Submitted:
02 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. History, Taxonomy and Classification
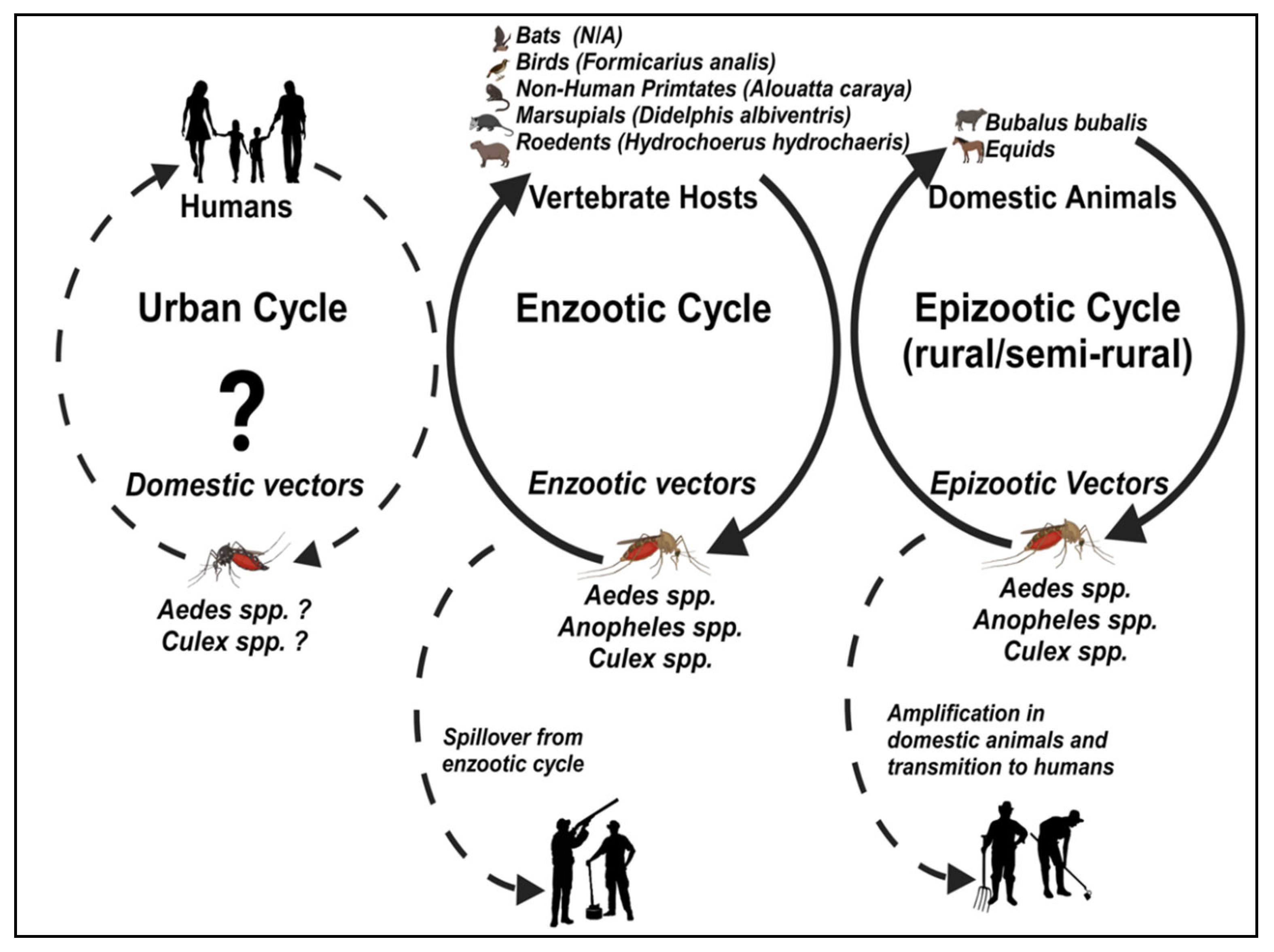
3. Ecology, Vectors, and Vertebrate Hosts
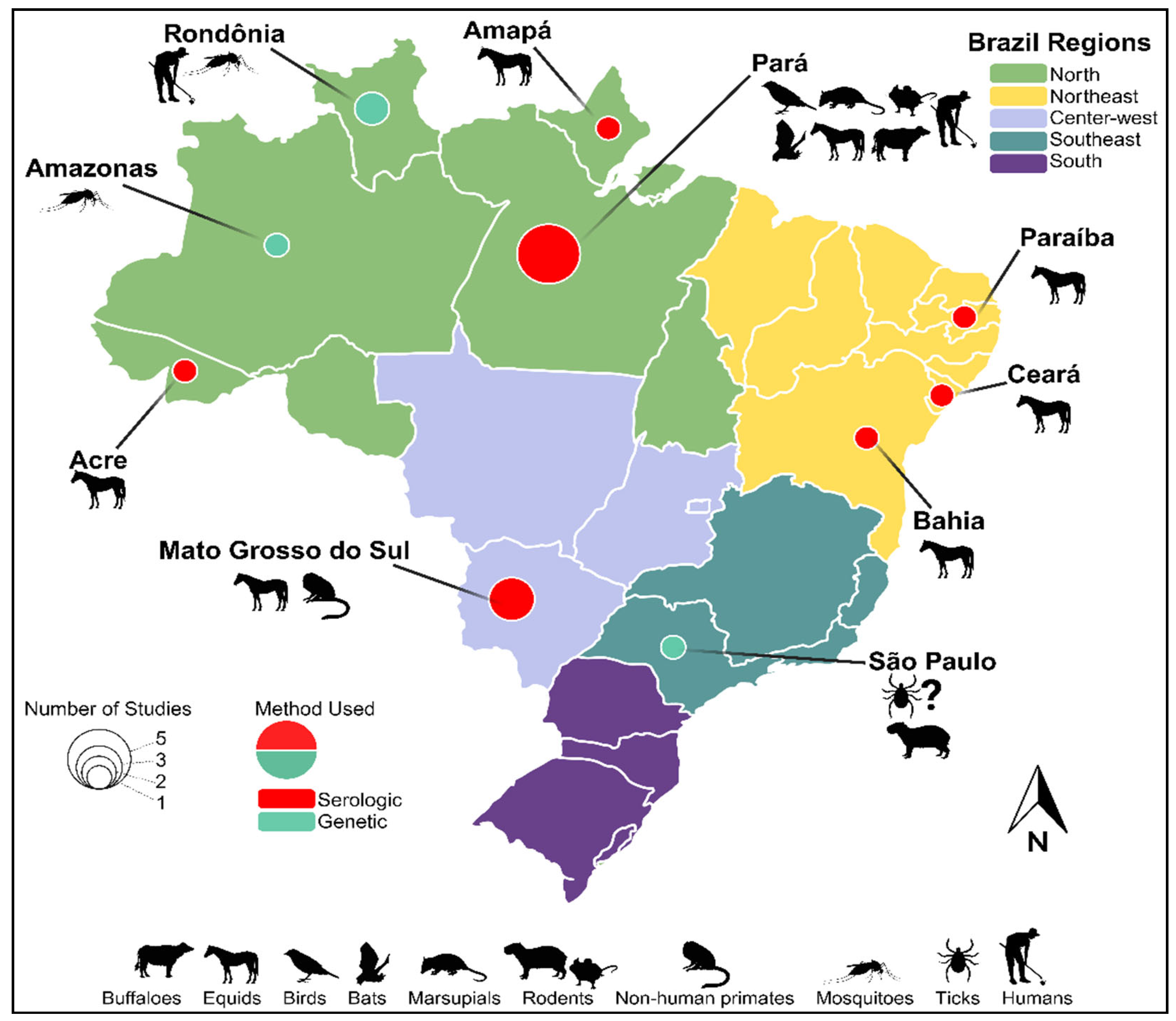
| Year | State | # of cases | Species/Animal | Tests Performed | Ref |
|---|---|---|---|---|---|
| 1977 | Pará | 1 | Formicarius analis | Viral isolation | [46,47] |
| 1976-1979 | 14 | Birds | HI | [47] | |
| 1 | Rodent | ||||
| 1977-1980 | 2 | Humans | |||
| 1978 | 2 | Birds | |||
| 1979 | 1 | Bird | |||
| 1979-1980 | 8 | Birds | |||
| 1 | Bat | ||||
| 1997 | São Paulo | 1 | Hydrochoerus hydrochaeris | RT-PCR | [49] |
| 1 pool | Amblyomma cajennense* | ||||
| 2002 | Rondônia | 1 | Human | RT-PCR and Semi-Nested-PCR | [67,68] |
| 2002 | Rondônia | 1 pool (8 females) | Culex sp. | RT-PCR and Semi-Nested-PCR | [50] |
| 1 pool (9 females) | Anopheles sp. | ||||
