Submitted:
05 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Antibody-based radiopharmaceuticals targeting FAP
2.1. Iodine-131-labeled monoclonal antibody F19
2.2. Iodine-131-labeled sibrotuzumab
2.3. 177Lu-ESC11/ESC14
2.4. 89Zr-labeled F19 and B12 IgG
2.5. Bispecific Antibodies
3. Peptide-based FAPIs
3.1. Peptide-based FAP radiopharmaceuticals
3.2. Structure of the selective and specific peptide FAPI
3.3. 68Ga-FAP-2286 and 111In-FAP-2286 for nuclear imaging
3.4. 177Lu-FAP-2286 for radionuclide therapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CAF | Cancer-associated Fibroblast |
| CT | Computerized Tomography |
| DPP | Dipeptidyl Peptidase |
| FAP | Fibroblast Activation Protein |
| FAPI | FAP Inhibitor |
| FDA | Food and Drug Administration |
| IC50 | Half Maximal Inhibitory Concentration |
| ID/g | Injected Dose/Gram |
| MAb | Monoclonal Antibody |
| mCRPC | Metastatic Castration-resistant Prostate Cancer |
| MIP | Maximum Intensity Projection |
| NIR | Near-infrared |
| PET | Positron Emission Tomography |
| PK | Pharmacokinetic |
| SPECT | Single Photon Emission Computed Tomography |
| TME | Tumor Microenvironment |
| T/K | Tumor-to-kidney Ratio |
References
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2016, 387, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dart, Tumour microenvironment: Radical changes. Nat Rev Cancer 2018, 18, 65. [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Katheder, N.S.; Khezri, R.; O’farrell, F.; Schultz, S.W.; Jain, A.; Rahman, M.M.; Schink, K.O.; Theodossiou, T.A.; Johansen, T.; Juhász, G.; et al. Microenvironmental autophagy promotes tumour growth. Nature 2017, 541, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 1–23. [Google Scholar] [CrossRef]
- Gordon-Weeks, A.; Yuzhalin, A.E. Cancer Extracellular Matrix Proteins Regulate Tumour Immunity. Cancers 2020, 12, 3331. [Google Scholar] [CrossRef] [PubMed]
- Oudin, M.J.; Jonas, O.; Kosciuk, T.; Broye, L.C.; Guido, B.C.; Wyckoff, J.; Riquelme, D.; Lamar, J.M.; Asokan, S.B.; Whittaker, C.; et al. Tumor Cell–Driven Extracellular Matrix Remodeling Drives Haptotaxis during Metastatic Progression. Cancer Discov. 2016, 6, 516–531. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Berg, T.J.; Pietras, A. Radiotherapy-induced remodeling of the tumor microenvironment by stromal cells. Semin. Cancer Biol. 2022, 86, 846–856. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; Galassi, C.; Galluzzi, L. Stress responses in stromal cells and tumor homeostasis. Pharmacol. Ther. 2019, 200, 55–68. [Google Scholar] [CrossRef]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xie, F.; Zhang, L.; Zhou, X.; Huang, J.; Wang, F.; Jin, J.; Zhang, L.; Zeng, L.; Zhou, F. Targeted Anti-Tumor Immunotherapy Using Tumor Infiltrating Cells. Adv. Sci. 2021, 8, 2101672. [Google Scholar] [CrossRef] [PubMed]
- Melssen, M.M.; Sheybani, N.D.; Leick, K.M.; Slingluff, C.L. Barriers to immune cell infiltration in tumors. J. Immunother. Cancer 2023, 11, e006401. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yao, Y.; Tang, Y.; Xin, Z.; Wu, D.; Ni, C.; Huang, J.; Wei, Q.; Zhang, T. Radiation-induced tumor immune microenvironments and potential targets for combination therapy. Signal Transduct. Target. Ther. 2023, 8, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- CChen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015, 13, 1–14. [Google Scholar] [CrossRef]
- Jin, M.-Z.; Jin, W.-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef]
- Jiang, J.; Mei, J.; Ma, Y.; Jiang, S.; Zhang, J.; Yi, S.; Feng, C.; Liu, Y.; Liu, Y. Tumor hijacks macrophages and microbiota through extracellular vesicles. Exploration 2022, 2. [Google Scholar] [CrossRef]
- Liu, H.; Sun, B.; Zhu, P.; Liu, C.; Zhang, G.; Wang, D.; Song, X.; Shi, J.; Yang, Y.; Lu, J. Preparation of Three-Dimensional Porous Graphene by Hydrothermal and Chemical Reduction with Ascorbic Acid and its Electrochemical Properties. ChemistryOpen 2022, 11, e202200161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.X.; Vu, L.T.; Ismail, N.N.; Le, M.T.; Grimson, A. Landscape of extracellular vesicles in the tumour microenvironment: Interactions with stromal cells and with non-cell components, and impacts on metabolic reprogramming, horizontal transfer of neoplastic traits, and the emergence of therapeutic resistance. Semin. Cancer Biol. 2021, 74, 24–44. [Google Scholar] [CrossRef]
- Ni, Y.; Zhou, X.; Yang, J.; Shi, H.; Li, H.; Zhao, X.; Ma, X. The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Matsueda, S.; Saya, H. Significance of Cancer-Associated Fibroblasts in the Interactions of Cancer Cells with the Tumor Microenvironment of Heterogeneous Tumor Tissue. Cancers 2023, 15, 2536. [Google Scholar] [CrossRef]
- Li, Z.; Low, V.; Luga, V.; Sun, J.; Earlie, E.; Parang, B.; Ganesh, K.S.; Cho, S.; Endress, J.; Schild, T.; et al. Tumor-produced and aging-associated oncometabolite methylmalonic acid promotes cancer-associated fibroblast activation to drive metastatic progression. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Coller, H.A. Fibroblasts Prompt Tumors to Mobilize Their Glycogen Reserves. Trends Cell Biol. 2019, 29, 278–280. [Google Scholar] [CrossRef]
- M. Cully, Tumour microenvironment: Fibroblast subtype provides niche for cancer stem cells. Nat Rev Cancer 2018, 18, 136. [CrossRef]
- Roulis, M.; Kaklamanos, A.; Schernthanner, M.; Bielecki, P.; Zhao, J.; Kaffe, E.; Frommelt, L.-S.; Qu, R.; Knapp, M.S.; Henriques, A.; et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature 2020, 580, 524–529. [Google Scholar] [CrossRef]
- Park, D.; Sahai, E.; Rullan, A. SnapShot: Cancer-Associated Fibroblasts. Cell 2020, 181, 486–486. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2018, 18, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Lavie, D.; Ben-Shmuel, A.; Erez, N.; Scherz-Shouval, R. Cancer-associated fibroblasts in the single-cell era. Nat. Cancer 2022, 3, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J. Cancer-Associated Fibroblasts: Perspectives in Cancer Therapy. Trends Cancer 2016, 2, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Saidou, J.; Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. Landmark Ed. 2010, 15, 166–179. [Google Scholar] [CrossRef]
- Álvarez-Teijeiro, S.; García-Inclán, C.; Villaronga, M. Á; Casado, P.; Hermida-Prado, F.; Granda-Díaz, R.; Rodrigo, J.P.; Calvo, F.; Del-Río-Ibisate, N.; Gandarillas, A.; et al. Factors Secreted by Cancer-Associated Fibroblasts that Sustain Cancer Stem Properties in Head and Neck Squamous Carcinoma Cells as Potential Therapeutic Targets. Cancers 2018, 10, 334. [Google Scholar] [CrossRef]
- Piper, M.; Mueller, A.C.; Karam, S.D. The interplay between cancer associated fibroblasts and immune cells in the context of radiation therapy. Mol. Carcinog. 2020, 59, 754–765. [Google Scholar] [CrossRef]
- Allam, A.; Yakou, M.; Pang, L.; Ernst, M.; Huynh, J. Exploiting the STAT3 Nexus in Cancer-Associated Fibroblasts to Improve Cancer Therapy. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol. Cancer 2021, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2020, 101, 147–176. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef]
- Hausmann, C.; Zoschke, C.; Wolff, C.; Darvin, M.E.; Sochorová, M.; Kováčik, A.; Wanjiku, B.; Schumacher, F.; Tigges, J.; Kleuser, B.; et al. Fibroblast origin shapes tissue homeostasis, epidermal differentiation, and drug uptake. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.; Goumas, F.A.; Affeldt, M.; Sandtner, S.; Gehling, U.M.; Brilloff, S.; Walter, J.; Karnatz, N.; Lamszus, K.; Rogiers, X.; et al. Stromal Fibroblasts in Colorectal Liver Metastases Originate From Resident Fibroblasts and Generate an Inflammatory Microenvironment. Am. J. Pathol. 2007, 171, 1608–1618. [Google Scholar] [CrossRef]
- Li, A.; Chen, Y.-S.; Ping, X.-L.; Yang, X.; Xiao, W.; Yang, Y.; Sun, H.-Y.; Zhu, Q.; Baidya, P.; Wang, X.; et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017, 27, 444–447. [Google Scholar] [CrossRef]
- Sebastian, A.; Hum, N.R.; Martin, K.A.; Gilmore, S.F.; Peran, I.; Byers, S.W.; Wheeler, E.K.; Coleman, M.A.; Loots, G.G. Single-Cell Transcriptomic Analysis of Tumor-Derived Fibroblasts and Normal Tissue-Resident Fibroblasts Reveals Fibroblast Heterogeneity in Breast Cancer. Cancers 2020, 12, 1307. [Google Scholar] [CrossRef] [PubMed]
- N.M. Anderson, M.C. Simon, The tumor microenvironment. Curr Biol 2020, 30, R921–R925. [CrossRef] [PubMed]
- Shook, B.A.; Wasko, R.R.; Rivera-Gonzalez, G.C.; Salazar-Gatzimas, E.; López-Giráldez, F.; Dash, B.C.; Muñoz-Rojas, A.R.; Aultman, K.D.; Zwick, R.K.; Lei, V.; et al. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 2018, 362, 909. [Google Scholar] [CrossRef]
- Yu, J.; Seldin, M.M.; Fu, K.; Li, S.; Lam, L.; Wang, P.; Wang, Y.; Huang, D.; Nguyen, T.L.; Wei, B.; et al. Topological Arrangement of Cardiac Fibroblasts Regulates Cellular Plasticity. Circ. Res. 2018, 123, 73–85. [Google Scholar] [CrossRef]
- Jiang, D.; Rinkevich, Y. Converting fibroblastic fates leads to wound healing without scar. Signal Transduct. Target. Ther. 2021, 6, 1–3. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.H.-C. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef]
- Hutchenreuther, J.; Leask, A. A tale of two orgins: do myofibroblasts originate from different sources in wound healing and fibrosis? Cell Tissue Res. 2016, 365, 507–509. [Google Scholar] [CrossRef]
- Foster, D.S.; Januszyk, M.; Yost, K.E.; Chinta, M.S.; Gulati, G.S.; Nguyen, A.T.; Burcham, A.R.; Salhotra, A.; Ransom, R.C.; Henn, D.; et al. Integrated spatial multiomics reveals fibroblast fate during tissue repair. Proc. Natl. Acad. Sci. 2021, 118. [Google Scholar] [CrossRef]
- H.F. Dvorak, Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986, 315, 1650–1659.
