1. Introduction
Septic shock is a subset of sepsis, often a fulminant disease, and affected neonates deteriorate rapidly [
1]. Because neonatal sepsis can present with nonspecific symptoms, there has been a strong emphasis on identifying and treating it early to prevent mortality in this age group. Hence, to optimize outcomes and prevent irreversible multi-organ damage, future interventions will need to be applied early after presentation.
The use of corticosteroids in septic shock remains controversial, but the 2020 Surviving Sepsis Campaign Guidelines suggest using hydrocortisone in infants with septic shock who remain hemodynamically unstable after fluid resuscitation and vasopressor therapy [
2]. Of note, this is listed as a weak recommendation with low-quality evidence, respectively, because of contradictory results in the literature [
3,
4]. The disparity in results from clinical trials may be due to significant differences in when hydrocortisone was administered. The results of an adult study [
5] showed that initiation within 12 hours of treatment in patients receiving hydrocortisone for septic shock was associated with improved survival. Another clinical trial involving 1,241 patients also showed a reduction in mortality among those treated with hydrocortisone within 24 hours of the onset of sepsis [
3]. No benefit was found in patients who started hydrocortisone 24 hours after the onset of septic shock [
6]. These results suggest that patients may benefit from treatment with hydrocortisone early in sepsis. However, the current clinical data are mostly from adult sepsis patients, and whether early treatment with hydrocortisone in children and neonates is equally beneficial, and whether there is an appropriate time window for hydrocortisone administration is still unknown. In addition, the molecular mechanisms responsible for this difference also need to be explored.
Previous study has shown that inhibition of the NF-κB pathway can alleviate inflammation levels in mice with LPS induced sepsis [
7]. The activation of NF-κB is associated with higher mortality, higher nitric oxide levels, and higher levels of pro-inflammatory cytokines in LPS induced sepsis mice [
8]. Hydrocortisone plays a protective role in early septic shock and inhibits NF-κB p65 expression [
9]. Thus, we hypothesized that hydrocortisone allows for improved survival in patients with more severe sepsis or septic shock by decreasing the expression of NF-κB pathway.
Accordingly, we conducted this animal study to determine the impact of the different timing of hydrocortisone administration on the expression of NF-κB pathway and survival rate of newborn mice with sepsis to find the appropriate administration time window for clinical neonatal sepsis patients. Our study reveals initiation of hydrocortisone therapy within 12 h of sepsis onset improves survival, mediated through NF-κB pathway down-regulation. It is of guiding value for the treatment of hydrocortisone in clinical neonatal sepsis.
Materials and Methods
2.1. Reagents
Hydrocortisone sodium succinate for injection was purchased from Tianjin Biochemical Pharmaceutical Co., Ltd.(Tianjin, China). Anti-NF-κB p65 (phospho S536) antibody was purchased from Abcam (Cambridge, MA, USA).
2.2. Mice
Eight pregnant C57BL/6 mice were purchased from Guangdong Medical Laboratory Center (Guangzhou, China) and bred in Guangzhou Jennio Biological Technology Co., Ltd. All subjects were born into the study over 3 days from 8 pregnant mice. All newborn mice were housed under specific pathogen-free conditions at the Guangzhou Jennio Animal Center. All animal experiments were performed according to the guidelines of the Helsinki Declaration of 1975 (revised in 2008) and the Animal Research Committee of the Guangdong Women and Children Hospital (the animal experiment ethics number: No. 202001115).
