1. Introduction
Visual perception is the culmination of neural processes triggered by the retinal image and this has long been known to be affected by age. Depth perception, estimated by stereoacuity tests, is reduced in the older population [
1,
2,
3,
4,
5,
6,
7] and has been cited as contributing to the higher incidence of falls in the elderly.[
8,
9] Several factors influence stereoacuity including aniseikonia and interocular differences in visual acuity.[
2,
3,
4,
5,
6,
7,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21] Aniseikonia describes the condition when the two retinal images are unequal in size and/or shape.[
15] Therefore, an increase in the disparity between the two retinal images should enhance aniseikonia and affect stereoacuity. Interocular differences in the refractive errors and eyeball lengths augment this disparity and carry the potential to reduce stereoacuity.[
2,
3,
4,
5,
6,
7,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21] A person staring at a stationary target remains oblivious to the involuntary eye movements that cause the retinal images to shift by upto 20µ.[
22] In addition, involuntary blinking, changes in the tear film and oscillations in the optical properties of the intraocular structures further contribute to changes in the dimensions and overall qualities of the retinal images.[
23,
24,
25] The constantly changing retinal images may not affect the measurement of visual acuity,[
22] but any interocular differences in the dynamics and rates of change of these images could impact on the assessment of stereoacuity. The higher order optical aberrations of the eyes (HOAs) have been cited as affecting aniseikonia.[
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29] The HOAs are objective measurements and are pupil size related whereas the clinical assessment of aniseikonia, and stereoacuity, is subjective. Both pupil size and refractive error change with age.[
30,
31,
32,
33,
34] Therefore, interocular differences in the objectively derived values of HOAs may contribute to any association between the subjective measures of stereoacuity and age. However, if these objective factors are not correlated with stereoacuity then, any age-related in this psychological aspect of vision must stem from other sources.
If a correlation between stereoacuity and age occurs in asymptomatic older people in the absence of aniseikonia and other interocular differences then, by a process of elimination, the cause must be due to other factors. The aim of this study was to determine if there were detectable relationships between clinical measures of stereoacuity and age in habitual spectacle wearing presbyopes free of any symptoms linked to binocular vision, and to determine if the stereoacuity correlated with aniseikonia, interocular differences in refractive error, HOAs, pupil size and visual acuities.
2. Methods
2.1. Study Design
A partially masked, semi-randomized, case-by-case, clinical investigation, approved by the Ethics committee at the Speciality Eye Hospital Svjetlost in Zagreb. The tenets of the Helsinki agreement were followed throughout. All subjects provided signed consent after they were fully informed of the purpose of the study. All subjects were examined, where appropriate, by the same clinical team. The subjects were patients attending for routine eye examinations.
2.2. Inclusion Criteria
Subjects with significant distance refractive error (sphere and/or astigmatic correction outside the range ±0.50D), requiring a presbyopic correction, free of any signs or symptoms associated with binocular vision anomalies and corrected distance visual acuity of logMAR 0.3 or better.
2.3. Exclusion Criteria
Subjects with a history of ocular surgery, binocular vision anomalies, systemic conditions known to affect quality of mono- or binocular vision, corneal dystrophies and degenerations, age related macular degeneration, glaucoma, macular disease, diabetic retinopathy, orthoptic treatment, tropias, amblyopia, unusual pupil shape or anisicoria. Anisometropia >2D sphere and/or astigmatism >1D. Emmetropia or corrected distance visual acuity worse than logMAR 0.3.
2.4. Refraction
All subjects underwent subjective refraction and astigmatism was assessed using a Jackson cross-cylinder. The best corrected logMAR visual acuity was measured using distance and near charts (CSO Visio Chart Mod. CVC 01, Firenze, Italy).
2.5. Stereoacuity
Stereoacuity, with the subject’s best spectacle correction in place, was assessed at distance with a Randot Stereotest having a range from 20 to 640 arcsecs (ʺ) in steps of 20ʺ [CSO, Visio Chart CVC03 v2.0.0, Firenze, Italy], then at 40cm with a binocular +2.50D near addition in place using a near Randot Stereotest with a range from 20 to 400ʺ in steps of 20ʺ [Precision Vision, Woodstock, USA]. These tests are stereograms constructed using a polarizing vectograph process resulting in a disparity between binocularly viewed targets. The just noticeable change in stereoacuity associated with these tests is 20” or more. The subject was asked to point out which target stood out as appearing either closer or further away from the rest, whilst wearing the test cross-polarized glasses. The test was halted once the subject made two consecutive errors. The stereoacuity value recorded was the value of the setting where the subject correctly identified the target (closer or further away) prior to making the two mistakes.
2.6. Aniseikonia
Aniseikonia was measured at 6m using the Awaya test [
35] with the subject wearing customised red-green glasses over their spectacle correction. The subject was asked to look at two red and green vertically orientated semi-circles of different sizes, the size of one of the semi-circles was adjusted until the subject reported they both appeared to be the same size. The changes in size in this test are in steps of 1%, therefore the noticeable value of aniseikonia is 1% or more. The magnitude of aniseikonia was the size of the image viewed by the right eye as a percentage (larger or smaller) compared with the size of the image viewed by the left eye. The percentage value of any aniseikonia was recorded as positive when the semi-circle seen by the right eye was larger than the semi-circle seen by the left eye, and negative if it was smaller. The test is constructed to measure aniseikonia in the vertical meridian. The test was modified to measure aniseikonia along the vertical meridian and then along the horizontal meridian.