- Byun, J.S.; Gardner, K. Wounds That Will Not Heal: Pervasive Cellular Reprogramming in Cancer. Am. J. Pathol. 2013, 182, 1055–1064. [Google Scholar] [CrossRef]
- Koustoulidou, S.; Hoorens, M.W.H.; Dalm, S.U.; Mahajan, S.; Debets, R.; Seimbille, Y.; de Jong, M. Cancer-Associated Fibroblasts as Players in Cancer Development and Progression and Their Role in Targeted Radionuclide Imaging and Therapy. Cancers 2021, 13, 1100. [Google Scholar] [CrossRef] [PubMed]
- Hosein, A.N.; Wu, M.; Arcand, S.L.; Lavallée, S.; Hébert, J.; Tonin, P.N.; Basik, M. Breast Carcinoma–Associated Fibroblasts Rarely Contain p53 Mutations or Chromosomal Aberrations. Cancer Res 2010, 70, 5770–5777. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, X.; Wan, Z.; Ge, M.; Ding, Y.; Gu, J.; Hua, J.; Guo, D.; Tan, M.; Xu, D. Identification of the novel therapeutic targets and biomarkers associated of prostate cancer with cancer-associated fibroblasts (CAFs). Front. Oncol. 2023, 13, 1136835. [Google Scholar] [CrossRef] [PubMed]
- C.D. van der Heide, S.U. Dalm. Radionuclide imaging and therapy directed towards the tumor microenvironment: a multi-cancer approach for personalized medicine. Eur J Nucl Med Mol Imaging 2022, 49, 4616–4641. [Google Scholar] [CrossRef] [PubMed]
- Imlimthan, S.; Moon, E.S.; Rathke, H.; Afshar-Oromieh, A.; Rösch, F.; Rominger, A.; Gourni, E. New Frontiers in Cancer Imaging and Therapy Based on Radiolabeled Fibroblast Activation Protein Inhibitors: A Rational Review and Current Progress. Pharmaceuticals 2021, 14, 1023. [Google Scholar] [CrossRef] [PubMed]
- Robb, M.A.; McInnes, P.M.; Califf, R.M. Biomarkers and Surrogate Endpoints. JAMA 2016, 315, 1107–1108. [Google Scholar] [CrossRef]
- Liu, D. Cancer biomarkers for targeted therapy. Biomark. Res. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Song, Q.; Zhu, M.; Xu, Y.; Xiao, M.; Zheng, W. Identifying cancer-associated fibroblasts as emerging targets for hepatocellular carcinoma. Cell Biosci. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Hu, D.; Li, Z.; Zheng, B.; Lin, X.; Pan, Y.; Gong, P.; Zhuo, W.; Hu, Y.; Chen, C.; Chen, L.; et al. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun. 2022, 42, 401–434. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.-W.; Qiu, S.-Q.; Zhang, G.-J. Molecular and functional imaging in cancer-targeted therapy: current applications and future directions. Signal Transduct. Target. Ther. 2023, 8, 1–32. [Google Scholar] [CrossRef]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. – Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef]
- Brennen, W.N.; Rosen, D.M.; Wang, H.; Isaacs, J.T.; Denmeade, S.R. Targeting Carcinoma-Associated Fibroblasts Within the Tumor Stroma With a Fibroblast Activation Protein-Activated Prodrug. JNCI J. Natl. Cancer Inst. 2012, 104, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Choyke, P.L. PET of Fibroblast-Activation Protein for Cancer Staging: What We Know and What We Need to Learn. Radiology 2022, 304, 658–659. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Liu, T.; Yin, R. Biomarkers for cancer-associated fibroblasts. Biomark. Res. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Mayola, M.F.; Thackeray, J.T. The Potential of Fibroblast Activation Protein-Targeted Imaging as a Biomarker of Cardiac Remodeling and Injury. Curr. Cardiol. Rep. 2023, 25, 515–523. [Google Scholar] [CrossRef]
- Ebert, L.M.; Yu, W.; Gargett, T.; Toubia, J.; Kollis, P.M.; Tea, M.N.; Ebert, B.W.; Bardy, C.; Hurk, M.v.D.; Bonder, C.S.; et al. Endothelial, pericyte and tumor cell expression in glioblastoma identifies fibroblast activation protein (FAP) as an excellent target for immunotherapy. Clin. Transl. Immunol. 2020, 9, e1191. [Google Scholar] [CrossRef]
- Rezaei, S.; Gharapapagh, E.; Dabiri, S.; Heidari, P.; Aghanejad, A. Theranostics in targeting fibroblast activation protein bearing cells: Progress and challenges. Life Sci. 2023, 329, 121970. [Google Scholar] [CrossRef]
- Levy, M.T.; McCaughan, G.W.; Abbott, C.A.; Park, J.E.; Cunningham, A.M.; Müller, E.; Rettig, W.J.; Gorrell, M.D. Fibroblast activation protein: A cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. J. Hepatol. 1999, 29, 1768–1778. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L.; Tafelmeyer, P.; Golshayan, D. Fibroblast activation protein-α in fibrogenic disorders and cancer: more than a prolyl-specific peptidase? Expert Opin. Ther. Targets 2017, 21, 977–991. [Google Scholar] [CrossRef]
- Zhang, T.; Tong, X.; Zhang, S.; Wang, D.; Wang, L.; Wang, Q.; Fan, H. The Roles of Dipeptidyl Peptidase 4 (DPP4) and DPP4 Inhibitors in Different Lung Diseases: New Evidence. Front. Pharmacol. 2021, 12, 731453. [Google Scholar] [CrossRef]
- R. Han, X. Wang, W. Bachovchin, Z. Zukowska, J.W. Osborn, Inhibition of dipeptidyl peptidase 8/9 impairs preadipocyte differentiation. Sci Rep 2015, 5, 12348. [CrossRef]
- Brennen, W.N.; Isaacs, J.T.; Denmeade, S.R. Rationale Behind Targeting Fibroblast Activation Protein–Expressing Carcinoma-Associated Fibroblasts as a Novel Chemotherapeutic Strategy. Mol. Cancer Ther. 2012, 11, 257–266. [Google Scholar] [CrossRef]
- M. Zubal, B. Vymolova, I. Matrasova, P. Vymola, J. Veprkova, M. Syrucek, R. Tomas, Z. Vanickova, E. Krepela, D. Konecna, P. Busek, A. Sedo, Fibroblast activation protein as a potential theranostic target in brain metastases of diverse solid tumours. Pathology 2023, 55, 806–817.
- Wonganu, B.; Berger, B.W. A specific, transmembrane interface regulates fibroblast activation protein (FAP) homodimerization, trafficking and exopeptidase activity. Biochim. et Biophys. Acta (BBA) - Biomembr. 2016, 1858, 1876–1882. [Google Scholar] [CrossRef]
- Dendl, K.; Koerber, S.A.; Kratochwil, C.; Cardinale, J.; Finck, R.; Dabir, M.; Novruzov, E.; Watabe, T.; Kramer, V.; Choyke, P.L.; et al. FAP and FAPI-PET/CT in Malignant and Non-Malignant Diseases: A Perfect Symbiosis? Cancers 2021, 13, 4946. [Google Scholar] [CrossRef]
- Park, J.E.; Lenter, M.C.; Zimmermann, R.N.; Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Fibroblast Activation Protein, a Dual Specificity Serine Protease Expressed in Reactive Human Tumor Stromal Fibroblasts. J. Biol. Chem. 1999, 274, 36505–36512. [Google Scholar] [CrossRef]
- Kalaei, Z.; Manafi-Farid, R.; Rashidi, B.; Kiani, F.K.; Zarei, A.; Fathi, M.; Jadidi-Niaragh, F. The Prognostic and therapeutic value and clinical implications of fibroblast activation protein-α as a novel biomarker in colorectal cancer. Cell Commun. Signal. 2023, 21, 1–17. [Google Scholar] [CrossRef]
- Aertgeerts, K.; Levin, I.; Shi, L.; Snell, G.P.; Jennings, A.; Prasad, G.S.; Zhang, Y.; Kraus, M.L.; Salakian, S.; Sridhar, V.; et al. Structural and Kinetic Analysis of the Substrate Specificity of Human Fibroblast Activation Protein α. J. Biol. Chem. 2005, 280, 19441–19444. [Google Scholar] [CrossRef]
- Niedermeyer, J.; Garin-Chesa, P.; Kriz, M.; Hilberg, F.; Mueller, E.; Bamberger, U.; Rettig, W.J.; Schnapp, A. Expression of the fibroblast activation protein during mouse embryo development. The International journal of developmental biology 2001, 45, 445–447. [Google Scholar]
- Niedermeyer, J.; Kriz, M.; Hilberg, F.; Garin-Chesa, P.; Bamberger, U.; Lenter, M.C.; Park, J.; Viertel, B.; Püschner, H.; Mauz, M.; et al. Targeted Disruption of Mouse Fibroblast Activation Protein. Mol. Cell. Biol. 2000, 20, 1089–1094. [Google Scholar] [CrossRef]
- Brown, D.D.; Wang, Z.; Furlow, J.D.; Kanamori, A.; A Schwartzman, R.; Remo, B.F.; Pinder, A. The thyroid hormone-induced tail resorption program during Xenopus laevis metamorphosis. Proc. Natl. Acad. Sci. 1996, 93, 1924–1929. [Google Scholar] [CrossRef] [PubMed]
- Rettig, W.J.; Su, S.L.; Fortunato, S.R.; Scanlan, M.J.; Raj, B.K.M.; Garin-Chesa, P.; Healey, J.H.; Old, L.J. Fibroblast activation protein: Purification, epitope mapping and induction by growth factors. Int. J. Cancer 1994, 58, 385–392. [Google Scholar] [CrossRef]
- Dolznig, H.; Schweifer, N.; Puri, C.; Kraut, N.; Rettig, W.J.; Kerjaschki, D.; Garin-Chesa, P. Characterization of cancer stroma markers: in silico analysis of an mRNA expression database for fibroblast activation protein and endosialin. Cancer immunity 2005, 5, 10. [Google Scholar] [PubMed]
- Egger, C.; Cannet, C.; Gérard, C.; Suply, T.; Ksiazek, I.; Jarman, E.; Beckmann, N. Effects of the fibroblast activation protein inhibitor, PT100, in a murine model of pulmonary fibrosis. Eur. J. Pharmacol. 2017, 809, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Tillmanns, J.; Hoffmann, D.; Habbaba, Y.; Schmitto, J.D.; Sedding, D.; Fraccarollo, D.; Galuppo, P.; Bauersachs, J. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J. Mol. Cell. Cardiol. 2015, 87, 194–203. [Google Scholar] [CrossRef]
- S. Uitte de Willige, J.J. S. Uitte de Willige, J.J. Malfliet, H.L. Janssen, F.W. Leebeek, D.C. Rijken, Increased N-terminal cleavage of alpha-2-antiplasmin in patients with liver cirrhosis, J Thromb Haemost, 11 (2013) 2029-2036.