The study was performed on healthy, 7-day-old newborn mice with a mean weight of 12.5 grams. All experimental procedures were performed by the same operators. Before sepsis modeling, newborn mice were allocated into six groups, using quasi-randomization based on a list of experimental animals available, as follows: 1. Treatment group: standard treatment and injected intraperitoneally (IP) with hydrocortisone (2mg/kg, once); 2. Control group: standard treatment. Based on the initiation of hydrocortisone therapy at various time points, Treatment groups were then assigned to subgroups of five-time points: one hour (1 h group), six hours (6 h group), 12 h (12 h group), 24 h (24 h group) and 48 h (48 h group) of hydrocortisone administration. Physiological data were analyzed at baseline, 1 h, 6 h, 12 h, 24 h and 48 h and the mice were sacrificed 72 h after the intraperitoneal injection of LPS 0.25mg/kg once by the same researchers. Researchers could not be blinded to the treated group, due to the need to deliver hydrocortisone to the treated group. The survival rates of the mice were monitored every day until 72 h after dosing, and mice were sacrificed by cervical dislocation, followed by cardiac blood collection and centrifugation to collect serum and heart and lung tissue for further experiments.
2.3. Western immunoblotting
Collected heart and lung tissues were embedded in TissueTek OCT compound (SAKURA Finetek, Tokyo) and snap frozen in liquid nitrogen. Heart and lung tissues were collected for western blotting analysis to relative expression levels of phosphorylated NF-κB p65. The frozen tissues are ground into powder and resuspended in lysis buffer. Protein concentrations were determined with the Protein assay kit. Lysates were analyzed by SDS/PAGE and immunoblotted. Primary antibodies used include the following: NF-κB (P-P65) (Abcam, 1:1000), GAPDH (Shanghai Kangcheng Biologics, 1:10000). secondary antibody dilution ratio: 1:10000.
