors of New World hantavirus infection. Cell Rep 2021, 35, 109086. [Google Scholar] [CrossRef]
- Hörling, J.; Lundkvist, A.; Huggins, J.W.; Niklasson, B. Antibodies to Puumala virus in humans determined by neutralization test. J Virol Methods 1992, 39, 139–147. [Google Scholar] [CrossRef]
- Iheozor-Ejiofor, R.; Vapalahti, K.; Sironen, T.; Levanov, L.; Hepojoki, J.; Lundkvist, Å.; Mäkelä, S.; Vaheri, A.; Mustonen, J.; Plyusnin, A.; et al. Neutralizing Antibody Titers in Hospitalized Patients with Acute Puumala Orthohantavirus Infection Do Not Associate with Disease Severity. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Valdivieso, F.; Vial, P.; Ferres, M.; Ye, C.; Goade, D.; Cuiza, A.; Hjelle, B. Neutralizing antibodies in survivors of Sin Nombre and Andes hantavirus infection. Emerg Infect Dis 2006, 12, 166–168. [Google Scholar] [CrossRef]
- Bharadwaj, M.; Nofchissey, R.; Goade, D.; Koster, F.; Hjelle, B. Humoral Immune Responses in the Hantavirus Cardiopulmonary Syndrome. The Journal of Infectious Diseases 2000, 182, 43–48. [Google Scholar] [CrossRef]
- Pettersson, L.; Thunberg, T.; Rocklöv, J.; Klingström, J.; Evander, M.; Ahlm, C. Viral load and humoral immune response in association with disease severity in Puumala hantavirus-infected patients--implications for treatment. Clin Microbiol Infect 2014, 20, 235–241. [Google Scholar] [CrossRef]
- Tuiskunen Bäck, A.; Rasmuson, J.; Thunberg, T.; Rankin, G.; Wigren Byström, J.; Andersson, C.; Sjödin, A.; Forsell, M.; Ahlm, C. Clinical and genomic characterisation of a fatal Puumala orthohantavirus case with low levels of neutralising antibodies. Infect Dis (Lond) 2022, 54, 766–772. [Google Scholar] [CrossRef]
- Liu, R.; Ma, H.; Shu, J.; Zhang, Q.; Han, M.; Liu, Z.; Jin, X.; Zhang, F.; Wu, X. Vaccines and Therapeutics Against Hantaviruses. Front Microbiol 2019, 10, 2989. [Google Scholar] [CrossRef]
- Bertolotti-Ciarlet, A.; Smith, J.; Strecker, K.; Paragas, J.; Altamura, L.A.; McFalls, J.M.; Frias-Stäheli, N.; García-Sastre, A.; Schmaljohn, C.S.; Doms, R.W. Cellular localization and antigenic characterization of crimean-congo hemorrhagic fever virus glycoproteins. J Virol 2005, 79, 6152–6161. [Google Scholar] [CrossRef]
- Zivcec, M.; Guerrero, L.I.W.; Albariño, C.G.; Bergeron, É.; Nichol, S.T.; Spiropoulou, C.F. Identification of broadly neutralizing monoclonal antibodies against Crimean-Congo hemorrhagic fever virus. Antiviral Res 2017, 146, 112–120. [Google Scholar] [CrossRef]
- Li, N.; Rao, G.; Li, Z.; Yin, J.; Chong, T.; Tian, K.; Fu, Y.; Cao, S. Cryo-EM structure of glycoprotein C from Crimean-Congo hemorrhagic fever virus. Virol Sin 2022, 37, 127–137. [Google Scholar] [CrossRef]
- Zhang, J.; Simayi, A.; Wang, M.; Moming, A.; Xu, W.; Wang, C.; Li, Y.; Ding, J.; Deng, F.; Zhang, Y.; et al. Fine mapping epitope on glycoprotein Gc from Crimean-Congo hemorrhagic fever virus. Comp Immunol Microbiol Infect Dis 2019, 67, 101371. [Google Scholar] [CrossRef]