2.7. Axial Length and Pupil Diameter
The eyeball lengths and pupil diameters were measured using IOL master 700 (Carl Zeiss Meditec AG, Jena, Germany). The mean value of 6 consecutive readings, each separated by a blink, were recorded for the right eye then the left.
2.8. Abberometry
The ocular higher order aberrations (HOAs) were measured using an aberrometer based on Hartman-Shack principles [L80 wave+TM, Luneau SAS, Prunay-le-Gillon, France]. The subject was dark adapted for at least ten minutes to allow the pupils to dilate naturally prior to measurement of HOAs. The specific HOAs recorded were coma, trefoil and spherical aberration for pupil diameters of 3mm and 5mm. Three consecutive readings, separated by a blink, were taken for the right eye then the left. For each eye, the average values for coma, trefoil and spherical aberration for 3mm and 5mm pupils were recorded for later analysis.
2.9. Data and Statistical Analysis
All data were stored on an Excel spreadsheet [Microsoft, Redmond, WA] prior to analysis.
2.10. Treatment of Refractive Data
Each spectacle prescription was reduced to the corresponding B, M, J
0 and J
45 vectors, in accordance with standard methods described elsewhere.[
36,
37] Values describing anisometropia were indicated by the root mean square (RMS) interocular differences in these vectors (ΔB, ΔM, ΔJ
0 and ΔJ
45).
2.11. Treatment of Aniseikonia Data
The vertical and horizontal measurements of aniseikonia were treated as mutually perpendicular sides of a right-angled triangle and the length of the hypotenuse was interpreted as a single figure representing the resultant aniseikonia perceived by the subject.
Data were assessed to determine the significance of any association, where appropriate, using either Spearman’s rho [rs] or Pearson correlation coefficient [r], between:
1) Stereoacuity, at distance and near, and i) subject age; ii) aniseikonia; iii) anisometropia as indicated by the ΔB,ΔM, ΔJ0, and ΔJ45 difference vectors; iv) interocular differences in HOAs, axial lengths, pupil diameters or corrected visual acuities.
2) Aniseikonia and i) subject age, ii) anisometropia as indicated by the ΔB, ΔM, ΔJ0, and ΔJ45 difference vectors; iii) interocular differences in HOAs, axial lengths, pupil diameters or corrected visual acuities.
In addition, data would be subjected to multiple linear regression if stereoacuity and/or aniseikonia values were significantly associated with more than one factor. Data sets were assessed for normality using the Kolmogorov Smirnov test and subjected to non-parametric tests where appropriate. A comparison was considered statistically significant when p˂.05. This was adjusted using a modification of the basic Bonferroni correction.[
38]
3. Results
Ninety-one subjects, 61 females and 30 males, were recruited during this study. The mean (±sd, 95% CI) age of the subjects was 56.2 years (±8.10, 54.6 to 57.9). Chief details of the refractive errors, the corrected visual acuities, stereoacuities (distance and near), aniseikonia (vertical, horizontal, and resultant), eyeball lengths, HOAs and results of vector analysis are shown in