2.4. Meso Scale Diagnostics
Blood was obtained by cardiac puncture, and sera were prepared by centrifugation of blood at 3000 rpm for 20 min. Pro-inflammatory factors (TNF-α and IL-6) were measured using electrochemiluminescent assay (Meso Scale Diagnostics).
2.5. Statistical analyses
Continuous variables were expressed as median (IQR) and categorical variables were presented as percentages (%). Differences between groups were analyzed using the Kruskal-Wallis test and analysis of variance (ANOVA). Analysis was performed using SPSS software (SPSS version 23, SPSS Inc). A p-value < 0.05 was considered significant.
3. Results
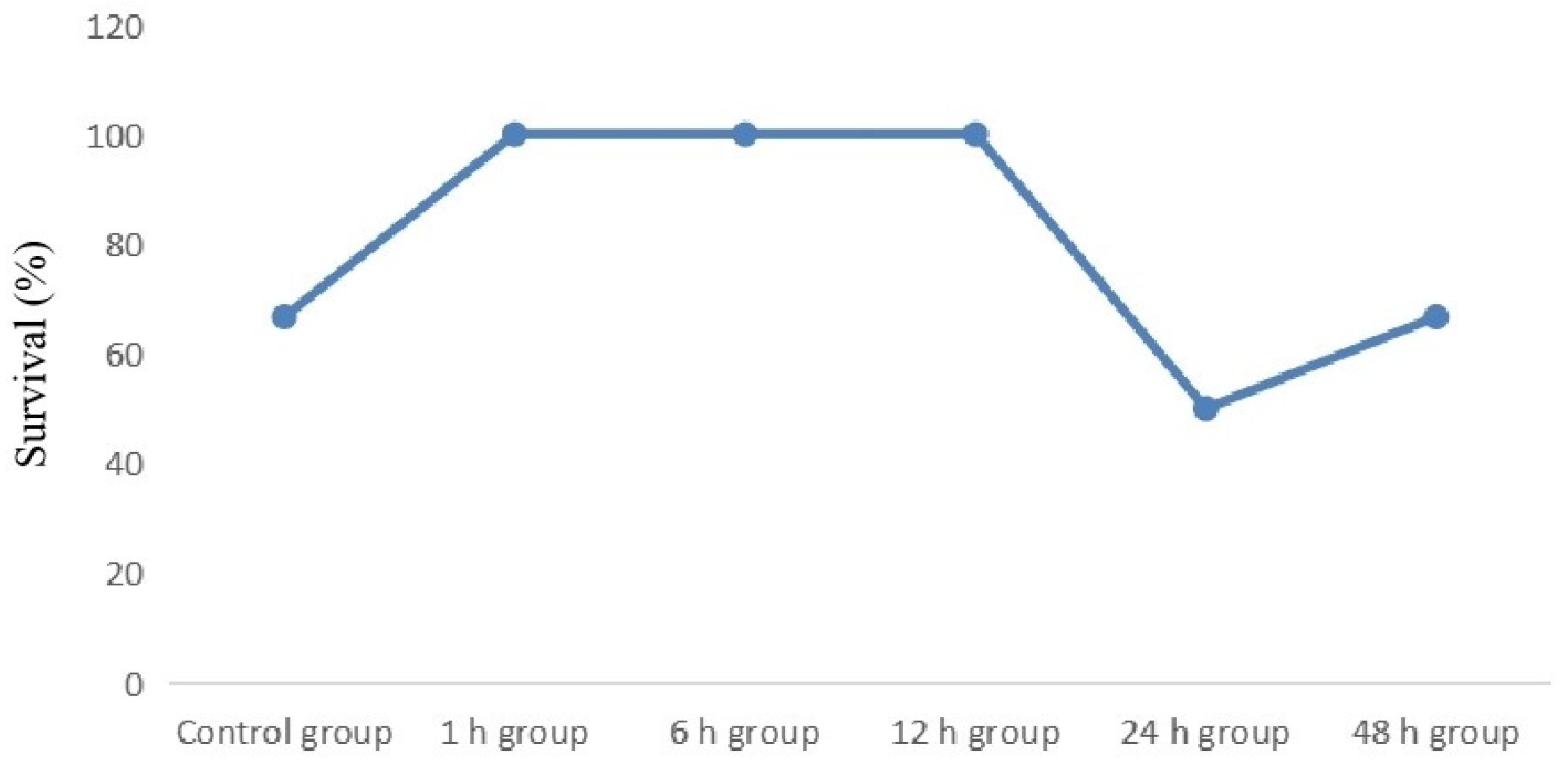
3.1. Initiation of hydrocortisone therapy within 12 h of sepsis onset improves the survival of LPS-induced newborn septic mice
36 newborn mice with sepsis were randomly divided into one control group and five treatment groups, with six mice in each group. First, we evaluated the effect of initiation of hydrocortisone therapy at various time points on the 72 h survival of LPS-induced newborn septic mice. As shown in Fig. 1, 2 newborn mice died in the control group and 5 in the treatment groups. Newborn mice in the control group (4/6, 66.7%) exhibited a lower survival rate (
p=0.573) than that of the treatment group (25/30, 83.3%), although this was not statistically significant. Interestingly, initiation of hydrocortisone therapy within 12 h of sepsis onset improved the survival rate to 100.0%. In contrast, 24 h (3/6, 50.0%) and 48 h (4/6, 66.7%) groups produced no improvement in the survival rate compared with the control. (
Figure 1).