- Nagaraju, C.K.; Dries, E.; Popovic, N.; Singh, A.A.; Haemers, P.; Roderick, H.L.; Claus, P.; Sipido, K.R.; Driesen, R.B. Global fibroblast activation throughout the left ventricle but localized fibrosis after myocardial infarction. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rettig, W.J.; Garin-Chesa, P.; Beresford, H.R.; Oettgen, H.F.; Melamed, M.R.; Old, L.J. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc. Natl. Acad. Sci. 1988, 85, 3110–3114. [Google Scholar] [CrossRef]
- Bauer, S.; Jendro, M.C.; Wadle, A.; Kleber, S.; Stenner, F.; Dinser, R.; Reich, A.; Faccin, E.; Gödde, S.; Dinges, H.; et al. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res. Ther. 2006, 8, R171–R171. [Google Scholar] [CrossRef]
- Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl. Acad. Sci. USA 1990, 87, 7235–7239. [Google Scholar] [CrossRef] [PubMed]
- Mentlein, R.; Hattermann, K.; Hemion, C.; Jungbluth, A.A.; Held-Feindt, J. Expression and role of the cell surface protease seprase/fibroblast activation protein-α (FAP-α) in astroglial tumors. Biol. Chem. 2011, 392, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Brokopp, C.E.; Schoenauer, R.; Richards, P.; Bauer, S.; Lohmann, C.; Emmert, M.Y.; Weber, B.; Winnik, S.; Aikawa, E.; Graves, K.; et al. Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur. Hear. J. 2011, 32, 2713–2722. [Google Scholar] [CrossRef]
- Wang, X.M.; Yu, D.M.T.; McCaughan, G.W.; Gorrell, M.D. Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cell line. J. Hepatol. 2005, 42, 935–945. [Google Scholar] [CrossRef]
- Park, J.E.; Lenter, M.C.; Zimmermann, R.N.; Garin-Chesa, P.; Old, L.J.; Rettig, W.J. Fibroblast Activation Protein, a Dual Specificity Serine Protease Expressed in Reactive Human Tumor Stromal Fibroblasts. J. Biol. Chem. 1999, 274, 36505–36512. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, M.J.; Raj, B.K.; Calvo, B.; Garin-Chesa, P.; Sanz-Moncasi, M.P.; Healey, J.H.; Old, L.J.; Rettig, W.J. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc. Natl. Acad. Sci. USA 1994, 91, 5657–5661. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.R.; Lee, H.-O.; Lee, J.S.; Klein-Szanto, A.; Watts, P.; Ross, E.A.; Chen, W.-T.; Cheng, J.D. Clinical Implications of Fibroblast Activation Protein in Patients with Colon Cancer. Clin. Cancer Res. 2007, 13, 1736–1741. [Google Scholar] [CrossRef]
- Cohen, S.J.; Alpaugh, R.K.; Palazzo, I.; Meropol, N.J.; Rogatko, A.; Xu, Z.; Hoffman, J.P.; Weiner, L.M.; Cheng, J.D. Fibroblast Activation Protein and Its Relationship to Clinical Outcome in Pancreatic Adenocarcinoma. Pancreas 2008, 37, 154–158. [Google Scholar] [CrossRef]
- Ju, M.-J.; Qiu, S.-J.; Fan, J.; Xiao, Y.-S.; Gao, Q.; Zhou, J.; Li, Y.-W.; Tang, Z.-Y. Peritumoral Activated Hepatic Stellate Cells Predict Poor Clinical Outcome in Hepatocellular Carcinoma After Curative Resection. Am. J. Clin. Pathol. 2009, 131, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, H.; Cai, J.; Zhang, T.; Guo, J.; Feng, D.; Wang, Z. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011, 303, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Teichgräber, V.; Monasterio, C.; Chaitanya, K.; Boger, R.; Gordon, K.; Dieterle, T.; Jäger, D.; Bauer, S. Specific inhibition of fibroblast activation protein (FAP)-alpha prevents tumor progression in vitro. Adv. Med Sci. 2015, 60, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huangfu, Z.; Yang, J.; Wang, G.; Hu, K.; Gao, M.; Zhong, Z. Imaging-guided targeted radionuclide tumor therapy: From concept to clinical translation. Adv. Drug Deliv. Rev. 2022, 190, 114538. [Google Scholar] [CrossRef] [PubMed]
- Fonti, R.; Conson, M.; Del Vecchio, S. PET/CT in radiation oncology. Semin. Oncol. 2019, 46, 202–209. [Google Scholar] [CrossRef]
- Ostermann, E.; Garin-Chesa, P.; Heider, K.H.; Kalat, M.; Lamche, H.; Puri, C.; Kerjaschki, D.; Rettig, W.J.; Adolf, G.R. Effective Immunoconjugate Therapy in Cancer Models Targeting a Serine Protease of Tumor Fibroblasts. Clin. Cancer Res. 2008, 14, 4584–4592. [Google Scholar] [CrossRef]
- Chen, M.; Lei, X.; Shi, C.; Huang, M.; Li, X.; Wu, B.; Li, Z.; Han, W.; Du, B.; Hu, J.; et al. Pericyte-targeting prodrug overcomes tumor resistance to vascular disrupting agents. J. Clin. Investig. 2017, 127, 3689–3701. [Google Scholar] [CrossRef]
- Wang, L.-C.S.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting Fibroblast Activation Protein in Tumor Stroma with Chimeric Antigen Receptor T Cells Can Inhibit Tumor Growth and Augment Host Immunity without Severe Toxicity. Cancer Immunol. Res. 2013, 2, 154–166. [Google Scholar] [CrossRef]
- Fu, H.; Guo, W.; Huang, J.; Wu, H.; Chen, H. Clinical applications of fibroblast activation protein-targeted theranostics in oncologic and nononcologic disease: Current status and future directions. iRADIOLOGY 2023, 1, 340–361. [Google Scholar] [CrossRef]
- M. Ying, Q. Yang, X. Xu, S. Wu, W. Yin, S. Liang, G. Pan, C. Zuo, Z. Guo, C. Cheng, S. Liu, Value of [68Ga]Ga-FAPI-04 PET imaging in acute coronary syndrome complicated by suspected gastrointestinal malignancies. Journal of Nuclear Medicine 2023, 64, 411.
