Introduction
Since the outbreak of the first novel coronavirus caused severe acute respiratory syndrome (SARS-CoV) in Guandong, China, in November 2002 [
1], another novel coronavirus emerged in Wuhan, China, in December 2019 [
2,
3] and rapidly caused the global pandemic. The virus, officially designated as SARS-CoV-2, is an enveloped single-stranded RNA virus belonging to a b-coronavirus family [
4]. The SARS-CoV-2 infection occurred directly in the lung tissue through an angiotensin-converting enzyme (ACE)-II as a primary receptor [
5], thus rapidly developing severe pneumonia. The disease caused by SARS-CoV-2 is called COVID-19.
The SARS-CoV-2 accumulated mutations continuously during human-to-human transmission and in chronic infections [
6]. The WHO worked with the reported genetic mutation of the virus and assigned simple labels for key variants as variants of interest (VOIs) and variants of concern (VOC) in May 2021 (
https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/). From the original virus defined as B.1, Alpha (B.1.1 lineage) and Beta (B.1.35 lineage) variants were diverged, followed by the Delta (B.1.617 lineage) variant in October 2020 in India. At the end of 2021, the Omicron (B.1.1.529 lineage) variant emerged in South Africa and became a significant variant worldwide. Although Omicron continues to expand as various sub-lineages, they have changed to preferably infect the upper respiratory tract (versus lower respiratory tract), as compared to pre-Omicron VOCs (
https://www.who.int/news/item/16-03-2023-statement-on-the-update-of-who-s-working-definitions-and-tracking-system-for-sars-cov-2-variants-of-concern-and-variants-of-interest) resulting in the attenuated phenotype. Currently, we need to be concerned about the virus spreading mainly among individuals with comorbidity and older people.
The rapid development of novel vaccine technology has helped us achieve herd immunity against SARS-CoV-2 infection in the general population. The COVID-19 vaccine, which started in late 2020, substantially altered the course of the pandemic [
7]. In Japan, mRNA-based vaccines such as BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna), as well as a defective adenovirus-based vaccine called ChAdOx1-S (Oxford), were introduced in The government first provided the vaccination program to medical workers and eligibility for free vaccines has since been extended to all age groups to achieve herd immunity in Japan (
https://www.niid.go.jp/niid/ja/diseases/ka/corona-virus/2019-ncov/2484-idsc/10569-covid19-53.html#). However, decay of vaccine-induced neutralizing antibody response and the increase of SARS-CoV-2 re-infection were of great concern [
8]. In this context, based on the experimental coronavirus infection study [
9], the re-infection of human common-cold coronaviruses has been known to occur. The COVID-19 Forecasting Team recently showed that past-infection-induced protection against re-infection from pre-omicron variants was very high [
10]. However, the protection was substantially lower and shorter for the Omicron BA.1 variant [
10]. Of note, the natural infection appears to link to a lower incidence of SARS-CoV-2 infection than mRNA primary series vaccination [
11]. Despite nationwide vaccinations, we have encountered eight epidemic peaks in Japan at the end of 2023 (
https://www.niid.go.jp/niid/ja/basic-science/epidemi/12252-epi-2023-02.html). Thus, these findings indicate that re-infection frequently occurs after vaccinations and even after natural infections, probably associated with continuous virus mutations. While the COVID-19 vaccine cannot prevent infection, it can reduce the severity of the disease [
7,
8,
10,
12].
During the COVID-19 pandemic in early 2020, the government restricted all social activity in Japan: offices and schools were closed, and people could not go outside their homes. This strategy was successful for an initial epidemic wave for some time in Japan, but the second wave soon started in the summer of When vaccination began in 2021, we monitored the immune status of university students to understand the vaccine's effectiveness. The students in our university were relatively free from COVID-19 during several waves of epidemics in 2020 and 2021, probably by avoiding close contact among students and staff. On the other hand, when an omicron strain became dominant, we had a substantial number of students infected with SARS-CoV-2, mostly from family members. However, significant gaps in our understanding remain regarding the differential dynamics of both IgG and IgA antibodies in blood and saliva following SARS-CoV-2 infection and vaccination, highlighting a critical area of research yet to be thoroughly explored.
To gain a deeper understanding of how immunity develops against SARS-CoV-2 during the pandemic period of 2020 to 2022, we analyzed humoral and cellular immune responses in vaccinated and infected individuals, including close contact individuals who lived with infected family members and remained PCR or antigen-test-negative. We previously showed that the saliva IgA was induced early after influenza virus infection and could help evaluate mucosal immune responses [
13]. Therefore, we also analyzed saliva IgA antibody responses in SARS-CoV-2 infection, including close contact with the infected.
Materials and Methods
2.Subject and sample collection
In 2020, one family member of a staff working in a hospital was infected with SARS-CoV-2, and samples were collected periodically. In 2021, university staff members recruited volunteers, including their families and students, before vaccination. We collected blood and saliva samples before (pre) and two weeks after (1st, second, and third post-vaccination) from twenty-two vaccinated donors (mostly primed from June to August 2021), as shown in Table We obtained some peripheral blood mononuclear cells (PBMCs) as described previously [
13].
Since February 2022, SARS-CoV-2 infection has spread among young students who received vaccination in 2021, the information of which is shown in Table The samples from infected donors (17 donors) were collected early (10~14 days) and late (~1 month) post-infection. We collected the samples from individuals who were in close contact with infected families (PCR+) but test-negative, asymptomatic (10 donors) at two similar time points (early and late). Their information is shown in Table
Additionally, a member of one of the authors’ family had a mild fever and sore throat, which was followed by her sister, who developed a high fever. Their infection was confirmed through an antigen test. In this specific case, we were able to collect saliva samples in the early days after the infection.
