Submitted:
03 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population
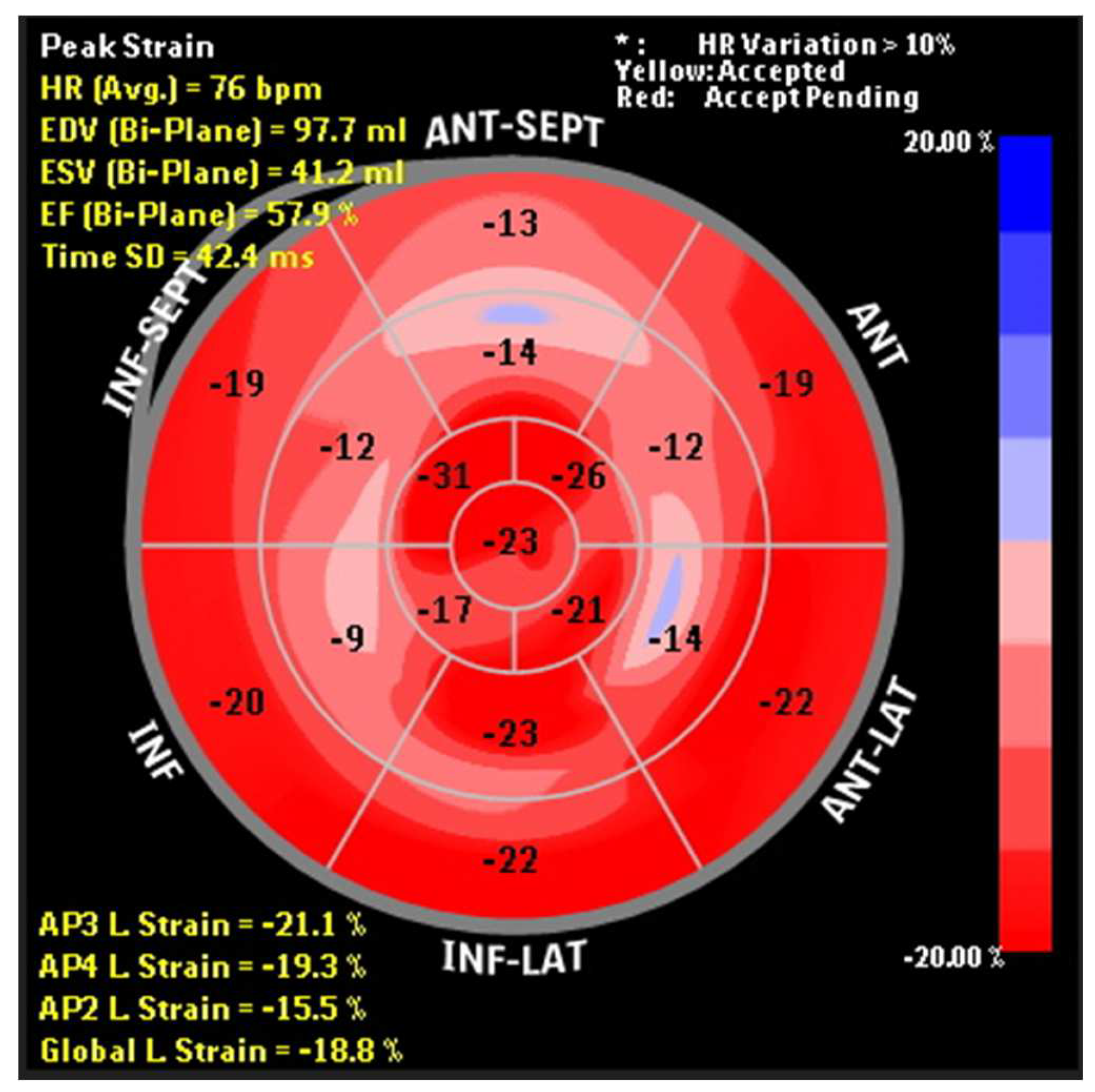
2.2. Analysis of Sistolic and Diastolic Cardiac Function
2.3. Ethics
2.4. Statistical Analysis
2.5. Chemotherapy Protocol and Monitoring
3. Results
4. Discussion
4.1. Limitations of Our Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coza DD, Bucurenci DM. ANALIZA CAZUISTICII REGISTRULUI NATIONAL AL CANCERELOR LA COPIL IN ROMANIA 2010-2017 n.d.:19.
- Cancer in Children and Adolescents—NCI 2021. https://www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet (accessed July 9, 2022).