Table 1,
Table 2,
Table 3 and
Table 4. The ranges of distance sphere and astigmatic powers, in positive format, were -13.00DS to +5.00DS and 0.00DC to +4.00DC respectively.
3.1. Stereoacuity
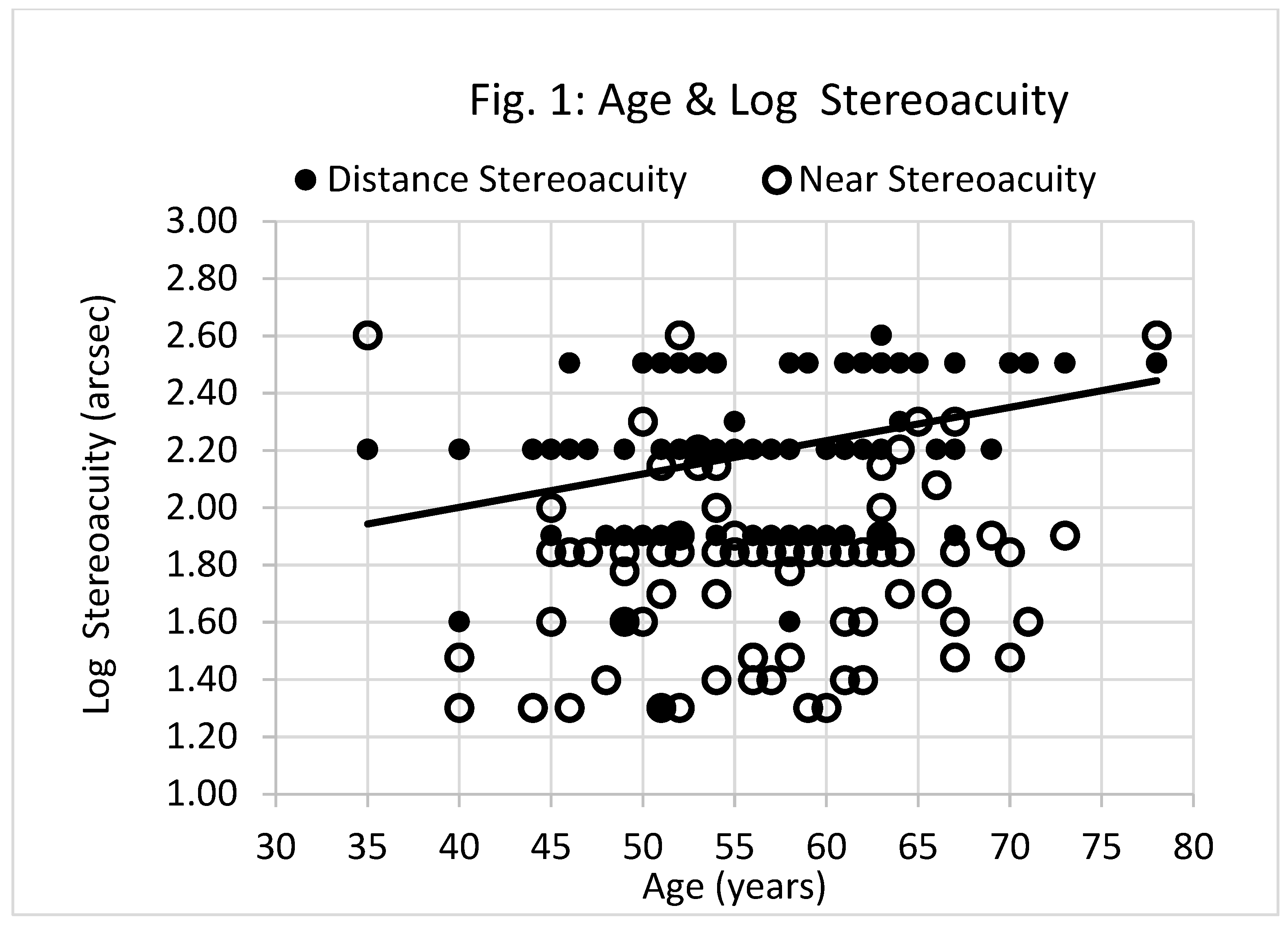
Figure 1 shows there was a significant association between subject age and log distance stereoacuity acuity (Spearman Rank r
s = 0.372, p<.01), but not between age and near stereoacuity acuity (Spearman Rank r
s = 0.169, p=.106). The least squares regression line describing the relationship between age and log distance stereoacuity acuity is shown in
Figure 1.
Stereoacuity (both distance and near) and aniseikonia were not associated with any descriptors of anisometropia, root mean square (RMS) interocular differences in eyeball lengths, pupil diameters or HOAs (p>0.05). The Holm’s sequential Bonferroni procedure [
38], formulated to improve the statistical power of the Bonferroni procedure for multiple hypothesis tests, revealed significant correlations between,
a) log distance stereoacuity and age (p<0.01), the RMS interocular difference in the corrected logMAR distance visual acuities (Spearman Rank rs = 0.246, p=.036),
b) log near stereoacuity and RMS interocular difference in the corrected logMAR distance (Spearman Rank rs = 0.400, p<.01), and near visual acuities (Spearman Rank rs = 0.421, p<0.01).
Multiple-linear regression revealed the association between log distance stereoacuity (y
1), age (x
1) and RMS interocular difference in the corrected logMAR distance visual acuities (x
2) is described by:
The association between log near stereoacuity (y
2), x
2 and RMS interocular difference in the corrected logMAR near visual acuities (x
3) is described by:
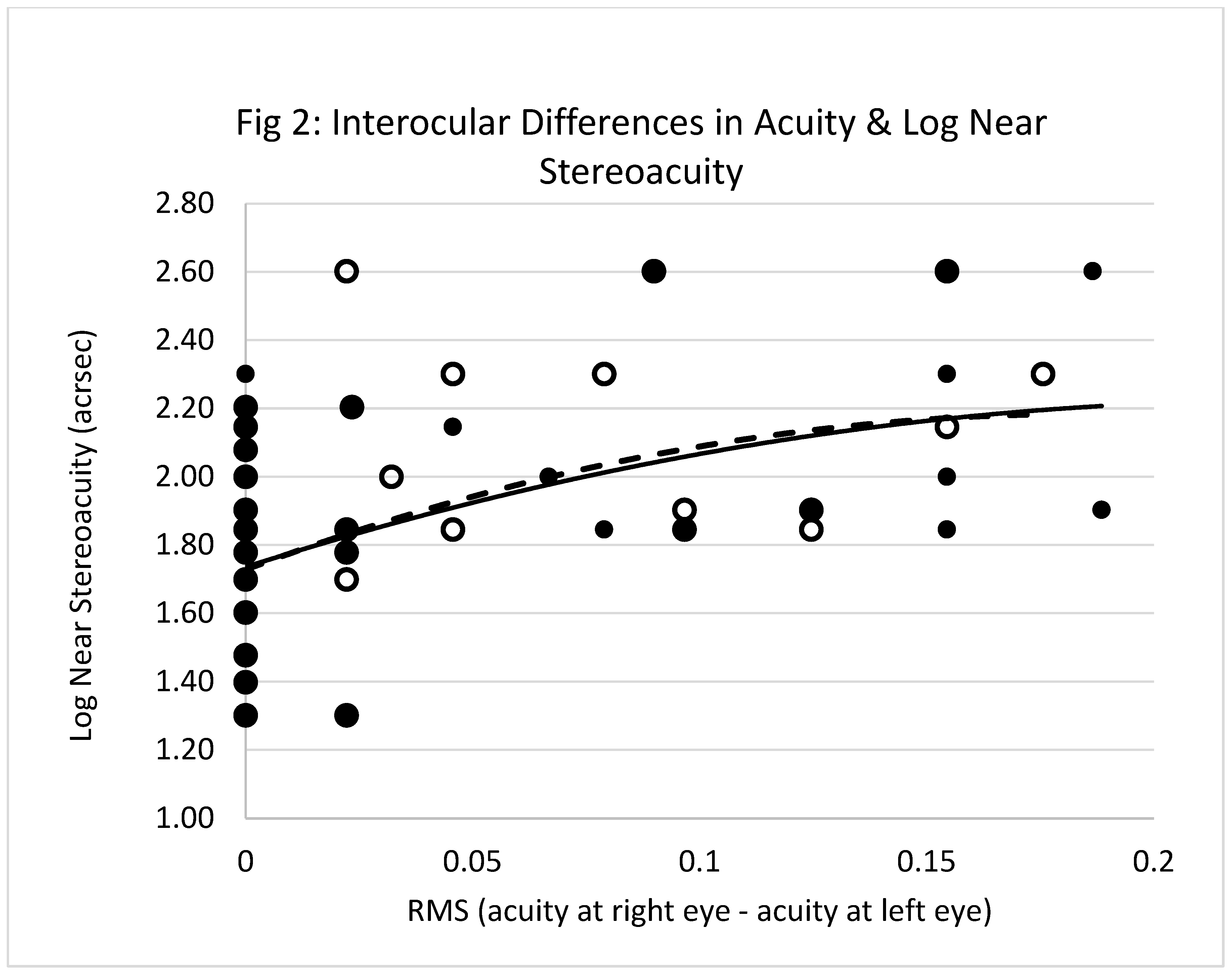
Figure 2 shows the best fit least squares single regression lines describing the relationships between, x
2, x
3 and y
2. These are described by:
3.2. Aniseikonia
Linear regression analysis did not reveal any significant associations between aniseikonia and age, any descriptors of anisometropia, interocular differences in eyeball lengths, pupil diameters or HOAs.
4. Discussion
The association between distance stereoacuity and age, as shown in
Figure 1, supports previous findings.[
1,
2,
3,
4,
5,
6,
7] There was a similar trend between near stereoacuity and age, but this was not statistically significant.
Table 1,
Table 3 and
Table 4show, there were no significant inter-ocular differences in the average refractive errors, axial lengths, pupil diameters, CDVA, CNVA, HOAs and descriptors of anisometropia. Furthermore, the RMS interocular differences in the objectively derived measures were not associated with either stereoacuity or aniseikonia. Measurements of the HOAs in the 91 asymptomatic older subjects did not reveal any model that could be used to predict stereoacuity. The repeatability of the Awaya test is about ±2% [
39,
40] and this is on par with the median values of aniseikonia noted in
