Submitted:
03 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Frankincense (Boswellia Serrata Extract)
2.2. Bacterial Strains, Human Oral Cells and Growth Conditions
2.3. Minimum Inhibitory Concentration (MIC)
2.4. Biofilm Formation Assay
2.5. Biofilm Reduction Assay
2.6. Lactate Dehydrogenase Quantification
2.7. Antibiotic Protection Assay
2.8. Immunostaining for P. gingivalis
2.9. Statistical Analysis
3. Results
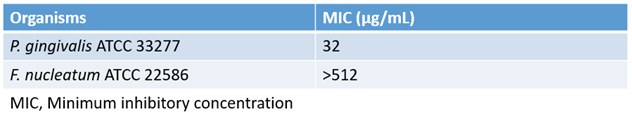
3.1. B. serrata Extract Differentially Impacts the Growth of P. gingivalis and F. nucleatum
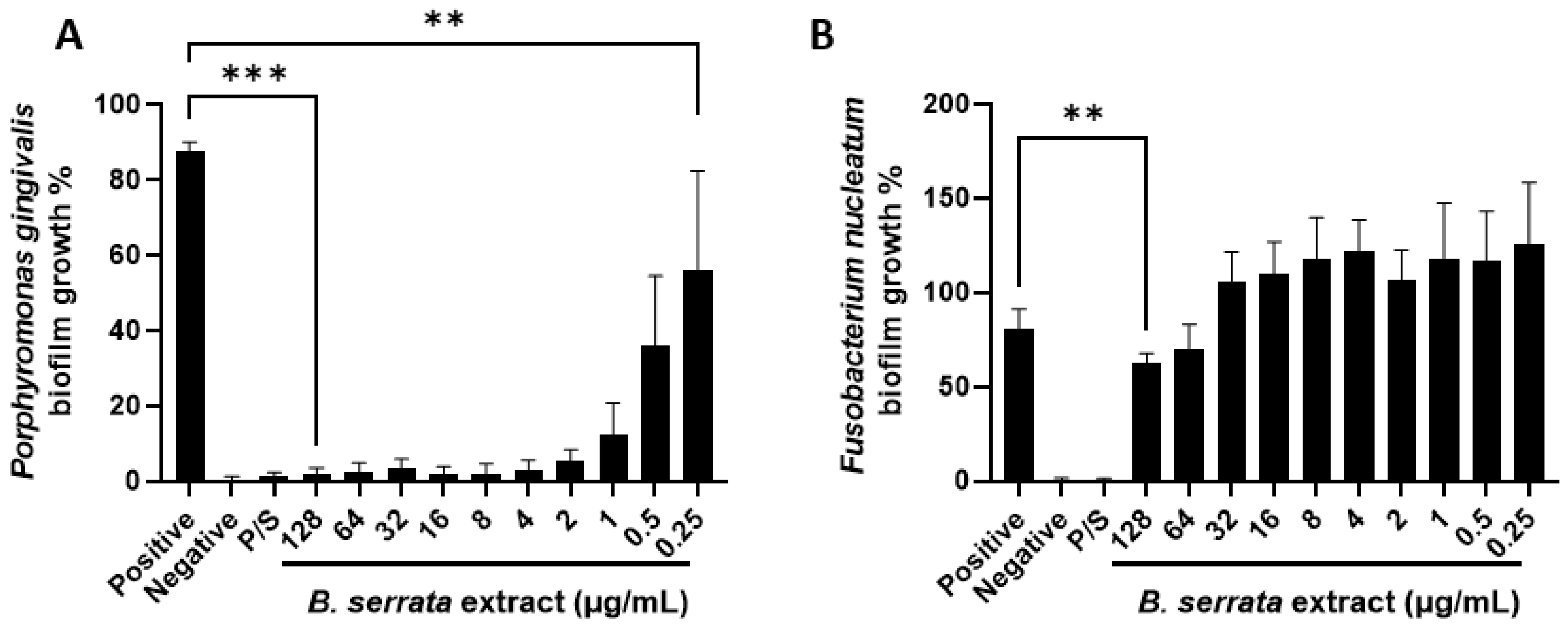
3.2. B. serrata Extract Inhibits P. gingivalis and F. nucleatum Biofilm Formation
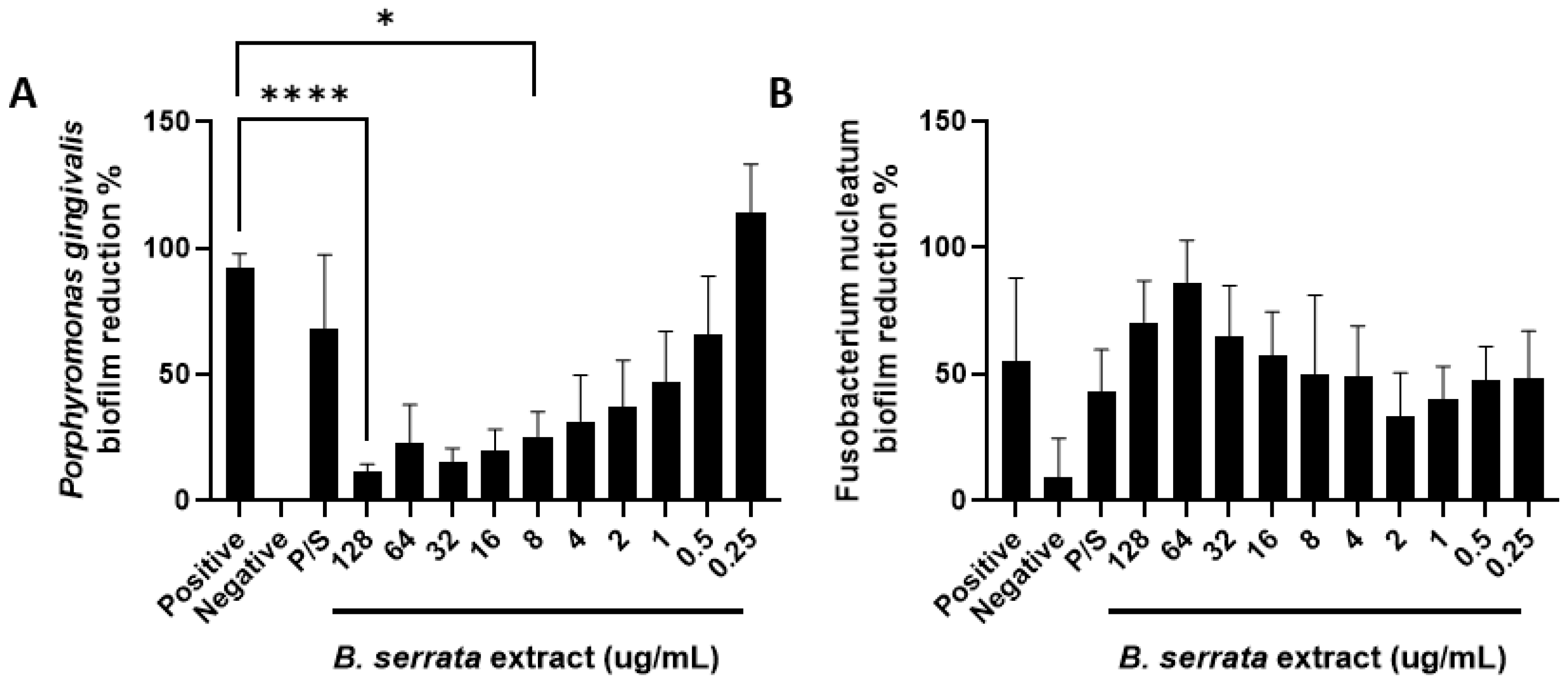
