Submitted:
02 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction to coronavirus
1.1. Virion composition of coronaviruses
1.3. SARS-CoV-2 infection
2. Endothelium
2.1. Angiopoietin family
2.2. The function of Angiopoietin 1
2.3. The function of Angiopoietin 2
2.4. Tie receptors
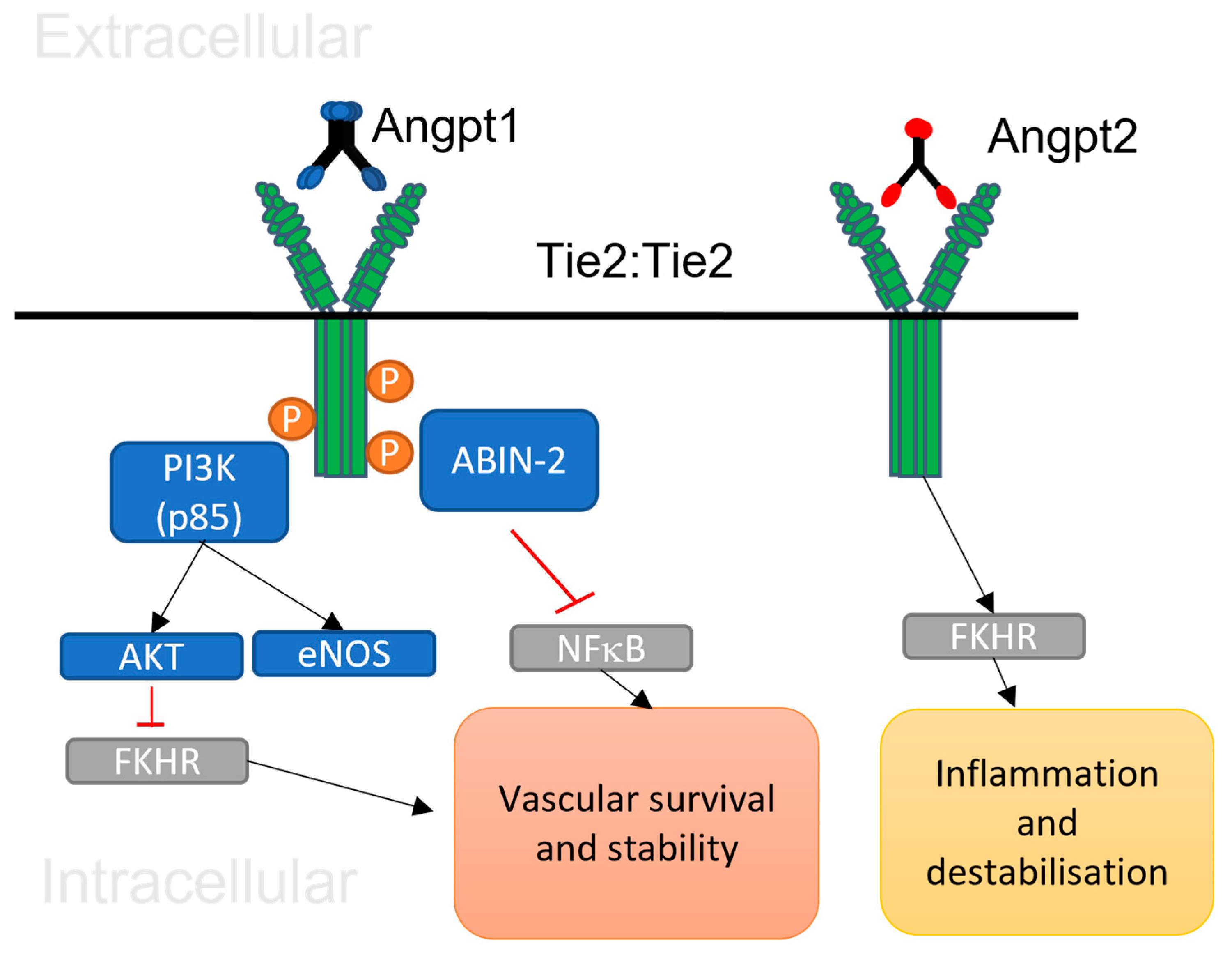
2.5. Angiopoietin 1/Tie 2 signalling
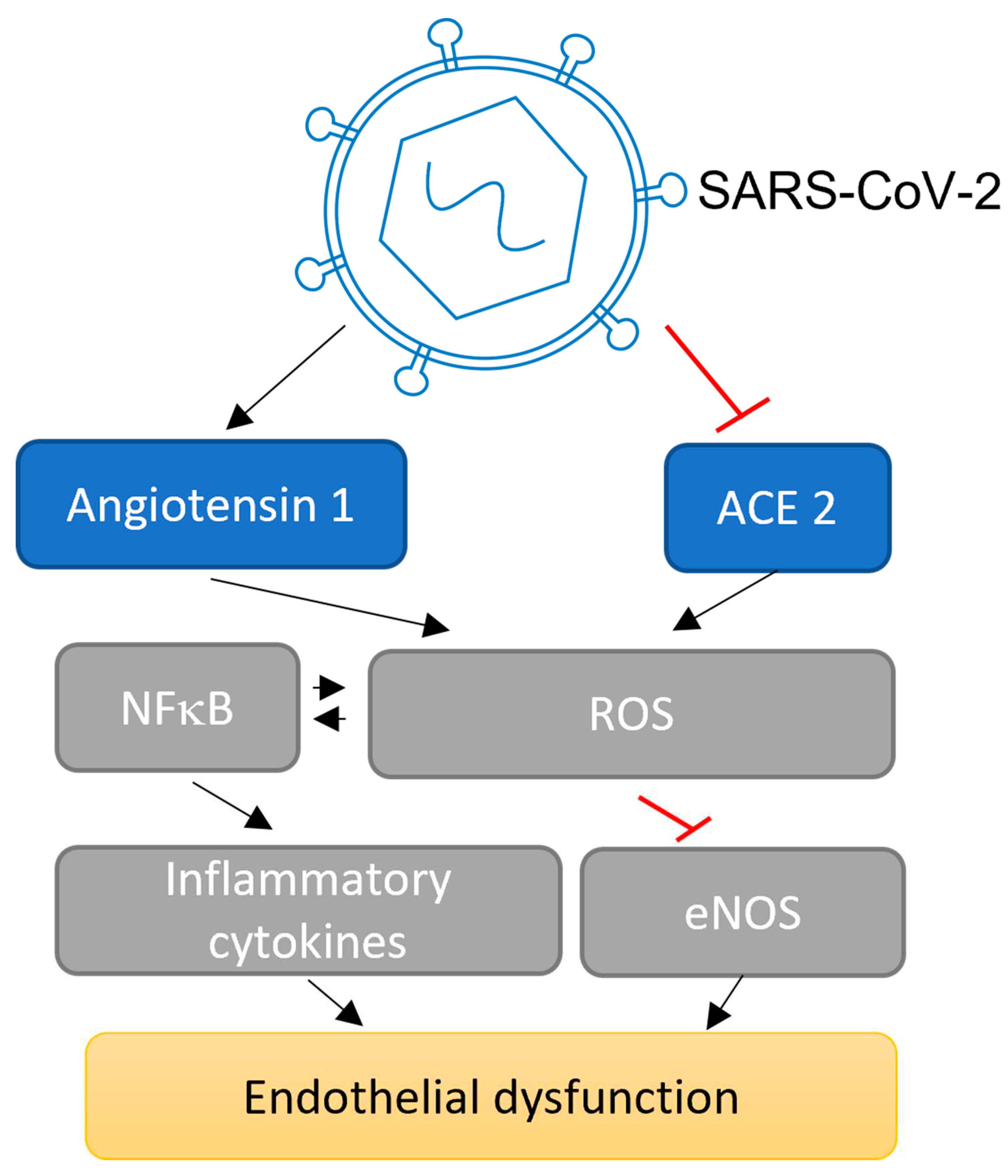
3. The impact of SARS-CoV-2 on endothelial cells
3.1. Potential impact of SARS-CoV-2 on Angpt/Tie signalling
4. Conclusion
Conflicts of Interest
References
- Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., Zhao, X., Huang, B., Shi, W., Lu, R., Niu, P., Zhan, F., Ma, X., Wang, D., Xu, W., Wu, G., Gao, G.F., Tan, W. and China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China. The New England journal of medicine, 2020, 382(8), 727-733. [CrossRef]
- Banerjee, A., Kulcsar, K., Misra, V., Frieman, M. and Mossman, K. Bats and Coronaviruses. Viruses. 2019, 11(1), 41.
- Philip V’kovski, Annika Kratzel, Silvio Steiner, Hanspeter Stalder & Volker Thiel. Coronavirus biology and replication: implications for SARS-CoV-2. Nature review microbiology. 2020, 19, 155-170.