- Jansen, K.; Heirbaut, L.; Verkerk, R.; Cheng, J.D.; Joossens, J.; Cos, P.; Maes, L.; Lambeir, A.-M.; De Meester, I.; Augustyns, K.; et al. Extended Structure–Activity Relationship and Pharmacokinetic Investigation of (4-Quinolinoyl)glycyl-2-cyanopyrrolidine Inhibitors of Fibroblast Activation Protein (FAP). J. Med. Chem. 2014, 57, 3053–3074. [Google Scholar] [CrossRef]
- Li, M.; Younis, M.H.; Zhang, Y.; Cai, W.; Lan, X. Clinical summary of fibroblast activation protein inhibitor-based radiopharmaceuticals: cancer and beyond. Eur. J. Nucl. Med. 2022, 49, 2844–2868. [Google Scholar] [CrossRef]
- Huang, R.; Pu, Y.; Huang, S.; Yang, C.; Yang, F.; Pu, Y.; Li, J.; Chen, L.; Huang, Y. FAPI-PET/CT in Cancer Imaging: A Potential Novel Molecule of the Century. Front. Oncol. 2022, 12, 854658. [Google Scholar] [CrossRef]
- Rettig, W.J.; Chesa, P.G.; Beresford, H.R.; Feickert, H.J.; Jennings, M.T.; Cohen, J.; Oettgen, H.F.; Old, L.J. Differential expression of cell surface antigens and glial fibrillary acidic protein in human astrocytoma subsets. Cancer research 1986, 46, 6406–12. [Google Scholar]
- Rettig, W.J.; Garin-Chesa, P.; Healey, J.H.; Su, S.L.; Ozer, H.L.; Schwab, M.; Albino, A.P.; Old, L.J. Regulation and heteromeric structure of the fibroblast activation protein in normal and transformed cells of mesenchymal and neuroectodermal origin. Cancer research 1993, 53, 3327–35. [Google Scholar] [PubMed]
- O’Brien, P.; O’Connor, B.F. Seprase: An overview of an important matrix serine protease. Biochim. et Biophys. Acta (BBA) - Proteins Proteom. 2008, 1784, 1130–1145. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.-C.; Chen, J.; Stefflova, K.; Warren, M.S.; Navab, R.; Bandarchi, B.; Mullins, S.; Tsao, M.; Cheng, J.D.; Zheng, G. Photodynamic Molecular Beacon Triggered by Fibroblast Activation Protein on Cancer-Associated Fibroblasts for Diagnosis and Treatment of Epithelial Cancers. J. Med. Chem. 2008, 52, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Peltier, A.; Seban, R.-D.; Buvat, I.; Bidard, F.-C.; Mechta-Grigoriou, F. Fibroblast heterogeneity in solid tumors: From single cell analysis to whole-body imaging. Semin. Cancer Biol. 2022, 86, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T. Fibroblast activation protein-α and dipeptidyl peptidase IV (CD26): Cell-surface proteases that activate cell signaling and are potential targets for cancer therapy. Drug Resist. Updat. 2005, 8, 51–58. [Google Scholar] [CrossRef]
- Privé, B.M.; Boussihmad, M.A.; Timmermans, B.; van Gemert, W.A.; Peters, S.M.B.; Derks, Y.H.W.; van Lith, S.A.M.; Mehra, N.; Nagarajah, J.; Heskamp, S.; et al. Fibroblast activation protein-targeted radionuclide therapy: background, opportunities, and challenges of first (pre)clinical studies. Eur. J. Nucl. Med. 2023, 50, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Nam, E.H.; Park, J.Y.; Ghosh, P.; Kim, I.S. Identification of BR102910 as a selective fibroblast activation protein (FAP) inhibitor. Bioorganic Med. Chem. Lett. 2021, 37, 127846. [Google Scholar] [CrossRef]
- Aoyama, A.; Chen, W.T. A 170-kDa membrane-bound protease is associated with the expression of invasiveness by human malignant melanoma cells. Proc. Natl. Acad. Sci. 1990, 87, 8296–8300. [Google Scholar] [CrossRef] [PubMed]
- Welt, S.; Divgi, C.R.; Scott, A.M.; Garin-Chesa, P.; Finn, R.D.; Graham, M.; A Carswell, E.; Cohen, A.; Larson, S.M.; Old, L.J. Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J. Clin. Oncol. 1994, 12, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wiseman, G.; Adjei, A.; Lee, F.-T.; Hopkins, W.; Divgi, C.R.; Hanson, L.H.; Mitchell, P.; Gansen, D.N.; Larson, S.M.; et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res. 2003, 9, 1639–1647. [Google Scholar]
- Fischer, E.; Chaitanya, K.; Wüest, T.; Wadle, A.; Scott, A.M.; van den Broek, M.; Schibli, R.; Bauer, S.; Renner, C. Radioimmunotherapy of Fibroblast Activation Protein Positive Tumors by Rapidly Internalizing Antibodies. Clin. Cancer Res. 2012, 18, 6208–6218. [Google Scholar] [CrossRef]
- Pandya, D.N.; Sinha, A.; Yuan, H.; Mutkus, L.; Stumpf, K.; Marini, F.C.; Wadas, T.J. Imaging of Fibroblast Activation Protein Alpha Expression in a Preclinical Mouse Model of Glioma Using Positron Emission Tomography. Molecules 2020, 25, 3672. [Google Scholar] [CrossRef] [PubMed]
- Hintz, H.M.; Gallant, J.P.; Griend, D.J.V.; Coleman, I.M.; Nelson, P.S.; LeBeau, A.M. Imaging Fibroblast Activation Protein Alpha Improves Diagnosis of Metastatic Prostate Cancer with Positron Emission Tomography. Clin. Cancer Res. 2020, 26, 4882–4891. [Google Scholar] [CrossRef]
- Brünker, P.; Wartha, K.; Friess, T.; Grau-Richards, S.; Waldhauer, I.; Koller, C.F.; Weiser, B.; Majety, M.; Runza, V.; Niu, H.; et al. RG7386, a Novel Tetravalent FAP-DR5 Antibody, Effectively Triggers FAP-Dependent, Avidity-Driven DR5 Hyperclustering and Tumor Cell Apoptosis. Mol. Cancer Ther. 2016, 15, 946–957. [Google Scholar] [CrossRef]
- E.E. Kim, Therapeutic Nuclear Medicine, Journal of nuclear medicine: official publication. Society of Nuclear Medicine 2016, 57, 163.
- Hofheinz, R.-D.; Al-Batran, S.-E.; Hartmann, F.; Hartung, G.; Jäger, D.; Renner, C.; Tanswell, P.; Kunz, U.; Amelsberg, A.; Kuthan, H.; et al. Stromal Antigen Targeting by a Humanised Monoclonal Antibody: An Early Phase II Trial of Sibrotuzumab in Patients with Metastatic Colorectal Cancer. Onkologie 2003, 26, 44–48. [Google Scholar] [CrossRef]
- P. Tanswell, P. Garin-Chesa, W.J. Rettig, S. Welt, C.R. Divgi, E.S. Casper, R.D. Finn, S.M. Larson, L.J. Old, A.M. Scott, Population pharmacokinetics of antifibroblast activation protein monoclonal antibody F19 in cancer patients. British journal of clinical pharmacology 2001, 51, 177–180. [CrossRef]
- B. Ingelheim, Single Dose Escalation Study of 131I-Sibrotuzumab in Patients With Advanced or Metastatic Non-small Cell Lung Cancer, 2014-08-06.
- Jain, T.; Sun, T.; Durand, S.; Hall, A.; Houston, N.R.; Nett, J.H.; Sharkey, B.; Bobrowicz, B.; Caffry, I.; Yu, Y.; et al. Biophysical properties of the clinical-stage antibody landscape. Proc. Natl. Acad. Sci. 2017, 114, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Starr, C.G.; Tessier, P.M. Selecting and engineering monoclonal antibodies with drug-like specificity. Curr. Opin. Biotechnol. 2019, 60, 119–127. [Google Scholar] [CrossRef] [PubMed]
- J.-F. Chatal, C.A. Hoefnagel, Radionuclide therapy. Lancet 1999, 354, 931–935. [CrossRef]
- DeNardo, S.J.; DeNardo, G.L. Targeted radionuclide therapy for solid tumors: An overview. Endocrine 2006, 66, S89–S95. [Google Scholar] [CrossRef] [PubMed]
- Ledsgaard, L.; Ljungars, A.; Rimbault, C.; Sørensen, C.V.; Tulika, T.; Wade, J.; Wouters, Y.; McCafferty, J.; Laustsen, A.H. Advances in antibody phage display technology. Drug Discov. Today 2022, 27, 2151–2169. [Google Scholar] [CrossRef]
- Mimmi, S.; Maisano, D.; Quinto, I.; Iaccino, E. Phage Display: An Overview in Context to Drug Discovery. Trends Pharmacol. Sci. 2019, 40, 87–91. [Google Scholar] [CrossRef]
- Saw, P.E.; Song, E.-W. Phage display screening of therapeutic peptide for cancer targeting and therapy. Protein Cell 2019, 10, 787–807. [Google Scholar] [CrossRef]
- Schofield, D.J.; Pope, A.R.; Clementel, V.; Buckell, J.; Chapple, S.D.; Clarke, K.F.; Conquer, J.S.; Crofts, A.M.; Crowther, S.R.; Dyson, M.R.; et al. Application of phage display to high throughput antibody generation and characterization. Genome Biol. 2007, 8, R254–R254. [Google Scholar] [CrossRef]
- Frenzel, A.; Kügler, J.; Helmsing, S.; Meier, D.; Schirrmann, T.; Hust, M.; Dübel, S. Designing Human Antibodies by Phage Display. Transfus. Med. Hemotherapy 2017, 44, 312–318. [Google Scholar] [CrossRef]
- Nagano, K.; Tsutsumi, Y. Phage Display Technology as a Powerful Platform for Antibody Drug Discovery. Viruses 2021, 13, 178. [Google Scholar] [CrossRef]
- Peissert, F.; Plüss, L.; Giudice, A.M.; Ongaro, T.; Villa, A.; Elsayed, A.; Nadal, L.; Plaza, S.D.; Scietti, L.; Puca, E.; et al. Selection of a PD-1 blocking antibody from a novel fully human phage display library. Protein Sci. 2022, 31, e4486. [Google Scholar] [CrossRef]
- S. Gupta, S. Batra, M. Jain, Antibody Labeling with Radioiodine and Radiometals. Drug Delivery System 2014, 1141, 147–157.
- Stein, R.; Govindan, S.V.; Mattes, M.J.; Chen, S.; Reed, L.; Newsome, G.; McBride, B.J.; Griffiths, G.L.; Hansen, H.J.; Goldenberg, D.M. Improved iodine radiolabels for monoclonal antibody therapy. Cancer Res. 2003, 63, 111–118. [Google Scholar]
- Anderson, W.T.; Strand, M. Radiolabeled antibody: iodine versus radiometal chelates. NCI Monogr. 1987, 149–151. [Google Scholar]
- Sun, J.; Huangfu, Z.; Yang, J.; Wang, G.; Hu, K.; Gao, M.; Zhong, Z. Imaging-guided targeted radionuclide tumor therapy: From concept to clinical translation. Adv. Drug Deliv. Rev. 2022, 190, 114538. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, A.H.; Eerd, J.E.M.V.; Frielink, C.; Oosterwijk, E.; Oyen, W.J.G.; Corstens, F.H.M.; Boerman, O.C. Optimization of radioimmunotherapy of renal cell carcinoma: labeling of monoclonal antibody cG250 with 131I, 90Y, 177Lu, or 186Re. J. Nucl. Med. 2004, 45, 327–37. [Google Scholar] [PubMed]
- Hoffmann, R.M.; Mele, S.; Cheung, A.; Larcombe-Young, D.; Bucaite, G.; Sachouli, E.; Zlatareva, I.; Morad, H.O.J.; Marlow, R.; McDonnell, J.M.; et al. Rapid conjugation of antibodies to toxins to select candidates for the development of anticancer Antibody-Drug Conjugates (ADCs). Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Morais, M.; Ma, M.T. Site-specific chelator-antibody conjugation for PET and SPECT imaging with radiometals. Drug Discov. Today: Technol. 2018, 30, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: a review and a discussion. EJNMMI Phys. 2016, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with 89Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 2012, 40, 3–14. [Google Scholar] [CrossRef] [PubMed]
- De Feo, M.S.; Pontico, M.; Frantellizzi, V.; Corica, F.; De Cristofaro, F.; De Vincentis, G. 89Zr-PET imaging in humans: a systematic review. Clin. Transl. Imaging 2021, 10, 23–36. [Google Scholar] [CrossRef]
- Fischer, G.; Seibold, U.; Schirrmacher, R.; Wängler, B.; Wängler, C. 89Zr, a Radiometal Nuclide with High Potential for Molecular Imaging with PET: Chemistry, Applications and Remaining Challenges. Molecules 2013, 18, 6469–6490. [Google Scholar] [CrossRef] [PubMed]
- S. Sandhu, C.M. Moore, E. Chiong, H. Beltran, R.G. Bristow, S.G. Williams, Prostate cancer. Lancet 2021, 398, 1075–1090.