| 2005-2006 | Amazonas | 3 pools (33 females) | Aedes aegypti | ||
| 2005 | Pará Amapá Acre |
16 | Equids | HI | [60] |
| 2007 | Pará | 1 | Didelphis albiventris | HI | [69] |
| 2007-2009 | Paraíba Ceará |
1 | Equids | HI | [70] |
| 10 | |||||
| 2009 | Mato Grosso do Sul | 5 | Horses | ELISA | [61] |
| 2009-2010 | 50 | Horses | PRNT | ||
| 2009 | Pará | 8 | Bubalus bubalis | HI | [64] |
| 2009-2010 | Mato Grosso do Sul | 139 | Equids | PRNT | [62] |
| 2012 | Mato Grosso do Sul | 1 | Alouatta caraya | HI | [65] |
| 2013 | Bahia | 4 | Horses | PRNT | [63] |
| 2015 | Bahia | 1 | Horses | PRNT | [63] |
| 2017 | 2 | ||||
| 2018 | 6 |
4. Human Epidemiology
5. Clinical Disease, Diagnosis and Treatment
6. Prevention Options
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genus: Orthoflavivirus | ICTV Available online: https://ictv.global/report/chapter/flaviviridae/flaviviridae/orthoflavivirus (accessed on 29 November 2023).