- Durie, I.A.; Tehrani, Z.R.; Karaaslan, E.; Sorvillo, T.E.; McGuire, J.; Golden, J.W.; Welch, S.R.; Kainulainen, M.H.; Harmon, J.R.; Mousa, J.J.; et al. Structural characterization of protective non-neutralizing antibodies targeting Crimean-Congo hemorrhagic fever virus. Nature Communications 2022, 13, 7298. [Google Scholar] [CrossRef]
- Lasecka, L.; Bin-Tarif, A.; Bridgen, A.; Juleff, N.; Waters, R.A.; Baron, M.D. Antibodies to the core proteins of Nairobi sheep disease virus/Ganjam virus reveal details of the distribution of the proteins in infected cells and tissues. PLoS One 2015, 10, e0124966. [Google Scholar] [CrossRef]
- Lombe, B.P.; Saito, T.; Miyamoto, H.; Mori-Kajihara, A.; Kajihara, M.; Saijo, M.; Masumu, J.; Hattori, T.; Igarashi, M.; Takada, A. Mapping of Antibody Epitopes on the Crimean-Congo Hemorrhagic Fever Virus Nucleoprotein. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Burt, F.J.; Samudzi, R.R.; Randall, C.; Pieters, D.; Vermeulen, J.; Knox, C.M. Human defined antigenic region on the nucleoprotein of Crimean-Congo hemorrhagic fever virus identified using truncated proteins and a bioinformatics approach. J Virol Methods 2013, 193, 706–712. [Google Scholar] [CrossRef]
- Golden, J.W.; Shoemaker, C.J.; Lindquist, M.E.; Zeng, X.; Daye, S.P.; Williams, J.A.; Liu, J.; Coffin, K.M.; Olschner, S.; Flusin, O.; et al. GP38-targeting monoclonal antibodies protect adult mice against lethal Crimean-Congo hemorrhagic fever virus infection. Sci Adv 2019, 5, eaaw9535. [Google Scholar] [CrossRef]
- Mishra, A.K.; Moyer, C.L.; Abelson, D.M.; Deer, D.J.; El Omari, K.; Duman, R.; Lobel, L.; Lutwama, J.J.; Dye, J.M.; Wagner, A.; et al. Structure and Characterization of Crimean-Congo Hemorrhagic Fever Virus GP38. J Virol 2020, 94. [Google Scholar] [CrossRef]
- Fels, J.M.; Maurer, D.P.; Herbert, A.S.; Wirchnianski, A.S.; Vergnolle, O.; Cross, R.W.; Abelson, D.M.; Moyer, C.L.; Mishra, A.K.; Aguilan, J.T.; et al. Protective neutralizing antibodies from human survivors of Crimean-Congo hemorrhagic fever. Cell 2021, 184, 3486–3501. [Google Scholar] [CrossRef]
- Mishra, A.K.; Hellert, J.; Freitas, N.; Guardado-Calvo, P.; Haouz, A.; Fels, J.M.; Maurer, D.P.; Abelson, D.M.; Bornholdt, Z.A.; Walker, L.M.; et al. Structural basis of synergistic neutralization of Crimean-Congo hemorrhagic fever virus by human antibodies. Science 2022, 375, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J.; Swanepoel, R.; Leman, P.A. Antibody Response in Crimean-Congo Hemorrhagic Fever. Reviews of Infectious Diseases 1989, 11, S801–S806. [Google Scholar] [CrossRef]
