Submitted:
03 February 2024
Posted:
05 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Etiology:
1.2. Classification:
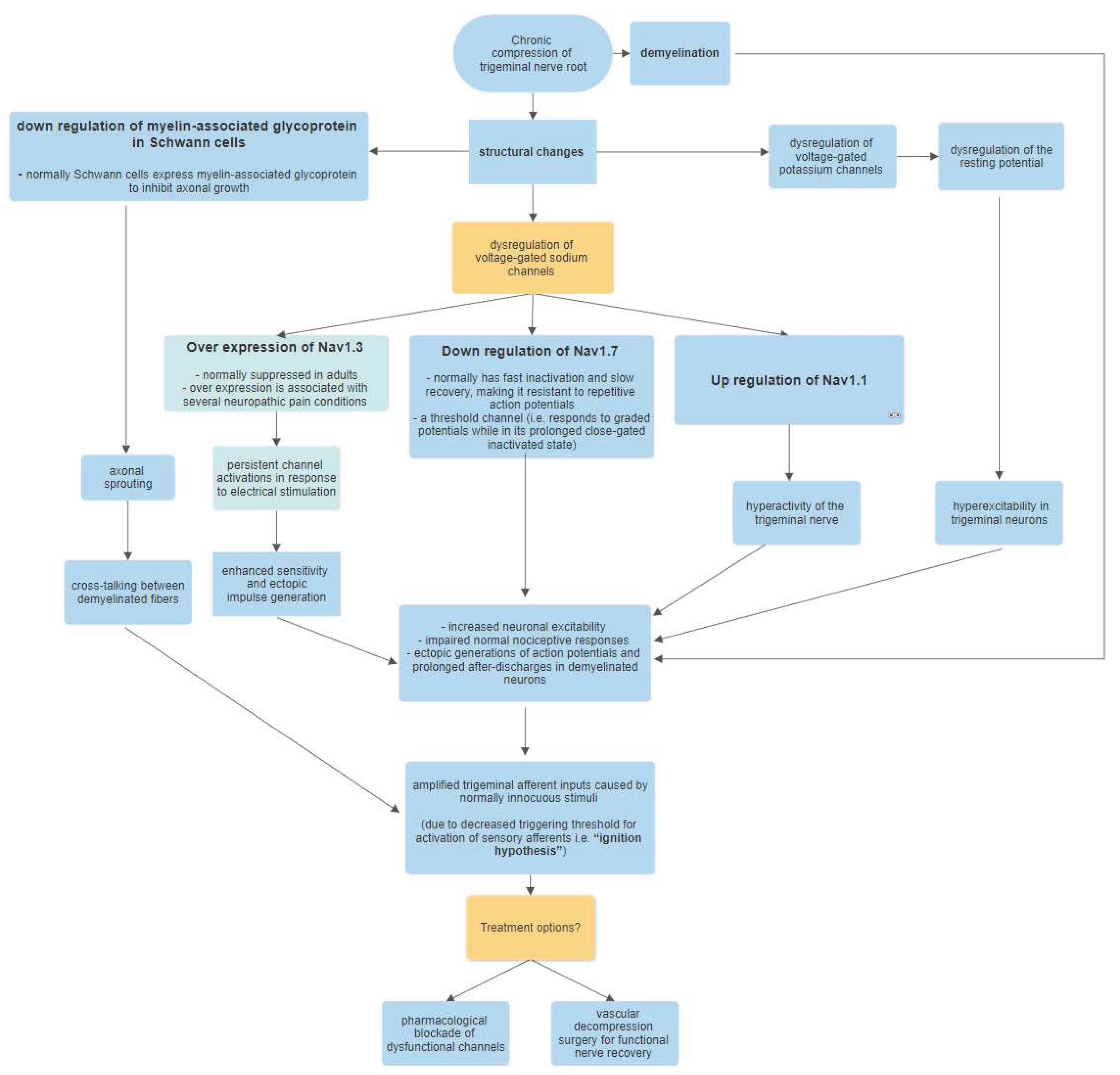
2. Pathophysiology, Molecular Process:
3. Diagnosis
- A.