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3, . [CrossRef]
- Pierson, T.C.; Diamond, M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020, 5, 796–812, . [CrossRef]
- Donalisio, M.R.; Freitas, A.R.R.; Zuben, A.P.B.V. Arboviruses Emerging in Brazil: Challenges for Clinic and Implications for Public Health. Rev. Saúde Pública 2017, 51, 30, . [CrossRef]
- Lima-Camara, T.N. Emerging Arboviruses and Public Health Challenges in Brazil. Rev. Saúde Pública 2016, 50, 36, . [CrossRef]
- Ribeiro, L.S.; Marques, R.E.; Jesus, A.M.R. de; Almeida, R.P. de; Teixeira, M.M. Zika Crisis in Brazil: Challenges in Research and Development. Curr. Opin. Virol. 2016, 18, 76–81, . [CrossRef]
- Figueiredo, P.O.; Dutra, A.G.S.; Costa, G.B.; Oliveira, J.S. de; Amaral, C.D.; Santos, J.D.; Rocha, K.L.S.; Júnior, J.P.A.; Nogueira, M.L.; Borges, M.A.Z.; et al. Re-Emergence of Yellow Fever in Brazil during 2016–2019: Challenges, Lessons Learned, and Perspectives. Viruses 2020, 12, 1233, . [CrossRef]
- Magalhaes, T.; Chalegre, K.D.M.; Braga, C.; Foy, B.D. The Endless Challenges of Arboviral Diseases in Brazil. Trop. Med. Infect. Dis. 2020, 5, 75, . [CrossRef]
- Lowe, R.; Barcellos, C.; Brasil, P.; Cruz, O.G.; Honório, N.A.; Kuper, H.; Carvalho, M.S. The Zika Virus Epidemic in Brazil: From Discovery to Future Implications. Int. J. Environ. Res. Public. Health 2018, 15, 96, . [CrossRef]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886, . [CrossRef]
- Périssé, A.R.S.; Souza-Santos, R.; Duarte, R.; Santos, F.; Andrade, C.R. de; Rodrigues, N.C.P.; Schramm, J.M. de A.; Silva, E.D. da; Jacobson, L. da S.V.; Lemos, M.C.F.; et al. Zika, Dengue and Chikungunya Population Prevalence in Rio de Janeiro City, Brazil, and the Importance of Seroprevalence Studies to Estimate the Real Number of Infected Individuals. PLOS ONE 2020, 15, e0243239, . [CrossRef]
- Tunali, M.; Radin, A.A.; Başıbüyük, S.; Musah, A.; Borges, I.V.G.; Yenigun, O.; Aldosery, A.; Kostkova, P.; dos Santos, W.P.; Massoni, T.; et al. A Review Exploring the Overarching Burden of Zika Virus with Emphasis on Epidemiological Case Studies from Brazil. Environ. Sci. Pollut. Res. 2021, 28, 55952–55966, . [CrossRef]
- Rios, F.G.F.; Alves do Nascimento, V.; Naveca, F.G.; Vieira, D.S.; Julião, G.R. Arbovirus Detection in Synanthropic Mosquitoes from the Brazilian Amazon and in Mosquito Saliva Using Flinders Technology Associates Cards. Microbes Infect. 2023, 25, 105046, . [CrossRef]
- Vieira, C.J. da S.P.; Andrade, C.D. de; Kubiszeski, J.R.; Silva, D.J.F. da; Barreto, E.S.; Massey, A.L.; Canale, G.R.; Bernardo, C.S.S.; Levi, T.; Peres, C.A.; et al. Detection of Ilheus Virus in Mosquitoes from Southeast Amazon, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 424–427, . [CrossRef]
- Carvalho, V.L.; Azevedo, R.S.S.; Carvalho, V.L.; Azevedo, R.S.; Henriques, D.F.; Cruz, A.C.R.; Vasconcelos, P.F.C.; Martins, L.C. Arbovirus Outbreak in a Rural Region of the Brazilian Amazon. J. Clin. Virol. 2022, 150–151, 105155, . [CrossRef]
- Mourão, M.P.G.; Bastos, M. de S.; Figueiredo, R.M.P. de; Gimaque, J.B. de L.; Alves, V. do C.R.; Saraiva, M. das G.G.; Figueiredo, M.L.G.; Ramasawmy, R.; Nogueira, M.L.; Figueiredo, L.T.M. Arboviral Diseases in the Western Brazilian Amazon: A Perspective and Analysis from a Tertiary Health & Research Center in Manaus, State of Amazonas. Rev. Soc. Bras. Med. Trop. 2015, 48, 20–26, . [CrossRef]