- Ly, H. Differential Immune Responses to New World and Old World Mammalian Arenaviruses. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Borenstein-Katz, A.; Shulman, A.; Hamawi, H.; Leitner, O.; Diskin, R. Differential Antibody-Based Immune Response against Isolated GP1 Receptor-Binding Domains from Lassa and Junín Viruses. J Virol 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, Z.; Zhang, L.; Wang, S.; Xiao, G. Structure-function relationship of the mammarenavirus envelope glycoprotein. Virol Sin 2016, 31, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Sommerstein, R.; Flatz, L.; Remy, M.M.; Malinge, P.; Magistrelli, G.; Fischer, N.; Sahin, M.; Bergthaler, A.; Igonet, S.; Ter Meulen, J.; et al. Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection. PLoS Pathog 2015, 11, e1005276. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.W.; Hastie, K.M.; Mire, C.E.; Robinson, J.E.; Geisbert, T.W.; Branco, L.M.; Ollmann Saphire, E.; Garry, R.F. Antibody therapy for Lassa fever. Current Opinion in Virology 2019, 37, 97–104. [Google Scholar] [CrossRef]
- Buck, T.K.; Enriquez, A.S.; Schendel, S.L.; Zandonatti, M.A.; Harkins, S.S.; Li, H.; Moon-Walker, A.; Robinson, J.E.; Branco, L.M.; Garry, R.F.; et al. Neutralizing Antibodies against Lassa Virus Lineage I. mBio 2022, 13, e01278–01222. [Google Scholar] [CrossRef]
- Robinson, J.E.; Hastie, K.M.; Cross, R.W.; Yenni, R.E.; Elliott, D.H.; Rouelle, J.A.; Kannadka, C.B.; Smira, A.A.; Garry, C.E.; Bradley, B.T.; et al. Most neutralizing human monoclonal antibodies target novel epitopes requiring both Lassa virus glycoprotein subunits. Nat Commun 2016, 7, 11544. [Google Scholar] [CrossRef]
- Perrett, H.R.; Brouwer, P.J.M.; Hurtado, J.; Newby, M.L.; Liu, L.; Müller-Kräuter, H.; Müller Aguirre, S.; Burger, J.A.; Bouhuijs, J.H.; Gibson, G.; et al. Structural conservation of Lassa virus glycoproteins and recognition by neutralizing antibodies. Cell Rep 2023, 42, 112524. [Google Scholar] [CrossRef]
- Enriquez, A.S.; Buck, T.K.; Li, H.; Norris, M.J.; Moon-Walker, A.; Zandonatti, M.A.; Harkins, S.S.; Robinson, J.E.; Branco, L.M.; Garry, R.F.; et al. Delineating the mechanism of anti-Lassa virus GPC-A neutralizing antibodies. Cell Rep 2022, 39, 110841. [Google Scholar] [CrossRef] [PubMed]
- Hastie, K.M.; Zandonatti, M.A.; Kleinfelter, L.M.; Heinrich, M.L.; Rowland, M.M.; Chandran, K.; Branco, L.M.; Robinson, J.E.; Garry, R.F.; Saphire, E.O. Structural basis for antibody-mediated neutralization of Lassa virus. Science 2017, 356, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Hastie, K.M.; Cross, R.W.; Harkins, S.S.; Zandonatti, M.A.; Koval, A.P.; Heinrich, M.L.; Rowland, M.M.; Robinson, J.E.; Geisbert, T.W.; Garry, R.F.; et al. Convergent Structures Illuminate Features for Germline Antibody Binding and Pan-Lassa Virus Neutralization. Cell 2019, 178, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Mahmutovic, S.; Clark, L.; Levis, S.C.; Briggiler, A.M.; Enria, D.A.; Harrison, S.C.; Abraham, J. Molecular Basis for Antibody-Mediated Neutralization of New World Hemorrhagic Fever Mammarenaviruses. Cell Host Microbe 2015, 18, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wu, Y.; Wang, W.; Zhang, L.; Xiao, G. Novel neutralizing monoclonal antibodies against Junin virus. Antiviral Res 2018, 156, 21–28. [Google Scholar] [CrossRef]