Table 2. Therefore, the measured aniseikonia values had a small, if any, influence on measured stereoacuity. All psycho-physical tests are fraught with limitations and the estimation of stereoacuity can be instrument dependent.[
41] This is not unusual considering the outcomes of most such tests depend on factors such as prevailing test conditions,[
42] intricacies of instrument design,[
40] subject alertness, duration of the test, the process used to elicit responses from subjects and ambient factors.[
43] Nevertheless, stereoacuity was confirmed to be significantly associated with the interocular differences in the corrected visual acuities.
The HOAs, refractive errors and visual acuities are pupil dependent, and it could be argued that not controlling pupil size obscured the detection of real associations. The mean pupil sizes vary between 2.6mm and 3.5mm in presbyopes when reading under well-lit conditions.[
44]
Table 1 shows the pupil diameters of the subjects assessed during the study fell within this range, yet it could be argued that interocular variations in the shapes of pupils could have contributed to the variance in the data. None of the subjects had any obvious signs of anisocoria. Recruiting then training subjects, observing them under rigid laboratory conditions using fine precision-controlled techniques for evaluating both stereoacuity and aniseikonia could have resulted in establishing significant associations. However, as stated from the outset, the aim of this study was to determine if such associations were detectable in asymptomatic habitual spectacle wearing presbyopes assessed in a real-world clinical setting, not an artificial tightly controlled laboratory environment.
Near stereoacuity was influenced by interocular differences in both CDVA and CNVA.
Figure 2 shows there was a gradual decline in near stereoacuity as the gap between right and left eye corrected acuities widened. This further supports previous findings though these other reports were more centred on interocular differences in retinal blur rather than visual acuity per se.[
2,
3,
4,
5,
6,
7,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20] This interpretation depends on our understanding and definition of blur. Interocular differences in low contrast acuity, or contrast sensitivity curves, could be considered as indicative of interocular differences in blur may also be associated with stereoacuity. A drawback of this investigation is that neither low contrast acuity measurements nor contrast sensitivity assessments were included from the outset. A recent, and extensive, review paper by Horwood claimed that
‘Blur is the subjective awareness that the edges of a high contrast image are indistinct.’ [45] Thus, differences in high contrast acuity can be translated as differences in blur. Therefore, it is reasonable to interpret the associations between stereoacuity and interocular differences in acuity as indicative of the
relationship between stereoacuity and interocular differences in blur.