For saliva sample collection, SalivaBio (Salimetris, Carlsbad, CA, USA) was used and centrifuged at 1,710g for 15 min, and the supernatants were transferred to new tubes as previously described [
13]. All these samples were collected with written informed consent and kept in a −80˚C freezer until use. In a previous study, we stocked blood and saliva samples from volunteers in our division until before the influenza season 2019 [
13]. We utilized some of them as pre-COVID-19 samples. The study followed the Helsinki Declaration and was approved by the ethical committee of the Tokyo University of Technology (No. E18HS-023).
2.Antigens
The nucleocapsid (N) is relatively conserved among human -coronaviruses in terms of amino acid sequences and structures [
14]. We used frozen stock of SARS-CoV-1 N protein, which was produced in 2007 as described before using the pET-SUMO system [
15], because the N protein of SARS-CoV-2 was shown to have approximately 90% homology with SARS-CoV-1 [
16]. Wen et al. recently showed that COVID-19 patient-derived monoclonal antibodies were cross-reactive to the N protein of SARS-CoV-1 but little to other human b-coronaviruses [
17]. The Receptor binding domain (RBD) derived from SARS-CoV-2 Wuhan-1 spike antigen was obtained commercially (Sino Biological, cat. 40592-VNAH). We used these antigens for the ELISA test.
For the in vitro stimulation of PBMC, a codon-humanized SARS-CoV-2 N DNA with Strep-tagII peptide coding sequence (WSHPQFEK) at 3' end was synthesized and cloned into an expression plasmid produced by GenScript Japan Co.Ltd. (Tokyo, Japan). The Expi293TM Expression System Kit (Thermo Fisher Scientific, Waltham, MA, USA) made recombinant N protein under the company's instruction. Both cell supernatant and lysates were combined, and we purified SARS-CoV-2 N using Strep-Tactin® Sepharose® (IBA Lifesciences GmbH, Goettingen, Germany) using the protocol provided by the company.
2.Standard antibodies
We obtained the RBD-specific IgG S309 (PMID: 32422645) by transfecting Expi293 cells with plasmids expressing S309 IgG heavy chain and light chain using the 293fectin transfection reagent (Thermo Fisher Scientific). On day 5, after transfection, the cell supernatant was clarified by centrifugation and filtered. The S309 antibody was purified from the supernatant by HiTrap rProtein A FF Column (Cytiva, Tokyo, Japan) using the AKTA go (Cytiva). We also utilized these heavy and light chain genes to produce RBD-specific IgA expression plasmid as described previously [
13]. We had the recombinant anti-RBD-IgA antibody by transfecting this plasmid as the IgG antibody described above. The culture supernatant was purified using Peptide M/agarose (InvivoGen Inc., San Diego, CA, USA). These RBD-specific IgG and IgA antibodies were served as a standard to measure RBD-specific antibodies. IgG and IgA antibodies were purified from the patient's serum in
Figure 1 at one-month post-infection and used to assess the level of N-specific IgG and IgA antibodies as standard.
2.ELISA
The amount of RBD or N-specific IgA and IgG in each sample was measured using the ELISA system described before [
13]. In brief, a Nunc 96-well microtiter plate (Thermo Fisher Scientific) was coated with RBD-Spike or N protein at 1 μg/mL in PBS and kept at 4˚C overnight. The plate was washed and blocked with PBS/0.5% BSA at RT for 1.5 hrs, and the samples were added to the plate. Then, the plate was incubated for 1 hr with a biotinylated anti-human IgG or IgA antibody (Southern Bio-Tech, Birmingham, UK), followed by HRP–streptavidin (1:2000 dilution with PBST, BioLegend) for 30 min. Finally, the TMB substrate (Sigma-Aldrich, St. Louis, MO, USA) was added, and the color development was measured at OD450 using a microplate reader iMARKTM (BioRad, Hercules, CA, USA).
The amount of total IgA in saliva was measured as described previously [
13]. As a standard, purified myeloma IgA1 protein (Sigma-Aldrich) was used. Note that the amount of total IgA was determined for saliva samples, and antigen-specific IgA titer was normalized with total IgA as previously described [
13].
2.In vitro stimulation and flow cytometry
Frozen PBMCs were thawed, and 1 million cells were distributed to wells of 96-flat-bottom culture plate (Corning Inc., Corning, NY, USA) in 0.1 ml of RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1/100 volume of Glutamax and Penicillin/Streptomycin (Thermo Fisher Scientific). The medium only or the medium containing 10 µg/ml of purified SARS-N antigen protein was added to make a final volume of 0.2 ml/well. A well containing SEB (Streptococcus enterotoxin B: Sigma-Aldrich) antigen at 1 µg/ml was prepared for each donor sample as a positive control. The plate was placed in a CO2 incubator (5% CO2) at 37ºC overnight. Sixteen hours later, brefeldin A (BFA) and monensin (both from Sigma-Aldrich) were added at a final concentration of 5 µg/mL and two µM, respectively. Simultaneously, 1 µl of PE-conjugated anti-CD107a antibody (BioLegend, San Diego, CA, USA) was added to each well, and the plate was incubated further for 5 hrs.