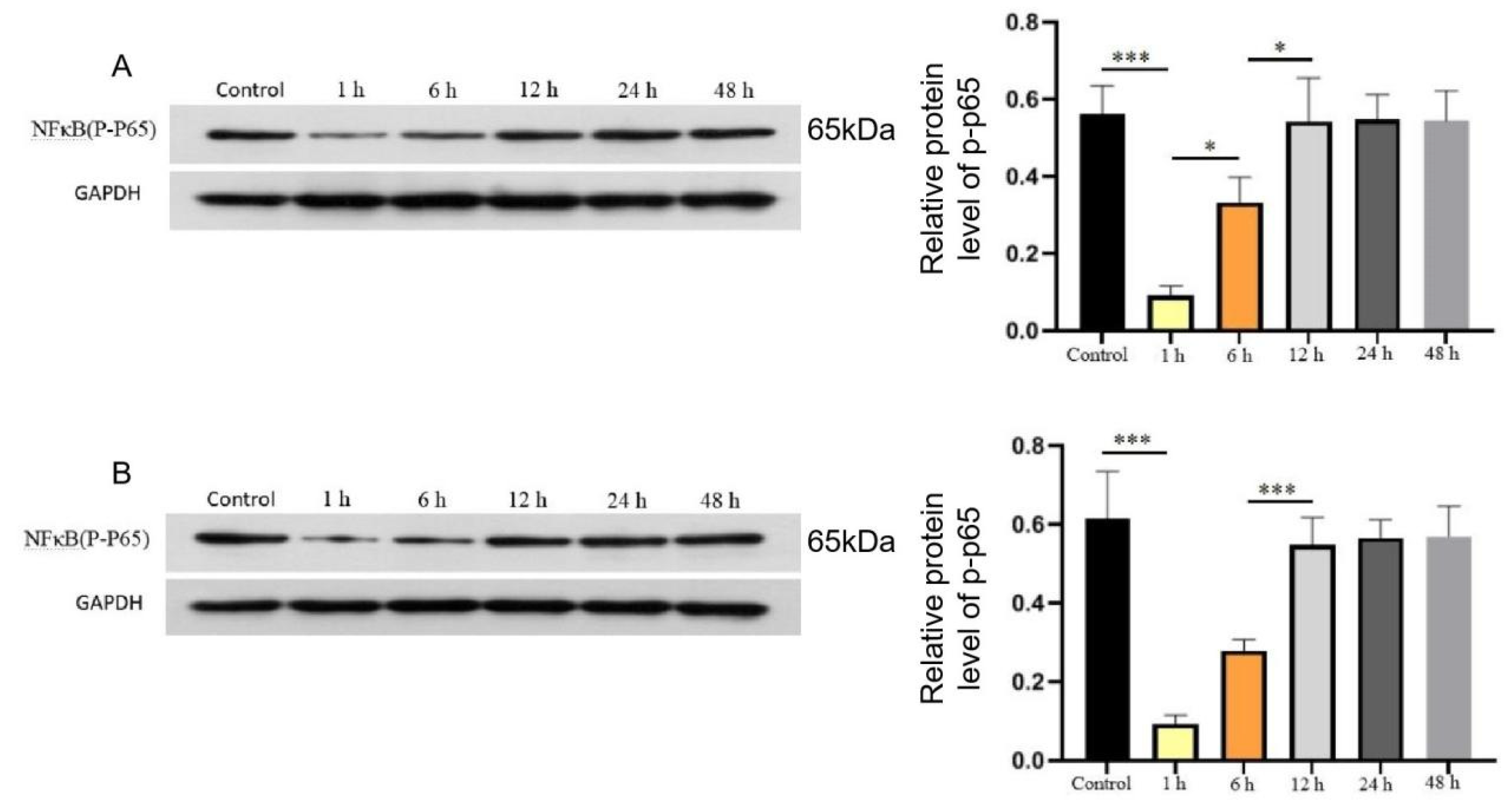
3.2. Initiation of hydrocortisone therapy within 6 h of sepsis onset inhibits NF-κB expression
Next, to evaluate the effect of initiation of hydrocortisone therapy at various time points on NF-κB expression, the heart and the lung were collected after 72 hours of hydrocortisone administration, and the tissues were assessed the expression levels of phosphorylated NF-κB p65 by Western blot. Panels A and B in
Figure 2 show the phosphorylation level of NF-κB in the heart and lung tissues, respectively. A marked decrease was observed in the phosphorylation level of NF-κB in 1 h and 6 h groups compared with the levels in control groups, but not in 12 h, 24 h and 48 h groups. (
Figure 2).
Panels A and B show the phosphorylation level of NF-κB in the heart and lung tissues, respectively. Data are the median of 3 separate experiments, and values are compared between the control and different treatment groups. * p<0.05, ** p<0.01 *** p<0.005.