One of the axioms governing regression analysis requires the dependant variable (x) to be standardized, that is, controlled. Clearly,
Figure 1 and
Figure 2 show there maybe trends between stereoacuity, age and interocular differences in the corrected visual acuities, and the corresponding r
s values confirm the significance of some of these trends.
Hence, it is practical to apply linear regression analysis to determine some form of valuation that signifies the relevance of any combination of trends.
The value of r2 in eq.1 implies that age and interocular differences in CDVA contribute towards 17% of the variance in distance stereoacuity, and the value of r2 in eq.2 suggests the interocular differences in CNVA account for about 24% of the variance in near stereoacuity. Interocular differences in the corrected visual acuities influence the clinical measurement of stereoacuity, but other factors are also influencing this measurement.
Eq.1 predicts distance stereoacuity values of 98.5ʺ and 210ʺ at ages of 40 and 70 years when the interocular difference in the CDVA is zero. Distance depth perception is approximately halved over a span of 30 years. This change is not related to any of the ocular factors considered in the study. These values more than double when the interocular difference in the CDVA is 0.3. Eq. 2 predicts near stereoacuity of 53ʺ when the interocular differences in CDVA and CNVA are zero. The clinical assessment of near stereoacuity deteriorates by more than 3 times when the interocular difference in CDVA and CNVA are 0.3 and zero respectively. Such differences in the perception of depth may predispose the healthy aged individual to mislocate objects in visual space and, in extreme cases, misstep leading to falls.
5. Conclusion
In asymptomatic presbyopic spectacle wearers, a) an association between distance stereoacuity and age is influenced by interocular differences in CDVA, b) near stereoacuity is associated with interocular differences in both CDVA and CNVA, c) stereoacuity is not linked with interocular differences in pupil sizes, HOAs or clinical measures of aniseikonia.
Author Contributions
Conceptualization and validation, I.M.,S.L.,A.B.,S.P.,M.B.,A.B., N.G. Results and analysis, I.M., S.L., S.P.A.B.,M.B. Collection of clinical data, I.M., S.L., A.B., M.B., A.B., N.G. Original manuscript preparation, I.M., S.P. Review, comments and editing, I.M.,S.L.,A.B.,S.P.,M.B.,A.B.,N.G. All authors have read and agreed to the publication of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee at the Speciality Eye Hospital Svjetlost, Zagreb. Signed consent was obtained from all subjects after they were fully informed of the purpose of the study.
Data Availability Statement
Data are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pesala, V.; Garg, P.; Bharadwaj, S.R. Image quality analysis of pseudophakic eyes with uncorrected astigmatism. Optom Vis Sci. 2014, 91, 444–451. [Google Scholar] [CrossRef]
- Westheimer, G.; McKee, S.P. Stereogram design for testing local stereopsis. Invest Ophthalmol Vis Sci. 1980, 19, 802–809. [Google Scholar]
- Lovasik, J.V.; Szymkiw, M. Effects of aniseikonia, anisometropia, accommodation, retinal illuminance, and pupil size on stereopsis. Invest Ophthalmol Vis Sci. 1985, 26, 741–750. [Google Scholar] [PubMed]
- Evans, B.J. Monovision: a review. Ophthalmic Physiol Opt. 2007, 27, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.P. Sensitivity of random dot stereoacuity and Snellen acuity to optical blur. Optom Vis Sci. 1994, 71, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.I.; Hove, M.; McCloskey, C.L.; Kaye, S.B. The effect of monocularly and binocularly induced astigmatic blur on depth discrimination is orientation dependent. Optom Vis Sci. 2005, 82, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.E.; Johnson, D.; Fischer, N. Anisometropia and binocularity. Ophthalmology 1996, 103, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Saftari, L.N.; Kwon, O.S. Ageing vision and falls: a review. J Physiol Anthropol. 2018, 37, 11. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Czanner, G.; Harding, S.; Newsham, D.; Robinson, J. Visual risk factors for falls in older adults: a case-control study. BMC Geriatr. 2022, 22, 134. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Bansal, A.; Prakash, P. Randot stereoacuity at various binocular combinations of Snellen acuity. Indian J Ophthalmol. 1997, 45, 169–171. [Google Scholar]
- Laframboise, S.; De Guise, D.; Faubert, J. Effect of aging on stereoscopic interocular correlation. Optom Vis Sci. 2006, 83, 589–593. [Google Scholar] [CrossRef]
- Larson, W.L.; Lachance, A. Stereoscopic acuity with induced refractive errors. Am J Optom Physiol Opt. 1983, 60, 509–513. [Google Scholar] [CrossRef]
- Atchison, D.A.; Schmid, K.L.; Haley, E.C.; Liggett, E.M.; Lee, S.J.; Lu, J.; Moon, H.J.; Baldwin, A.S.; Hess, R.F. Comparison of blur and magnification effects on stereopsis: overall and meridional, monocularly- and binocularly-induced. Ophthalmic Physiol Opt. 2020, 40, 660–668. [Google Scholar] [CrossRef]
- Donzis, P.B.; Rappazzo, J.A.; Burde, R.M.; Gordon, M. Effect of binocular variations of Snellen’s visual acuity on Titmus stereoacuity. Arch Ophthalmol. 1983, 101, 930–932. [Google Scholar] [CrossRef]
- Borish, I.M. Anisometropia and Aniseikonia. In Clinical Refraction, 3rd ed.; Professional Press Inc Chicago, 1970; p. 270. [Google Scholar]