- Saletta F, Seng MS, Lau LMS. Advances in paediatric cancer treatment. Transl Pediatr 2014;3:156–82. [CrossRef]
- Ruggiero A, Rizzo D, Catalano M, Coccia P, Triarico S, Attiná G. Acute chemotherapy-induced nausea and vomiting in children with cancer: Still waiting for a common consensus on treatment. J Int Med Res 2018;46:2149–56. [CrossRef]
- Ehrhardt MJ, Skinner R, Castellino SM. Renal and Hepatic Health After Childhood Cancer. Pediatr Clin North Am 2020;67:1203–17. [CrossRef]
- Ruggiero A, Skinner R, Khaled Zekri WZ. Editorial: Adverse and Toxic Effects of Childhood Cancer Treatments. Front Oncol 2021;11:795664. [CrossRef]
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–801. [CrossRef]
- Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J 2013;34:1102–11. [CrossRef]
- Murabito A, Hirsch E, Ghigo A. Mechanisms of Anthracycline-Induced Cardiotoxicity: Is Mitochondrial Dysfunction the Answer? Front Cardiovasc Med 2020;7:35. [CrossRef]
- Manrique CR, Park M, Tiwari N, Plana JC, Garcia MJ. Diagnostic Strategies for Early Recognition of Cancer Therapeutics–Related Cardiac Dysfunction. Clin Med Insights Cardiol 2017;11:1179546817697983. [CrossRef]
- Oechsle K, Bokemeyer C. Kardiotoxizitäten bei Chemo- und Radiotherapie. Onkol 2009;15:157–62. [CrossRef]
- Ammon M, Arenja N, Leibundgut G, Buechel RR, Kuster GM, Kaufmann BA, et al. Cardiovascular management of cancer patients with chemotherapy-associated left ventricular systolic dysfunction in real-world clinical practice. J Card Fail 2013;19:629–34. [CrossRef]
- Germanakis I, Anagnostatou N, Kalmanti M. Troponins and natriuretic peptides in the monitoring of anthracycline cardiotoxicity. Pediatr Blood Cancer 2008;51:327–33. [CrossRef]
- Schlitt A, Jordan K, Vordermark D, Schwamborn J, Langer T, Thomssen C. Cardiotoxicity and Oncological Treatments. Dtsch Ärztebl Int 2014;111:161–8. [CrossRef]
- Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 2000;36:517–22. [CrossRef]
- Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 2014;27:911–39. [CrossRef]
- Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol Off J Am Soc Clin Oncol 2010;28:1276–81. [CrossRef]
- Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009;339:b4606. [CrossRef]
- Li VW-Y, So EK-F, Wong WH-S, Cheung Y-F. Myocardial Deformation Imaging by Speckle-Tracking Echocardiography for Assessment of Cardiotoxicity in Children during and after Chemotherapy: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 2022;35:629–56. [CrossRef]
- Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 2010;23:465–95; quiz 576–7. [CrossRef]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [CrossRef]
- Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 2013;61:77–84. [CrossRef]
- Otterstad JE, Froeland G, St John Sutton M, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J 1997;18:507–13. [CrossRef]
- Yoldaş T, Yeşil Ş, Karademir S, Şahin G, Arman Örün U, Doğan V, et al. Evaluation of long-term cardiac side effects of anthracycline chemotherapy by conventional and non-conventional echocardiographic methods in childhood cancer survivors. Cardiol Young 2019;29:904–9. [CrossRef]
- López-Fernández T, Martín García A, Santaballa Beltrán A, Montero Luis Á, García Sanz R, Mazón Ramos P, et al. Cardio-Onco-Hematology in Clinical Practice. Position Paper and Recommendations. Rev Espanola Cardiol Engl Ed 2017;70:474–86. [CrossRef]
- Merkx R, Leerink JM, De Baat EC, Feijen EAM, Kok WEM, Mavinkurve-Groothuis AMC, et al. Asymptomatic systolic dysfunction on contemporary echocardiography in anthracycline-treated long-term childhood cancer survivors: a systematic review. J Cancer Surviv 2022;16:338–52. [CrossRef]
- Ardelean AM, Olariu IC, Isac R, Jurac R, Stolojanu C, Murariu M, et al. Correlation of Speckle-Tracking Echocardiography with Traditional Biomarkers in Predicting Cardiotoxicity among Pediatric Hemato-Oncology Patients: A Comprehensive Evaluation of Anthracycline Dosages and Treatment Protocols. Children 2023;10:1479. [CrossRef]
- Sex-Specific Cardiovascular Risks of Cancer and Its Therapies | Circulation Research n.d. https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.121.319901 (accessed January 13, 2024).
- Female Sex and Higher Drug Dose as Risk Factors for Late Cardiotoxic Effects of Doxorubicin Therapy for Childhood Cancer | NEJM n.d. https://www.nejm.org/doi/full/10.1056/NEJM199506293322602 (accessed January 13, 2024).