- Araújo, P.A.; Freitas, M.O.; Chiang, J.O.; Silva, F.A.; Chagas, L.L.; Casseb, S.M.; Silva, S.P.; Nunes-Neto, J.P.; Rosa-Júnior, J.W.; Nascimento, B.S.; et al. Investigation about the Occurrence of Transmission Cycles of Arbovirus in the Tropical Forest, Amazon Region. Viruses 2019, 11, 774, . [CrossRef]
- Catenacci, L.S.; Ferreira, M.; Martins, L.C.; De Vleeschouwer, K.M.; Cassano, C.R.; Oliveira, L.C.; Canale, G.; Deem, S.L.; Tello, J.S.; Parker, P.; et al. Surveillance of Arboviruses in Primates and Sloths in the Atlantic Forest, Bahia, Brazil. EcoHealth 2018, 15, 777–791, . [CrossRef]
- de Miranda, R.M.; Ferreira-de-Brito, A.; Silva, J. dos S.; Xavier, A. da S.; Freitas Silva, S.O.; Alencar, J.; Lourenço-de-Oliveira, R. Mosquito Fauna and Spatial Distribution in an Atlantic Forest Area in Rio de Janeiro State, Brazil, Reveal a High Risk of Transmission of Yellow Fever and Other Arboviruses. Trop. Med. Infect. Dis. 2022, 7, 410, . [CrossRef]
- Silva, S.O.F.; de Mello, C.F.; Campos, J.A.R. dos; Leite, P.J.; Sabino, R.; Alencar, J. Report of Mosquito Vectors of Arboviruses from a Federal Conservation Unit in the Atlantic Forest, Rio de Janeiro State, Brazil. Life 2022, 12, 1597, . [CrossRef]
- Abreu, F.V.S. de; de Andreazzi, C.S.; Neves, M.S.A.S.; Meneguete, P.S.; Ribeiro, M.S.; Dias, C.M.G.; de Albuquerque Motta, M.; Barcellos, C.; Romão, A.R.; Magalhães, M. de A.F.M.; et al. Ecological and Environmental Factors Affecting Transmission of Sylvatic Yellow Fever in the 2017–2019 Outbreak in the Atlantic Forest, Brazil. Parasit. Vectors 2022, 15, 23, . [CrossRef]
- Lopes, O.D.S.; Sacchetta, L.D.A.; Coimbra, T.L.M.; Pinto, G.H.; Glasser, C.M. Emergence of a New Arbovirus Disease in Brazil: II. Epidemiologic Studies on 1975 Epidemic. Am. J. Epidemiol. 1978, 108, 394–401, . [CrossRef]
- Pauvolid-Corrêa, A.; Tavares, F.N.; Alencar, J.; Silva, J. dos S.; Murta, M.; Serra-Freire, N.M.; Pellegrin, A.O.; Gil-Santana, H.; Guimarães, A.É.; Silva, E.E. da Preliminary Investigation of Culicidae Species in South Pantanal, Brazil and Their Potential Importance in Arbovirus Transmission. Rev. Inst. Med. Trop. São Paulo 2010, 52, 17–24, . [CrossRef]
- Pauvolid-Corrêa, A.; Campos, Z.; Soares, R.; Nogueira, R.M.R.; Komar, N. Neutralizing Antibodies for Orthobunyaviruses in Pantanal, Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0006014, . [CrossRef]
- Pauvolid-Corrêa, A.; Tavares, F.N.; Costa, E.V. da; Burlandy, F.M.; Murta, M.; Pellegrin, A.O.; Nogueira, M.F.; Silva, E.E. da Serologic Evidence of the Recent Circulation of Saint Louis Encephalitis Virus and High Prevalence of Equine Encephalitis Viruses in Horses in the Nhecolândia Sub-Region in South Pantanal, Central-West Brazil. Mem. Inst. Oswaldo Cruz 2010, 105, 829–833, . [CrossRef]
- Pauvolid-Corrêa, A.; Kenney, J.L.; Couto-Lima, D.; Campos, Z.M.S.; Schatzmayr, H.G.; Nogueira, R.M.R.; Brault, A.C.; Komar, N. Ilheus Virus Isolation in the Pantanal, West-Central Brazil. PLoS Negl. Trop. Dis. 2013, 7, e2318, . [CrossRef]
- Pauvolid-Corrêa, A.; Juliano, R.S.; Campos, Z.; Velez, J.; Nogueira, R.M.R.; Komar, N. Neutralising Antibodies for Mayaro Virus in Pantanal, Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 125–133, . [CrossRef]
- Iversson, L.B.; Silva, R.A.M.S.; Rosa, A.P.A.T. da; Barros, V.L.R.S. Circulation of Eastern Equine Encephalitis, Western Equine Encephalitis, Ilhéus, Maguari and Tacaiuma Viruses in Equines of the Brazilian Pantanal, South America. Rev. Inst. Med. Trop. São Paulo 1993, 35, 355–359, . [CrossRef]