- Mravicic, I.; Bohac, M.; Lukacevic, S.; Jagaric, K.; Maja, M.; Patel, S. The relationship between clinical measures of aniseikonia and stereoacuity before and after LASIK. J Optom. 2020, 13, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Corliss, D.A.; Rutstein, R.P.; Than, T.P.; Hopkins, K.B.; Edwards, C. Aniseikonia testing in an adult population using a new computerized test, "the Aniseikonia Inspector". Binocul Vis Strabismus Q. 2005, 20, 205–215, discussion 216. [Google Scholar] [PubMed]
- Liu, C.; Liu, Y.; Hou, C.; Yan, M.; Luo, Q. Stereopsis of patients after cataract extraction and intraocular lens implantation. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003, 34, 539–540. [Google Scholar] [PubMed]
- Liu, S.; Zhang, P.; Wu, X.; Hu, S.; Tan, X. Clinical analysis of binocular aniseikonia after laser in situ keratomileusis on myopic patients. Yan Ke Xue Bao. 2003, 19, 107–109. [Google Scholar] [PubMed]
- Krarup, T.; Nisted, I.; Kjaerbo, H.; Christensen, U.; Kiilgaard, J.F.; la Cour, M. Measuring aniseikonia tolerance range for stereoacuity - a tool for the refractive surgeon. Acta Ophthalmol. 2021, 99, e43–e53. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.F.; Ding, R.; Clavagnier, S.; Liu, C.; Guo, C.; Viner, C.; Barrett, B.T.; Radia, K.; Zhou, J. A Robust and Reliable Test to Measure Stereopsis in the Clinic. Invest Ophthalmol Vis Sci. 2016, 57, 798–804. [Google Scholar] [CrossRef]
- Keesey, U.T. Effects of involuntary eye movements on visual acuity. J Opt Soc Am. 1960, 50, 769–774. [Google Scholar] [CrossRef]
- Davson, H. The Physiology of the Eye, 3rd ed.; Churchill-Livingstone: Edinburgh, UK, 1972; pp. 343–344. [Google Scholar]
- Charman, W.N.; Heron, G. Microfluctuations in accommodation: an update on their characteristics and possible role. Ophthalmic Physiol Opt. 2015, 35, 476–499. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Chan, T.C.; Chow, S.S.; Di Zazzo, A.; Inomata, T.; Shih, K.C.; Tong, L.A. Systematic Review on the Association Between Tear Film Metrics and Higher Order Aberrations in Dry Eye Disease and Treatment. Ophthalmol Ther. 2022, 11, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, J.R.; Anera, R.G.; Jiménez, R.; Salas, C. Impact of interocular differences in corneal asphericity on binocular summation. Am J Ophthalmol. 2003, 135, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.R.; Villa, C.; Anera, R.G.; Gutiérrez, R.; del Barco, L.J. Binocular visual performance after LASIK. J Refract Surg. 2006, 22, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.R.; Castro, J.J.; Jiménez, R.; Hita, E. Interocular differences in higher-order aberrations on binocular visual performance. Optom Vis Sci. 2008, 85, 174–179. [Google Scholar] [CrossRef]
- Arba Mosquera, S.; Verma, S. Bilateral symmetry in vision and influence of ocular surgical procedures on binocular vision: A topical review. J Optom. 2016, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Birren, J.E.; Casperson, R.C.; Botwinick, J. Age changes in pupil Size. J Gerontology 1950, 5, 216–221. [Google Scholar] [CrossRef]
- Loewenfeld, I.E. Pupillary changes related to age. In Topics in Neuro-Ophthalmology; Thompson, S.H., Ed.; Williams & Wilkins Baltimore, 1970; pp. 124–150. [Google Scholar]
- Saunders, H. Age-dependence of human refractive errors. Ophthalmic Physiol Opt. 1981, 1, 159–174. [Google Scholar]
- Saunders, H. A longitudinal study of the age-dependence of human ocular refraction—I. Age-dependent changes in the equivalent sphere. Ophthalmic Physiol Opt. 1986, 6, 39–46. [Google Scholar]
- Sayegh, F.N. Age and refraction in 46,000 patients as a potential predictor of refractive stability after refractive surgery. J Refract Surg. 2009, 25, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Awaya, S.; Sugawara, M.; Horibe, F.; Torii, F. The new aniseikonia tests and its clinical applications. Nippon Ganka Gakkai Zasshi. 1982, 86, 217–222. [Google Scholar] [PubMed]
- Thibos, L.N.; Wheeler, W.; Horner, D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997, 74, 367–375. [Google Scholar] [CrossRef]
- Thibos, L.N.; Horner, D. Power vector analysis of the optical outcome of refractive surgery. J Cataract Refract Surg. 2001, 27, 80–85. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand J Statistics. 1979, 6, 65–70. [Google Scholar]
- Antona, B.; Barra, F.; Barrio, A.; Gonzalez, E.; Sanchez, I. Validity and repeatability of a new test for aniseikonia. Invest Ophthalmol Vis Sci. 2001, 48, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Rutstein, R.P.; Corliss, D.A.; Fullard, R.J. Comparison of aniseikonia as measured by the aniseikonia inspector and the space eikonometer. Optom Vis Sci. 2006, 83, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, B.; Wu, H. Evaluating the mechanism by which the TNO stereo test overestimates stereo thresholds. J Ophthalmol. 2021, 18, 6665638. [Google Scholar] [CrossRef]
- Larson, W.L.; Lachance, A. Stereoscopic acuity with induced refractive errors. Am J Optom Physiol Opt. 1983, 60, 509–513. [Google Scholar] [CrossRef]
- García-Pérez, M.A.; Peli, E. Aniseikonia Tests: The role of viewing mode, response bias, and size-color illusions. Transl Vis Sci Technol. 2015, 4, 9. [Google Scholar] [CrossRef]
- Koch, D.D.; Samuelson, S.W.; Haft, E.A.; Merin, L.M. Pupillary size and responsiveness. Implications for selection of a bifocal intraocular lens. Ophthalmology. 1991, 98, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Horwood, A.M. When does blur matter? A narrative review and commentary. J Binocul Vis Ocul Motil. 2022, 72, 57–68. [Google Scholar] [PubMed]
Figure 1.