- Recurrent paroxysms of unilateral facial pain in the distribution(s) of one or more divisions of the trigeminal nerve, with no radiation beyond, and fulfilling criteria B and C.
- B.
- Pain has all of the following characteristics:
- Lasting from a fraction of a second to 2 min.
- Severe intensity.
- Electric shock-like shooting, stabbing, or sharp in quality.
- C.
- Precipitated by innocuous stimuli within the affected trigeminal distribution.
- D.
- Not better accounted for by another ICHD-3 diagnosis.
4. Pharmacological Treatment
5. Neurosurgical Treatments and Outcomes
5.1. Glycerol Injection
5.2. Balloon compression
5.3. Ablation using Radiofrequency
5.4. Transcutaneous Electrical Nerve Stimulation
5.5. Peripheral Nerve Stimulation
5.6. Deep Brain Stimulation
5.7. Stereotactic Radiosurgery
5.8. Microvascular Decompression (MVD)
6. Discussion
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Katusic, S. , Beard C.M., Bergstralh E., Kurland L.T. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann. Neurol. 1990, 27, 89–95. [Google Scholar] [CrossRef]
- Cruccu, G. , Di Stefano, G., & Truini, A. Trigeminal Neuralgia. The New England journal of medicine 2020, 383, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, S. N. , & Scrivani, S. J. Trigeminal Neuralgia. Dental clinics of North America 2023, 67, 99–115. [Google Scholar] [CrossRef]
- Headache Classification Subcommittee of The International Headache Society. The International classification of headache disorders. 38. 3rd edn. Cephalalgia, 2018: 1–211.
- Siqueira SRDT, Alves B, Malpartida HMG, et al. . Abnormal expression of voltage-gated sodium channels Nav1.7, NaV1.3 and Nav1.8 in trigeminal neuralgia. Neuroscience 2009, 164, 573–7. [Google Scholar] [CrossRef]
- Obermann M, Yoon M-S, Ese D, et al. . Impaired trigeminal nociceptive processing in patients with trigeminal neuralgia. Neurology 2007, 69, 835–41. [Google Scholar] [CrossRef]
- Zakrzewska JM, Wu J, Mon-Williams M, et al. . Evaluating the impact of trigeminal neuralgia. Pain 2017, 158, 1166–74. [Google Scholar] [CrossRef] [PubMed]
- Ferneini E., M. Trigeminal Neuralgia. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 2021, 79, 2370–2371. [Google Scholar] [CrossRef]
- May, A. , & Hoffmann, J. Facial pain beyond trigeminal neuralgia. Current opinion in neurology 2021, 34, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A. , & Kondziolka, D. Trigeminal Neuralgia and Other Facial Neuralgias. Progress in neurological surgery 2019, 34, 273–278. [Google Scholar] [CrossRef]
- Liao, J. Y. , Zhou, T. H., Chen, B. K., & Liu, Z. X. Schwann cells and trigeminal neuralgia. Molecular pain 2020, 16, 1744806920963809. [Google Scholar] [CrossRef]
- Alwardian, M. , Chrysikos, D., Samolis, A., Papachristou, A., Spartalis, E., Piagkou, M., & Troupis, T. Trigeminal Neuralgia and Potential Correlations with Anatomical Variations of the Trigeminal Nerve. Acta medica academica 2021, 50, 292–299. [Google Scholar] [CrossRef]
- Bendtsen, L. , Zakrzewska, J. M., Abbott, J., Braschinsky, M., Di Stefano, G., Donnet, A., Eide, P. K., Leal, P. R. L., Maarbjerg, S., May, A., Nurmikko, T., Obermann, M., Jensen, T. S., & Cruccu, G. European Academy of Neurology guideline on trigeminal neuralgia. European journal of neurology 2019, 26, 831–849. [Google Scholar] [CrossRef]