3.3. B. serrata Extract Induces P. gingivalis Biofilm Reduction
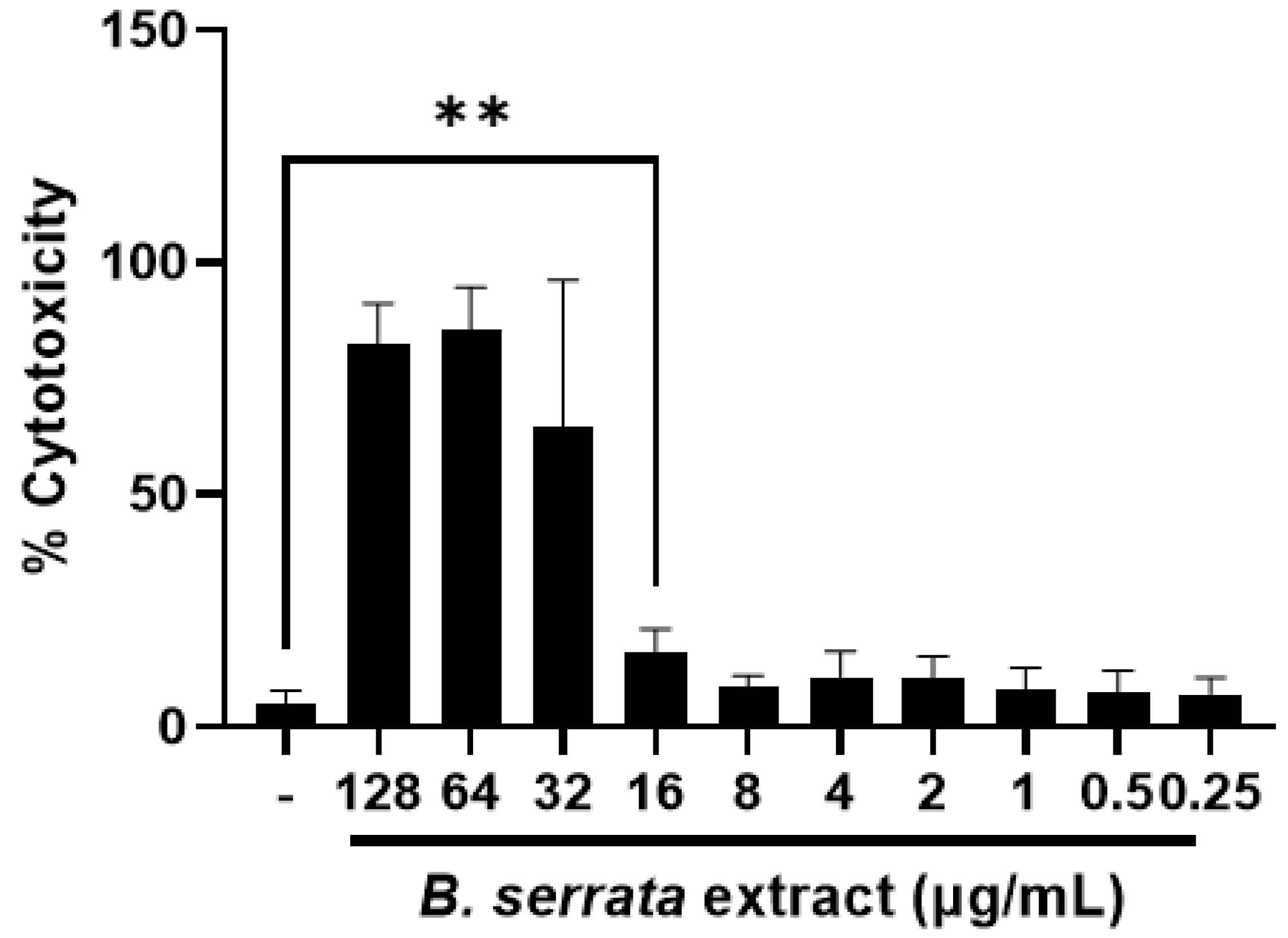
3.4. B. serrata Extract at Low Doses Is Not Toxic to Human Gingival Epithelial Cells
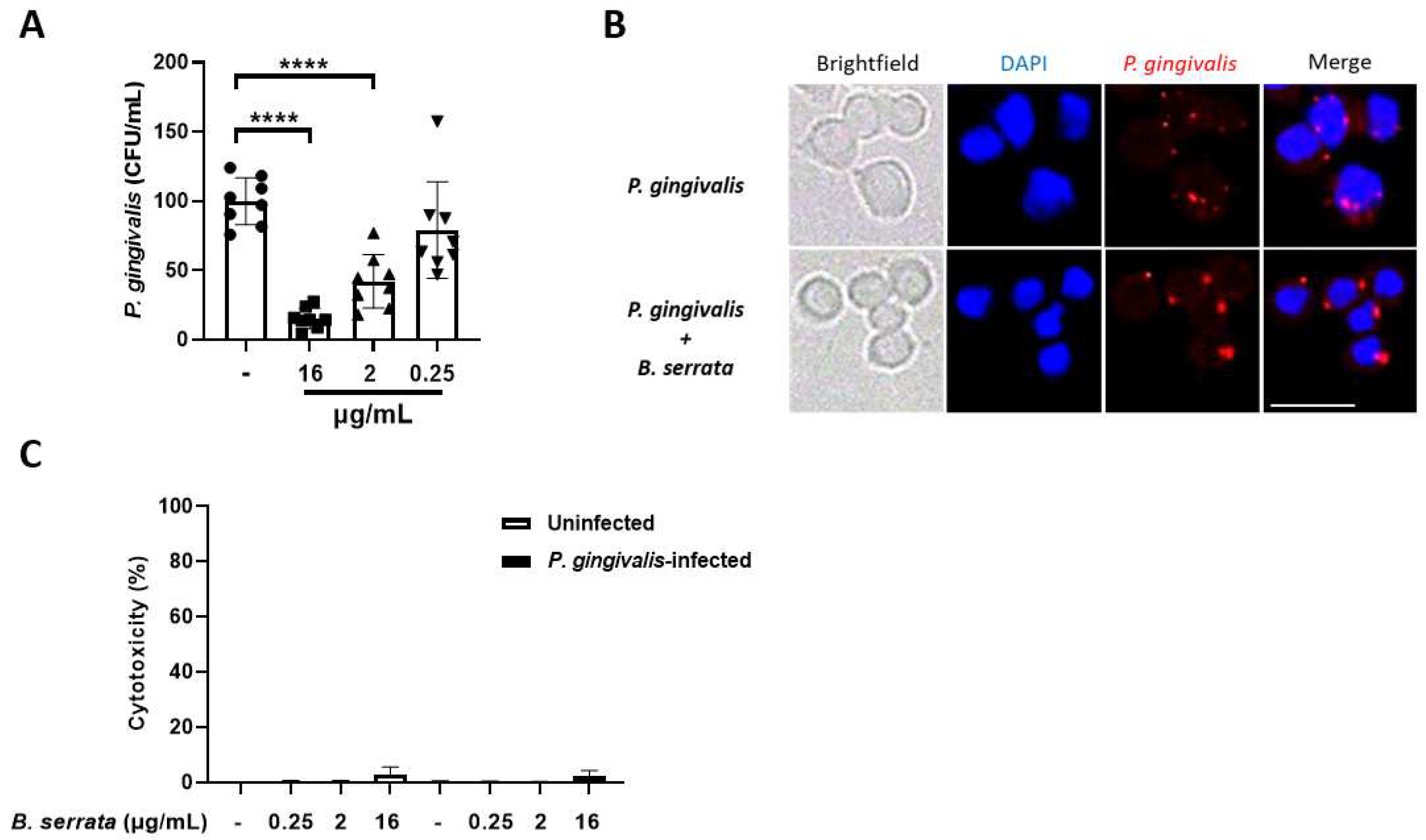
3.5. B. serrata Extract Decreases Intracellular P. gingivalis Load in Human Gingival Epithelial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miethke, M., et al., Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem, 2021. 5(10): p. 726-749. [CrossRef]
- Gasmi, A., et al., Natural Ingredients to Improve Immunity. Pharmaceuticals (Basel), 2023. 16(4). [CrossRef]
- Nazir, M.A., Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim), 2017. 11(2): p. 72-80.