- York, J.; Berry, J.D.; Ströher, U.; Li, Q.; Feldmann, H.; Lu, M.; Trahey, M.; Nunberg, J.H. An antibody directed against the fusion peptide of Junin virus envelope glycoprotein GPC inhibits pH-induced membrane fusion. J Virol 2010, 84, 6119–6129. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Duehr, J.; Huang, C.; Paessler, S.; Tan, G.S.; Krammer, F. Monoclonal Antibodies with Neutralizing Activity and Fc-Effector Functions against the Machupo Virus Glycoprotein. J Virol 2020, 94. [Google Scholar] [CrossRef]
- Oestereich, L.; Müller-Kräuter, H.; Pallasch, E.; Strecker, T. Passive Transfer of Animal-Derived Polyclonal Hyperimmune Antibodies Provides Protection of Mice from Lethal Lassa Virus Infection. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Abreu-Mota, T.; Hagen, K.R.; Cooper, K.; Jahrling, P.B.; Tan, G.; Wirblich, C.; Johnson, R.F.; Schnell, M.J. Non-neutralizing antibodies elicited by recombinant Lassa-Rabies vaccine are critical for protection against Lassa fever. Nat Commun 2018, 9, 4223. [Google Scholar] [CrossRef]
- Ronk, A.J.; Lloyd, N.M.; Zhang, M.; Atyeo, C.; Perrett, H.R.; Mire, C.E.; Hastie, K.M.; Sanders, R.W.; Brouwer, P.J.M.; Saphire, E.O.; et al. A Lassa virus mRNA vaccine confers protection but does not require neutralizing antibody in a guinea pig model of infection. Nat Commun 2023, 14, 5603. [Google Scholar] [CrossRef]
- Battegay, M.; Moskophidis, D.; Waldner, H.; Bründler, M.A.; Fung-Leung, W.P.; Mak, T.W.; Hengartner, H.; Zinkernagel, R.M. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J Immunol 1993, 151, 5408–5415. [Google Scholar] [CrossRef]
- Eschli, B.; Zellweger, R.M.; Wepf, A.; Lang, K.S.; Quirin, K.; Weber, J.; Zinkernagel, R.M.; Hengartner, H. Early antibodies specific for the neutralizing epitope on the receptor binding subunit of the lymphocytic choriomeningitis virus glycoprotein fail to neutralize the virus. J Virol 2007, 81, 11650–11657. [Google Scholar] [CrossRef] [PubMed]
- Bergthaler, A.; Flatz, L.; Verschoor, A.; Hegazy, A.N.; Holdener, M.; Fink, K.; Eschli, B.; Merkler, D.; Sommerstein, R.; Horvath, E.; et al. Impaired antibody response causes persistence of prototypic T cell-contained virus. PLoS Biol 2009, 7, e1000080. [Google Scholar] [CrossRef]
- Buchmeier, M. Arenaviruses: protein structure and function. Arenaviruses I: The Epidemiology, Molecular and Cell Biology of Arenaviruses 2002, 159-173. [CrossRef]
- McCormick, J.B.; King, I.J.; Webb, P.A.; Scribner, C.L.; Craven, R.B.; Johnson, K.M.; Elliott, L.H.; Belmont-Williams, R. Lassa Fever. New England Journal of Medicine 1986, 314, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Hoch, S.P.; McCormick, J.B.; Auperin, D.; Brown, B.G.; Castor, M.; Perez, G.; Ruo, S.; Conaty, A.; Brammer, L.; Bauer, S. Protection of rhesus monkeys from fatal Lassa fever by vaccination with a recombinant vaccinia virus containing the Lassa virus glycoprotein gene. Proceedings of the National Academy of Sciences 1989, 86, 317–321. [Google Scholar] [CrossRef]