- Maia, L.M.S.; Pinto, A.Z. de L.; Carvalho, M.S. de; Melo, F.L. de; Ribeiro, B.M.; Slhessarenko, R.D. Novel Viruses in Mosquitoes from Brazilian Pantanal. Viruses 2019, 11, 957, . [CrossRef]
- Pauvolid-Corrêa, A.; Solberg, O.; Couto-Lima, D.; Kenney, J.; Serra-Freire, N.; Brault, A.; Nogueira, R.; Langevin, S.; Komar, N. Nhumirim Virus, a Novel Flavivirus Isolated from Mosquitoes from the Pantanal, Brazil. Arch. Virol. 2015, 160, 21–27, . [CrossRef]
- de Oliveira, C.H.; Andrade, M.S.; Campos, F.S.; da C. Cardoso, J.; Gonçalves-dos-Santos, M.E.; Oliveira, R.S.; Aquino-Teixeira, S.M.; Campos, A.A.; Almeida, M.A.; Simonini-Teixeira, D.; et al. Yellow Fever Virus Maintained by Sabethes Mosquitoes during the Dry Season in Cerrado, a Semiarid Region of Brazil, in 2021. Viruses 2023, 15, 757, . [CrossRef]
- Dias, H.G.; Lima, R.C. de; Barbosa, L.S.; Souza, T.M.A. de; Badolato-Correa, J.; Maia, L.M.S.; Ferreira, R. da S.; Neves, N.A. da S.; Costa, M.C. de S.; Martins, L.R.; et al. Retrospective Molecular Investigation of Mayaro and Oropouche Viruses at the Human-Animal Interface in West-Central Brazil, 2016–2018. PLOS ONE 2022, 17, e0277612, . [CrossRef]
- Costa, V.G. da; Féres, V.C. de R.; Saivish, M.V.; Gimaque, J.B. de L.; Moreli, M.L. Silent Emergence of Mayaro and Oropouche Viruses in Humans in Central Brazil. Int. J. Infect. Dis. 2017, 62, 84–85, . [CrossRef]
- Pinto, A.Z. de L.; Carvalho, M.S. de; Melo, F.L. de; Ribeiro, A.L.M.; Ribeiro, B.M.; Slhessarenko, R.D. Novel Viruses in Salivary Glands of Mosquitoes from Sylvatic Cerrado, Midwestern Brazil. PLOS ONE 2017, 12, e0187429, . [CrossRef]
- Junior, J.B.S.; Massad, E.; Lobao-Neto, A.; Kastner, R.; Oliver, L.; Gallagher, E. Epidemiology and Costs of Dengue in Brazil: A Systematic Literature Review. Int. J. Infect. Dis. 2022, 122, 521–528, . [CrossRef]
- Figueiredo, L.T.M. Dengue in Brazil. Rev. Soc. Bras. Med. Trop. 2012, 45, 285–285, . [CrossRef]
- Brasil, P.; Calvet, G.A.; Siqueira, A.M.; Wakimoto, M.; Sequeira, P.C. de; Nobre, A.; Quintana, M. de S.B.; Mendonça, M.C.L. de; Lupi, O.; Souza, R.V. de; et al. Zika Virus Outbreak in Rio de Janeiro, Brazil: Clinical Characterization, Epidemiological and Virological Aspects. PLoS Negl. Trop. Dis. 2016, 10, e0004636, . [CrossRef]
- Heukelbach, J.; Alencar, C.H.; Kelvin, A.A.; Oliveira, W.K. de; Cavalcanti, L.P. de G. Zika Virus Outbreak in Brazil. J. Infect. Dev. Ctries. 2016, 10, 116–120, . [CrossRef]
- Nunes, M.R.T.; Faria, N.R.; de Vasconcelos, J.M.; Golding, N.; Kraemer, M.U.; de Oliveira, L.F.; Azevedo, R. do S. da S.; da Silva, D.E.A.; da Silva, E.V.P.; da Silva, S.P.; et al. Emergence and Potential for Spread of Chikungunya Virus in Brazil. BMC Med. 2015, 13, 102, . [CrossRef]
- Figueiredo, L.T.M. Large Outbreaks of Chikungunya Virus in Brazil Reveal Uncommon Clinical Features and Fatalities. Rev. Soc. Bras. Med. Trop. 2017, 50, 583–584, . [CrossRef]
- Cunha, M. dos P.; Santos, C.A. dos; Neto, D.F. de L.; Schanoski, A.S.; Pour, S.Z.; Passos, S.D.; Souza, M.S.F. de; Costa, D.D.; Zanotto, P.M. de A. Outbreak of Chikungunya Virus in a Vulnerable Population of Sergipe, Brazil—A Molecular and Serological Survey. J. Clin. Virol. 2017, 97, 44–49, . [CrossRef]
- Rodrigues Faria, N.; Lourenço, J.; Marques de Cerqueira, E.; Maia de Lima, M.; Pybus, O.; Carlos Junior Alcantara, L. Epidemiology of Chikungunya Virus in Bahia, Brazil, 2014-2015. PLoS Curr. 2016, 8.