Cells were collected into 5 ml polystyrene round tubes (Falcon®352008, Corning), washed once with PBS, reacted with LIVE/DEAD™ Fixable Aqua Dead Cell Stain (Thermo Fisher Scientific) for 15 min at room temperature (RT), and then, incubated for 20 min on ice with the following antibody mixture: FITC-conjugated anti-human CD69, PerCP-Cy5.5-conjugated anti-human CD8, PE-Cy7-conjugated anti-human CD45RA, APC-Cy7-conjugated anti-human CD27, BV421-conjugated anti-human CD3 and FcReceptor blocker. For intracellular staining, cells were washed twice with PBS and fixed by 100 µl/sample of fixation buffer supplied in eBioscience™ Intracellular Fixation & Permeabilization kit (Thermo Fisher Scientific) for 20 min on ice. These cells were washed twice with 500 µl/sample of permeabilization buffer in the kit and incubated with APC-conjugated anti-human IFN-γ. APC-anti-mouse IgG1 was used as an isotype control. After 20 min incubation on ice, cells were washed with PBS/2%FBS/0.05%NaN3 (SB) and resuspended in SB for flow cytometer analysis. All these mouse monoclonal antibodies were purchased from BioLegend.
Finally, stained cells were acquired using FACSVerse™ Cell Analyzer (Becton Dickinson, and Co.(BD), Franklin Lakes, NJ, USA) and re-analyzed by a Flowjo ver 10.8.1 (BD). After removing doublets, live and CD3-positive T cells were gated for analysis. Then, the CD8+ and CD8- T cells were separately analyzed.
2.Statistical analysis
The amount of SARS-CoV-2 specific IgA and IgG antibodies was calculated based on standard IgG and IgA antibody titers using Microplate Manager 6 (BioRad). Statistical analysis was performed using GraphPad Prism version 10.1.The differences between groups were analyzed using the Non-parametric T-test of the Wilcoxon matched-pairs signed rank test. A P-value <0.05* or <0.01** was considered statistically significant.
Discussion
The outbreak of SARS-CoV-2 pandemic compelled us to change our lifestyle, but it proved to be a beneficial opportunity to study the immunological process in humans who have never encountered an emerging virus before. In this study, we chronologically analyzed immune responses to SARS-CoV-2 infection, by vaccination, and to post-vaccination infections.
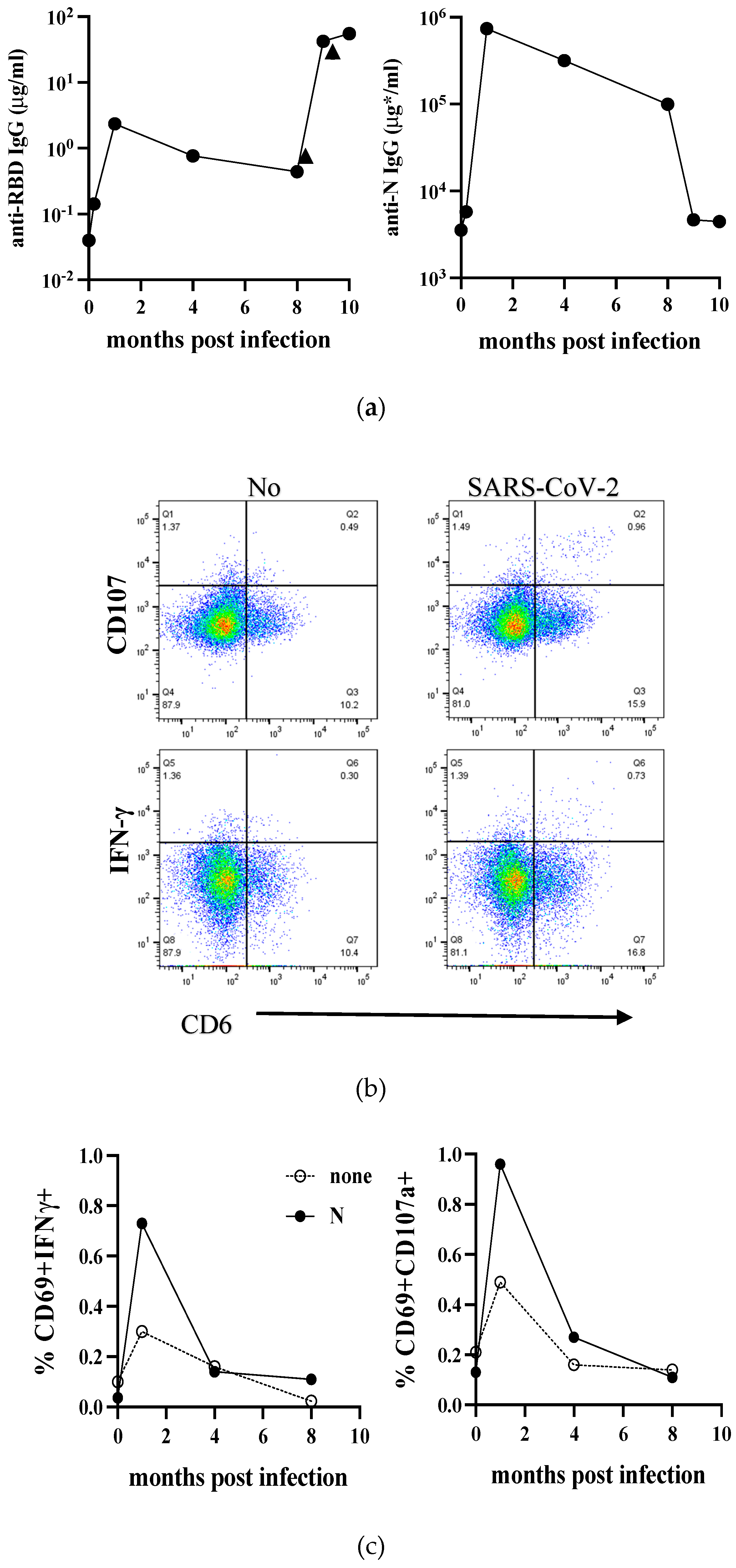
The study of a single first case of COVID-19 infection in our surroundings has revealed some interesting findings. We observed a typical antibody response against acute virus infection that peaked at one month, gradually declining. Anti-N IgG returned to near the basal level at nine months. Feng et al. reported that RBD- and full-length spike-IgG decreased during the first six months but remained stable up to 1 year after hospital discharge, then declined [