- Jahrling, P.B.; Peters, C.J. Passive antibody therapy of Lassa fever in cynomolgus monkeys: importance of neutralizing antibody and Lassa virus strain. Infect Immun 1984, 44, 528–533. [Google Scholar] [CrossRef]
- Branco, L.M.; Grove, J.N.; Boisen, M.L.; Shaffer, J.G.; Goba, A.; Fullah, M.; Momoh, M.; Grant, D.S.; Garry, R.F. Emerging trends in Lassa fever: redefining the role of immunoglobulin M and inflammation in diagnosing acute infection. Virology journal 2011, 8, 1–15. [Google Scholar] [CrossRef]
- Mire, C.E.; Cross, R.W.; Geisbert, J.B.; Borisevich, V.; Agans, K.N.; Deer, D.J.; Heinrich, M.L.; Rowland, M.M.; Goba, A.; Momoh, M.; et al. Human-monoclonal-antibody therapy protects nonhuman primates against advanced Lassa fever. Nat Med 2017, 23, 1146–1149. [Google Scholar] [CrossRef]
- Grant, A.; Seregin, A.; Huang, C.; Kolokoltsova, O.; Brasier, A.; Peters, C.; Paessler, S. Junín Virus Pathogenesis and Virus Replication. Viruses 2012, 4, 2317–2339. [Google Scholar] [CrossRef]
- Enria, D.A.; Briggiler, A.M.; Sánchez, Z. Treatment of Argentine hemorrhagic fever. Antiviral research 2008, 78, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Enria, D.; Fernandez, N.; Briggiler, A.; Levis, S.; Maiztegui, J. Importance of dose of neutralising antibodies in treatment of Argentine haemorrhagic fever with immune plasma. The Lancet 1984, 324, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Maiztegui, J.I.; McKee Jr, K.T.; Oro, J.G.B.; Harrison, L.H.; Gibbs, P.H.; Feuillade, M.R.; Enria, D.A.; Briggiler, A.M.; Levis, S.C.; Ambrosio, A.M. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. Journal of Infectious Diseases 1998, 177, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, L.; Geisbert, J.B.; Deer, D.J.; Fenton, K.A.; Bohorov, O.; Bohorova, N.; Goodman, C.; Kim, D.; Hiatt, A.; Pauly, M.H.; et al. Monoclonal antibody therapy for Junin virus infection. Proceedings of the National Academy of Sciences 2016, 113, 4458–4463. [Google Scholar] [CrossRef] [PubMed]
- CEPI awards funding agreement worth up to US$9.5 million to Colorado State University to develop a human vaccine against Rift Valley fever .
- Stedman, A.; Wright, D.; Wichgers Schreur, P.J.; Clark, M.H.A.; Hill, A.V.S.; Gilbert, S.C.; Francis, M.J.; van Keulen, L.; Kortekaas, J.; Charleston, B.; et al. Safety and efficacy of ChAdOx1 RVF vaccine against Rift Valley fever in pregnant sheep and goats. npj Vaccines 2019, 4, 44. [Google Scholar] [CrossRef]
- Safety and Immunogenicity of a Candidate RVFV Vaccine (RVF001) .
- Song, J.Y.; Jeong, H.W.; Yun, J.W.; Lee, J.; Woo, H.J.; Bae, J.Y.; Park, M.S.; Choi, W.S.; Park, D.W.; Noh, J.Y.; et al. Immunogenicity and safety of a modified three-dose priming and booster schedule for the Hantaan virus vaccine (Hantavax): A multi-center phase III clinical trial in healthy adults. Vaccine 2020, 38, 8016–8023. [Google Scholar] [CrossRef]
- Song, J.Y.; Woo, H.J.; Cheong, H.J.; Noh, J.Y.; Baek, L.J.; Kim, W.J. Long-term immunogenicity and safety of inactivated Hantaan virus vaccine (Hantavax™) in healthy adults. Vaccine 2016, 34, 1289–1295. [Google Scholar] [CrossRef]