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. New Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; Phung, D.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. New. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef]
- ESMO Congress 2022, 2022-09.
- A.J.W. Murtha, E.; van der Eecken, K.; Kwan, E.; Herberts, C.; Sipola, J.; Ng, S.; Chen, E.; Fonseca, N.; Schönlau, E.; Bernales, C.; Donnellan, G.; Verbeke, S.; Lumen, N.; Van Dorpe, J.; De Laere, B.; Annala, M.; Vandekerkhove, G.; Ost, P.; Wyatt, A. W. 4MO - Multi-focal genomic dissection of synchronous primary and metastatic tissue from de novo metastatic prostate cancer.
- Lappalainen, T.; Scott, A.J.; Brandt, M.; Hall, I.M. Genomic Analysis in the Age of Human Genome Sequencing. Cell 2019, 177, 70–84. [Google Scholar] [CrossRef]
- De Smet, F.; Martinez, A.A.; Bosisio, F.M. Next-Generation Pathology by Multiplexed Immunohistochemistry. Trends Biochem. Sci. 2020, 46, 80–82. [Google Scholar] [CrossRef]
- Pflueger, D.; Terry, S.; Sboner, A.; Habegger, L.; Esgueva, R.; Lin, P.-C.; Svensson, M.A.; Kitabayashi, N.; Moss, B.J.; MacDonald, T.Y.; et al. Discovery of non-ETS gene fusions in human prostate cancer using next-generation RNA sequencing. Genome Res. 2010, 21, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Bhanvadia, R.R.; VanOpstall, C.; Brechka, H.; Barashi, N.S.; Gillard, M.; McAuley, E.M.; Vasquez, J.M.; Paner, G.; Chan, W.-C.; Andrade, J.; et al. MEIS1 and MEIS2 Expression and Prostate Cancer Progression: A Role For HOXB13 Binding Partners in Metastatic Disease. Clin. Cancer Res. 2018, 24, 3668–3680. [Google Scholar] [CrossRef] [PubMed]
- Klarmann, G.J.; Hurt, E.M.; Mathews, L.A.; Zhang, X.; Duhagon, M.A.; Mistree, T.; Thomas, S.B.; Farrar, W.L. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin. Exp. Metastasis 2009, 26, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Hintz, H.M.; Cowan, A.E.; Shapovalova, M.; LeBeau, A.M. Development of a Cross-Reactive Monoclonal Antibody for Detecting the Tumor Stroma. Bioconjugate Chem. 2019, 30, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Sum, E.; Rapp, M.; Fröbel, P.; Le Clech, M.; Dürr, H.; Giusti, A.M.F.; Perro, M.; Speziale, D.; Kunz, L.; Menietti, E.; et al. Fibroblast Activation Protein α-Targeted CD40 Agonism Abrogates Systemic Toxicity and Enables Administration of High Doses to Induce Effective Antitumor Immunity. Clin. Cancer Res. 2021, 27, 4036–4053. [Google Scholar] [CrossRef]
- Labiano, S.; Roh, V.; Godfroid, C.; Hiou-Feige, A.; Romero, J.; Sum, E.; Rapp, M.; Boivin, G.; Wyss, T.; Simon, C.; et al. CD40 Agonist Targeted to Fibroblast Activation Protein α Synergizes with Radiotherapy in Murine HPV-Positive Head and Neck Tumors. Clin. Cancer Res. 2021, 27, 4054–4065. [Google Scholar] [CrossRef]
- A Dose Escalation Study of RO6874813 in Participants With Locally Advanced or Metastatic Solid Tumors, 2018.
- A Study to Evaluate Safety, Pharmacokinetics and Anti-Tumor Activity of RO7300490, as Single Agent or in Combination With Atezolizumab in Participants With Advanced Solid Tumors, (2022).
- Wang, F.; Tsai, J.C.; Davis, J.H.; Chau, B.; Dong, J.; West, S.M.; Hogan, J.M.; Wheeler, M.L.; Bee, C.; Morishige, W.; et al. Design and characterization of mouse IgG1 and IgG2a bispecific antibodies for use in syngeneic models. mAbs 2019, 12, 1685350. [Google Scholar] [CrossRef]
- Meermeier, E.W.; Welsh, S.J.; Sharik, M.E.; Du, M.T.; Garbitt, V.M.; Riggs, D.L.; Shi, C.-X.; Stein, C.K.; Bergsagel, M.; Chau, B.; et al. Tumor Burden Limits Bispecific Antibody Efficacy through T-cell Exhaustion Averted by Concurrent Cytotoxic Therapy. Blood Cancer Discov. 2021, 2, 354–369. [Google Scholar] [CrossRef]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Theruvath, J.; Menard, M.; Smith, B.A.H.; Linde, M.H.; Coles, G.L.; Dalton, G.N.; Wu, W.; Kiru, L.; Delaidelli, A.; Sotillo, E.; et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med. 2022, 28, 333–344. [Google Scholar] [CrossRef]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef] [PubMed]
- P. Hingorani, M.D. Krailo, A. Buxton, P.R. Hutson, J. Davis, K.A. Janeway, R.G. Gorlick, M. Isakoff, Phase II study of antidisialoganglioside antibody, dinutuximab, in combination with GM-CSF in patients with recurrent osteosarcoma (AOST1421): A report from the Children’s Oncology Group. Journal of Clinical Oncology 2020, 38, 10508–10508.
- Edelman, M.; Dvorkin, M.; Laktionov, K.K.; Navarro, A.; Juan-Vidal, O.; Kozlov, V.; Golden, G.; Jordan, O.; Deng, C. The anti-disialoganglioside (GD2) antibody dinutuximab (D) for second-line treatment (2LT) of patients (pts) with relapsed/refractory small cell lung cancer (RR SCLC): Results from part II of the open-label, randomized, phase II/III distinct study. J. Clin. Oncol. 2020, 38, 9017–9017. [Google Scholar] [CrossRef]
- Sikic, B.I.; Narayanan, S.; Colevas, A.D.; Padda, S.K.; Fisher, G.A.; Supan, D.; Wakelee, H.A.; Aoki, R.; Pegram, M.D.; Villalobos, V.M.; et al. A first-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J. Clin. Oncol. 2016, 34, 3019–3019. [Google Scholar] [CrossRef]
- F. Pharmaceuticals, Fusion Pharmaceuticals Announces IND Clearance For FPI-2068, A Jointly Developed Novel Targeted Alpha Therapy, 2023.
- Moores, S.L.; Chiu, M.L.; Bushey, B.S.; Chevalier, K.; Luistro, L.; Dorn, K.; Brezski, R.J.; Haytko, P.; Kelly, T.; Wu, S.-J.; et al. A Novel Bispecific Antibody Targeting EGFR and cMet Is Effective against EGFR Inhibitor–Resistant Lung Tumors. Cancer Res 2016, 76, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Hernandez, R.; Carlson, P.; Grudzinski, J.; Bates, A.M.; Jagodinsky, J.C.; Erbe, A.; Marsh, I.R.; Arthur, I.; Aluicio-Sarduy, E.; et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef]
- Altunay, B.; Morgenroth, A.; Beheshti, M.; Vogg, A.; Wong, N.C.L.; Ting, H.H.; Biersack, H.-J.; Stickeler, E.; Mottaghy, F.M. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur. J. Nucl. Med. 2020, 48, 1371–1389. [Google Scholar] [CrossRef]
- K. Hu, X. Ma, L. Xie, Y. Zhang, M. Hanyu, H. Obata, L. Zhang, K. Nagatsu, H. Suzuki, R. Shi, W. Wang, M.-R. Zhang, Development of a Stable Peptide-Based PET Tracer for Detecting CD133-Expressing Cancer Cells. ACS Omega 2021, 7, 334–341.