- Di Stefano, G. , Maarbjerg, S., & Truini, A. Trigeminal neuralgia secondary to multiple sclerosis: from the clinical picture to the treatment options. The journal of headache and pain 2019, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J. L. , Domingo, R. A., Rowland, N. C., & Vandergrift Iii, W. A. Trigeminal Neuralgia Secondary to Meckel's Cave Meningoencephaloceles: A Systematic Review and Illustrative Case. Neurology India 2022, 70, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Gerwin, R. Chronic Facial Pain: Trigeminal Neuralgia, Persistent Idiopathic Facial Pain, and Myofascial Pain Syndrome-An Evidence-Based Narrative Review and Etiological Hypothesis. International journal of environmental research and public health 2020, 17, 7012. [Google Scholar] [CrossRef] [PubMed]
- Boeddinghaus, R. , & Whyte, A. Imaging of Trigeminal Neuralgia and Other Facial Pain. Neuroimaging clinics of North America 2021, 31, 485–508. [Google Scholar] [CrossRef] [PubMed]
- Ganz J., C. Trigeminal neuralgia and other cranial pain syndromes. Progress in brain research 2022, 268, 347–378. [Google Scholar] [CrossRef] [PubMed]
- Obermann, M. (2019). Recent advances in understanding/managing trigeminal neuralgia. F1000Research. [CrossRef]
- Dong, B. , Xu, R., & Lim, M. The pathophysiology of trigeminal neuralgia: a molecular review. Journal of neurosurgery 2023, 139, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A. , Maxwell, C., Gofman, N., Liebman, K., & Veznedaroglu, E. The management of trigeminal neuralgia with triptans, a narrative review of the literature. Headache 2022, 62, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. , Bansal, R. N., Singh Sodhi, S. P., & Brar, G. K. Animal models - Mimicking the pain of trigeminal neuralgia. Indian journal of pharmacology 2022, 54, 138–145. [Google Scholar] [CrossRef]
- Maltez, N. , Choi, M. Y., Troyanov, Y., Wang, M., Jantz, M., Fritzler, M. J., Baron, M., Hudson, M., & Canadian Scleroderma Research Group Trigeminal neuralgia in systemic sclerosis. Seminars in arthritis and rheumatism 2021, 51, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Kobata, H. , Kondo, A., Iwasaki, K., & Nishioka, T. Combined hyperactive dysfunction syndrome of the cranial nerves: trigeminal neuralgia, hemifacial spasm, and glossopharyngeal neuralgia: 11-year experience and review. Neurosurgery 1998, 43, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Chen Q, Yi DI, Perez JNJ, Liu M, Chang SD, Barad MJ, Lim M, Qian X. The Molecular Basis and Pathophysiology of Trigeminal Neuralgia. Int J Mol Sci. 2022, 23, 3604. [CrossRef]
- Liu, M.; Zhong, J.; Xia, L.; Dou, N.; Li, S. The expression of voltage-gated sodium channels in trigeminal nerve following chronic constriction injury in rats. Int. J. Neurosci. 2019, 129, 955–962. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, J.; Wang, Y.; Wang, L.; Wang, X. Changes in the expression of voltage-gated sodium channels Nav1.3, Nav1.7, Nav1.8, and Nav1.9 in rat trigeminal ganglia following chronic constriction injury. Neuroreport 2016, 27, 929–934. [Google Scholar] [CrossRef]
- Siqueira, S.R.; Alves, B.; Malpartida, H.M.; Teixeira, M.J.; Siqueira, J.T. Abnormal expression of voltage-gated sodium channels Nav1.7, Nav1.3 and Nav1.8 in trigeminal neuralgia. Neuroscience 2009, 164, 573–577. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. From genes to pain: Na v 1.7 and human pain disorders. Trends Neurosci. 2007, 30, 555–563. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.A.; Ikeda, R.; Jia, Z.; Ling, J.; Zuo, X.; Li, M.; Gu, J.G. KCNQ channels in nociceptive cold-sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia. Mol. Pain 2015, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Rowshan, K.; Chao, T.; Mozaffar, T.; Steward, O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp. Neurol. 2004, 187, 500–508. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Dellon, A.L.; Hudson, A.R.; Hunter, D.A. Chronic human nerve compression—A histological assessment. Neuropathol. Appl. Neurobiol. 1986, 12, 547–565. [Google Scholar] [CrossRef]