- Li, Q., Guan, X., Wu, P., Wang, X., Zhou, L., Tong, Y., Ren, R., Leung, K.S.M., Lau, E.H.Y., Wong, J.Y., Xing, X., Xiang, N., Wu, Y., Li, C., Chen, Q., Li, D., Liu, T., Zhao, J., Liu, M., Tu, W., Chen, C., Jin, L., Yang, R., Wang, Q., Zhou, S., Wang, R., Liu, H., Luo, Y., Liu, Y., Shao, G., Li, H., Tao, Z., Yang, Y., Deng, Z., Liu, B., Ma, Z., Zhang, Y., Shi, G., Lam, T.T.Y., Wu, J.T., Gao, G.F., Cowling, B.J., Yang, B., Leung, G.M. and Feng, Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. The New England journal of medicine. 2020, 382(13), 1199-1207. [CrossRef]
- Gaunt, E.R., Hardie, A., Claas, E.C., Simmonds, P. and Templeton, K.E. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. Journal of clinical microbiology. 2010. 48(8), 2940-2947. [CrossRef]
- Naskalska, A., Dabrowska, A., Szczepanski, A., Milewska, A., Jasik, K.P. and Pyrc, K. Membrane Protein of Human Coronavirus NL63 Is Responsible for Interaction with the Adhesion Receptor. Journal of virology. 2019, 93(19), e00355-19. [CrossRef]
- Corman, V.M., Muth, D., Niemeyer, D. and Drosten, C. Hosts and Sources of Endemic Human Coronaviruses. Advances in Virus Research. 2018, 100, 163-188. [CrossRef]
- Lalchhandama K. The chronicles of coronaviruses: the bronchitis, the hepatitis and the common cold. Science vision. 2020, 20 (1), 43-53. [CrossRef]
- Gierer, S., Bertram, S., Kaup, F., Wrensch, F., Heurich, A., Kramer-Kuhl, A., Welsch, K., Winkler, M., Meyer, B., Drosten, C., Dittmer, U., von Hahn, T., Simmons, G., Hofmann, H. and Pohlmann, S. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. Journal of virology. 2013, 87(10), 5502-5511. [CrossRef]
- Neuman, B.W., Kiss, G., Kunding, A.H., Bhella, D., Baksh, M.F., Connelly, S., Droese, B., Klaus, J.P., Makino, S., Sawicki, S.G., Siddell, S.G., Stamou, D.G., Wilson, I.A., Kuhn, P. and Buchmeier, M.J. A structural analysis of M protein in coronavirus assembly and morphology. Journal of structural biology. 2011, 174(1), 11-22. [CrossRef]
- Mart M. Lamers and Bart L. Haagmans. SARS-CoV-2 pathogenesis. Nature Reviews Microbiology. 2022, 20, 270–284.
- Markus Hoffmann , Hannah Kleine-Weber , Simon Schroeder , Nadine Krüger , Tanja Herrler , Sandra Erichsen , Tobias S Schiergens , Georg Herrler , Nai-Huei Wu, Andreas Nitsche , Marcel A Müller, Christian Drosten , Stefan Pöhlmann. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020, 181(2), 271-280. [CrossRef]
- Ou, X., Liu, Y., Lei, X., Li, P., Mi, D., Ren, L., Guo, L., Guo, R., Chen, T., Hu, J., Xiang, Z., Mu, Z., Chen, X., Chen, J., Hu, K., Jin, Q., Wang, J. and Qian, Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature communications. 2020, 11(1), 1620-9.
- Kawase, M., Shirato, K., van der Hoek, L., Taguchi, F. and Matsuyama, S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. Journal of virology. 2012, 86(12), 6537-6545. [CrossRef]
- Letko, M., Marzi, A. and Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature microbiology. 2020, 5(4), 562-569. [CrossRef]
- W. Wang, L. Ye, L. Ye, B. Li, B. Gao, Y. Zeng, L. Kong, X. Fang, H. Zheng, Z. Wu, Y. She. Up-regulation of IL-6 and TNF-α induced by SARS-coronavirus spike protein in murine macrophages via NF-κB pathway. Virus Res. 2007. 128 (1–2), pp. 18, 10.1016/j. [CrossRef]
- Xin Yin, Laura Riva, Yuan Pu, Laura Martin-Sancho, Jun Kanamune, Yuki Yamamoto, Kouji Sakai, Shimpei Gotoh, Lisa Miorin, Paul D. De Jesus, Chih-Cheng Yang, Kristina M. Herbert, Sunnie Yoh, Judd F. Hultquist, Adolfo García-Sastre, and Sumit K. Chanda. MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Reports. 2021, 34(2), 108628.
- Lokugamage, K.G., Hage, A., de Vries, M., Valero-Jimenez, A.M., Schindewolf, C., Dittmann, M., Rajsbaum, R. and Menachery, V.D. Type I Interferon Susceptibility Distinguishes SARS-CoV-2 from SARS-CoV. Journal of virology. 2020, 94(23), e01410-20.
- Ann-Kathrin Reuschl , Lucy G Thorne, Matthew V X Whelan , Roberta Ragazzini , Wilhelm Furnon , Vanessa M Cowton , Giuditta De Lorenzo , Dejan Mesner , Jane L E Turner , Giulia Dowgier , Nathasha Bogoda , Paola Bonfanti, Massimo Palmarini , Arvind H Patel, Clare Jolly , Greg J Towers. Evolution of enhanced innate immune suppression by SARS-CoV-2 Omicron subvariants.Nature Microbiology. 2024. [CrossRef]
- Kim, Y. and Shin, E. Type I and III interferon responses in SARS-CoV-2 infection. Experimental & molecular medicine. 2021, 53(5), 750-760.
- Donghyuk Shin, Rukmini Mukherjee, Diana Grewe, Denisa Bojkova, Kheewoong Baek, Anshu Bhattacharya, Laura Schulz, Marek Widera, Ahmad Reza Mehdipour, Georg Tascher, Paul P. Geurink, Alexander Wilhelm, Gerbrand J. van der Heden van Noort, Huib Ovaa, Stefan Müller, Klaus-Peter Knobeloch, Krishnaraj Rajalingam, Brenda A. Schulman, Jindrich Cinatl, Gerhard Hummer, Sandra Ciesek & Ivan Dikic. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020. 587, 657-662.
- Shang, J., Ye, G., Shi, K., Wan, Y., Luo, C., Aihara, H., Geng, Q., Auerbach, A. and Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020, 581(7807), 221-224.