- Almeida-da-Silva, C.L.C., et al., Effects of Frankincense Compounds on Infection, Inflammation, and Oral Health. Molecules, 2022. 27(13). [CrossRef]
- Sanz, M., et al., Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol, 2020. 47(3): p. 268-288. [CrossRef]
- Sugawara, S., et al., Proteolysis of human monocyte CD14 by cysteine proteinases (gingipains) from Porphyromonas gingivalis leading to lipopolysaccharide hyporesponsiveness. J Immunol, 2000. 165(1): p. 411-8. [CrossRef]
- Shahoumi, L.A., M.H.A. Saleh, and M.M. Meghil, Virulence Factors of the Periodontal Pathogens: Tools to Evade the Host Immune Response and Promote Carcinogenesis. Microorganisms, 2023. 11(1). [CrossRef]
- Al-Yasiry, A.R. and B. Kiczorowska, Frankincense--therapeutic properties. Postepy Hig Med Dosw (Online), 2016. 70: p. 380-91. [CrossRef]
- Hajishengallis, G., R.P. Darveau, and M.A. Curtis, The keystone-pathogen hypothesis. Nat Rev Microbiol, 2012. 10(10): p. 717-25. [CrossRef]
- Efferth, T. and F. Oesch, Anti-inflammatory and anti-cancer activities of frankincense: Targets, treatments and toxicities. Semin Cancer Biol, 2020. [CrossRef]
- Bui, F.Q., et al., Association between periodontal pathogens and systemic disease. Biomed J, 2019. 42(1): p. 27-35. [CrossRef]
- Socransky, S.S., et al., Microbial complexes in subgingival plaque. Journal of clinical periodontology, 1998. 25(2): p. 134-44. [CrossRef]
- de Andrade, K.Q., C.L.C. Almeida-da-Silva, and R. Coutinho-Silva, Immunological Pathways Triggered by Porphyromonas gingivalis and Fusobacterium nucleatum: Therapeutic Possibilities? Mediators Inflamm, 2019. 2019: p. 7241312. [CrossRef]
- Hajishengallis, G., Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect, 2009. 11(6-7): p. 637-45. [CrossRef]
- Hajishengallis, G., Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol, 2015. 15(1): p. 30-44. [CrossRef]
- Makkawi, H., et al., Porphyromonas gingivalis Stimulates TLR2-PI3K Signaling to Escape Immune Clearance and Induce Bone Resorption Independently of MyD88. Front Cell Infect Microbiol, 2017. 7: p. 359. [CrossRef]
- Hung, S.C., et al., NLRX1 modulates differentially NLRP3 inflammasome activation and NF-kappaB signaling during Fusobacterium nucleatum infection. Microbes Infect, 2018. 20(9-10): p. 615-625. [CrossRef]
- Yilmaz, O., et al., Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infec. Immun., 2004. 72: p. 3743-3751. [CrossRef]
- Yilmaz, O., et al., Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect. Immun., 2006. 74: p. 703-710. [CrossRef]
- Yilmaz, O., et al., ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X(7)-mediated host-cell apoptosis. Cell. Microbiol., 2008. 10(): p. 863-875. [CrossRef]
- Johnson, L., et al., Porphyromonas gingivalis attenuates ATP-mediated inflammasome activation and HMGB1 release through expression of a nucleoside-diphosphate kinase. Microbes Infect, 2015. 17(5): p. 369-77. [CrossRef]
- Lee, K., et al., Porphyromonas gingivalis traffics into endoplasmic reticulum-rich-autophagosomes for successful survival in human gingival epithelial cells. Virulence, 2018. 9(1): p. 845-859. [CrossRef]