- Hu, K.; Shang, J.; Xie, L.; Hanyu, M.; Zhang, Y.; Yang, Z.; Xu, H.; Wang, L.; Zhang, M.-R. PET Imaging of VEGFR with a Novel 64Cu-Labeled Peptide. ACS Omega 2020, 5, 8508–8514. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Wu, W.; Xie, L.; Geng, H.; Zhang, Y.; Hanyu, M.; Zhang, L.; Liu, Y.; Nagatsu, K.; Suzuki, H.; et al. Whole-body PET tracking of a d-dodecapeptide and its radiotheranostic potential for PD-L1 overexpressing tumors. Acta Pharm. Sin. B 2021, 12, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- L. Au - Zhang, S. Au - Zhang, W. Au - Wu, X. Au - Wang, J. Au - Shen, D. Au - Wang, K. Au - Hu, M.-R. Au - Zhang, F. Au - Wang, R. Au - Wang, Development of a 68Gallium-Labeled D-Peptide PET Tracer for Imaging Programmed Death-Ligand 1 Expression. J Vis Exp. 2023, e65047. [CrossRef]
- Zhang, L.; Zhang, S.; Wu, J.; Wang, Y.; Wu, Y.; Sun, X.; Wang, X.; Shen, J.; Xie, L.; Zhang, Y.; et al. Linear Peptide-Based PET Tracers for Imaging PD-L1 in Tumors. Mol. Pharm. 2023, 20, 4256–4267. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Xie, L.; Hanyu, M.; Zhang, Y.; Li, L.; Ma, X.; Nagatsu, K.; Suzuki, H.; Wang, W.; Zhang, M.-R. Harnessing the PD-L1 interface peptide for positron emission tomography imaging of the PD-1 immune checkpoint. RSC Chem. Biol. 2020, 1, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Okarvi, S. Peptide-based radiopharmaceuticals and cytotoxic conjugates: Potential tools against cancer. Cancer Treat. Rev. 2008, 34, 13–26. [Google Scholar] [CrossRef]
- Zhu, Z.; Miao, W.; Li, Q.; Dai, H.; Ma, Q.; Wang, F.; Yang, A.; Jia, B.; Jing, X.; Liu, S.; et al. 99mTc-3PRGD2 for Integrin Receptor Imaging of Lung Cancer: A Multicenter Study. J. Nucl. Med. 2012, 53, 716–722. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Lin, K.-S.; Lau, J.; Zeisler, J.; Colpo, N.; Perrin, D.M.; Bénard, F. Melanoma Imaging Using 18F-Labeled α-Melanocyte-Stimulating Hormone Derivatives with Positron Emission Tomography. Mol. Pharm. 2018, 15, 2116–2122. [Google Scholar] [CrossRef]
- H. Ma, S.Y. Liu, Z.W. Zhang, G.H. Tang, G.J. Yuan, J. Zhao, S. Su, Preliminary biological evaluation of Ga-68-labeled cyclic RGD dimer as an integrin alpha(v)beta(3)-targeting radiotracer for tumor PET imaging. Journal of Radioanalytical and Nuclear Chemistry 2019, 321, 857–865. [CrossRef]
- Sakai, K.; Passioura, T.; Sato, H.; Ito, K.; Furuhashi, H.; Umitsu, M.; Takagi, J.; Kato, Y.; Mukai, H.; Warashina, S.; et al. Macrocyclic peptide-based inhibition and imaging of hepatocyte growth factor. Nat. Chem. Biol. 2019, 15, 598–606. [Google Scholar] [CrossRef]
- R. Siitonen, E. Peuhu, A. Autio, H. Liljenback, E. Mattila, O. Metsala, M. Kakela, T. Saanijoki, I. Dijkgraaf, S. Jalkanen, J. Ivaska, A. Roivainen, (68)Ga-DOTA-E[c(RGDfK)]2 PET Imaging of SHARPIN-Regulated Integrin Activity in Mice. J Nucl Med 2019, 60, 1380–1387.
- Cooper, B.M.; Iegre, J.; Donovan, D.H.O.; Halvarsson, M..; Spring, D.R. Peptides as a platform for targeted therapeutics for cancer: peptide–drug conjugates (PDCs). Chem. Soc. Rev. 2020, 50, 1480–1494. [CrossRef]
- X. Feng, Y.P. Wang, D.H. Lu, X.X. Xu, X. Zhou, H.Y. Zhang, T. Zhang, H. Zhu, Z. Yang, F. Wang, N. Lie, Z.F. Liu, Clinical Translation of a Ga-68-Labeled Integrin alpha(v)beta(6)-Targeting Cyclic Radiotracer for PET Imaging of Pancreatic Cancer. Journal of Nuclear Medicine 2020, 61, 1461–1467.
- Y. Gai, Y. Jiang, Y. Long, L. Sun, Q. Liu, C. Qin, Y. Zhang, D. Zeng, X. Lan, Evaluation of an Integrin αvβ3 and Aminopeptidase N Dual-Receptor Targeting Tracer for Breast Cancer Imaging. Molecular Pharmaceutics 2020, 17, 349–358. [CrossRef] [PubMed]
- N. Pirooznia, K. Abdi, D. Beiki, F. Emami, S.S. Arab, O. Sabzevari, S. Soltani-Gooshkhaneh, yyy Lu-177-labeled cyclic RGD peptide as an imaging and targeted radionuclide therapeutic agent in non-small cell lung cancer: Biological evaluation and preclinical study. Bioorganic Chemistry 2020, 102, 104100.
- Uehara, T.; Sensui, A.; Ishioka, S.; Mizuno, Y.; Takahashi, S.; Takemori, H.; Suzuki, H.; Arano, Y. Manipulating Pharmacokinetics of Purification-Free 99mTc-Labeled Bivalent Probes for In Vivo Imaging of Saturable Targets. Mol. Pharm. 2020, 17, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- M. Zhao, J.N. Ding, Q.L. Mao, Y.Q. Zhang, Y.J. Gao, S.Y. Ye, H.N. Qin, H.B. Shi, A novel alpha(v)beta(3) integrin-targeted NIR-II nanoprobe for multimodal imaging-guided photothermal therapy of tumors in vivo. Nanoscale 2020, 12, 6953–6958. [CrossRef] [PubMed]
- Kwon, D.; Lozada, J.; Zhang, Z.; Zeisler, J.; Poon, R.; Zhang, C.; Roxin; Lin, K.-S.; Perrin, D.; Benard, F. High-Contrast CXCR4-Targeted 18F-PET Imaging Using a Potent and Selective Antagonist. Mol. Pharm. 2020, 18, 187–197. [CrossRef]
- Yang, Y.; Zhang, J.; Zou, H.; Shen, Y.; Deng, S.; Wu, Y. Synthesis and evaluation of 68Ga-labeled dimeric cNGR peptide for PET imaging of CD13 expression with ovarian cancer xenograft. J. Cancer 2021, 12, 244–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Wong, C.T.T.; Li, X. Chemoselective Peptide Cyclization and Bicyclization Directly on Unprotected Peptides. J. Am. Chem. Soc. 2019, 141, 12274–12279. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Meibohm, B. Pharmacokinetics and Pharmacokinetic–Pharmacodynamic Correlations of Therapeutic Peptides. Clin. Pharmacokinet. 2013, 52, 855–868. [Google Scholar] [CrossRef] [PubMed]
- K. Hu, H. Geng, Q. Zhang, Q. Liu, M. Xie, C. Sun, W. Li, H. Lin, F. Jiang, T. Wang, Y.-D. Wu, Z. Li, An In-tether Chiral Center Modulates the Helicity, Cell Permeability, and Target Binding Affinity of a Peptide. Angewandte Chemie International Edition 2016, 55, 8013–8017. [CrossRef]
- Hu, K.; Li, W.; Yu, M.; Sun, C.; Li, Z. Investigation of Cellular Uptakes of the In-Tether Chiral-Center-Induced Helical Pentapeptides. Bioconjugate Chem. 2016, 27, 2824–2827. [Google Scholar] [CrossRef]
- Hu, K.; Sun, C.; Li, Z. Reversible and Versatile On-Tether Modification of Chiral-Center-Induced Helical Peptides. Bioconjugate Chem. 2017, 28, 2001–2007. [Google Scholar] [CrossRef]
- K. Hu, W. Xiong, C. Sun, C. Wang, J. Li, F. Yin, Y. Jiang, M.-R. Zhang, Z. Li, X. Wang, Z. Li, Self-Assembly of Constrained Cyclic Peptides Controlled by Ring Size. CCS Chemistry 2020, 2, 42–51. [CrossRef]
- Li, W.; Hu, K.; Zhang, Q.; Wang, D.; Ma, Y.; Hou, Z.; Yin, F.; Li, Z. N terminal N-methylation modulates chiral centre induced helical (CIH) peptides’ biophysical properties. Chem. Commun. 2018, 54, 1865–1868. [Google Scholar] [CrossRef]
- K. Hu, F. Yin, M. Yu, C. Sun, J. Li, Y. Liang, W. Li, M. Xie, Y. Lao, W. Liang, Z.-g. Li, In-Tether Chiral Center Induced Helical Peptide Modulators Target p53-MDM2/MDMX and Inhibit Tumor Growth in Stem-Like Cancer Cell. Theranostics 2017, 7, 4566–4576. [CrossRef]
- F.Z.D. Osterkamp, E. Schneider, C. Haase, M. Paschke, A. Höhne, J. Ungewiss, C. Smerling, U. Reineke, B. A.;, Compounds comprising a fibroblast activation protein ligand and use thereof, 2021.
- Calais, J. FAP: The Next Billion Dollar Nuclear Theranostics Target? J. Nucl. Med. 2020, 61, 163–165. [Google Scholar] [CrossRef] [PubMed]
- F.Z.D. Osterkamp, E. Schneider, C. Haase, M. Paschke, A. Höhne, J. Ungewiss, C. Smerling, U. Reineke, Bredenbeck, A.Compounds Comprising a Fibroblast Activation Protein Ligand and Use Thereof., 2021.
- E Perico, M.; Chinol, M.; Nacca, A.; Luison, E.; Paganelli, G.; Canevari, S. The humoral immune response to macrocyclic chelating agent DOTA depends on the carrier molecule. J. Nucl. Med. 2001, 42, 1697–703. [Google Scholar]
- D. Trujillo-Benitez, M. Luna-Gutierrez, G. Ferro-Flores, B. Ocampo-Garcia, C. Santos-Cuevas, G. Bravo-Villegas, E. Morales-Avila, P. Cruz-Nova, L. Diaz-Nieto, J. Garcia-Quiroz, E. Azorin-Vega, A. Rosato, L. Melendez-Alafort, Design, Synthesis and Preclinical Assessment of Tc-99m-iFAP for In Vivo Fibroblast Activation Protein (FAP) Imaging. Molecules 2022, 27, 264.
- Zboralski, D.; Hoehne, A.; Bredenbeck, A.; Schumann, A.; Nguyen, M.; Schneider, E.; Ungewiss, J.; Paschke, M.; Haase, C.; von Hacht, J.L.; et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur. J. Nucl. Med. 2022, 49, 3651–3667. [Google Scholar] [CrossRef] [PubMed]
- R.P. Baum, C. Schuchardt, A. Singh, M. Chantadisai, F.C. Robiller, J. Zhang, D. Mueller, A. Eismant, F. Almaguel, D. Zboralski, F. Osterkamp, A. Hoehne, U. Reineke, C. Smerling, H.R. Kulkarni, Feasibility, Biodistribution, and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy of Diverse Adenocarcinomas Using (177)Lu-FAP-2286: First-in-Humans Results. J Nucl Med 2022, 63, 415–423.