- Devor, M.; Amir, R.; Rappaport, Z.H. Pathophysiology of trigeminal neuralgia: The ignition hypothesis. Clin. J. Pain 2002, 18, 4–13. [Google Scholar] [CrossRef]
- Di Stefano G, De Stefano G, Leone C, et al. . Concomitant continuous pain in patients with trigeminal neuralgia is associated with trigeminal nerve root atrophy. Cephalalgia 2020, 40, 1502–10. [Google Scholar] [CrossRef]
- Peker, S.; Kurtkaya, O.; Uzun, I.; Pamir, M.N. Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery 2006, 59, 354–359. [Google Scholar] [CrossRef]
- Prasad, S.; Galetta, S. Trigeminal neuralgia: Historical notes and current concepts. Neurologist 2009, 15, 87–94. [Google Scholar] [CrossRef]
- Berger, B.L.; Gupta, R. Demyelination secondary to chronic nerve compression injury alters Schmidt-Lanterman incisures. J. Anat. 2006, 209, 111–118. [Google Scholar] [CrossRef]
- Di Stefano, G. , Maarbjerg, S., Nurmikko, T., Truini, A., & Cruccu, G. Triggering trigeminal neuralgia. Cephalalgia : an international journal of headache 2018, 38, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, J. M. , & Linskey, M. E. Trigeminal Neuralgia. American family physician 2016, 94, 133–135. [Google Scholar]
- Nurmikko, T. J. , & Eldridge, P. R. Trigeminal neuralgia--pathophysiology, diagnosis and current treatment. British journal of anaesthesia 2001, 87, 117–132. [Google Scholar] [CrossRef]
- Krafft R., M. Trigeminal neuralgia. American family physician 2008, 77, 1291–1296. [Google Scholar] [PubMed]
- Korczeniewska, O. A. , Kohli, D., Benoliel, R., Baddireddy, S. M., & Eliav, E. Pathophysiology of Post-Traumatic Trigeminal Neuropathic Pain. Biomolecules 2022, 12, 1753. [Google Scholar] [CrossRef]
- Stienen, M. N. , Cadosch, D., Seule, M. A., Fournier, J. Y., Hildebrandt, G., & Gautschi, O. P. Trigeminusneuralgie - Pathophysiologie, klinische Aspekte und Therapie [Trigeminal neuralgia - pathophysiology, clinical aspects and treatment]. Praxis 2010, 99, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Edlich, R. F. , Winters, K. L., Britt, L., & Long, W. B., 3rd. Trigeminal neuralgia. Journal of long-term effects of medical implants 2006, 16, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Nurmikko T., J. Pathophysiology of MS-related trigeminal neuralgia. Pain 2009, 143, 165–166. [Google Scholar] [CrossRef]
- Loeser, J. D. , Calvin, W. H., & Howe, J. F. Pathophysiology of trigeminal neuralgia. Clinical neurosurgery 1977, 24, 527–537. [Google Scholar] [CrossRef]
- Boto G., R. Neuralgia del trigémino [Trigeminal neuralgia]. Neurocirugia (Asturias, Spain) 2010, 21, 361–372. [Google Scholar] [CrossRef]
- Gardner W., J. Trigeminal neuralgia. Clinical neurosurgery 1968, 15, 1–56. [Google Scholar] [CrossRef]
- Zakrzewska, J. M. , & Linskey, M. E. Trigeminal neuralgia. BMJ (Clinical research ed.) 2015, 350, h1238. [Google Scholar] [CrossRef]
- Comi G, Filippi M, Rovaris M, Leocani L, Medaglini S, Locatelli T. Clinical, neurophysiological, and magnetic resonance imaging correlations in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998, 64 (Suppl 1), S21–S25. [Google Scholar]
- uck K, Christensen H, Bazinski M. Systemic Lidocaine for the Treatment of Pain — Adult/Pediatric — Inpatient/Ambulatory/Emergency Department Clinical Practice Guideline, 2019.