- Bui, F.Q., et al., Fusobacterium nucleatum infection of gingival epithelial cells leads to NLRP3 inflammasome-dependent secretion of IL-1β and the danger signals ASC and HMGB1. Cellular Microbiology, 2016. 18: p. 970-981. [CrossRef]
- De Andrade, K.Q., et al., Differential involvement of the canonical and noncanonical inflammasomes in the immune response against infection by the periodontal bacteria Porphyromonas gingivalis and Fusobacterium nucleatum. Curr Res Microb Sci, 2021. 2: p. 100023. [CrossRef]
- Sharma, S., et al., Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms, 2023. 11(6). [CrossRef]
- Oluwole, O.M., Biofilm: Formation and Natural Products’ Approach to Control - a Review. Afr J Infect Dis, 2022. 16(2 Suppl): p. 59-71.
- Siddiqui, M.Z., Boswellia serrata, a potential antiinflammatory agent: an overview. Indian J Pharm Sci, 2011. 73(3): p. 255-61.
- Khan, A., et al., Anti-diabetic potential of beta-boswellic acid and 11-keto-beta-boswellic acid: Mechanistic insights from computational and biochemical approaches. Biomed Pharmacother, 2022. 147: p. 112669. [CrossRef]
- Hussain, H., et al., Frankincense diterpenes as a bio-source for drug discovery. Expert Opin Drug Discov, 2022. 17(5): p. 513-529. [CrossRef]
- Efferth, T. and F. Oesch, Anti-inflammatory and anti-cancer activities of frankincense: Targets, treatments and toxicities. Semin Cancer Biol, 2022. 80: p. 39-57. [CrossRef]
- Hajishengallis, G., et al., Intracellular signaling and cytokine induction upon interactions of Porphyromonas gingivalis fimbriae with pattern-recognition receptors. Immunol. Invest., 2004. 33: p. 157-172. [CrossRef]
- Eskan, M.A., G. Hajishengallis, and D.F. Kinane, Differential activation of human gingival epithelial cells and monocytes by Porphyromonas gingivalis fimbriae. Infect. Immun., 2007. 75. [CrossRef]
- Ramos-Junior, E.S., et al., A Dual Role for P2X7 Receptor during Porphyromonas gingivalis Infection. Journal of Dental Research, 2015. 94(9): p. 1233-1242. [CrossRef]
- Almeida-da-Silva, C.L.C., et al., P2X7 receptor-mediated leukocyte recruitment and Porphyromonas gingivalis clearance requires IL-1beta production and autocrine IL-1 receptor activation. Immunobiology, 2019. 224(1): p. 50-59. [CrossRef]
- Nakhjiri, S.F., et al., Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol Lett, 2001. 200(2): p. 145-9. [CrossRef]
- Yilmaz, O., et al., Gingival epithelial cell signaling and cytoskeletal responses to Porphyromonas gingivalis invasion. Microbiology, 2003. 149: p. 2417-2426. [CrossRef]
- Avila, M., D.M. Ojcius, and O. Yilmaz, The oral microbiota: living with a permanent guest. DNA Cell Biol, 2009. 28(8): p. 405-11. [CrossRef]
- Yilmaz, O., et al., ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell. Microbiol., 2010. 28: p. 243-255. [CrossRef]
- Taxman, D.J., et al., Porphyromonas gingivalis mediates inflammasome repression in polymicrobial cultures through a novel mechanism involving reduced endocytosis. J Biol Chem, 2012. 287(39): p. 32791-9. [CrossRef]
- Vahabi, S., M. Hakemi-Vala, and S. Gholami, In vitro Antibacterial Effect of Hydroalcoholic Extract of Lawsonia inermis, Malva sylvestris, and Boswellia serrata on Aggregatibacter actinomycetemcomitans. Adv Biomed Res, 2019. 8: p. 22. [CrossRef]