3.3. Hydrocortisone therapy downregulates the levels of IL-6 and TNF-α in the sera
Furthermore, we measured the serum levels of IL-6 and TNF-α in each groups. Compared with the serum level of IL-6 and TNF-α in the control group, those levels significantly decreased in the treatment group (
p<0.05). Although hydrocortisone administration reduced serum IL-6 and TNF-α levels at all time points, since mortality was lower in the 1 h, 6 h and 12 h treatment group than in the 24 h and 48 h treatment group, we speculated that IL-6 and TNF-α levels might be different between the 1-12 h group and the 24-48 h group. Indeed, it is. In comparisons between treatment groups, initiation of hydrocortisone within 12 h significantly reduces serum levels of IL-6 and TNF-α in newborn mice with sepsis (
p<0.01). In addition, we also noticed that the earlier the use of hydrocortisone, the lower the TNF-α concentration, with a statistically significant difference (
p=0.026). This suggests that hydrocortisone can rapidly act on the NF-κB signaling pathway, thereby regulating the level of inflammatory factors. For newborn mice with sepsis, the earlier treatment with hydrocortisone, the greater the benefit (
Table 1).
4. Discussion
In this study, we revealed that initiation of hydrocortisone therapy within 12 h of onset improves the survival of LPS-induced newborn septic mice by possibly suppressing NF-κB pathway expression and production of inflammatory cytokines (TNF-α and IL-6). These results suggest that hydrocortisone suppressed NF-κB pathway expression, but was no longer inhibited when given 12 h after onset, suggesting possible the establishment of a quick glucocorticoid resistance after sepsis. Thus, in newborns in whom hydrocortisone is prescribed for sepsis, timing is crucial and hydrocortisone should be started within the first 12 hours after onset.
There are still no effective management strategies for treating neonatal septic shock because of the complex changes in hemodynamics and its poor prognosis. The latest guidelines recommend using hydrocortisone in infants with septic shock who remain hemodynamically unstable after fluid resuscitation and vasopressor therapy [
2]. However, no clear consensus exists on the timing of glucocorticoid treatment in the management of neonatal septic shock. In this work, we demonstrated that early hydrocortisone administration (within 12 h of sepsis onset) was effective in increasing the survival rate of neonatal mice with sepsis. By comparing the levels of the NF-κB pathway and the levels of serum inflammatory cytokines IL-6 and TNF-α, we observed that the earlier the neonatal mice with sepsis received hydrocortisone treatment (within 12 h), the lower the level of tissue and organ inflammation. It is suggested that the more timely treatment with hydrocortisone, the lower the tissue damage and the greater the benefit of treatment. We saw that although the mice in the 24 h treatment group had a lower survival rate than those in the 48 h group, the administration of hydrocortisone at 12 h did not inhibit NF-κB expression, so it was thought that the administration of hydrocortisone after 12 h did not have a protective effect. The survival rate of the 48 h group was consistent with the survival rate of the control group, which may be related to a small sample size. In addition, since the increase of serum IL-6 level was observed in the 24 h group compared with the 12 h and 48 h group, we cannot rule out that hydrocortisone may regulate the content of IL-6 through other ways, thus affecting the survival of mice.
Corticosteroids are important for preserving cardiovascular integrity and function. During septic shock, corticosteroids are beneficial for shock resolution compared with placebo [
10]. This effect is due partly to a restoration of the sensitivity of myocardial and peripheral receptors to catecholamines [
11]. These findings were also confirmed in our study. We found that early hydrocortisone treatment significantly decreases inflammatory factors such as IL-6 and TNF-α.
Our study demonstrated that initiation of hydrocortisone therapy within 12 h of sepsis onset improves survival in newborn mice, which was associated with NF-κB pathway down-regulation. Early hydrocortisone treatment may improve the therapeutic effect of sepsis by reducing the level of NF-κB expression in cardiopulmonary tissue, while late-stage sepsis develops glucocorticoid resistance may lead to reduced or even complete elimination of the inhibitory effect of hydrocortisone on NF-κB pathway.