- Silva, N.I.O.; Albery, G.F.; Arruda, M.S.; Oliveira, G.F.G.; Costa, T.A.; Mello, É.M. de; Moreira, G.D.; Reis, E.V.; Silva, S.A. da; Silva, M.C.; et al. Ecological Drivers of Sustained Enzootic Yellow Fever Virus Transmission in Brazil, 2017–2021. PLoS Negl. Trop. Dis. 2023, 17, e0011407, . [CrossRef]
- Rosser, J.I.; Nielsen-Saines, K.; Saad, E.; Fuller, T. Reemergence of Yellow Fever Virus in Southeastern Brazil, 2017–2018: What Sparked the Spread? PLoS Negl. Trop. Dis. 2022, 16, e0010133, . [CrossRef]
- Aliota, M.T.; Bassit, L.; Bradrick, S.S.; Cox, B.; Garcia-Blanco, M.A.; Gavegnano, C.; Friedrich, T.C.; Golos, T.G.; Griffin, D.E.; Haddow, A.D.; et al. Zika in the Americas, Year 2: What Have We Learned? What Gaps Remain? A Report from the Global Virus Network. Antiviral Res. 2017, 144, 223–246, . [CrossRef]
- Rosa, J.F.S.T. da; Rosa, A.P. de A.T. da; Vasconcelos, P.F. da C.; Pinheiro, F. de P.; Dias, L.B.; Cruz, A.C.R. Arboviruses Isolated in the Evandro Chagas Institute, Including Some Described for the First Time in the Brazilian Amazon Region, Their Known Hosts, and Their Pathology for Man Available online: https://patua.iec.gov.br/items/7bd58f72-1478-482f-a252-ad7ec9b6528f (accessed on 29 November 2023).
- Karabatsos, N. The International Catalog of Arboviruses—ArboCat Virus: Cacipacore Virus (CPCV) Available online: https://wwwn.cdc.gov/arbocat/VirusDetails.aspx?ID=89 (accessed on 30 November 2023).
- Moureau, G.; Cook, S.; Lemey, P.; Nougairede, A.; Forrester, N.L.; Khasnatinov, M.; Charrel, R.N.; Firth, A.E.; Gould, E.A.; De Lamballerie, X. New Insights into Flavivirus Evolution, Taxonomy and Biogeographic History, Extended by Analysis of Canonical and Alternative Coding Sequences. PLOS ONE 2015, 10, e0117849, . [CrossRef]
- de Figueiredo, G.G.; Amarilla, A.A.; de Souza, W.M.; Fumagalli, M.J.; de Figueiredo, M.L.G.; Szabó, M.P.J.; Badra, S.J.; Setoh, Y.X.; Khromykh, A.A.; Aquino, V.H.; et al. Genetic Characterization of Cacipacoré Virus from Ticks Collected in São Paulo State, Brazil. Arch. Virol. 2017, 162, 1783–1786, . [CrossRef]
- Figueiredo, M.L.G. de; Amarilla, A.A.; Figueiredo, G.G. de; Alfonso, H.L.; Lippi, V.; Maia, F.G.M.; Morais, F.A.; Costa, C.A. da; Henriques, D.A.; Durigon, E.L.; et al. Cacipacore Virus as an Emergent Mosquito-Borne Flavivirus. Rev. Soc. Bras. Med. Trop. 2017, 50, 539–542, . [CrossRef]
- Alaniz, A.J.; Carvajal, M.A.; Bacigalupo, A.; Cattan, P.E. Global Spatial Assessment of Aedes Aegypti and Culex Quinquefasciatus: A Scenario of Zika Virus Exposure. Epidemiol. Infect. 2018, 147, e52, . [CrossRef]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Marm Kilpatrick, A. “Bird Biting” Mosquitoes and Human Disease: A Review of the Role of Culex Pipiens Complex Mosquitoes in Epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585, . [CrossRef]
- Hamer, G.L.; Kitron, U.D.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Goldberg, T.L.; Walker, E.D. Culex Pipiens (Diptera: Culicidae): A Bridge Vector of West Nile Virus to Humans. J. Med. Entomol. 2008, 45, 125–128, . [CrossRef]