- Parker, M.F.; Blecha, J.; Rosenberg, O.; Ohliger, M.; Flavell, R.R.; Wilson, D.M. Cyclic 68Ga-Labeled Peptides for Specific Detection of Human Angiotensin-Converting Enzyme 2. J. Nucl. Med. 2021, 62, 1631–1637. [Google Scholar] [CrossRef]
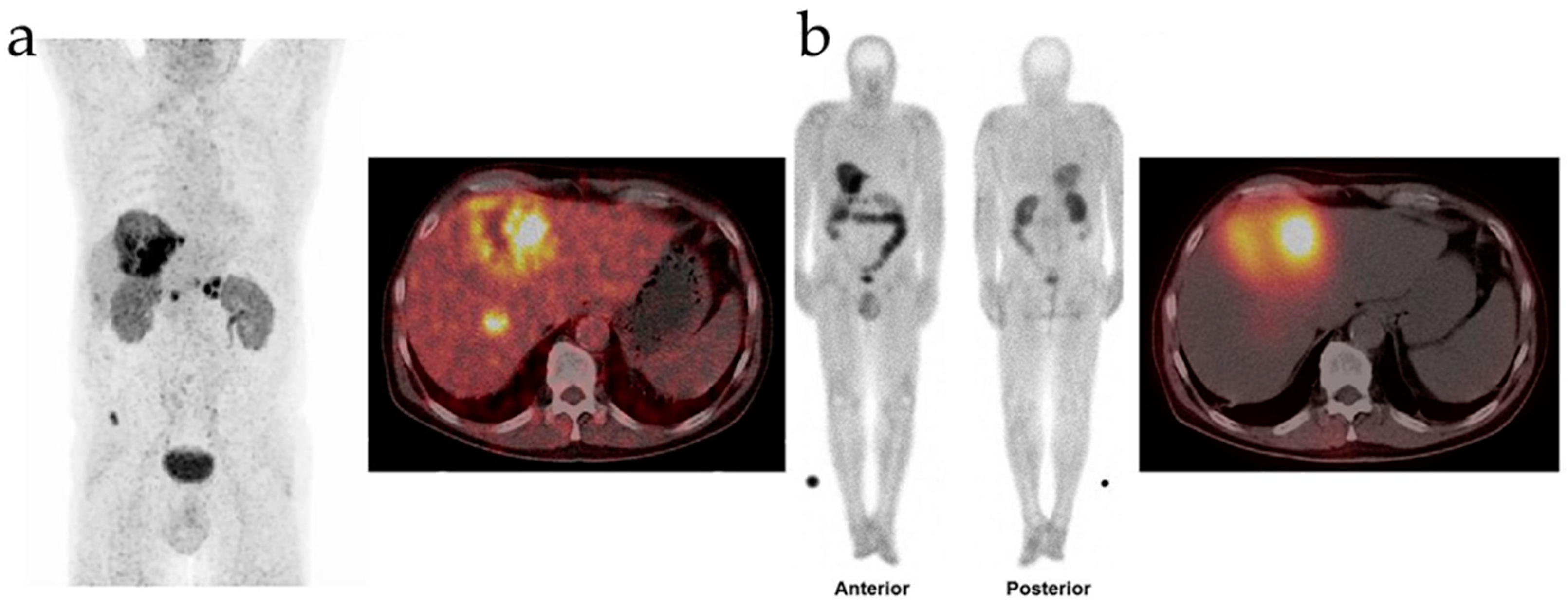
- Y. Pang, L. Zhao, T. Meng, W. Xu, Q. Lin, H. Wu, J. Zhang, X. Chen, L. Sun, H. Chen, PET imaging of fibroblast activation protein in various types of cancers by using (68)Ga-FAP-2286: Comparison with (18)F-FDG and (68)Ga-FAPI-46 in a single-center, prospective study. Journal of Nuclear Medicine 2023, 64, 386–394.
- Meng, L.; Fang, J.; Zhao, L.; Wang, T.; Yuan, P.; Zhao, Z.; Zhuang, R.; Lin, Q.; Chen, H.; Chen, X.; et al. Rational Design and Pharmacomodulation of Protein-Binding Theranostic Radioligands for Targeting the Fibroblast Activation Protein. J. Med. Chem. 2022, 65, 8245–8257. [Google Scholar] [CrossRef] [PubMed]
- FAP-2286 and Clovis’ Targeted Radionuclide Therapy Development Program.
- C. Oncology, A Study of 177Lu-FAP-2286 in Advanced Solid Tumors (LuMIERE) (LuMIERE), 2022.
- Rao, Z.; Zhang, Y.; Liu, L.; Wang, M.; Zhang, C. [177Lu]Lu-FAP-2286 therapy in a case of right lung squamous cell carcinoma with systemic metastases. Eur. J. Nucl. Med. 2022, 50, 1266–1267. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Herrmann, K.; Schöder, H.; Scott, A.M.; Lewis, J.S. Radiotheranostics in oncology: current challenges and emerging opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 534–550. [Google Scholar] [CrossRef] [PubMed]
- Privé, B.M.; Peters, S.M.; Muselaers, C.H.; van Oort, I.M.; Janssen, M.J.; Sedelaar, J.M.; Konijnenberg, M.W.; Zámecnik, P.; Uijen, M.J.; Schilham, M.G.; et al. Lutetium-177-PSMA-617 in Low-Volume Hormone-Sensitive Metastatic Prostate Cancer: A Prospective Pilot Study. Clin. Cancer Res. 2021, 27, 3595–3601. [Google Scholar] [CrossRef]
- J.R. Strosberg, M.E. Caplin, P.L. Kunz, P.B. Ruszniewski, L. Bodei, A. Hendifar, E. Mittra, E.M. Wolin, J.C. Yao, M.E. Pavel, E. Grande, E. Van Cutsem, E. Seregni, H. Duarte, G. Gericke, A. Bartalotta, M.F. Mariani, A. Demange, S. Mutevelic, E.P. Krenning, 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. The Lancet Oncology 2021, 22, 1752–1763.
- Larimer, B.M.; Wehrenberg-Klee, E.; Caraballo, A.; Mahmood, U. Quantitative CD3 PET Imaging Predicts Tumor Growth Response to Anti-CTLA-4 Therapy. J. Nucl. Med. 2016, 57, 1607–1611. [Google Scholar] [CrossRef]
- E. Jagoda, F. Basuli, M. Williams, A. Opina, K. Wong, S. Adler, A. Ton, L. Szajek, O. Vasalatiy, B. Xu, R. Swenson, P. Choyke, Immuno-PET imaging of the programmed cell death-1 ligand (PD-L1) using the therapeutic mAb, avelumab. Journal of Nuclear Medicine 2017, 58, 178.
- Geller, J. Yan, H. Guo, 89Zr-Df-anti-PD-L1 for evaluating in vivo PD-L1 level in lung cancer mouse model. Journal of Nuclear Medicine 2018, 59, 1114.
- Vermeulen, K.; Vandamme, M.; Bormans, G.; Cleeren, F. Design and Challenges of Radiopharmaceuticals. Semin. Nucl. Med. 2019, 49, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lu, C.; Yang, Y.; Shi, X.; Wang, H.; Yang, N.; Yang, K.; Zhang, X. Current Status and Trends in Peptide Receptor Radionuclide Therapy in the Past 20 Years (2000–2019): A Bibliometric Study. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Minczeles, N.; Hofland, J.; de Herder, W.; Brabander, T. Strategies Towards Improving Clinical Outcomes of Peptide Receptor Radionuclide Therapy. Curr. Oncol. Rep. 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Scott, A.; Bodei, L. Pharmacogenomics in Radionuclide Therapy: Impact on Response to Theranostics. J. Nucl. Med. 2020, 62, 884–885. [Google Scholar] [CrossRef]
- Wahl, R.L.; Chareonthaitawee, P.; Clarke, B.; Drzezga, A.; Lindenberg, L.; Rahmim, A.; Thackeray, J.; Ulaner, G.A.; Weber, W.; Zukotynski, K.; et al. Mars Shot for Nuclear Medicine, Molecular Imaging, and Molecularly Targeted Radiopharmaceutical Therapy. J. Nucl. Med. 2020, 62, 6–14. [Google Scholar] [CrossRef]