23]. In our case, the earlier decay of anti-N IgG could be due to the disease severity, which is very mild without any pulmonary damage. By vaccination at eight months after infection, the level of anti-RBD IgG became 1-log higher than the peak achieved by infection. Thus, vaccination can significantly enhance the antibody response elicited by SARS-CoV-2 infection. The long-term follow-up study by Koerber et al. showed that virus-neutralizing antibody titers rapidly declined in convalescents after asymptomatic or mild SARS-CoV-2 infection over nine months. Still, vaccination enhanced both antibody and cellular responses, especially against SARS-CoV-2 spike [
22]. Unfortunately, because of the lack of RBD protein for in vitro stimulation, we followed only N-specific T-cell responses starting from one month after infection. We detected activated (CD69
+) CD107a
+CD8
+ T cells only transiently at one month, not IFN-γ
+ CD8
+ T cells. While our research detected weak T-cell memory responses one month after infection, recent technology [
21,
22,
23,
27] can help improve our assay sensitivity and detect such responses earlier. These findings highlight the importance of vaccination as a critical strategy in the fight against COVID-19, especially for individuals who have previously been infected with the virus. These findings emphasize the importance of immunization as a vital strategy in the battle against COVID-19, especially for individuals who have previously been infected with the virus.
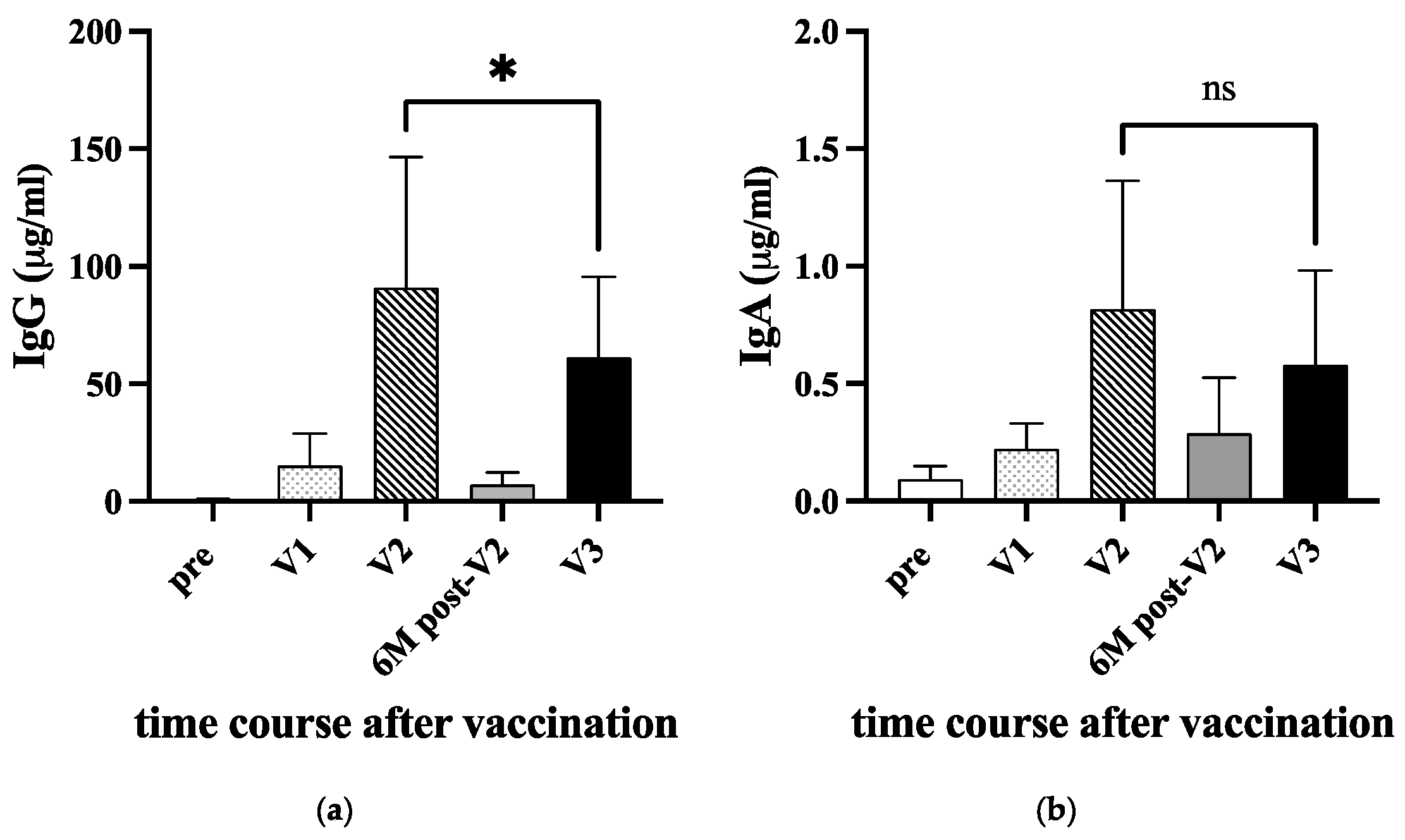
The level of RBD-IgG is important for protection against COVID-19, as it positively correlates with serum neutralizing capacity [
28]. To assess the effectiveness of mRNA vaccines coding for the Spike gene, we analyzed the level of anti-RBD IgG and IgA in uninfected individuals' serum. Following the second booster, both IgG and IgA levels in vaccinees significantly increased. However, the IgG level gradually decreased over time, as reported earlier [
22]. With the emergence of the delta variant, a third booster vaccination has been introduced [
29,
30,
31]. In our cohort, the level of IgG increased again after the third vaccination but did not increase beyond the level seen after the second vaccination, which was also noted in a study by Kesharvarz et al. [
18]. It is important to note that vaccine effectiveness increases significantly after the third vaccination [
29,
30,
31,
32]. Therefore, the difference in IgG levels between the second and third doses may have little impact. This data could be influenced by factors such as the age distribution of vaccine recipients, the timing of sampling, and the method of measurement.