- Morse, J.S., Lalonde, T., Xu, S. and Liu, W. Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV. ChemRxiv: the preprint server for chemistry. 2020, 2,21 (5), 730-738. [CrossRef]
- Roberts, K.A., Colley, L., Agbaedeng, T.A., Ellison-Hughes, G.M. and Ross, M.D. Vascular Manifestations of COVID-19 - Thromboembolism and Microvascular Dysfunction. Frontiers in cardiovascular medicine. 2020, 7, 598400. [CrossRef]
- Pugsley, M.K. and Tabrizchi, R. The vascular system. An overview of structure and function. Journal of pharmacological and toxicological methods. 2000, 44(2), 333-340.
- Aird, W.C. 'Endothelium and haemostasis', Hamostaseologie, 2015, 35(1), 11-16. [CrossRef]
- G. Jia, A. R. Aroor, C. Jia, J. R. Sowers,Biochim. Biophys. Acta - Mol.Basis Dis. 2019,1865, 1802.
- Lo Gullo,C.O. Aragona,M.Scuruchi,A.G.Versace,A.Saitta,E.Imbalzano, S. Loddo, G. M. Campo, G. Mandraffino,Vascul. Phar-macol.2018,108, 8.
- Deanfield, J.E., Halcox, J.P. and Rabelink, T.J. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007. 115(10), 1285-1295.
- Pearson, J.D. 'Normal endothelial cell function'. Lupus. 2000, 9(3), 183-188. [CrossRef]
- Buhimschi, C.S., Bhandari, V., Dulay, A.T., Thung, S., Razeq, S.S.A., Rosenberg, V., Han, C.S., Ali, U.A., Zambrano, E., Zhao, G., Funai, E.F. and Buhimschi, I.A. Amniotic fluid angiopoietin-1, angiopoietin-2, and soluble receptor tunica interna endothelial cell kinase-2 levels and regulation in normal pregnancy and intraamniotic inflammation-induced preterm birth. The Journal of clinical endocrinology and metabolism. (2010). 95(7), 3428-3436. [CrossRef]
- Daly, C., Eichten, A., Castanaro, C., Pasnikowski, E., Adler, A., Lalani, A.S., Papadopoulos, N., Kyle, A.H., Minchinton, A.I., Yancopoulos, G.D. and Thurston, G. Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer research. 2013, 73(1), 108-118. [CrossRef]
- Bilimoria, J. and Singh, H. The Angiopoietin ligands and Tie receptors: potential diagnostic biomarkers of vascular disease, Journal of receptor and signal transduction research. 2019, 39(3), 187-193. [CrossRef]
- Maisonpierre, P.C., Suri, C., Jones, P.F., Bartunkova, S., Wiegand, S.J., Radziejewski, C., Compton, D., McClain, J., Aldrich, T.H., Papadopoulos, N., Daly, T.J., Davis, S., Sato, T.N. and Yancopoulos, G.D. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science (New York, N.Y.). 1997, 277(5322), 55-60. [CrossRef]
- Witzenbichler, B., Maisonpierre, P.C., Jones, P., Yancopoulos, G.D. and Isner, J.M. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. The Journal of biological chemistry. 1998, 273(29), 18514-18521. [CrossRef]
- Suri, C., Jones, P.F., Patan, S., Bartunkova, S., Maisonpierre, P.C., Davis, S., Sato, T.N. and Yancopoulos, G.D. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell, 1996, 87(7), 1171-1180. [CrossRef]
- Chae, J.K., Kim, I., Lim, S.T., Chung, M.J., Kim, W.H., Kim, H.G., Ko, J.K. and Koh, G.Y. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000, 20(12), 2573-2578. [CrossRef]
- Saharinen, P., Eklund, L., Miettinen, J., Wirkkala, R., Anisimov, A., Winderlich, M., Nottebaum, A., Vestweber, D., Deutsch, U., Koh, G.Y., Olsen, B.R. and Alitalo, K. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nature cell biology. 2008, 10(5), 527-537. [CrossRef]
- Papapetropoulos, A., Fulton, D., Mahboubi, K., Kalb, R.G., O'Connor, D.S., Li, F., Altieri, D.C. and Sessa, W.C. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. The Journal of biological chemistry. 2000, 275(13), 9102-9105. [CrossRef]
- Cai, J., Kehoe, O., Smith, G.M., Hykin, P. and Boulton, M.E. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Investigative ophthalmology & visual science. 2000, 49(5), 2163-2171. [CrossRef]
- Stratmann, A., Risau, W. and Plate, K.H. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. The American journal of pathology. 1998. 153(5), 1459-1466. [CrossRef]
- Holash, J., Maisonpierre, P.C., Compton, D., Boland, P., Alexander, C.R., Zagzag, D., Yancopoulos, G.D. and Wiegand, S.J. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science (New York, N.Y.). 1999, 284(5422), 1994-1998. [CrossRef]
- Lobov, I.B., Brooks, P.C. and Lang, R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002, 99(17), 11205-11210. [CrossRef]
- Dumont, D.J., Gradwohl, G., Fong, G.H., Puri, M.C., Gertsenstein, M., Auerbach, A. and Breitman, M.L. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes & development. 1994. 8(16), 1897-1909. [CrossRef]