- Congestive Heart Failure After Treatment for Wilms’ Tumor: A Report From the National Wilms’ Tumor Study Group | Journal of Clinical Oncology n.d. https://ascopubs.org/doi/10.1200/JCO.2001.19.7.1926 (accessed January 13, 2024).
- Diaz ANR, Hurtado GP, Manzano AAA, Keyes MJ, Turissini C, Choudhary A, et al. Sex Differences in the Development of Anthracycline-Associated Heart Failure. J Card Fail 2023;0. [CrossRef]
- Kouwenberg TW, Van Dalen EC, Feijen EAM, Netea SA, Bolier M, Slieker MG, et al. Acute and early-onset cardiotoxicity in children and adolescents with cancer: a systematic review. BMC Cancer 2023;23:866. [CrossRef]
- Gherbesi E, Bergamaschi L, Cusmano I, Tien TT, Paolisso P, Foà A, et al. The usefulness of speckle tracking echocardiography in identifying subclinical myocardial dysfunction in young adults recovered from mild COVID-19. Echocardiogr Mt Kisco N 2022. [CrossRef]
- Cui C, Zheng Q, Li Y, Huang D, Hu Y, Wang Y, et al. Reference Values of Noninvasive Myocardial Work Indices Measured by Echocardiography in Healthy Children. Front Pediatr 2022;10:792526. [CrossRef]