The COVID-19 vaccines have shown efficacy in clinical trials and effectiveness studies. However, the level and duration of antibody response may not be high enough. A study conducted by Wei et al. indicated that the protection against infection would last for 5-8 months after two BNT162b2 doses without prior infection, compared to 1-2 years in unvaccinated individuals after natural infection [
33]. Additionally, almost all symptoms were reported less frequently in infected and vaccinated individuals than in infected unvaccinated individuals. The vaccinated were also more likely to be completely asymptomatic [
34]. Carazo et al. showed in their study of healthcare workers that those who had two doses of mRNA vaccine and previous Omicron infection were better protected from re-infection compared to a third vaccine dose without infection, indicating the limited benefit from additional vaccine doses for people with hybrid immunity [
35]. In other words, current vaccination may not provide better immunity than natural infection.
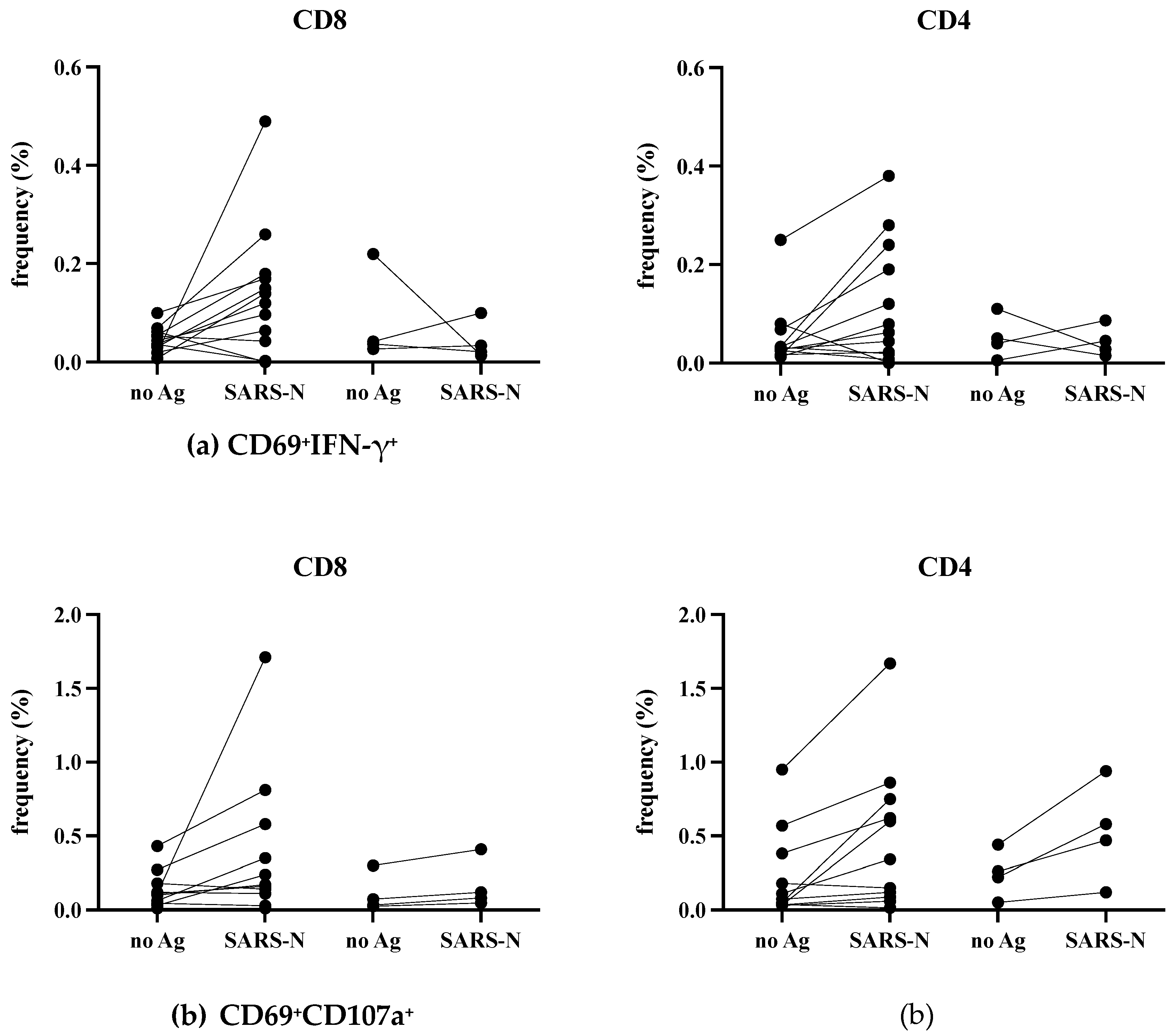
It has been observed that cellular immune responses, specifically the spike-specific CD8
+ T-cell responses that were produced by the original vaccines, are still very effective in responding to the Omicron variant [
36]. This highlights the significance of T-cell responses in providing protection. During our study, we detected low but significant N-specific CD4
+ and CD8
+ T-cell responses two weeks after infection, but these responses were not detected 3-5 months later. Due to the small number of samples and limitations in our methodology, we are unable to determine the longevity of T-cell responses. However, T-cells triggered by vaccination or infection against SARS can help in reducing the severity of the disease, as previously reported [
36].