- Chapman, N.S.; Hulswit, R.J.G.; Westover, J.L.B.; Stass, R.; Paesen, G.C.; Binshtein, E.; Reidy, J.X.; Engdahl, T.B.; Handal, L.S.; Flores, A.; et al. Multifunctional human monoclonal antibody combination mediates protection against Rift Valley fever virus at low doses. Nature Communications 2023, 14, 5650. [Google Scholar] [CrossRef]
- Hooper, J.W.; Brocato, R.L.; Kwilas, S.A.; Hammerbeck, C.D.; Josleyn, M.D.; Royals, M.; Ballantyne, J.; Wu, H.; Jiao, J.A.; Matsushita, H.; et al. DNA vaccine-derived human IgG produced in transchromosomal bovines protect in lethal models of hantavirus pulmonary syndrome. Sci Transl Med 2014, 6, 264ra162. [Google Scholar] [CrossRef]
- Bryden, S.R.; Dunlop, J.I.; Clarke, A.T.; Fares, M.; Pingen, M.; Wu, Y.; Willett, B.J.; Patel, A.H.; Gao, G.F.; Kohl, A.; et al. Exploration of immunological responses underpinning severe fever with thrombocytopenia syndrome virus infection reveals IL-6 as a therapeutic target in an immunocompromised mouse model. PNAS Nexus 2022, 1, pgac024. [Google Scholar] [CrossRef]
- Friebe, S.; van der Goot, F.G.; Bürgi, J. The Ins and Outs of Anthrax Toxin. Toxins (Basel) 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Akanmu, S.; Herrera, B.B.; Chaplin, B.; Ogunsola, S.; Osibogun, A.; Onawoga, F.; John-Olabode, S.; Akase, I.E.; Nwosu, A.; Hamel, D.J.; et al. High SARS-CoV-2 seroprevalence in Lagos, Nigeria with robust antibody and cellular immune responses. J Clin Virol Plus 2023, 3, 100156. [Google Scholar] [CrossRef]
- Herrera, B.B.; Hamel, D.J.; Oshun, P.; Akinsola, R.; Akanmu, A.S.; Chang, C.A.; Eromon, P.; Folarin, O.; Adeyemi, K.T.; Happi, C.T.; et al. A modified anthrax toxin-based enzyme-linked immunospot assay reveals robust T cell responses in symptomatic and asymptomatic Ebola virus exposed individuals. PLoS Negl Trop Dis 2018, 12, e0006530. [Google Scholar] [CrossRef]
- Herrera, B.B.; Tsai, W.Y.; Chang, C.A.; Hamel, D.J.; Wang, W.K.; Lu, Y.; Mboup, S.; Kanki, P.J. Sustained Specific and Cross-Reactive T Cell Responses to Zika and Dengue Virus NS3 in West Africa. J Virol 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.D.; Chaubal, G.Y.; Shete, A.M.; Mourya, D.T. A mini-review of Bunyaviruses recorded in India. Indian J Med Res 2017, 145, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Gaillet, M.; Pichard, C.; Restrepo, J.; Lavergne, A.; Perez, L.; Enfissi, A.; Abboud, P.; Lambert, Y.; Ma, L.; Monot, M.; et al. Outbreak of Oropouche Virus in French Guiana. Emerg Infect Dis 2021, 27, 2711–2714. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Arikawa, J. [Bunyavirus and its ecology]. Uirusu 2012, 62, 239–250. [Google Scholar] [CrossRef]
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley fever: biology and epidemiology. J Gen Virol 2019, 100, 1187–1199. [Google Scholar] [CrossRef]