- Felcht, M., Luck, R., Schering, A., Seidel, P., Srivastava, K., Hu, J., Bartol, A., Kienast, Y., Vettel, C., Loos, E.K., Kutschera, S., Bartels, S., Appak, S., Besemfelder, E., Terhardt, D., Chavakis, E., Wieland, T., Klein, C., Thomas, M., Uemura, A., Goerdt, S. and Augustin, H.G. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. The Journal of clinical investigation. 2012, 122(6), 1991-2005. [CrossRef]
- Fiedler, U., Scharpfenecker, M., Koidl, S., Hegen, A., Grunow, V., Schmidt, J.M., Kriz, W., Thurston, G. and Augustin, H.G. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004, 103(11), 4150-4156. [CrossRef]
- Scholz, A., Plate, K.H. and Reiss, Y. Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation. Annals of the New York Academy of Sciences. 2015, 1347, 45-51. [CrossRef]
- Kontos, C.D., Cha, E.H., York, J.D. and Peters, K.G. The endothelial receptor tyrosine kinase Tie1 activates phosphatidylinositol 3-kinase and Akt to inhibit apoptosis. Molecular and cellular biology. 2002, 22(6), 1704-1713. [CrossRef]
- Fiedler, U., Krissl, T., Koidl, S., Weiss, C., Koblizek, T., Deutsch, U., Martiny-Baron, G., Marme, D. and Augustin, H.G. Angiopoietin-1 and angiopoietin-2 share the same binding domains in the Tie-2 receptor involving the first Ig-like loop and the epidermal growth factor-like repeats. The Journal of biological chemistry. 2003, 278(3), 1721-1727. [CrossRef]
- Koblizek, T.I., Weiss, C., Yancopoulos, G.D., Deutsch, U. and Risau, W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Current biology: CB. 1998, 8(9), 529-532. [CrossRef]
- Korhonen, E.A., Lampinen, A., Giri, H., Anisimov, A., Kim, M., Allen, B., Fang, S., D'Amico, G., Sipila, T.J., Lohela, M., Strandin, T., Vaheri, A., Yla-Herttuala, S., Koh, G.Y., McDonald, D.M., Alitalo, K. and Saharinen, P. Tie1 controls angiopoietin function in vascular remodeling and inflammation. The Journal of clinical investigation. 2016, 126(9), 3495-3510. [CrossRef]
- Procopio, W.N., Pelavin, P.I., Lee, W.M. and Yeilding, N.M. Angiopoietin-1 and -2 coiled coil domains mediate distinct homo-oligomerization patterns, but fibrinogen-like domains mediate ligand activity. The Journal of biological chemistry. 1999, 274(42), 30196-30201. [CrossRef]
- Leppanen, V., Saharinen, P. and Alitalo, K. Structural basis of Tie2 activation and Tie2/Tie1 heterodimerization. Proceedings of the National Academy of Sciences of the United States of America. 2017, 114(17), 4376-4381.
- Jeansson M, Gawlik A, Anderson G, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest. 2011, 1 21, 2278–2289. [CrossRef]
- Kwak HJ, So J, Lee SJ, et al. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 1999, 448, 249–253. [CrossRef]
- Kwak HJ, Lee SJ, Lee Y, et al. Angiopoietin-1 inhibits irradiation and mannitol-induced apoptosis in endothelial cells. Circulation. 2000, 101, 2317. [CrossRef]
- Kim I, Kim HG, So J, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Circ Res. 2000, 8 6, 24–29. [CrossRef]
- Papapetropoulos A, Fulton D, Mahboubi K, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the akt/survivin pathway. J Biol Chem. 2000, 275, 9102–9105. [CrossRef]
- Kontos CD, Stauffer TP, Yang W, et al. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol Cell Biol. 1998, 18, 4131–4140. [CrossRef]
- Jones N, Master Z, Jones J, et al. Identification of tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem. 1999, 274, 30896–30905.
- Jones N, Chen SH, Sturk C, et al. A unique autophosphorylation site on Tie2/tek mediates dok-R phosphotyrosine binding domain binding and function. Mol Cell Biol. 2003, 23, 2658–2668.
- Harfouche R, Gratton J, Yancopoulos GD, et al. Angiopoietin-1 activates both anti- and proapoptotic mitogen-activated protein kinases. FASEB J. 2003, 17, 1523–1525.
- Kichina JV, Goc A, Al-Husein B, et al. PAK1 as a therapeutic target. Expert Opin Ther Tar. 2010, 14, pp 703–725. [CrossRef]
- Hughes DP, Dunmore BJ, et al. ABIN-2 protects endothelial cells from death and has a role in the antiapoptotic effect of Angiopoietin-1. Blood. 2003, 102, 4407–4409. [CrossRef]
- Tadros A, Hughes DP, Dunmore BJ, et al. ABIN-2 protects endothelial cells from death and has a role in the antiapoptotic effect of Angiopoietin-1. Blood. 2003, 102, 4407–4409. [CrossRef]
- Van Huffel S, Delaei F, Heyninck K, et al. Identification of a novel A20-binding inhibitor of nuclear factor-kappaB activation termed ABIN-2. J Biol Chem. 2001, 276, 30216–30223.