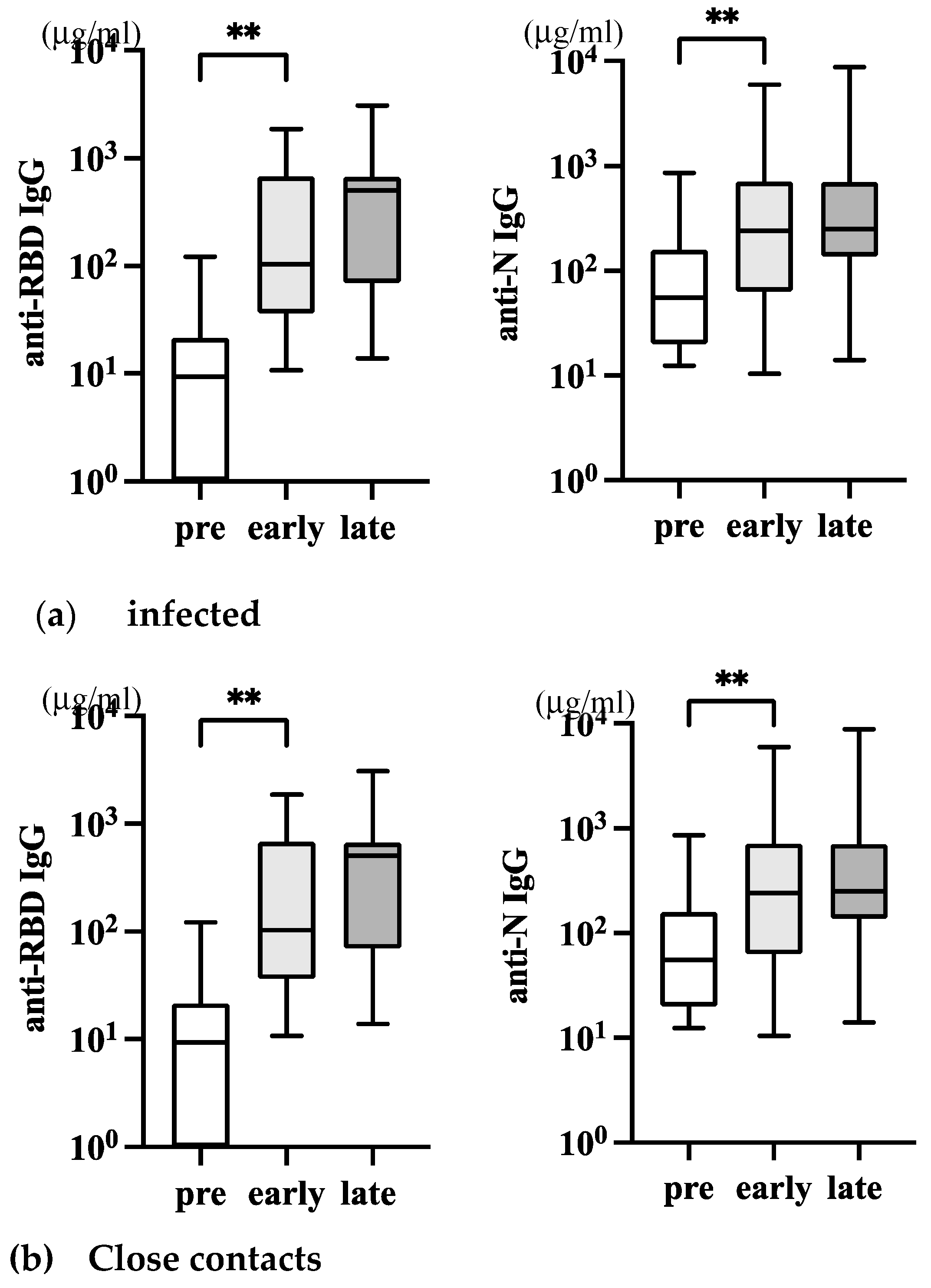
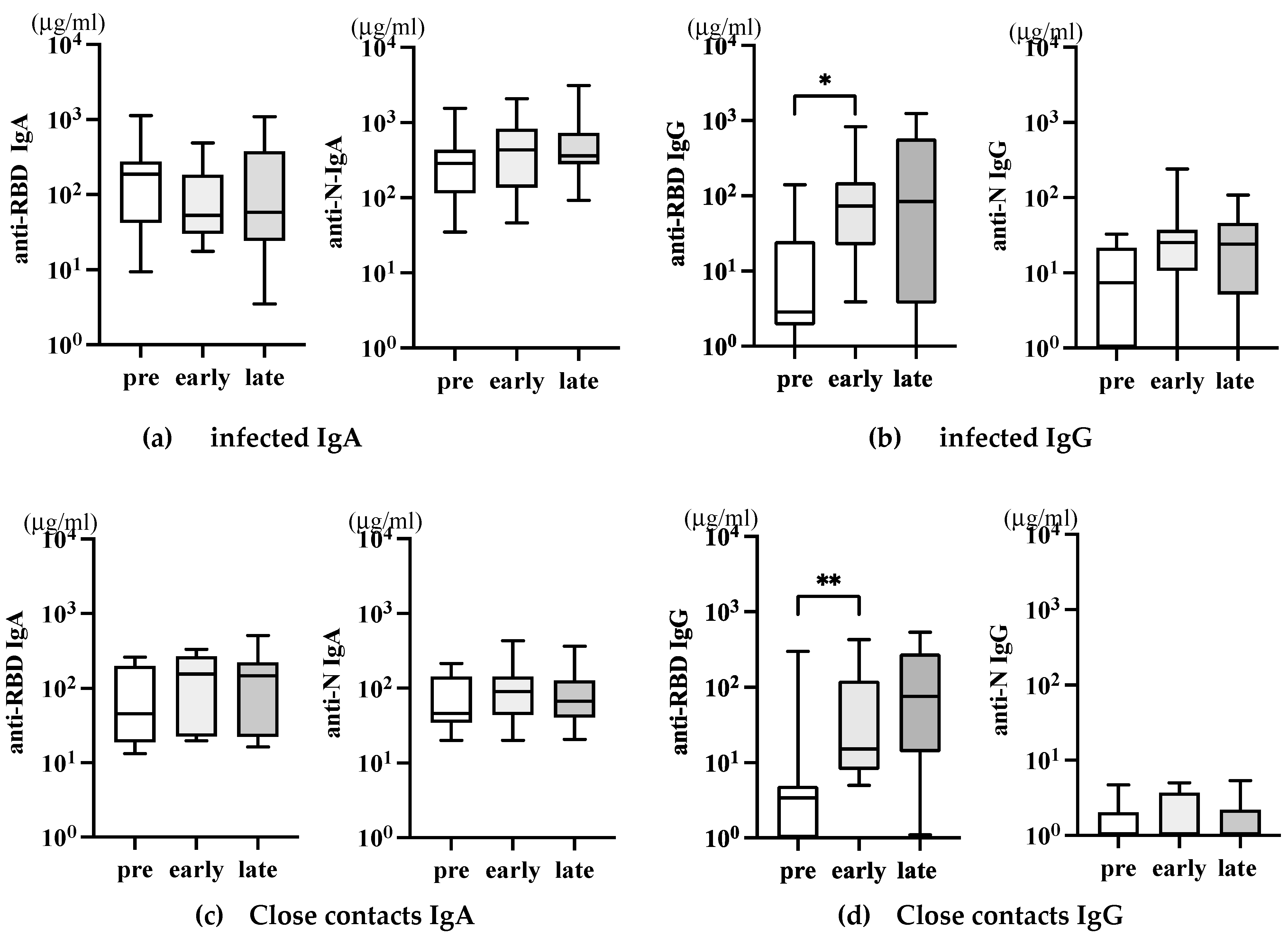
Reinfection of SARS-CoV-2 is becoming more common even after vaccination, particularly with the emergence of the Omicron variant. This is because the vaccine-elicited neutralizing antibodies have been greatly reduced [
37]. Additionally, the Omicron variant has reduced virus pathogenicity [
20], making reinfection or asymptomatic infection more likely than before [
6]. In our study, all infected individuals were previously vaccinated with original mRNA vaccines in 2021, and their disease was mild. It was unknown what the vaccine-induced anti-RBD IgG level was just before infection. However, the increased level of serum anti-N IgG shows the SARS-CoV-2 infection. Anti-N IgG increased early but was not augmented later, suggesting the transient nature of virus invasion. Close contacts of infected individuals did not show an increase in anti-N IgG, but their anti-RBD IgG levels were higher than pre-pandemic levels, likely due to previous mRNA vaccination. An interesting finding in the study was that close contacts who did not have detectable infection or antibodies gained T-cell immunity against SARS-CoV-2 [
38]. This was determined by stimulating PBMCs in vitro with peptide pools for ten days. The authors suggested that exposure to the virus led to T-cell immunity even without a successful infection. However, more research is needed to determine if these T-cells can provide protective immunity. Alternatively, early mucosal IgA responses may have contributed to the inhibition of spreading infection in these close contacts.
We were unable to find any SARS-CoV-2-specific IgA antibodies in the saliva samples. The levels of anti-RBD IgG found in the saliva could result from the systemic IgG produced after vaccination [
39]. In a previous study of influenza infection, we detected the presence of anti-flu IgA antibodies in saliva after 7 to 10 days of infection [
13]. Therefore, at more than two weeks after the SARS-CoV-2 infection, the saliva sample collection appeared unsuitable for detecting early IgA response. In fact, Shan et al. reported that N protein presents early (day 1~7 p.i.) in blood and saliva in SARS-CoV-2 infection [
40].