- Linskey, ME. 143 pediatric trigeminal neuralgia (TN): results with early microvascular decompression (MVD). Neurosurgery. 2017, 64, 234. [Google Scholar] [CrossRef]
- Resnick DK, Levy EI, Jannetta PJ. Microvascular decompression for pediatric onset trigeminal neuralgia. Neurosurgery. 1998, 43, 804–807, discussion 807–808. [Google Scholar] [CrossRef]
- Bendtsen L, Zakrzewska JM, Abbott J, et al. European Academy of Neurology guideline on trigeminal neuralgia. Eur J Neurol. 2019, 26, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Gambeta, E. , Chichorro, J. G., & Zamponi, G. W. Trigeminal neuralgia: An overview from pathophysiology to pharmacological treatments. Molecular pain 2020, 16, 1744806920901890. [Google Scholar] [CrossRef] [PubMed]
- Ruscheweyh, R. , Lutz, J., & Mehrkens, J. H. Trigeminusneuralgie : Moderne Diagnostik und Therapie [Trigeminal neuralgia : Modern diagnostic workup and treatment]. Schmerz (Berlin, Germany) 2020, 34, 486–494. [Google Scholar] [CrossRef]
- Wang, T. , Liu, L., Song, D., & Huang, D. Emerging roles of lncRNAs in the pathogenesis, diagnosis, and treatment of trigeminal neuralgia. Biochemical Society transactions 2022, 50, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M. , & Di Stefano, G. Novel ways of approaching the pharmacologic treatment of trigeminal neuralgia. Headache 2022, 62, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Paranathala, M. P. , Ferguson, L., Bowers, R., & Mukerji, N. Percutaneous retrogasserian glycerol rhizotomy for trigeminal neuralgia: an alternative technique. British journal of neurosurgery 2018, 32, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Piper, K. , Smith, T., Saez-Alegre, M., Jean, W., Bezchlibnyk, Y., & Van Loveren, H. Does Head Positioning After Percutaneous Glycerol Rhizotomy for Trigeminal Neuralgia Matter? World neurosurgery, 2023. [Google Scholar] [CrossRef]
- Joswig, H. , Staudt, M. D., MacDougall, K. W., & Parrent, A. G. Effect of Training on Percutaneous Glycerol Rhizotomy for Trigeminal Neuralgia: A Long-Term, Retrospective Comparison of Staff Neurosurgeon and Trainee Complications and Efficacy. World neurosurgery 2020, 134, e1001–e1007. [Google Scholar] [CrossRef] [PubMed]
- Xu-Hui W, Chun Z, Guang-Jian S, et al. Long-term outcomes of percutaneous retrogasserian glycerol rhizotomy in 3370 patients with trigeminal neuralgia. Turk Neurosurg. 2011, 21, 48–52. [Google Scholar]
- Piper, K. , George, Z., Gordon, J., Peto, I., Vakharia, K., & Van Loveren, H. Clival-Meckel's Cave Angle: A Predictor of Glycerol Displacement in Percutaneous Glycerol Rhizotomy for Trigeminal Neuralgia. Operative neurosurgery (Hagerstown, Md.), 2023. [Google Scholar] [CrossRef]
- Aljuboori, Z. , & Nauta, H. J. Multiple Recurrences of Trigeminal Neuralgia Caused by Deformation of the Trigeminal Nerve. Cureus 2019, 11, e6433. [Google Scholar] [CrossRef]
- Goel, A. , Kulkarni, G., Cotici, A., Paluzzi, A., Hayton, T., & Chelvarajah, R. Volume maximised glycerol rhizolysis for trigeminal neuralgia: a single centre analysis of outcomes. British journal of neurosurgery, 2023; 1–6. [Google Scholar] [CrossRef]
- Cordeiro, K. , Kim, J., Buckley, N., Kraemer, M., Pun, C., & Resnick, D. Pterygoid venous plexus anastomosis in trigeminal percutaneous glycerol rhizotomy: illustrative case. Journal of neurosurgery. Case lessons 2023, 6, CASE23173. [Google Scholar] [CrossRef]
- Krishnan, S. , Bigder, M., & Kaufmann, A. M. Long-term follow-up of multimodality treatment for multiple sclerosis-related trigeminal neuralgia. Acta neurochirurgica 2018, 160, 135–144. [Google Scholar] [CrossRef]