- Manguin, S.; Bangs, M.J.; Pothikasikorn, J.; Chareonviriyaphap, T. Review on Global Co-Transmission of Human Plasmodium Species and Wuchereria Bancrofti by Anopheles Mosquitoes. Infect. Genet. Evol. 2010, 10, 159–177, . [CrossRef]
- Corbet, P.S.; Williams, M.C.; Gillett, J.D. O’nyong-Nyong Fever: An Epidemic Virus Disease in East Africa. Trans. R. Soc. Trop. Med. Hyg. 1961, 55, 463–480, . [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes Aegypti and Ae. Albopictus. eLife 2015, 4, e08347, . [CrossRef]
- Coelho, G.E. Challenges in the Control of Aedes Aegypti. Rev. Inst. Med. Trop. São Paulo 2012, 54, 13–14, . [CrossRef]
- Ding, F.; Fu, J.; Jiang, D.; Hao, M.; Lin, G. Mapping the Spatial Distribution of Aedes Aegypti and Aedes Albopictus. Acta Trop. 2018, 178, 155–162, . [CrossRef]
- Kotsakiozi, P.; Gloria-Soria, A.; Caccone, A.; Evans, B.; Schama, R.; Martins, A.J.; Powell, J.R. Tracking the Return of Aedes Aegypti to Brazil, the Major Vector of the Dengue, Chikungunya and Zika Viruses. PLoS Negl. Trop. Dis. 2017, 11, e0005653, . [CrossRef]
- Rodrigues, S.; Oliva, O.; Araujo, F.; Martins, L.; Chiang, J.; Henriques, D.; Silva, E.; Rodrigues, D.; Prazeres, A.; Tavares-Neto, J.; et al. Epidemiology of Saint Louis Encephalitis Virus in the Brazilian Amazon Region and in the State of Mato Grosso Do Sul, Brazil: Elevated Prevalence of Antibodies in Horses. Rev. Pan-Amaz. Saúde 2010, 1, . [CrossRef]
- Corrêa, A.P. Investigação para a circulação do vírus do oeste do Nilo e outros flavivírus no Pantanal de Mato Grosso do Sul. PhD thesis, Instituto Oswaldo Cruz: Rio de Janeiro, Rio de Janeiro State, Brazil, 2012.
- Pauvolid-Corrêa, A.; Campos, Z.; Juliano, R.; Velez, J.; Nogueira, R.M.R.; Komar, N. Serological Evidence of Widespread Circulation of West Nile Virus and Other Flaviviruses in Equines of the Pantanal, Brazil. PLoS Negl. Trop. Dis. 2014, 8, e2706, . [CrossRef]
- de Oliveira-Filho, E.F.; Fischer, C.; Berneck, B.S.; Carneiro, I.O.; Kühne, A.; de Almeida Campos, A.C.; Ribas, J.R.L.; Netto, E.M.; Franke, C.R.; Ulbert, S.; et al. Ecologic Determinants of West Nile Virus Seroprevalence among Equids, Brazil. Emerg. Infect. Dis. 2021, 27, 2466–2470, . [CrossRef]
- Casseb, A.R.; Cruz, A.V.; Jesus, I.S.; Chiang, J.O.; Martins, L.C.; Silva, S.P.; Henriques, D.F.; Casseb, L.M.; Vasconcelos, P.F.C. Seroprevalence of Flaviviruses Antibodies in Water Buffaloes (Bubalus Bubalis) in Brazilian Amazon. J. Venom. Anim. Toxins Trop. Dis. 2014, 20, 9, . [CrossRef]
- Batista, P.M.; Andreotti, R.; Almeida, P.S. de; Marques, A.C.; Rodrigues, S.G.; Chiang, J.O.; Vasconcelos, P.F. da C. Detection of Arboviruses of Public Health Interest in Free-Living New World Primates (Sapajus Spp.; Alouatta Caraya) Captured in Mato Grosso Do Sul, Brazil. Rev. Soc. Bras. Med. Trop. 2013, 46, 684–690, . [CrossRef]
- Mueller, C.G.; Cao-Lormeau, V.-M. Chapter 8—Insect-Borne Viruses and Host Skin Interface. In Skin and Arthropod Vectors; Boulanger, N., Ed.; Academic Press, 2018; pp. 275–292 ISBN 978-0-12-811436-0.