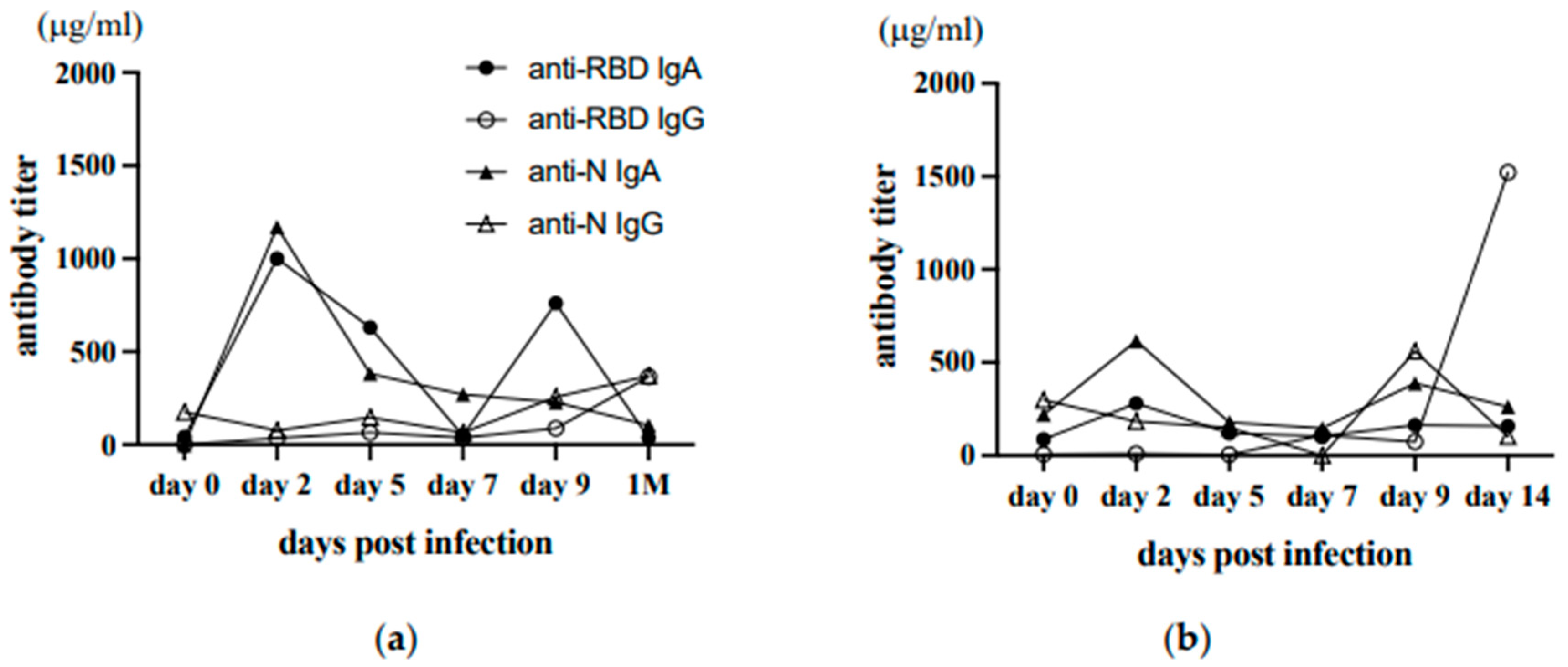
We had a valuable opportunity to obtain early saliva samples from two individuals at home. By analyzing SARS-specific antibodies in consecutive saliva samples starting from day 0, we found that SARS-CoV-2-specific IgA commonly peaked at day two, followed by a second peak around day nine p.i. These results suggest that the initial IgA-dominant mucosal response is weak and transient, whereas secondary memory responses involving systemic IgG are active after day nine p.i. Thus, if the self-collection of saliva samples is feasible, saliva will be more beneficial for detecting SARS-CoV-2 infection at mucosal sites. This may help us to understand how close contacts exposed to the virus are transiently infected but blocked the infection to a limited mucosal area by mucosal IgA or local tissue-resident T-cells, essentially conferring the protection as suggested by Wang et al. [
38]. Using bronchoalveolar lavage samples, Mitsui et al. demonstrated that donors with a history of both infection and vaccination have more airway mucosal SARS-CoV-2 antibodies and memory B cells than those only vaccinated, suggesting that peripheral vaccination alone fails to induce durable lung mucosal immunity against SARS-CoV-2 [
41]. Thus, as in the case of influenza vaccine [
42], mucosal priming must be considered.
During the SARS-CoV-2 pandemic, we gained a lot of knowledge about how the human body develops immunity against acute virus infections. This information is crucial for us to prepare for any future emergence of unknown viruses. Nowadays, vaccines can be developed quickly once the genetic structure of a virus is determined. However, current vaccines are not efficient in blocking upper respiratory mucosal infections and they don't provide long-lasting protection. Therefore, we need to put in more efforts to develop highly effective and long-lasting vaccines. We also need better ways to predict the emergence of new pathogens.
Author Contributions
Conceptualization, YTY; software, WI, YK, and YTY.; validation, ASM and TY.; formal analysis, WI, YK, and SO.; investigation, WI and YK.; resources, WI, YK and TN.; data curation, YTY.; writing—original draft preparation, YTY.; writing—review and editing, WI, ASM, TN and TY.; visualization, YTY.; project administration, YTY, ASM and TY.; funding acquisition, ASM. All authors have read and agreed to the published version of the manuscript.