- Zhan J, Van den Eynde J, Cordrey K, Long R, Danford DA, Hays AG, et al. Deterioration in myocardial work indices precedes changes in global longitudinal strain following anthracycline chemotherapy. Int J Cardiol 2022;363:171–8. [CrossRef]
- Adams MJ, Lipshultz SE. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer 2005;44:600–6. [CrossRef]
- Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2015;16:e123-136. [CrossRef]
- Bansal N, Blanco JG, Sharma UC, Pokharel S, Shisler S, Lipshultz SE. Cardiovascular diseases in survivors of childhood cancer. Cancer Metastasis Rev 2020;39:55–68. [CrossRef]
- Fulbright JM. Review of Cardiotoxicity in Pediatric Cancer Patients: During and after Therapy. Cardiol Res Pract 2011;2011:942090. [CrossRef]
- van der Pal HJ, van Dalen EC, Hauptmann M, Kok WE, Caron HN, van den Bos C, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med 2010;170:1247–55. [CrossRef]
- Amedro P, Vincenti M, Abassi H, Lanot N, De La Villeon G, Guillaumont S, et al. Use of speckle tracking echocardiography to detect late anthracycline-induced cardiotoxicity in childhood cancer: A prospective controlled cross-sectional study. Int J Cardiol 2022;354:75–83. [CrossRef]