There are currently few data on the use of hydrocortisone in the management of neonatal sepsis or septic shock. We acknowledge that this is also a limitation because hydrocortisone provides little insight into neonatal septic shock therapy`s effectiveness. Most of the current neonatal sepsis and septic shock guideline recommendations are based on the adult and children population. To our knowledge, this study is the first of its kind to explore the timing of hydrocortisone therapy for newborns with sepsis, even in newborn mice. Our results are similar to those of some other studies [
3,
4,
5,
6]. Neonates at an early stage of sepsis seem to profit from hydrocortisone administration; in contrast, neonates at a late stage of sepsis do not benefit from hydrocortisone treatment.
5. Conclusions
Taken together, our study demonstrated that the administration of hydrocortisone within 12 hours after the onset of sepsis in neonatal mice promotes survival rate by down-regulating the NF-κB pathway. The likely reason for this is that the anti-inflammatory effects of hydrocortisone are evident at the early stage of sepsis. It is of guiding value for the treatment of hydrocortisone in clinical neonatal sepsis.
Author Contributions
Conceptualization, X.Y.; methodology, J.Z., J.M.; investigation, J.Z.; data curation, J.Z., Y.L.; writing—original draft preparation, J.Z.; D.M.; writing—review and editing, X.Y.; supervision, J.M.. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program Grant Number 2021YFC2701700 (China) and the Foundation of Medical Science and Technology Research of Guangdong Province Grant Number A2022065 (China).
Institutional Review Board Statement
This study was performed according to the guidelines of the Helsinki Declaration of 1975 (revised in 2008) and the Animal Research Committee of the Guangdong Women and Children Hospital (the animal experiment ethics number: No. 202001115).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are contained within this manuscript.
Acknowledgments
Thank you very much for the technical support of Guangzhou Jennio Biological Technology Co., Ltd.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fleischmann-Struzek, C.; Goldfarb, D.M.; Schlattmann, P.; Schlapbach, L.J.; Reinhart, K.; Kissoon, N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Resp Med 2018, 6, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.L.; Peters, M.J.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schlapbach, L.J.; Tasker, R.C.; Argent, A.C.; et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med 2020, 21, 924–925. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Renault, A.; Brun-Buisson, C.; Megarbane, B.; Quenot, J.P.; Siami, S.; Cariou, A.; Forceville, X.; Schwebel, C.; et al. Hydrocortisone plus fludrocortisone for adults with septic shock. New Engl J Med 2018, 378, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive glucocorticoid therapy in patients with septic shock. New Engl J Med 2018, 378, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Sacha, G.L.; Chen, A.Y.; Palm, N.M.; Duggal, A. Evaluation of the initiation timing of hydrocortisone in adult patients with septic shock. Shock 2021, 55, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Sprung, C.L.; Annane, D.; Keh, D.; Moreno, R.; Singer, M.; Freivogel, K.; Weiss, Y.G.; Benbenishty, J.; Kalenka, A.; Forst, H.; et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008, 358, 111–24. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed Pharmacother 2020, 122, 109772. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.E.; Feng, Y.; Velazquez, H.; Sessa, W.C. Endothelial glucocorticoid receptor is required for protection against sepsis. P NATL ACAD USA 2013, 110, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Zhang, J.; Zhao, H.; Zhang, Z.; Yang, K. Protective effect of low-dose hydrocortisone on myocardium in early septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020, 32, 210–214. [Google Scholar] [PubMed]
- Annane, D.; Bellissant, E.; Bollaert, P.E.; Briegel, J.; Keh, D.; Kupfer, Y. Corticosteroids for treating sepsis. Cochrane Database Syst Rev 2015, CD002243. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Pastores, S.M.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; Marik, P.E.; et al. Critical illness-related corticosteroid insufficiency (CIRCI): a narrative review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Med 2017, 43, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).