Age and log stereoacuity. Filled circles represent data for distance stereoacuity, empty circles represent data for near stereoacuity. The solid line represents the linear relationship between subject age (years) and log distance stereoacuity (arcsec). The equation of this line is, y = 0.012x + 1.537 (r2= 0.131, p<.01, n=91). There was no significant correlation between subject age and log near stereoacuity (p=.106). NB, larger filled circles represent cases where distance and near data points overlap.
Figure 1.
Age and log stereoacuity. Filled circles represent data for distance stereoacuity, empty circles represent data for near stereoacuity. The solid line represents the linear relationship between subject age (years) and log distance stereoacuity (arcsec). The equation of this line is, y = 0.012x + 1.537 (r2= 0.131, p<.01, n=91). There was no significant correlation between subject age and log near stereoacuity (p=.106). NB, larger filled circles represent cases where distance and near data points overlap.
Figure 2.
RMS interocular difference in the corrected visual acuities and near stereoacuity. Empty circles represent interocular differences in the distance acuities and filled circles represent the differences in near acuities. The solid line is the relationship between RMS interocular difference in the corrected distance visual acuities (x) and log near stereoacuity (y). The equation of this line is, y = 1.735+ 4.227x -9.152x2 (r² = 0.204, p<.01, n=91). The hatched line is the relationship between RMS interocular difference in the corrected near visual acuities (x1) and log near stereoacuity (y). The equation of this line is, y = 1.727+ 4.951x1 -13.463x12 (r² = 0.204, p<.01, n=91). NB, larger filled circles represent cases where distance and near data points overlap.
Figure 2.
RMS interocular difference in the corrected visual acuities and near stereoacuity. Empty circles represent interocular differences in the distance acuities and filled circles represent the differences in near acuities. The solid line is the relationship between RMS interocular difference in the corrected distance visual acuities (x) and log near stereoacuity (y). The equation of this line is, y = 1.735+ 4.227x -9.152x2 (r² = 0.204, p<.01, n=91). The hatched line is the relationship between RMS interocular difference in the corrected near visual acuities (x1) and log near stereoacuity (y). The equation of this line is, y = 1.727+ 4.951x1 -13.463x12 (r² = 0.204, p<.01, n=91). NB, larger filled circles represent cases where distance and near data points overlap.
Table 1.
Breakdown of refractive errors, axial lengths, corrected distance and near acuities (n=91). Mean (±sd, 95% CI) sphere and astigmatic powers values in diopters, astigmatic axis values in degrees, eyeball lengths and pupil diameters in mm. Median and mode (interquartile range) of corrected distance (CDVA) and near (CNVA) logMAR visual acuities are shown. None of the interocular differences were significant (where appropriate, either paired t-test or Wilcoxon signed rank test, p>.05). The root mean (±sd, 95% CI) square differences between right (OD) and left (OS) eyes are listed under ΔRMS.
Table 1.
Breakdown of refractive errors, axial lengths, corrected distance and near acuities (n=91). Mean (±sd, 95% CI) sphere and astigmatic powers values in diopters, astigmatic axis values in degrees, eyeball lengths and pupil diameters in mm. Median and mode (interquartile range) of corrected distance (CDVA) and near (CNVA) logMAR visual acuities are shown. None of the interocular differences were significant (where appropriate, either paired t-test or Wilcoxon signed rank test, p>.05). The root mean (±sd, 95% CI) square differences between right (OD) and left (OS) eyes are listed under ΔRMS.
| |
OD |
OS |
ΔRMS |
| Sphere [D] |
0.62(±2.50, 0.11-1.13) |
0.62(±2.50, 0.11-1.13) |
0.66(±0.93, 0.47-0.85) |
| Astigmatic power [D] |
0.87(±0.70, 0.73-1.01) |
0.87(±0.70, 0.73-1.01) |
0.17(±0.27, 0.11-0.23) |
| Astigmatic axis [°] |
101.5(±67.5, 87.7-115.4) |
113.7(±61. 0,101.1-126.2) |
36.7(±59.1, 24.4-49.0) |
| Axial length [mm] |
23.27(±1.19, 23.03-23.51) |
23.23(±1.22, 22.98-23.48) |
0.24(±0.33, 0.17-0.31) |
| Pupil diameter [mm] |
2.83(±0.46, 2.73-2.93) |
2.84(±0.42, 2.75-2.93) |
0.15(±0.11, 0.12-0.17) |
| CDVA |
0.00,0.00 (0.00-0.02) |
0.00,0.00 (0.00-0.02) |
0.02(±0.05, 0.01-0.03) |
| CNVA |
0.00,0.00 (0.00-0.05) |
0.00,0.00 (0.00-0.00) |
0.02(±0.04, 0.01-0.03) |
Table 2.