- Xia, Y. , Yu, G., Min, F., Xiang, H., Huang, J., & Leng, J. The Focus and New Progress of Percutaneous Balloon Compression for the Treatment of Trigeminal Neuralgia. Journal of pain research 2022, 15, 3059–3068. [Google Scholar] [CrossRef]
- Nascimento, R. F. V. , Pipek, L. Z., & de Aguiar, P. H. P. Is percutaneous balloon compression better than microvascular decompression to treat trigeminal neuralgia? A systematic review and meta-analysis. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 2023, 109, 11–20. [Google Scholar] [CrossRef]
- Brown JA, Hoeflinger B, Long PB, et al. Axon and ganglion cell injury in rabbits after percutaneous trigeminal balloon compression. Neurosurgery. 1996, 38, 993–1003. [CrossRef]
- Skirving DJ, Dan NG. A 20-year review of percutaneous balloon compression of the trigeminal ganglion. J Neurosurg. 2001, 94, 913–917, discussion 1003–4. [Google Scholar] [CrossRef]
- Abdennebi B, Guenane L. Technical considerations and outcome assessment in retrogasserian balloon compression for treatment of trigeminal neuralgia. Series of 901 patients. Surg Neurol Int. 2014, 5, 118. [Google Scholar] [CrossRef]
- Leclerc, A. , Salkine, M. F., & Emery, E. Percutaneous balloon compression for trigeminal neuralgia: a how I do it. Acta neurochirurgica 2022, 164, 2939–2943. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R. B. , Ali, A., Mandel, M., Muhsen, B., Adada, B., Borghei-Razavi, H., & Obrzut, M. Trigeminal Neuralgia-Step-by-Step DYNA-Computed Tomography-Assisted Balloon Compression Rhizotomy. World neurosurgery 2023, 171, 84. [Google Scholar] [CrossRef] [PubMed]
- Sweet WH, Wepsic JG. Controlled thermocoagulation of trigeminal ganglion and rootlets for differential destruction of pain fibers. 1. Trigeminal neuralgia. J Neurosurg. 1974, 40, 143–156. [CrossRef]
- Eskandar, E. , Kumar, H., Boini, A., Velasquez Botero, F., El Hunjul, G. N., Nieto Salazar, M. A., Quinonez, J., Dinh, B., & Mouhanna, J. E. The Role of Radiofrequency Ablation in the Treatment of Trigeminal Neuralgia: A Narrative Review. Cureus 2023, 15, e36193. [Google Scholar] [CrossRef]
- Abd-Elsayed, A. , Martens, J. M., Fiala, K. J., & Izuogu, A. Pulsed Radiofrequency for the Treatment of Trigeminal Neuralgia. Current pain and headache reports 2022, 26, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Orhurhu, V. , Sidharthan, S., Roberts, J., Karri, J., Umukoro, N., Hagedorn, J. M., Odonkor, C. A., & Abd-Elsayed, A. Radiofrequency Ablation for Craniofacial Pain Syndromes. Physical medicine and rehabilitation clinics of North America 2021, 32, 601–645. [Google Scholar] [CrossRef] [PubMed]
- Wu CY, Meng FG, Xu SJ, Liu YG, Wang HW. Selective percutaneous radiofrequency thermocoagulation in the treatment of trigeminal neuralgia: report on 1860 cases. Chin Med J (Engl). 2004, 117, 467–470. [CrossRef]
- Kanpolat Y, Savas A, Bekar A, Berk C. Percutaneous controlled radiofrequency trigeminal rhizotomy for the treatment of idiopathic trigeminal neuralgia: 25-year experience with 1600 patients. Neurosurgery. 2001, 48, 524–532, discussion 532–524. [CrossRef]
- Chakole, V. , Sharma, K., Tople, J., Akre, S., & Wanjari, M. B. Radiofrequency Ablation of Gasserian Ganglion in Trigeminal Neuralgia With Multiple Sclerosis: A Rare Clinical Case. Cureus 2022, 14, e32595. [Google Scholar] [CrossRef]
- Gusmao S, Oliveira M, Tazinaffo U, Honey CR. Percutaneous trigeminal nerve radiofrequency rhizotomy guided by computerized tomography fluoroscopy. Technical note. J Neurosurg. 2003, 99, 785–786. [CrossRef]