- Elgh, F.; Linderholm, M.; Wadell, G.; Tärnvik, A.; Juto, P. Development of humoral cross-reactivity to the nucleocapsid protein of heterologous hantaviruses in nephropathia epidemica. FEMS Immunol Med Microbiol 1998, 22, 309–315. [Google Scholar] [CrossRef]
- Avižinienė, A.; Kučinskaitė-Kodzė, I.; Petraitytė-Burneikienė, R.; Žvirblienė, A.; Mertens, M.L.; Schmidt, S.; Schlegel, M.; Lattwein, E.; Koellner, B.; Ulrich, R.G. Characterization of a Panel of Cross-Reactive Hantavirus Nucleocapsid Protein-Specific Monoclonal Antibodies. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Kalkan-Yazıcı, M.; Karaaslan, E.; Çetin, N.S.; Hasanoğlu, S.; Güney, F.; Zeybek, Ü.; Doymaz, M.Z. Cross-Reactive anti-Nucleocapsid Protein Immunity against Crimean-Congo Hemorrhagic Fever Virus and Hazara Virus in Multiple Species. J Virol 2021, 95. [Google Scholar] [CrossRef]
- Sanchez*, A.; Pifat, D.Y.; Kenyon, R.H.; Peters, C.J.; McCormick, J.B.; Kiley, M.P. Junin Virus Monoclonal Antibodies: Characterization and Cross-reactivity with Other Arenaviruses. Journal of General Virology 1989, 70, 1125–1132. [Google Scholar] [CrossRef]
| Virus | T cell type | Epitope | Host | Approach |
|---|---|---|---|---|
| Peribunyaviridae | ||||
| OROV | CD8+ |
Glycoproteins TSSWGCEEY (1043–1051) CSMCGLIHY (48–56) LAIDTGCLY (4–12) |
Humans |
Immunoinformatics [28] |
| BUNV | CD8+/CD4+ | N protein KRSEWEVTL (55-63) AIGIYKVQRKEMEPK (161-75) |
Humans | Immunoinformatics [30] |
| Glycoproteins YQPTELTRS (716–724) YKAHDKEET (782–790) ILGTGTPKF (1172–1180) |
Immunoinformatics [31] | |||
| JCV | CD8+ | N protein AAKAKAALA (26-34) AALARKPER (152-161) ADHGESVSL (175-183) ADHGESVSLS (157-165) YPLTIGIYRV (108-117) |
Humans | Immunoinformatics [29] |
| CD4+ | N protein AALARKPER (146-154) ADHGESVSL (160-169) DVEQLKWGR (119-127) EIYLSFFPG (183-191) FLIKFGVKL (141-149) |
|||
| SBV | CD8+ |
N protein Glycoprotein Gc (678–947) |
IFNAR−/− mice | Ubiquitinated and non-ubiquitinated cDNA immunization [32] |
| LACV | CD4+ | Glycoprotein N protein |
IFNAR−/− mice | DNA vaccination [33] |
| SBV | CD8+ | N protein | IFNAR−/− mice | Bacterially expressed (SBV-N) [34] |
|
Phenuiviridae | ||||
| TOSV | CD4+ | N protein VKMMIVLNL (58–66) Glycoprotein VMILGLLSS (824–832) |
Humans |
Immunoinformatics [35] |
| SFTS | CD8+/CD4+ | Panel of peptides 8 peptides within RdRp 8 peptides within glycoprotein |
Humans | Immunoinformatics [36] |
| RVFV |
CD4+ | Glycoprotein LPALAVFALAPVVFA (139–153) PALAVFALAPVVFAE (140–154) GIAMTVLPALAVFAL (133–147) GSWNFFDWFSGLMSW (1138–1152) FFLLIYLGRTGLSKM (1174–1188) N protein HMMHPSFAGMVDPSL (143-158) |
Humans |
Immunoinformatics [37] |
| CD8+ | Glycoprotein AVFALAPVV (143–151) LAVFALAPV (142-150) FALAPVVFA (145–153) VFALAPVVF (144-152) IAMTVLPAL (134–142) FFDWFSGLM (1142–1150) FLLIYLGRT (1142–1150) N protein MMHPSFAGM (144–152) |
|||
| CD8+/CD4+ | Panel of peptides 14 peptides within N 13 peptides within Gn 16 peptides within Gc |
Humans | Ex vivo stimulation assays / immunoinformatics [38] | |
| CD8+ | N protein VLSEWLPVT (121–129) ILDAHSLYL (165–173) |