- De Caro E, Smeraldi A, Trocchio G, Calevo M, Hanau G, Pongiglione G. Subclinical cardiac dysfunction and exercise performance in childhood cancer survivors. Pediatr Blood Cancer 2011;56:122–6. [CrossRef]
- Serrano JM, González I, Del Castillo S, Muñiz J, Morales LJ, Moreno F, et al. Diastolic Dysfunction Following Anthracycline-Based Chemotherapy in Breast Cancer Patients: Incidence and Predictors. The Oncologist 2015;20:864–72. [CrossRef]
- Wolf CM, Reiner B, Kühn A, Hager A, Müller J, Meierhofer C, et al. Subclinical Cardiac Dysfunction in Childhood Cancer Survivors on 10-Years Follow-Up Correlates With Cumulative Anthracycline Dose and Is Best Detected by Cardiopulmonary Exercise Testing, Circulating Serum Biomarker, Speckle Tracking Echocardiography, and Tissue Doppler Imaging. Front Pediatr 2020;8:123. [CrossRef]
- Velensek V, Mazic U, Krzisnik C, Demšar D, Jazbec J, Jereb B. Cardiac damage after treatment of childhood cancer: A long-term follow-up. BMC Cancer 2008;8:141. [CrossRef]
- Stone JR, Kanneganti R, Abbasi M, Akhtari M. Monitoring for Chemotherapy-Related Cardiotoxicity in the Form of Left Ventricular Systolic Dysfunction: A Review of Current Recommendations. JCO Oncol Pract 2021;17:228–36. [CrossRef]