- Attallah, N.G.M., et al., Antibacterial Activity of Boswellia sacra Flueck. Oleoresin Extract against Porphyromonas gingivalis Periodontal Pathogen. Antibiotics (Basel), 2021. 10(7). [CrossRef]
- Raja, A.F., et al., Acetyl-11-keto-beta-boswellic acid (AKBA); targeting oral cavity pathogens. BMC Res Notes, 2011. 4: p. 406. [CrossRef]
- Perez-Pinero, S., et al., Efficacy of Boswellia serrata Extract and/or an Omega-3-Based Product for Improving Pain and Function in People Older Than 40 Years with Persistent Knee Pain: A Randomized Double-Blind Controlled Clinical Trial. Nutrients, 2023. 15(17). [CrossRef]
- Mohsenzadeh, A., et al., Evaluation of the effectiveness of topical oily solution containing frankincense extract in the treatment of knee osteoarthritis: a randomized, double-blind, placebo-controlled clinical trial. BMC Res Notes, 2023. 16(1): p. 28. [CrossRef]
- Kachouei, R.A., et al., Acetyl-11-Keto-Beta-Boswellic Acid Has Therapeutic Benefits for NAFLD Rat Models That Were Given a High Fructose Diet by Ameliorating Hepatic Inflammation and Lipid Metabolism. Inflammation, 2023. 46(5): p. 1966-1980. [CrossRef]
- Alkanat, H.O., U. Ozdemir, and F. Kulakli, The effects of massage with frankincense and myrrh oil in chronic low back pain: A three-arm randomised controlled trial. Explore (NY), 2023. 19(5): p. 761-767. [CrossRef]
- Talebi Ardakani, M., et al., Effect of an herbal mouthwash on periodontal indices in patients with plaque-induced gingivitis: A cross-over clinical trial. J Adv Periodontol Implant Dent, 2022. 14(2): p. 109-113. [CrossRef]
- Khoshbakht, Z., et al., Evaluation of Herbal Mouthwashes Containing Zataria Multiflora Boiss, Frankincense and Combination Therapy on Patients with Gingivitis: A Double-Blind, Randomized, Controlled, Clinical Trial. Galen Med J, 2019. 8: p. e1366. [CrossRef]
- Khosravi Samani, M., et al., The effect of Frankincense in the treatment of moderate plaque-induced gingivitis: a double blinded randomized clinical trial. Daru, 2011. 19(4): p. 288-94.
- Almeida-da-Silva, C.L.C., et al., Cigarette Smoke Stimulates SARS-CoV-2 Internalization by Activating AhR and Increasing ACE2 Expression in Human Gingival Epithelial Cells. Int J Mol Sci, 2021. 22(14). [CrossRef]
- Abdolhosseini, M., et al., Lysine substitutions convert a bacterial-agglutinating peptide into a bactericidal peptide that retains anti-lipopolysaccharide activity and low hemolytic activity. Peptides, 2012. 35(2): p. 231-8. [CrossRef]
- Ben Lagha, A., P. Maquera Huacho, and D. Grenier, A cocoa (Theobroma cacao L.) extract impairs the growth, virulence properties, and inflammatory potential of Fusobacterium nucleatum and improves oral epithelial barrier function. PLoS One, 2021. 16(5): p. e0252029. [CrossRef]
- Kormas, I., et al., Peri-Implant Diseases: Diagnosis, Clinical, Histological, Microbiological Characteristics and Treatment Strategies. A Narrative Review. Antibiotics (Basel), 2020. 9(11). [CrossRef]
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