Humans | Ex vivo assays using N-transduced dendritic cells primed with CD8 T cells from HLA-A2 donors [39] | |
| CD8+ | N protein NAAVNSNFI (201-210) |
Mice C57BL/6 | Ex vivo stimulation assay of vaccinated mice [40] | |
| CD4+ | N protein VREFAYQGFDARRVI (25-40) AYQGFDARRVIELLK (29-44) |
|||
|
Hantaviridae | ||||
| Orhtohantaviruses (multiple) | CD8+/CD4+ | A panel of cross-reactive epitopes between multiple orthohantaviruses 6 peptides within glycoprotein 2 peptides within nucleocapsid 2 peptides within RdRp 1 peptide within NS protein |
Humans | Immunoinformatics [41] |
| HTNV |
CD8+ |
N protein NAHEGQLVI (12-20) ISNQEPLKL (421-429) |
Humans | Ex vivo stimulation [42] |
| N protein TSFVVPILLKALYML (127-141) YMLTTRGRQTTKDNK (139-153) IEPCKLLPDTAAVSL (241-255) LRKKSSFYQSYLRRT (355-369) |
Humans | Ex vivo stimulation [43] | ||
| N protein RYRTAVCGL (197-205) KLLPDTAAV (245-253) GPATNRDYL (258-266) |
Humans | Ex vivo stimulation [44] | ||
| HTNV /SNV |
CD8+ | ILQDMRNTI (HTNV, aa 334-342; SNV, aa 333-341) |
Humans | In silico prediction of conserved epitopes, validation of peptides Ex vivo using patients PBMCs [45] |
| CD8+/CD4+ | N protein ERIDDFLAA (234-242) LPIILKALY (131-139) GIQLDQKIII (372-380) |
Humans | Ex vivo stimulation assays [46] | |
| HTNV | Glycoprotein LIWTGMIDL (358-366) |
Humans | In silico prediction and evaluation of efficacy in transgenic mice [47] | |
| ANDV | CD8+ | Glycoprotein Gn SLFSLMPDVAHSLAV (461–475) |
Humans | Ex vivo stimulation [48] |
| PUUV | CD8+ | Glycoprotein Gn HWMDATFNL (731–739) |
Humans | Ex vivo stimulation [49] |
|
Nairoviridae | ||||
| CCHFV |
CD8+/CD4+ |
Panel of peptides 3 peptides within Gc 2 peptides within Gn |
Humans | Immunoinformatics [50] |
| A panel of peptides 5 peptides within N protein 4 peptides within Glycoprotein |
Humans | Immunoinformatics [51] | ||
| RdRp DCSSTPPDR (197-202) |
Humans | Immunoinformatic [52] | ||
| CD8+ | A panel of peptides 4 peptides within NSm 5 peptides within GP38 |
Humans | Ex vivo stimulation [53] | |
| N protein Gc |
Humans | Ex vivo stimulation [54] | ||
| Gc NSm |
C57BL/6 mice | Ex vivo stimulation [55] | ||
|
Arenaviridae | ||||
| LASV |
CD8+ | Glycoprotein MRMAWGGSY (192-200) N protein ALTDLGLIY (201-210) |
Humans | Immunoinformatics [56] |
| CD4+ | A panel of peptides 4 peptides within glycoprotein 4 peptides within N protein |
Humans | Immunoinformatics [56] | |
| LASV |
CD8+ |
A panel of peptides 12 epitopes within glycoprotein and/or N |
Humans | Ex vivo stimulation [57] |
| N (1-91) N (411–491) GP 2 (92–172) |
Humans | Ex vivo stimulation [58] | ||
| LASV |
CD4+ |
4 highly conserved peptides between Old and New World arenaviruses within GP2 (289-301) |
Humans | Ex vivo stimulation [59] |
| 6 peptides within N protein | Humans | Ex vivo stimulation [60] | ||
| LASV/ cross reacts with LCMV | CD4+ | Glycoprotein IEQQADNMITEMLQK (403-417) |
C3H/HeJ mice | Ex vivo stimulation [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