- Mansano, A. M. , Frederico, T. N., Valentin, R. E. B., Carmona, M. J. C., & Ashmawi, H. A. Percutaneous Radiofrequency Ablation for Trigeminal Neuralgia Management: A Randomized, Double-Blinded, Sham-Controlled Clinical Trial. Pain medicine (Malden, Mass.) 2023, 24, 234–243. [Google Scholar] [CrossRef]
- Zheng, Y. , Liu, C. W., Hui Chan, D. X., Kai Ong, D. W., Xin Ker, J. R., Ng, W. H., & Wan, K. R. Neurostimulation for Chronic Pain: A Systematic Review of High-Quality Randomized Controlled Trials With Long-Term Follow-Up. Neuromodulation : journal of the International Neuromodulation Society 2023, 26, 1276–1294. [Google Scholar] [CrossRef]
- Yameen, F. , Shahbaz, N. N., Hasan, Y., Fauz, R., & Abdullah, M. Efficacy of transcutaneous electrical nerve stimulation and its different modes in patients with trigeminal neuralgia. JPMA. The Journal of the Pakistan Medical Association 2011, 61, 437–439. [Google Scholar]
- Zayan, K. , Felix, E. R., & Galor, A. Transcutaneous Electrical Nerve Stimulation for Facial Pain. Progress in neurological surgery 2020, 35, 35–44. [Google Scholar] [CrossRef]
- Singla, S. , Prabhakar, V., & Singla, R. K. Role of transcutaneous electric nerve stimulation in the management of trigeminal neuralgia. Journal of neurosciences in rural practice 2011, 2, 150–152. [Google Scholar] [CrossRef]
- Motwani, M. , Fadnavis, A., & Dhole, A. Efficacy of transcutaneous electrical nerve stimulation (TENS) in the management of trigeminal neuralgia: A systematic review and meta-analysis. Journal of clinical and experimental dentistry 2023, 15, e505–e510. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y. , Yang, L., Han, R., Guo, G., Huang, S., Weng, L., Wang, X., Li, Z., Huang, D., Hu, R., & Zhou, H. Implantable Peripheral Nerve Stimulation for Trigeminal Neuropathic Pain: A Systematic Review and Meta-Analysis. Neuromodulation : journal of the International Neuromodulation Society 2021, 24, 983–991. [Google Scholar] [CrossRef]
- Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967, 155, 108–109. [CrossRef]
- Meyerson BA, Hakansson S. Alleviation of atypical trigeminal pain by stimulation of the Gasserian ganglion via an implanted electrode. Acta Neurochir Suppl (Wien). 1980, 30, 303–309.
- Ellis JA, Mejia Munne JC, Winfree CJ. Trigeminal branch stimulation for the treatment of intractable craniofacial pain. J Neurosurg. 2015, 123, 283–288. [CrossRef]
- Klein, J. , Siepmann, T., Schackert, G., Ziemssen, T., & Juratli, T. A. Peripheral nerve field stimulation in medically refractory trigeminal neuralgia attributed to multiple sclerosis. Journal of neurosurgery 2020, 134, 1244–1250. [Google Scholar] [CrossRef]
- Chang M., C. Efficacy of Pulsed Radiofrequency Stimulation in Patients with Peripheral Neuropathic Pain: A Narrative Review. Pain physician 2018, 21, E225–E234. [Google Scholar] [CrossRef]
- Chung, M. , & Huh, R. Neuromodulation for Trigeminal Neuralgia. Journal of Korean Neurosurgical Society 2022, 65, 640–651. [Google Scholar] [CrossRef]
- Nandi D, Aziz T, Carter H, Stein J. Thalamic field potentials in chronic central pain treated by periventricular gray stimulation – a series of eight cases. Pain. 2003, 101, 97–107. [CrossRef]
- Franzini, A. , Messina, G., Cordella, R., Marras, C., & Broggi, G. Deep brain stimulation of the posteromedial hypothalamus: indications, long-term results, and neurophysiological considerations. Neurosurgical focus 2010, 29, E13. [Google Scholar] [CrossRef]
- Ben-Haim, S. , Mirzadeh, Z., & Rosenberg, W. S. Deep brain stimulation for intractable neuropathic facial pain. Neurosurgical focus 2018, 45, E15. [Google Scholar] [CrossRef]
- Leksell, L. Sterotaxic radiosurgery in trigeminal neuralgia. Acta chirurgica Scandinavica 1971, 137, 311–314. [Google Scholar]