- Perez IE, Taveras Alam S, Hernandez GA, Sancassani R. Cancer Therapy-Related Cardiac Dysfunction: An Overview for the Clinician. Clin Med Insights Cardiol 2019;13:1179546819866445. [CrossRef]
- Gershwin ME, Goetzl EJ, Steinberg AD. Cyclophosphamide: use in practice. Ann Intern Med 1974;80:531–40. [CrossRef]
- Simbre VC, Duffy SA, Dadlani GH, Miller TL, Lipshultz SE. Cardiotoxicity of cancer chemotherapy: implications for children. Paediatr Drugs 2005;7:187–202. [CrossRef]
- Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 2013;128:1927–95. [CrossRef]
- Alam S, Chandra S, Saran M, Chaudhary G, Sharma A, Bhandhari M, et al. To study the usefulness and comparison of myocardial strain imaging by 2D and 3D echocardiography for early detection of cardiotoxicity in patients undergoing cardiotoxic chemotherapy. Indian Heart J 2019;71:468–75. [CrossRef]
- Onishi T, Fukuda Y, Miyazaki S, Yamada H, Tanaka H, Sakamoto J, et al. Practical guidance for echocardiography for cancer therapeutics-related cardiac dysfunction. J Echocardiogr 2021;19:1–20. [CrossRef]