- Korhonen EA, Lampinen A, Giri H, et al. Tie1 controls angiopoietin function in vascular remodeling and inflammation. JCI. 2016, 126, 3495–3510. [CrossRef]
- Inoue M, Itoh H, Ueda M, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998, 98, 2108–2016.
- Singh H, Hansen TM, Patel N, et al. The molecular balance between receptor tyrosine kinases Tie1 and Tie2 is dynamically controlled by VEGF and TNFa and regulates angiopoietin signalling. PLoS ONE. 2012, 7, 29319.
- Marron MB, Singh H, Tahir TA, et al. Regulated proteolytic processing of Tie1 modulates ligand responsiveness of the receptortyrosine kinase Tie2. J Biol Chem. 2007, 282, 30509–30517. [CrossRef]
- Findley CM, Cudmore MJ, Ahmed A, et al. VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt-dependent pathway to modulate Tie2 signaling. Arterioscl Throm Vas Biol. 2007, 27, pp 2619–2626. [CrossRef]
- Seegar TC, Eller B, Tzvetkova-Robev D, et al. Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Mol Cell. 2010, 37, 643–655. [CrossRef]
- Schlosser K, Taha M, Deng Y, et al. High circulating Angiopoietin 2 levels exacerbate pulmonary inflammation but not vascular leak or mortality in endotoxin-induced lung injury in mice. Thorax. 2018, 73, 248–261. [CrossRef]
- Zhang ZG, Zhang L, Croll SD, et al. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002, 113, 683–687. [CrossRef]
- Zernecke A, Weber C. Inflammatory mediators in atherosclerotic vascular disease. Basic Res Cardiol. 2005, 100, 93–101. [CrossRef]
- Davis JS, Yeo TW, Piera KA, et al. Angiopoietin-2 is increased in sepsis and inversely associated with nitric oxide-dependent microvascular reactivity. Crit Care. 2010, 14, R89. [CrossRef]
- Ghosh CC, Thamm K, Berghelli AV, et al. Drug repurposing screen identifies Foxo1-dependent Angiopoietin-2 regulation in sepsis. Crit Care Med. 2015, 43, 230–240. [CrossRef]
- Leow CC, Coffman K, Inigo I, et al. MEDI3617, a human anti angiopoietin 2 monoclonal antibody, inhibits angiogenesis and tumor growth in human tumor xenograft models. Int J Oncol. 2012, 40, 1321–1330. [CrossRef]
- White RR, Shan S, Rusconi CP, et al. Inhibition of rat corneal angiogenesis by a nuclease-resistant RNA aptamer specific for Angiopoietin-2. PNAS. 2003, 100, 5028–5033. [CrossRef]
- Yang, P. et al. (2017) The ratio of serum Angiopoietin-1 to Angiopoietin-2 in patients with cervical cancer is a valuable diagnostic and prognostic biomarker. Peer J. 2017, 5, 3387. [CrossRef]
- Ackermann M, Verleden SE, Kuehnel M et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020, 383, 120–128.
- Goshua G, Pine AB, Meizlish ML et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [CrossRef]
- Smadja DM, Mentzer SJ, Fontenay M et al. COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis. 2021, 24, 755–788. [CrossRef]
- Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020, 20, 389–391.
- Varga, Z., Flammer, A.J., Steiger, P., Haberecker, M., Andermatt, R., Zinkernagel, A.S., Mehra, M.R., Schuepbach, R.A., Ruschitzka, F. and Moch, H. 'Endothelial cell infection and endotheliitis in COVID-19', Lancet (London, England). 2020, 395(10234), 1417-1418. [CrossRef]
- Garcia-Ponce, A., Chanez Paredes, S., Castro Ochoa, K.F. and Schnoor, M. Regulation of endothelial and epithelial barrier functions by peptide hormones of the adrenomedullin family. Tissue barriers. 2016, 4(4), e1228439. [CrossRef]
- Millar, F.R., Summers, C., Griffiths, M.J., Toshner, M.R. and Proudfoot, A.G. The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax. 2016. 71(5), 462-473. [CrossRef]
- Noris, M., Benigni, A. and Remuzzi, G. The case of complement activation in COVID-19 multiorgan impact. Kidney international. 2020, 98(2), 314-322. [CrossRef]
- Pelaia, C., Tinello, C., Vatrella, A., De Sarro, G. and Pelaia, G. Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications. Therapeutic advances in respiratory disease. 2020, 14, 1-9. [CrossRef]
- Freeman, T.L. and Swartz, T.H. Targeting the NLRP3 Inflammasome in Severe COVID-19. Frontiers in immunology. 2020, 11, 1518. [CrossRef]
- Bernard, I., Limonta, D., Mahal, L.K. and Hobman, T.C. Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19. Viruses. 2020, 13(1), 29.