- Batista, W.C. Mapeamento de arboviroses no Estado de Rondônia. PhD thesis, Universidade Federal do Amazonas: Manaus—Amazonas State. Brazil, 2007.
- Batista, W.C.; Tavares, G. da S.B.; Vieira, D.S.; Honda, E.R.; Pereira, S.S.; Tada, M.S. Notification of the First Isolation of Cacipacore Virus in a Human in the State of Rondônia, Brazil. Rev. Soc. Bras. Med. Trop. 2011, 44, 528–530, . [CrossRef]
- Monteiro, H.A.O. Avaliação da diversidade de insetos hematófagos da subordem nematocera e de vertebrados silvestres: transmissão de arbovírus na área de influência do projeto salobo, carajás e Pará. Masters dissertation, Universidade Federal do Pará: Instituto Evandro Chagas, Belém—Pará State, 2009.
- Araújo, F.A.A. Inquéritos Sorológicos Em Equídeos E Aves Silvestres Para Detecção De Anticorpos Anti- Arbovírus De Importância Em Saúde Pública No Brasil. PhD thesis, Universidade Federal de Goiás: Goiânia, Goiás State. Brazil, 2011.
- Weaver, S.C.; Reisen, W.K. Present and Future Arboviral Threats. Antiviral Res. 2010, 85, 328, . [CrossRef]
- Almeida, M.A.B. de; Santos, E. dos; Cardoso, J. da C.; Noll, C.A.; Lima, M. de M.; Silva, F. de A. e; Ferreira, M.S.; Martins, L.C.; Vasconcelos, P.F. da C.; Bicca-Marques, J.C. Detection of Antibodies against Icoaraci, Ilhéus, and Saint Louis Encephalitis Arboviruses during Yellow Fever Monitoring Surveillance in Non-Human Primates (Alouatta Caraya) in Southern Brazil. J. Med. Primatol. 2019, 48, 211–217, . [CrossRef]
- Figueiredo, L.T.; Batista, W.C.; Kashima, S.; Nassar, E.S. Identification of Brazilian Flaviviruses by a Simplified Reverse Transcription-Polymerase Chain Reaction Method Using Flavivirus Universal Primers. Am. J. Trop. Med. Hyg. 1998, 59, 357–362, . [CrossRef]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831, . [CrossRef]
- Tavares, M.; da Silva, M.R.M.; de Oliveira de Siqueira, L.B.; Rodrigues, R.A.S.; Bodjolle-d’Almeida, L.; dos Santos, E.P.; Ricci-Júnior, E. Trends in Insect Repellent Formulations: A Review. Int. J. Pharm. 2018, 539, 190–209, . [CrossRef]
- Prevent Mosquito Bites | Mosquitoes | CDC Available online: https://www.cdc.gov/mosquitoes/mosquito-bites/prevent-mosquito-bites.html (accessed on 3 December 2023).
- Chan, E.Y.Y.; Sham, T.S.T.; Shahzada, T.S.; Dubois, C.; Huang, Z.; Liu, S.; Hung, K.K.C.; Tse, S.L.A.; Kwok, K.O.; Chung, P.-H.; et al. Narrative Review on Health-EDRM Primary Prevention Measures for Vector-Borne Diseases. Int. J. Environ. Res. Public. Health 2020, 17, 5981, . [CrossRef]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33, 10.1128/cmr.00083-18, . [CrossRef]
- Costa, G.B.; Smithyman, R.; O’Neill, S.L.; Moreira, L.A. How to Engage Communities on a Large Scale? Lessons from World Mosquito Program in Rio de Janeiro, Brazil. Gates Open Res. 2021, 4, 109, . [CrossRef]
- Evans, M.V.; Dallas, T.A.; Han, B.A.; Murdock, C.C.; Drake, J.M. Data-Driven Identification of Potential Zika Virus Vectors. eLife 2017, 6, e22053, . [CrossRef]
- Han, B.A.; O’Regan, S.M.; Paul Schmidt, J.; Drake, J.M. Integrating Data Mining and Transmission Theory in the Ecology of Infectious Diseases. Ecol. Lett. 2020, 23, 1178–1188, . [CrossRef]
- Saivish, M.V.; Gomes da Costa, V.; de Lima Menezes, G.; Alves da Silva, R.; Dutra da Silva, G.C.; Moreli, M.L.; Sacchetto, L.; Pacca, C.C.; Vasilakis, N.; Nogueira, M.L. Rocio Virus: An Updated View on an Elusive Flavivirus. Viruses 2021, 13, 2293, . [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