- Guimarães LC, Fidale TM, Pereira TCR, Lopes PR, Ferreira-Junior MD, Deconte SR, et al. Cardioprotective Effects of Leucine Supplementation against Doxorubicin-Induced Cardiotoxicit. Cardiovasc Toxicol 2024. [CrossRef]
- Armenian SH, Hudson MM, Lindenfeld L, Chen S, Chow EJ, Colan S, et al. Effect of carvedilol versus placebo on cardiac function in anthracycline-exposed survivors of childhood cancer (PREVENT-HF): a randomised, controlled, phase 2b trial. Lancet Oncol 2024:S1470-2045(23)00637-X. [CrossRef]
- Rose-Felker K, Effinger K, Kelleman MS, Sachdeva R, Meacham LR, Border WL. Improving paediatric cardiologists’ awareness about the needs of childhood cancer survivors: results of a single-centre directed educational initiative. Cardiol Young 2019;29:808–12. [CrossRef]
| Patients included (n = 29) |
|
|---|---|
| Age (years) | 8.63 [1,16] |
| Gender (male) | 18 (62%) |
| Body Surface Area (m2) | 1.04 [0.46,1.81] |
| Saturation of oxygen (%) | 98 [98,100] |
| Systolic blood pressure (mmHg) | 94 [84,110] |
| Diastolic blood pressure (mmHg) | 71 [53,82] |
| Heart rate (bpm) | 94 ± 9.20 |
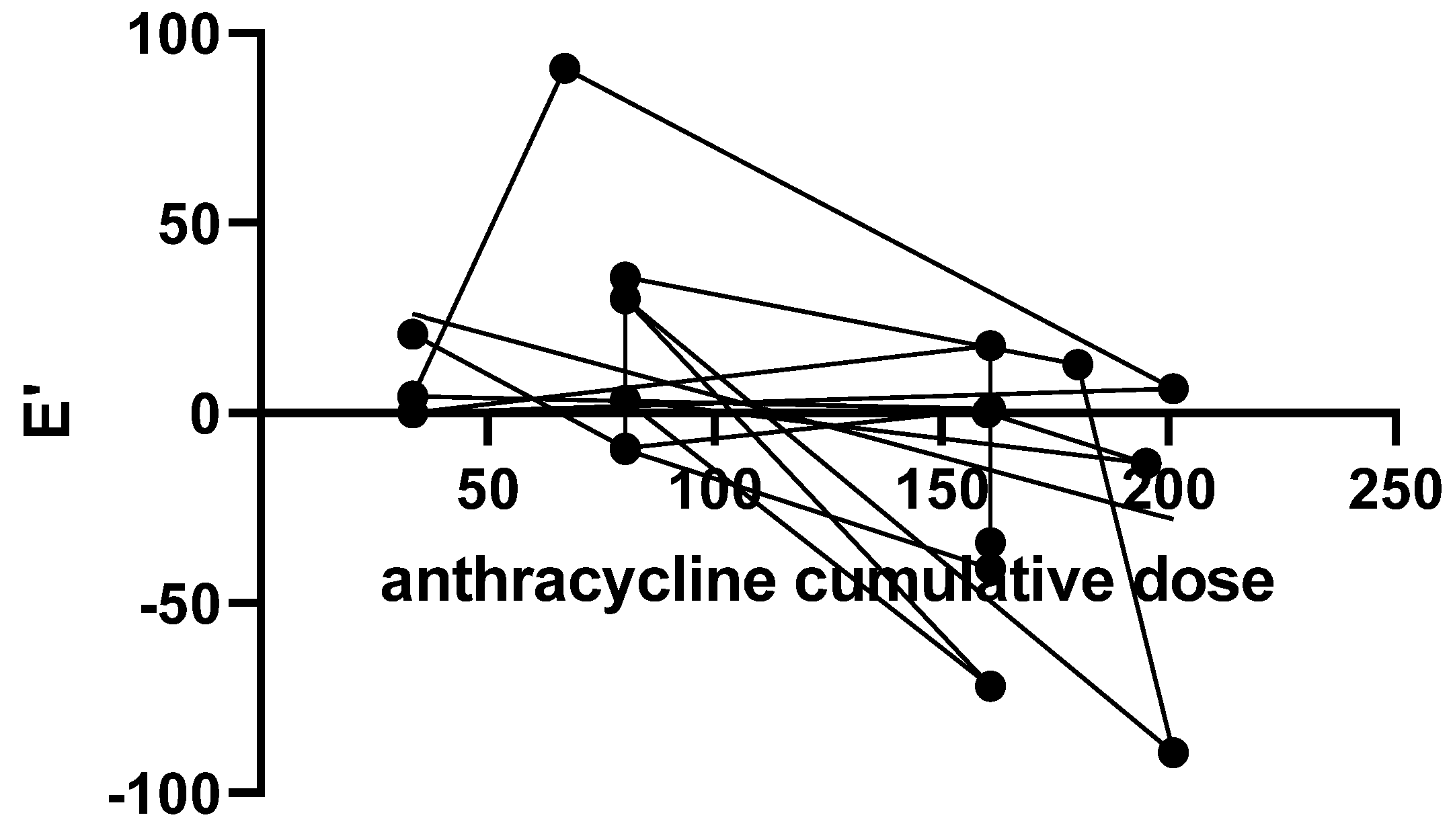
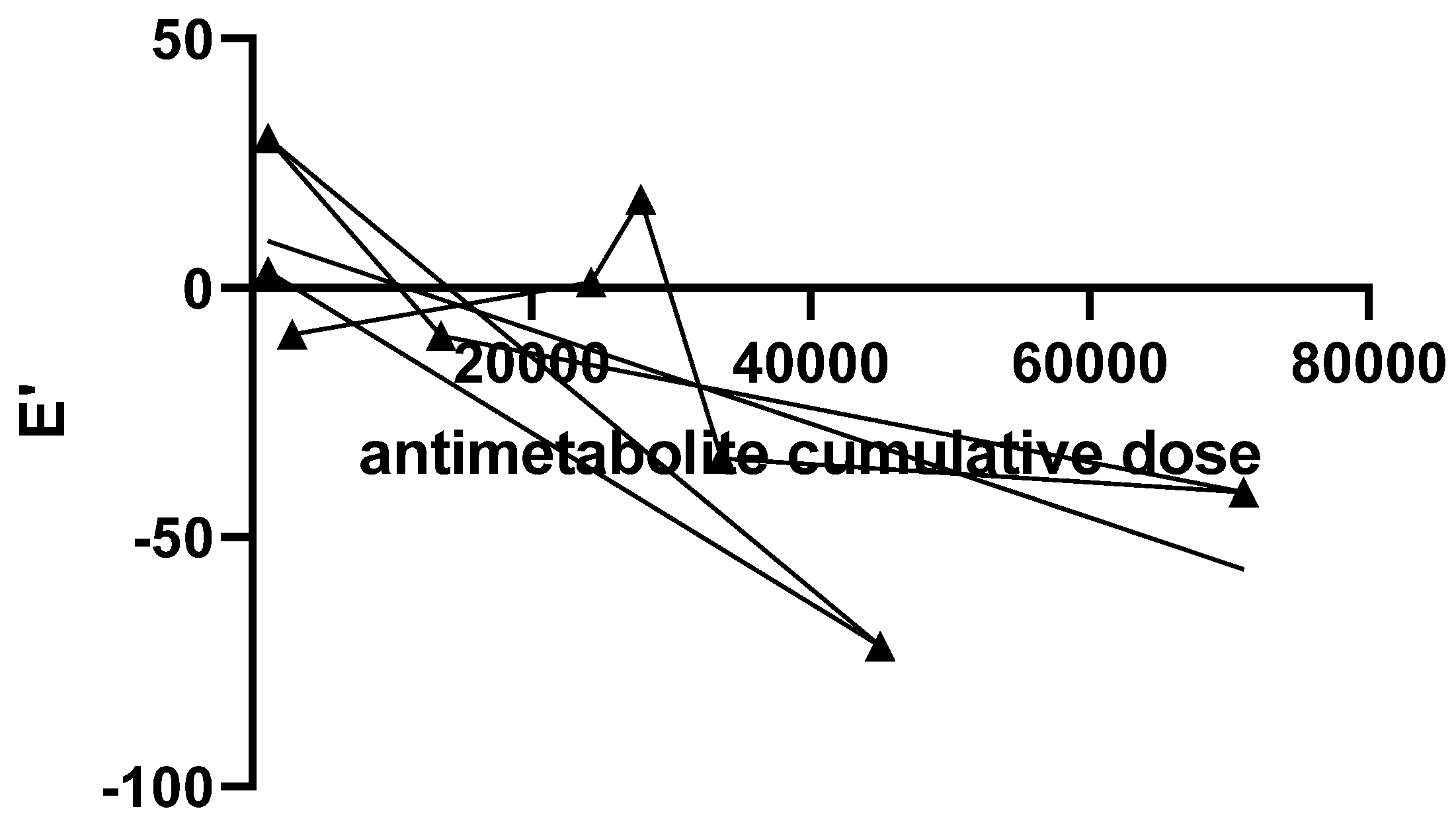
| Cumulative anthracycline dose (mg/m²) | 120 |
| Follow-up time, years | 1 |