- Grobler, C., Maphumulo, S.C., Grobbelaar, L.M., Bredenkamp, J.C., Laubscher, G.J., Lourens, P.J., Steenkamp, J., Kell, D.B. and Pretorius, E. Covid-19: The Rollercoaster of Fibrin (Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. International journal of molecular sciences. 2020, 21(14), 5168. [CrossRef]
- Nicin L, Abplanalp WT, Mellentin H et al. Cell type specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J. 2020, 41, 1804–1806.
- Sluimer JC, Gasc JM, Hamming I et al. Angiotensin converting enzyme 2 (ACE2) expression and activity in human carotid atherosclerotic lesions. J Pathol. 2008, 215, 273–279. [CrossRef]
- Lu R, Zhao X, Li J et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020, 395, 565–574. [CrossRef]
- Hikmet F, Mear L, Edvinsson A, Micke P, Uhlen M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020, 16, 9610. [CrossRef]
- Toni M. Delorey, Carly G. K. Ziegler, Graham Heimberg, Rachelly Normand, Yiming Yang, Åsa Segerstolpe, Domenic Abbondanza, Stephen J. Fleming, Ayshwarya Subramanian, Daniel T. Montoro, Karthik A. Jagadeesh, Kushal K. Dey, Pritha Sen, Michal Slyper, Yered H. Pita-Juárez, Devan Phillips, Jana Biermann, Zohar Bloom-Ackermann, Nikolaos Barkas, Andrea Ganna, James Gomez, Johannes C. Melms, Igor Katsyv, Erica Normandin, Aviv Regev. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021. 595, 107-113.
- Peng Wang, Ronghua Luo, Min Zhang, Yaqing Wang, Tianzhang Song, Tingting Tao, Zhongyu Li, Lin Jin, Hongyi Zheng, Wenwen Chen, Mengqian Zhao, Yongtang Zheng, Jianhua Qin. A cross-talk between epithelium and endothelium mediates human alveolar-capillary injury during SARS-CoV-2 infection. Cell death and disease. 2020. 11(12), 1042.
- Vanessa Monteil , Hyesoo Kwon, Patricia Prado, Astrid Hagelkrüys, Reiner A Wimmer, Martin Stahl, Alexandra Leopoldi, Elena Garreta, Carmen Hurtado Del Pozo, Felipe Prosper, Juan Pablo Romero, Gerald Wirnsberger, Haibo Zhang, Arthur S Slutsky, Ryan Conder, Nuria Montserrat, Ali Mirazimi, Josef M Penninger. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell. 2020. 14;181 (4), 905-913.
- Schimmel, L., Chew, K.Y., Stocks, C.J., Yordanov, T.E., Essebier, P., Kulasinghe, A., Monkman, J., Dos Santos Miggiolaro, A.F.R., Cooper, C., de Noronha, L., Schroder, K., Lagendijk, A.K., Labzin, L.I., Short, K.R. and Gordon, E.J. Endothelial cells are not productively infected by SARS-CoV-2. Clinical & translational immunology. 2021, 10(10), e1350.
- Andersson, M.I., Arancibia-Carcamo, C.V., Auckland, K., Baillie, J.K., Barnes, E., Beneke, T., Bibi, S., Brooks, T., Carroll, M., Crook, D., Dingle, K., Dold, C., Downs, L.O., Dunn, L., Eyre, D.W., Gilbert Jaramillo, J., Harvala, H., Hoosdally, S., Ijaz, S., James, T., James, W., Jeffery, K., Justice, A., Klenerman, P., Knight, J.C., Knight, M., Liu, X., Lumley, S.F., Matthews, P.C., McNaughton, A.L., Mentzer, A.J., Mongkolsapaya, J., Oakley, S., Oliveira, M.S., Peto, T., Ploeg, R.J., Ratcliff, J., Robbins, M.J., Roberts, D.J., Rudkin, J., Russell, R.A., Screaton, G., Semple, M.G., Skelly, D., Simmonds, P., Stoesser, N., Turtle, L., Wareing, S. and Zambon, M. SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome open research, 2020, 5, 181.
- Ranucci, M., Ballotta, A., Di Dedda, U., Baryshnikova, E., Dei Poli, M., Resta, M., Falco, M., Albano, G. and Menicanti, L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. Journal of thrombosis and haemostasis: JTH. 2020, 18(7), 1747-1751. [CrossRef]
- Fodor, A., Tiperciuc, B., Login, C., Orasan, O.H., Lazar, A.L., Buchman, C., Hanghicel, P., Sitar-Taut, A., Suharoschi, R., Vulturar, R. and Cozma, A. Endothelial Dysfunction, Inflammation, and Oxidative Stress in COVID-19-Mechanisms and Therapeutic Targets. Oxidative medicine and cellular longevity. 2021, 8671713.
- Xu, S., Ilyas, I. and Weng, J. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacologica Sinica. 2023, 44(4), 695-709. [CrossRef]
- Miesbach, W. and Makris, M. COVID-19: Coagulopathy, Risk of Thrombosis, and the Rationale for Anticoagulation. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2020, 26, 1076029620938149.
- Nguyen Dinh Cat, A., Montezano, A.C., Burger, D. and Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxidants & redox signaling. 2013, 19(10), 1110-1120.