Breakdown of stereoacuity and aniseikonia. Median (Me) and mode (Mo) values of distance and near stereoacuity (ʺarcsec), vertical horizontal and resultant aniseikonia (%) values are shown with the corresponding interquartile ranges (IQ) in parentheses.
Table 2.
Breakdown of stereoacuity and aniseikonia. Median (Me) and mode (Mo) values of distance and near stereoacuity (ʺarcsec), vertical horizontal and resultant aniseikonia (%) values are shown with the corresponding interquartile ranges (IQ) in parentheses.
| Test |
Me, Mo, IQ |
| Distance Stereoacuity [ʺ] |
160, 160, (80-320) |
| Near Stereoacuity [ʺ] |
70, 70, (40-80) |
| Aniseikonia Vertical [%] |
0.0, -1.0, (-1.0 to +1.0) |
| Aniseikonia Horizontal [%] |
0.0, 0.0, (-1.0 to +1.0) |
| Total Resultant Aniseikonia [%] |
2.8, 1.0, (1.3 to 4.0) |
Table 3.
Breakdown of higher order aberrations. Mean (±sd, 95% CI) values of the three higher order aberrations (HOA in units of µm) for right (OD) and left (OS) eyes, and root mean square interocular differences are listed under RMS Δ HOA. RMS ΔHOA = √ [(value of HOA at right eye – value of HOA at left eye)2]. SA is the abbreviation for spherical aberration. Apparent interocular differences of mean coma, trefoil and spherical aberration were not significant (paired t-test, p>.05 for 3mm & 5mm pupil sizes).
Table 3.
Breakdown of higher order aberrations. Mean (±sd, 95% CI) values of the three higher order aberrations (HOA in units of µm) for right (OD) and left (OS) eyes, and root mean square interocular differences are listed under RMS Δ HOA. RMS ΔHOA = √ [(value of HOA at right eye – value of HOA at left eye)2]. SA is the abbreviation for spherical aberration. Apparent interocular differences of mean coma, trefoil and spherical aberration were not significant (paired t-test, p>.05 for 3mm & 5mm pupil sizes).
| |
OD |
OS |
RMS ΔHOA |
| For 3mm pupil |
| Coma [µm] |
0.05(±0.05, 0.04-0.06) |
0.05(±0.05,0.04-0.06) |
0.04(±0.06,0.03-0.05) |
| Trefoil [µm] |
0.05(±0.06,0.04-0.06) |
0.05(±0.04,0.04-0.06) |
0.03(±0.06,0.02-0.04) |
| SA [µm] |
0.01(±0.05, 0.00-0.02) |
0.01(±0.04,0.00-0.02) |
0.02(±0.04,0.01-0.03) |
| For 5mm pupil |
| Coma [µm] |
0.11(±0.11,0.09-0.13) |
0.12(±0.08,0.10-0.14) |
0.08(±0.10,0.06-0.10) |
| Trefoil [µm] |
0.10(±0.10,0.08-0.12) |
0.10(±0.07,0.10-0.12) |
0.06(±0.10,0.04-0.08) |
| SA [µm] |
0.04(±0.07,0.03-0.06) |
0.03(±0.07,0.02-0.04) |
0.04(±0.05.02-0.05) |
Table 4.
Cases where astigmatism was present (n=53). Vectorial representations of refractive errors in the cases where astigmatism was present Mean (±sd, 95% CI) values of M, J0, J0 and B for right (OD) and left (OS) eyes, and the root mean square interocular differences (RMS Δ) of M, J0, J0 and B are shown. Interocular differences of mean M, J0, J45 and B values were not significant (paired t-test, p>.05).
Table 4.
Cases where astigmatism was present (n=53). Vectorial representations of refractive errors in the cases where astigmatism was present Mean (±sd, 95% CI) values of M, J0, J0 and B for right (OD) and left (OS) eyes, and the root mean square interocular differences (RMS Δ) of M, J0, J0 and B are shown. Interocular differences of mean M, J0, J45 and B values were not significant (paired t-test, p>.05).
| |
OD |
OS |
RMS Δ |
| M |
0.55(±2.87,-0.22 to 1.32) |
0.85(±2.63,0.14 to 1.56) |
0.88(±1.04,0.59 to 1.17) |
| J0
|
0.08(±0.42,-0.04 to 0.19) |
0.05(±0.50,-0.08 to 0.18) |
0.15(±0.13,0.12 to 0.19) |
| J45
|
0.04(±0.26,-0.03 to 0.11) |
0.01(±0.28,-0.0 to 0.09) |
0.22(±0.34,0.13 to 0.31) |
| B |
2.27(±1.88,1.76 to 2.78) |
2.54(±2.03,1.99 to 3.09) |
1.08(±1.30,0.73 to 1.43) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).