| Parameter | Initial evaluation (mean ± SD) |
Second evaluation (mean ± SD) |
P value |
| EF (Teichholz formula) | 61.56 ± 7.39 | 63.29 ± 7.31 | 0.87 |
| EF (modified Simpson method) |
62.21 ± 4.19 | 59.27 ± 7.14 | 0.03 |
| FS | 32.12 ± 5.90 | 29.99 ± 14.10 | 0.79 |
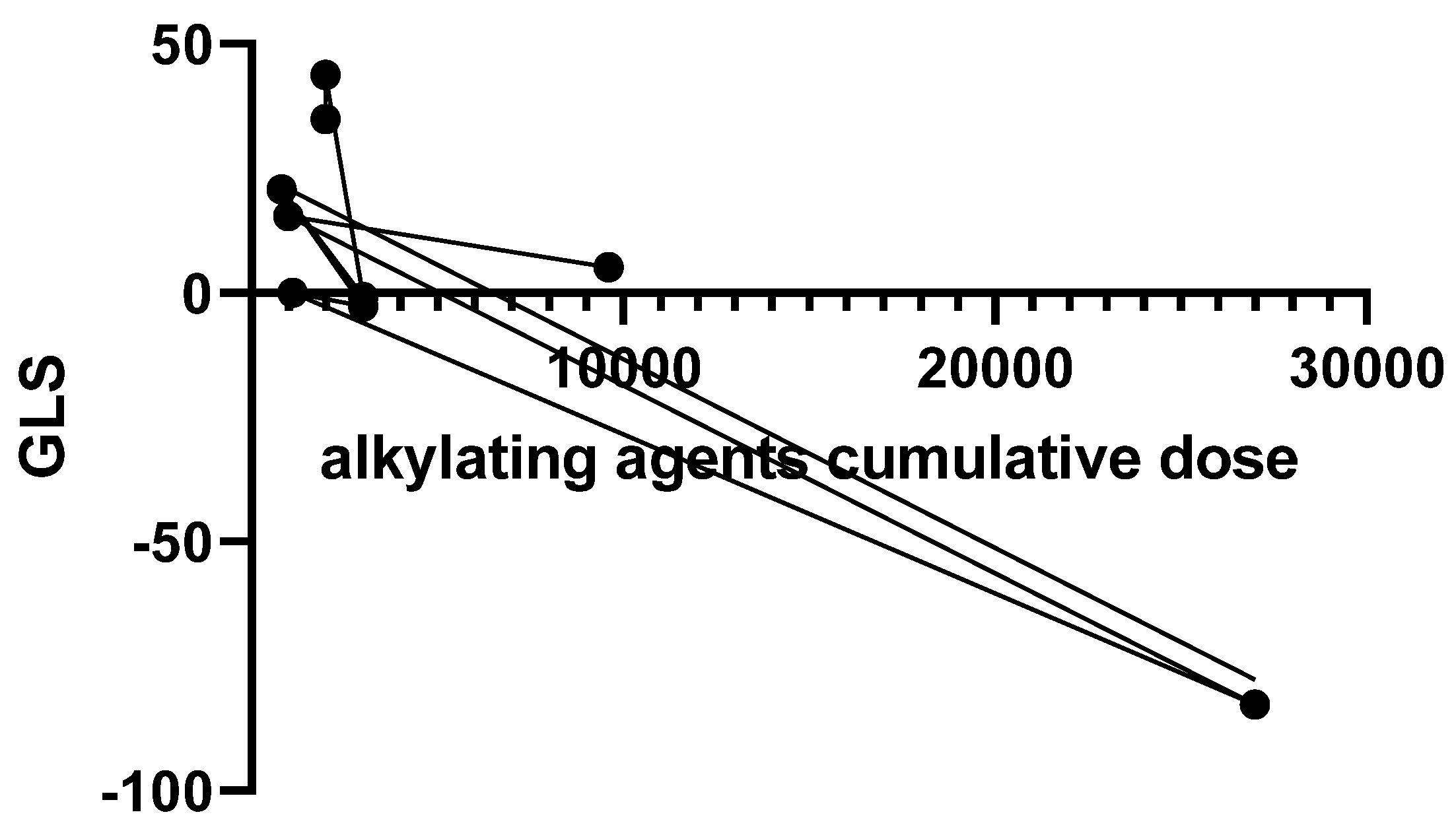
| GLS | -24.01 ± 5.61 | -22.87 ± 4.28 | 0.43 |
| GCS | -24.43 ± 4.47 | -25.23 ± 5.26 | 0.58 |
| AP2LS | -20.84 ± 11.14 | -21.88 ± 10.01 | 0.57 |
| AP3LS | -24.90 ± 5.59 | -23.37 ± 5.67 | 0.36 |
| AP4LS | -21.72 ± 11.76 | -21.87 ± 3.51 | 0.56 |
| MAPSE | 3.43 ± 4.51 | 2.83 ± 3.59 | 0.70 |
| TAPSE | 2.31 ± 1.47 | 1.95 ± 0.28 | 0.31 |
| S wave | 9.62 ± 2.09 | 8.98 ± 2.69 | 0.32 |
| E’ wave | 11.14 ± 3.47 | 10.80 ± 4.21 | 0.72 |
| A’ wave | 7.59 ± 2.03 | 8.17 ± 3.25 | 0.41 |
| E’/A’ | 1.52 ± 0.49 | 1.47 ± 0.69 | 0.36 |
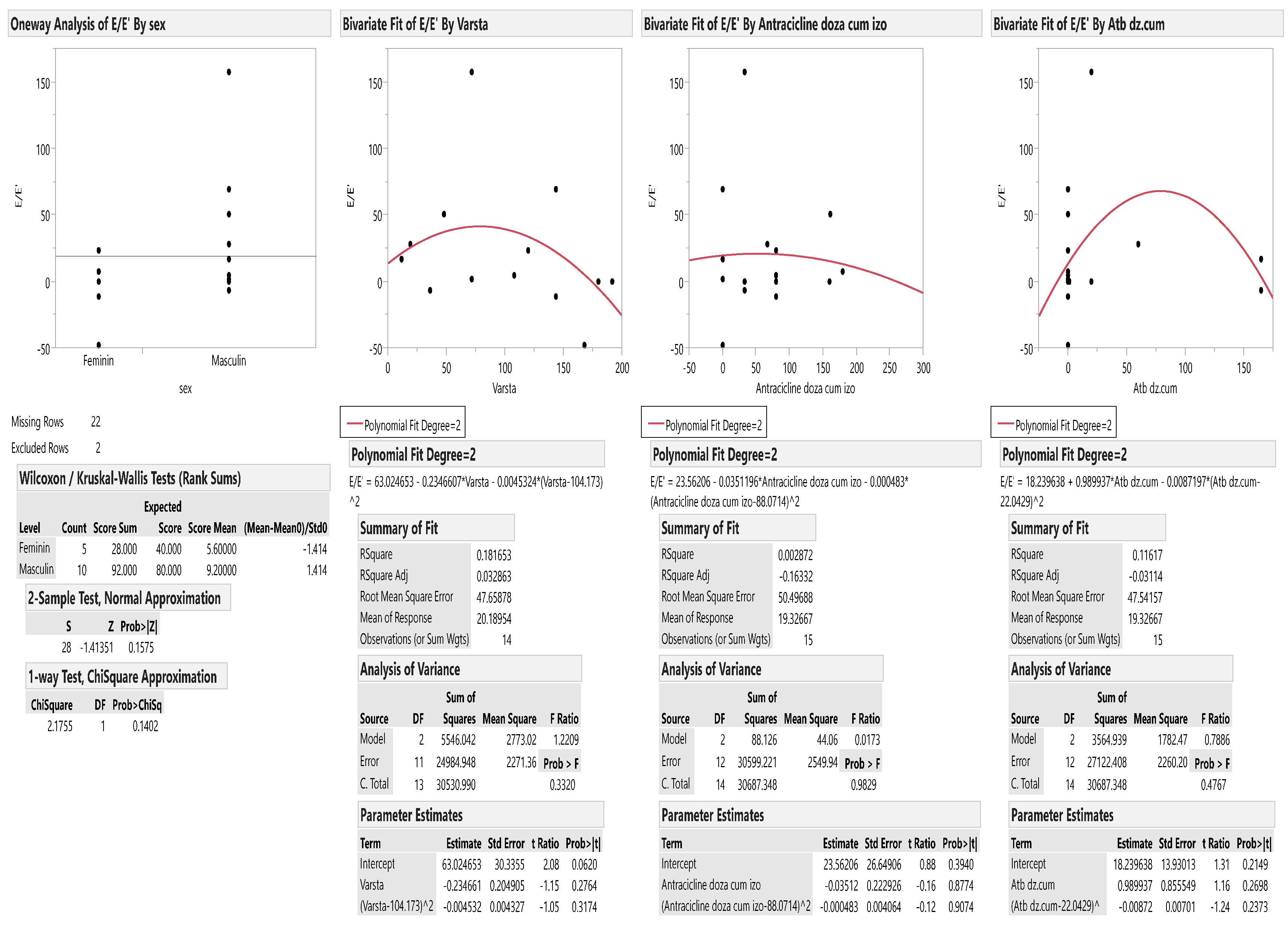
| E/E’ | 7.29 ± 1.92 | 8.27 ± 3.20 | 0.23 |
| SAXA | -24.59 ± 10.36 | -27.08 ± 8.83 | 0.87 |
| SAXB | -23.41 ± 10.73 | -24.49 ± 5.66 | 0.65 |
| SAXM | -20.52 ± 14.88 | -23.05 ± 4.72 | 0.83 |
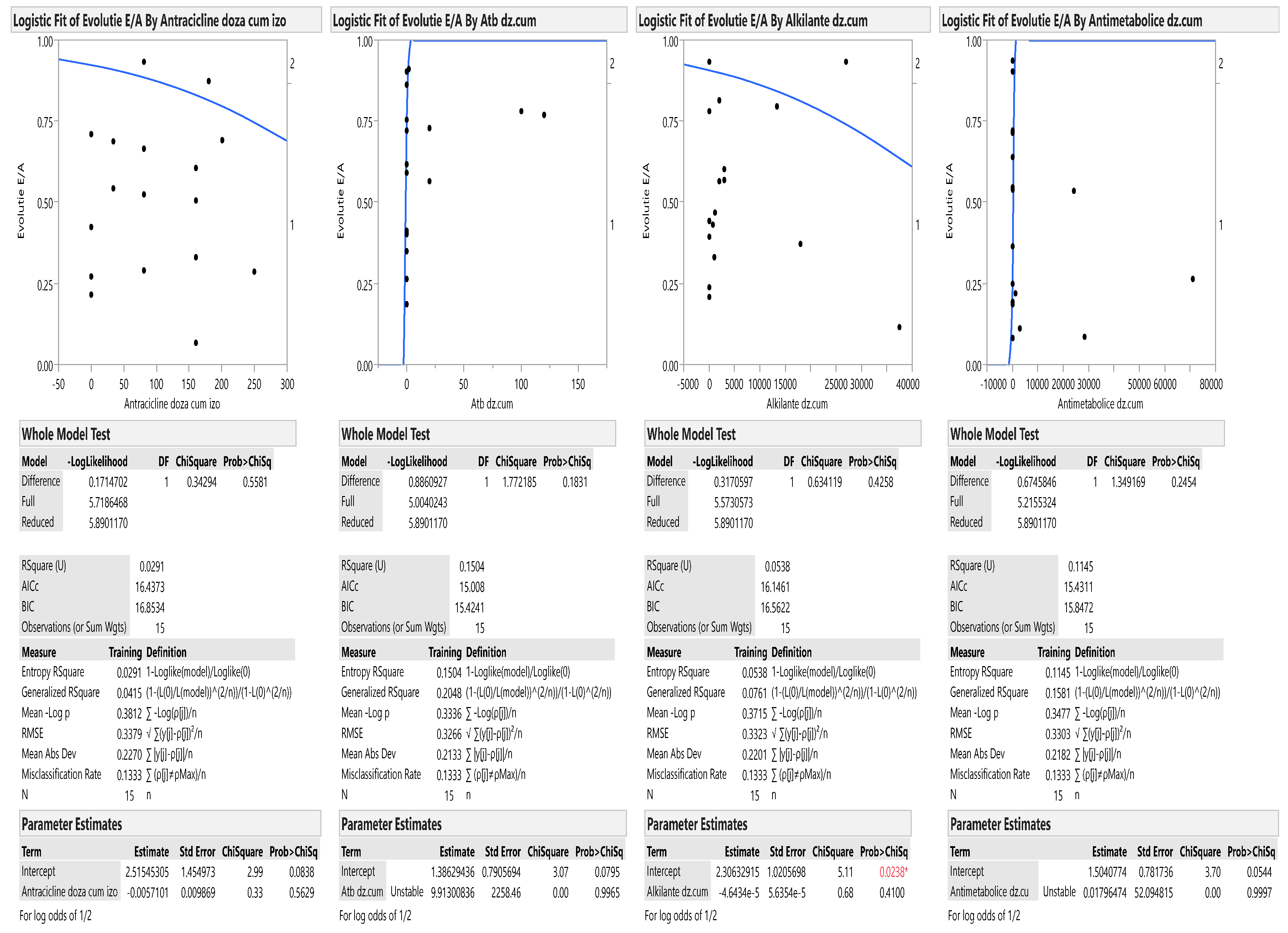
| E/A | 1.34 ± 0.17 | 1.47 ± 0.33 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