- Iba, T., Connors, J.M. and Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflammation research: official journal of the European Histamine Research Society ...[et al.]. 2020, 69(12), 1181-1189.
- Brandon Michael Henry, Maria Helena Santos de Oliveira, Isaac Cheruiyot, Justin L. Benoit, David S. Cooper, Giuseppe Lippi, Timothy D. Le Cras & Stefanie W. Benoit. Circulating level of Angiopoietin-2 is associated with acute kidney injury in coronavirus disease 2019 (Covid-19). Angiogenesis. 2021, 24, 403-406. [CrossRef]
- Lu, R.X.Z., Lai, B.F.L., Rafatian, N., Gustafson, D., Campbell, S.B., Banerjee, A., Kozak, R., Mossman, K., Mubareka, S., Howe, K.L., Fish, J.E. and Radisic, M. Vasculature-on-a-chip platform with innate immunity enables identification of angiopoietin-1 derived peptide as a therapeutic for SARS-CoV-2 induced inflammation. Lab on a chip. 2022, 22(6), 1171-1186.
- Del Valle, D.M., Kim-Schulze, S., Huang, H., Beckmann, N.D., Nirenberg, S., Wang, B., Lavin, Y., Swartz, T.H., Madduri, D., Stock, A., Marron, T.U., Xie, H., Patel, M., Tuballes, K., Van Oekelen, O., Rahman, A., Kovatch, P., Aberg, J.A., Schadt, E., Jagannath, S., Mazumdar, M., Charney, A.W., Firpo-Betancourt, A., Mendu, D.R., Jhang, J., Reich, D., Sigel, K., Cordon-Cardo, C., Feldmann, M., Parekh, S., Merad, M. and Gnjatic, S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature medicine. 2020, 26(10), 1636-1643. [CrossRef]
- Rendeiro, A.F., Ravichandran, H., Bram, Y., Chandar, V., Kim, J., Meydan, C., Park, J., Foox, J., Hether, T., Warren, S., Kim, Y., Reeves, J., Salvatore, S., Mason, C.E., Swanson, E.C., Borczuk, A.C., Elemento, O. and Schwartz, R.E. The spatial landscape of lung pathology during COVID-19 progression. Nature. 2021, 593(7860), 564-569. [CrossRef]
- Ramaiah, M.J. mTOR inhibition and p53 activation, microRNAs: The possible therapy against pandemic COVID-19. Gene reports. 2020, 20, 100765. [CrossRef]
- Lei, X., Shi, F., Basu, D., Huq, A., Routhier, S., Day, R. and Jin, W. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. The Journal of biological chemistry. 2011, 286(18), 15747-15756. [CrossRef]
- Bhatraju, P.K., Morrell, E.D., Stanaway, I.B., Sathe, N.A., Srivastava, A., Postelnicu, R., Green, R., Andrews, A., Gonzalez, M., Kratochvil, C.J., Kumar, V.K., Hsiang, T., Gale, M.J., Anesi, G.L., Wyles, D., Broadhurst, M.J., Brett-Major, D., Mukherjee, V., Sevransky, J.E., Landsittel, D., Hung, C., Altemeier, W.A., Gharib, S.A., Uyeki, T.M., Cobb, J.P., Liebler, J.M., Crosslin, D.R., Jarvik, G.P., Segal, L.N., Evans, L., Mikacenic, C. and Wurfel, M.M. 'Angiopoietin-Like4 Is a Novel Marker of COVID-19 Severity', Critical care explorations. 2022, 5(1), e0827. [CrossRef]
- Reyes, A., Corrales, N., Galvez, N.M.S., Bueno, S.M., Kalergis, A.M. and Gonzalez, P.A. Contribution of hypoxia inducible factor-1 during viral infections. Virulence. 2020, 11(1), 1482-1500. [CrossRef]
- Hadjadj, J., Yatim, N., Barnabei, L., Corneau, A., Boussier, J., Smith, N., Pere, H., Charbit, B., Bondet, V., Chenevier-Gobeaux, C., Breillat, P., Carlier, N., Gauzit, R., Morbieu, C., Pene, F., Marin, N., Roche, N., Szwebel, T., Merkling, S.H., Treluyer, J., Veyer, D., Mouthon, L., Blanc, C., Tharaux, P., Rozenberg, F., Fischer, A., Duffy, D., Rieux-Laucat, F., Kerneis, S. and Terrier, B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science (New York, N.Y.). 2020, 369(6504), 718-724. [CrossRef]
- Vassiliou, A.G., Keskinidou, C., Jahaj, E., Gallos, P., Dimopoulou, I., Kotanidou, A. and Orfanos, S.E. ICU Admission Levels of Endothelial Biomarkers as Predictors of Mortality in Critically Ill COVID-19 Patients. Cells. 2021, 10(1), pp. 186. [CrossRef]
- Osama Abou Arab, Youssef Bennis, Pierre Gauthier, Herve Dupont, Said Kamel, Yazine Mahjoub. Association between inflammation, angiopoietins, and disease severity in critically ill COVID-19 patients: a prospective study. British Journal of Anaesthesia. 2021, 126. (3), 127-130 . [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