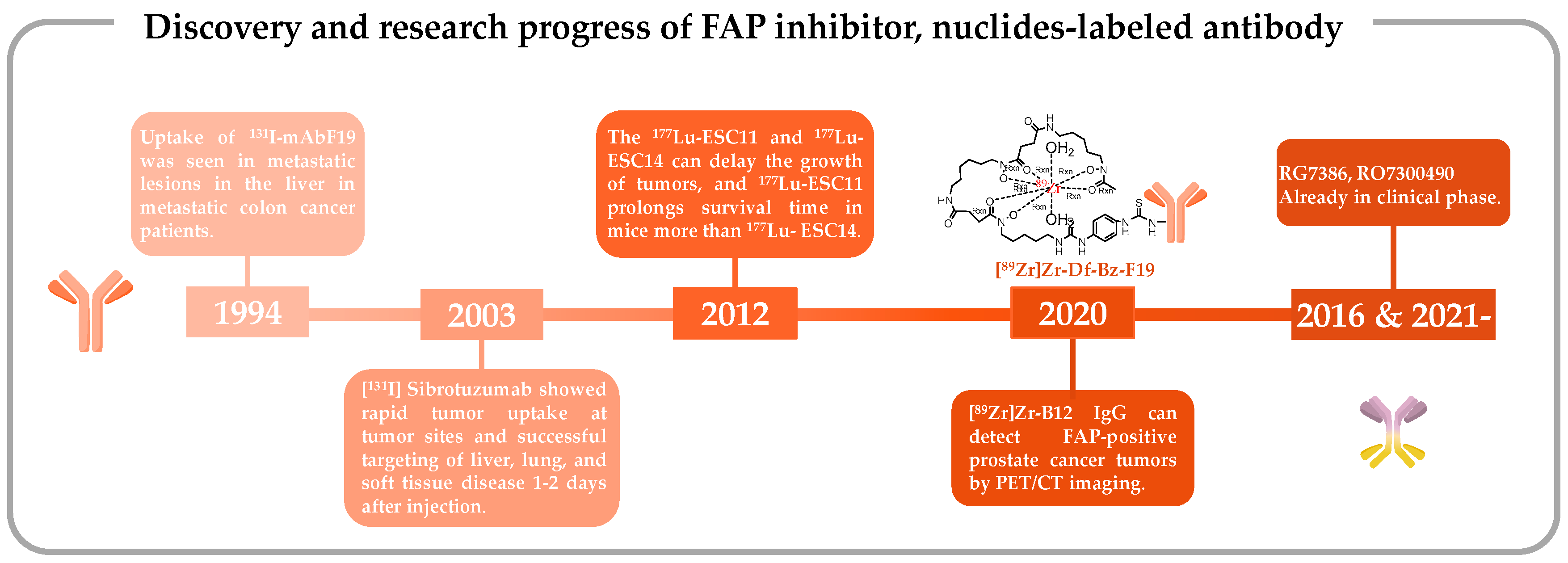
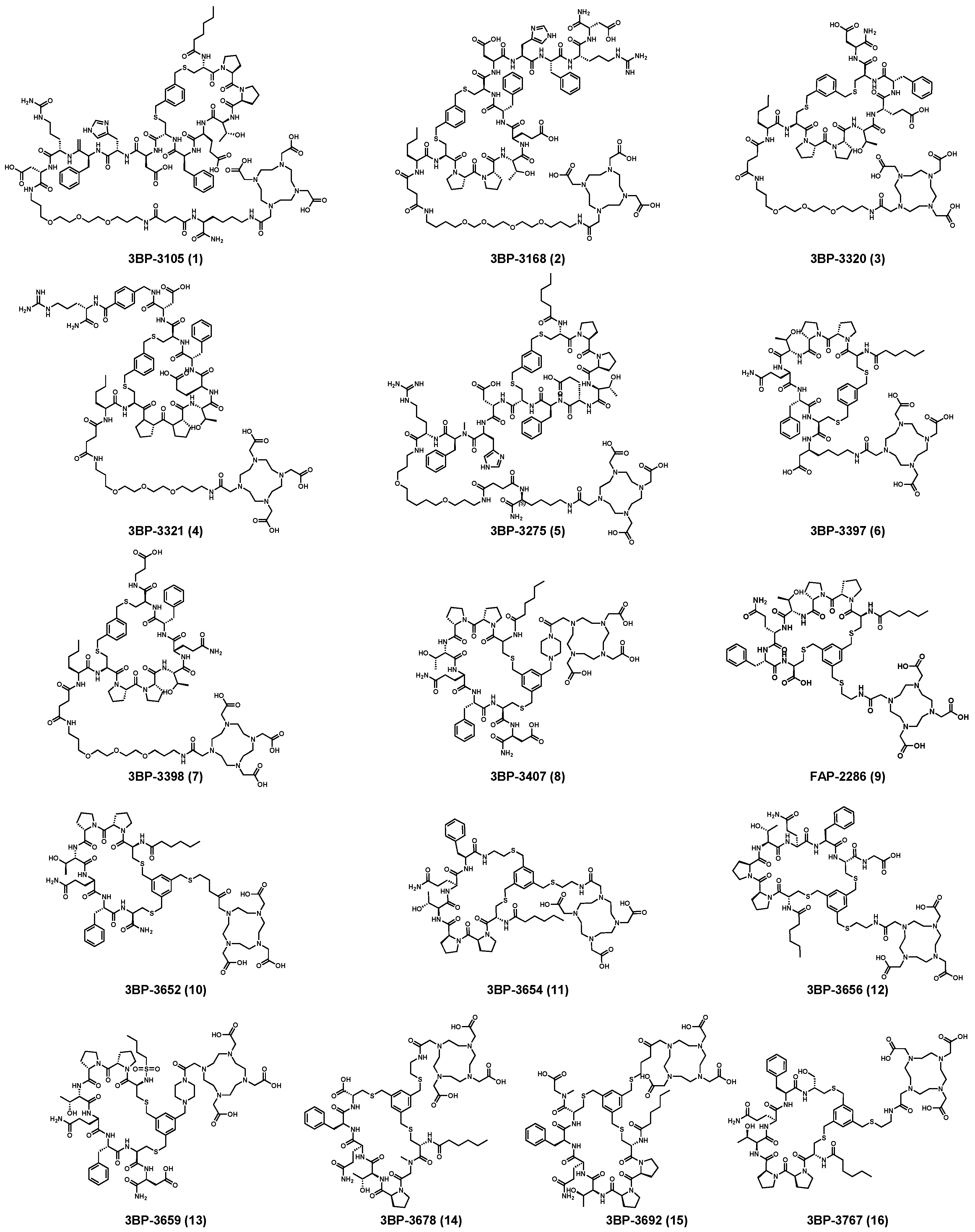
| Developer | Name | In vitro Assessment | Tumor Model | Indication | Phase | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|
| Boehringer Ingelheim | F19 (BIBH-1) & 131I-BIBH-1 |
NA | Colorectal Cancer Metastatic Cancer |
SPECT/CT | Completed | NCT00005616 |
| Boehringer Ingelheim |
131I- Sibrotuzumab |
Radiochemical purity: ≥ 95 % | Patients of colorectal carcinoma and non-small cell lung cancer | SPECT | Terminated | NCT02209727 |
| Eliane Fischer | 177Lu-ESC11/ESC14 | Kd: 10 nM/ 210 nM |
Melanoma xenograft nude mouse | SPECT/CT | Preclinical | NA |
| Darpan N. Pandya | [89Zr]Zr-Df-Bz-F19 | Radiochemical purity: ≥ 99.5 % | U87MG tumor bearing mice | PET/CT | Preclinical | NA |
| Hallie M. Hintz | [89Zr]Zr- B12-IgG |
NA | Mice bearing intratibial CWR-R1FAP | PET/CT | Preclinical | NA |
| Name | Structural Unit | HPLC Retention Time (min) | HPLC Area% Day 0 Post End of Synthesis | HPLC Area% Day 6 Post End of Synthesis |
|---|---|---|---|---|
| 177Lu-3BP-3407 | Hex-[C(tMeBn(DOTA-PP))-P-P-T-Q-F-C]-D-NH2 | 7.5 | 95.7 | 94.0 |
| 177Lu-3BP-3554 | Hex-[C(tMeBn(DOTA-AET))-P-P-T-Q-F-C]-OH | 7.6 | 97.2 | 95.6 |
| Name | Molecular Formula |
Molecular Weight |
Structural Unit | Structures (Simple) | HPLC Retention Time (min) | HEK-FAP Intake (Time) in %ID/g |
|---|---|---|---|---|---|---|
|
3BP- 3105 |
C114H169N25O33S2 | 2481.171 | Hex-[C(3MeBn)-P-P-T-E-F-C]-D-H-F-R-D-Ttds-K(DOTA)-NH2 | 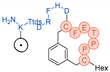 |
8.9 | + (6 h) |
|
3BP- 3168 |
C109H161N25O33S2 | 2412.110 | DOTA-Ttds-Nle-[C-(3MeBn)-P-P-T-E-F-C]-D-H-F-R-D-NH2 | 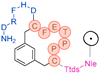 |
7.9 | + (3 h) |
|
3BP- 3320 |
C82H124N16O26S2 | 1812.841 | DOTA-Ttds-Nle-[C-(3MeBn)-P-P-T-E-F-C]-D-NH2 | 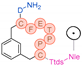 |
8.6 | + (1 h) |
|
3BP- 3321 |
C96H143N21O28S2 | 2101.993 | DOTA-Ttds-Nle-[C-(3MeBn)-P-P-T-E-F-C]-D-Pamb-R-NH2 | 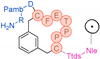 |
7.4 | + (3 h) |
|
3BP- 3275 |
C110H165N25O30S2 | 2379.820 | Hex-[C(3MeBn)-P-P-T-E-F-C]-D-H-Nmf-R-Ttds-K(DOTA)-NH2 | 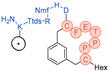 |
7.3 | + (3 h) |
|
3BP- 3397 |
C71H106N14O19S2 | 1522.720 | Hex-[C(3MeBn)-P-P-T-Q-F-C]-Bhk(DOTA)-OH | 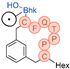 |
8.3 | ++ (24 h) |
|
3BP- 3398 |
C81H124N16O24S2 | 1768.842 | DOTA-Ttds-Nle-[C-(3MeBn)-P-P-T-Q-F-C]-Bal-OH | 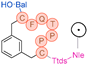 |
7.3 | + (3 h) |
|
3BP- 3407 |
C73H108N16O20S2 | 1592.737 | Hex-[C(tMeBn(DOTA-PP))-P-P-T-Q-F-C]-D-NH2 |  |
7.3 | ++ (1/24 h) |
|
3BP- 3554 |
C67H99N13O18S3 | 1469.640 | Hex-[C(tMeBn(DOTA-AET))-P-P-T-Q-F-C]-OH | 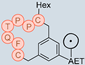 |
7.5 | ++++ (6 h) |
|
3BP- 3652 |
C67H100N14O17S3 | 1468.667 | Hex-[C(tMeBn(DOTA-AET))-P-Q-F-C]-NH2 |  |
7.3 | +++ (1 h) |
|
3BP- 3654 |
C66H99N13O16S3 | 1425.661 | Hex-[C(tMeBn(DOTA-AET))-P-P-T-Q-F-AET] | 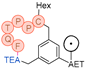 |
7.8 | + (1 h) |
|
3BP- 3656 |
C69H102N14O19S3 | 1526.665 | Hex-[C(tMeBn(DOTA-AET))-P-P-T-Q-F-C]-G-OH | 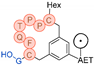 |
7.3 | - |
|
3BP- 3659 |
C70H104N14O19S3 | 1540.682 | Hex-[C(tMeBn(DOTA-AET))-P-P-T-Q-F-C]-Nmg-OH | 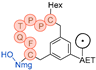 |
7.3 | - |
|
3BP- 3678 |
C65H97N13O18S3 | 1443.624 | Hex-[C(tMeBn(DOTA-AET))-Nmg-P-T-G-Q-C]-OH | 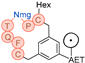 |
7.4 | + (1 h) |
|
3BP- 3692 |
C72H108N16O21S3 | 1628.706 | Pentyl-SO2-[C(tMeBn(DOTA-PP))-P-P-T-Q-F-C]-D-NH2 | 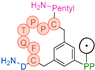 |
7.8 | + (3 h) |
|
3BP- 3767 |
C67H101N13O17S3 | 1455.666 | Hex-[C(tMeBn(DOTA-AET))-P-P-T-Q-F-Cysol] | 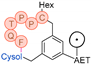 |
7.4 | ++ (1 h) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