- De La Peña, N. M. , Singh, R., Anderson, M. L., Koester, S. W., Sio, T. T., Ashman, J. B., Vora, S. A., & Patel, N. P. High-Dose Frameless Stereotactic Radiosurgery for Trigeminal Neuralgia: A Single-Institution Experience and Systematic Review. World neurosurgery 2022, 167, e432–e443. [Google Scholar] [CrossRef]
- Marchetti, M. , Pinzi, V., De Martin, E., Ghielmetti, F., & Fariselli, L. Radiosurgery for trigeminal neuralgia: the state of art. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 2019; 40, 153–157. [Google Scholar] [CrossRef]
- Tuleasca, C. , Régis, J., Sahgal, A., De Salles, A., Hayashi, M., Ma, L., Martínez-Álvarez, R., Paddick, I., Ryu, S., Slotman, B. J., & Levivier, M. Stereotactic radiosurgery for trigeminal neuralgia: a systematic review. Journal of neurosurgery 2018, 130, 733–757. [Google Scholar] [CrossRef]
- Tuleasca, C. , Régis, J., Sahgal, A., De Salles, A., Hayashi, M., Ma, L., Martínez-Álvarez, R., Paddick, I., Ryu, S., Slotman, B. J., & Levivier, M. Stereotactic radiosurgery for trigeminal neuralgia: a systematic review. Journal of neurosurgery 2018, 130, 733–757. [Google Scholar] [CrossRef]
- Matsuda, S. , Serizawa, T., Sato, M., & Ono, J. Gamma knife radiosurgery for trigeminal neuralgia: the dry-eye complication. Journal of neurosurgery, 2002; 97, 525–528. [Google Scholar] [CrossRef]
- Carnochan J., M. On Tic Douloureux: "The Painful Affection of the Face, Dolor Faciei Crucians," of Fothergill, with a New Operation for Its Cure. The American journal of dental science 1860, 10, 254–278. [Google Scholar] [PubMed]
- Tan, T. C. , & Black, P. M. Sir Victor Horsley (1857-1916): pioneer of neurological surgery. Neurosurgery 2002, 50, 607–612. [Google Scholar] [CrossRef]
- Patel, S. K. , & Liu, J. K. Overview and History of Trigeminal Neuralgia. Neurosurgery clinics of North America 2016, 27, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Dandy W., E. THE TREATMENT OF TRIGEMINAL NEURALGIA BY THE CEREBELLAR ROUTE. Annals of surgery 1932, 96, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A. M. , & Price, A. V. A history of the Jannetta procedure. Journal of neurosurgery 2019, 132, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Yang, L. , & Cheng, H. Surgical technique management of microvascular decompression for trigeminal neuralgia. Sebészi technikák a trigeminusneuralgia microvascularis dekompresszióval történő kezeléséhez. Ideggyogyaszati szemle 2022, 75, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Barker, F. G. , 2nd, Jannetta, P. J., Bissonette, D. J., Larkins, M. V., & Jho, H. D. The long-term outcome of microvascular decompression for trigeminal neuralgia. The New England journal of medicine 1996, 334, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D. T. , Benedetto, N., & Perrini, P. Clinical outcome after microvascular decompression for trigeminal neuralgia: a systematic review and meta-analysis. Neurosurgical review 2022, 46, 8. [Google Scholar] [CrossRef]
| Drug | Dose | Monitoring |
|---|---|---|
| Carbamazepine | 50 mg twice daily (for people over 65), 100 milligrams twice daily (for younger people) | Track baseline LFTs, CBC, and salt levels. |
| Oxcarbazepine | 150 mg twice daily (beginning) Twice daily, 300–600 mg | Monitor sodium, HLA-B*1502 variant screening |
| Levetiracetam | 3000–5000 mg per day, BID or TID | NA |
| Gabapentin | 300–1200 mg TID | NA |
| Valproate | 500–1500mg per day | Total and free valproate level, LFTs, CBC, ammonia |
| Lamotrigine | 100mg BID | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





