Submitted:
05 February 2024
Posted:
06 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Agriculture applications
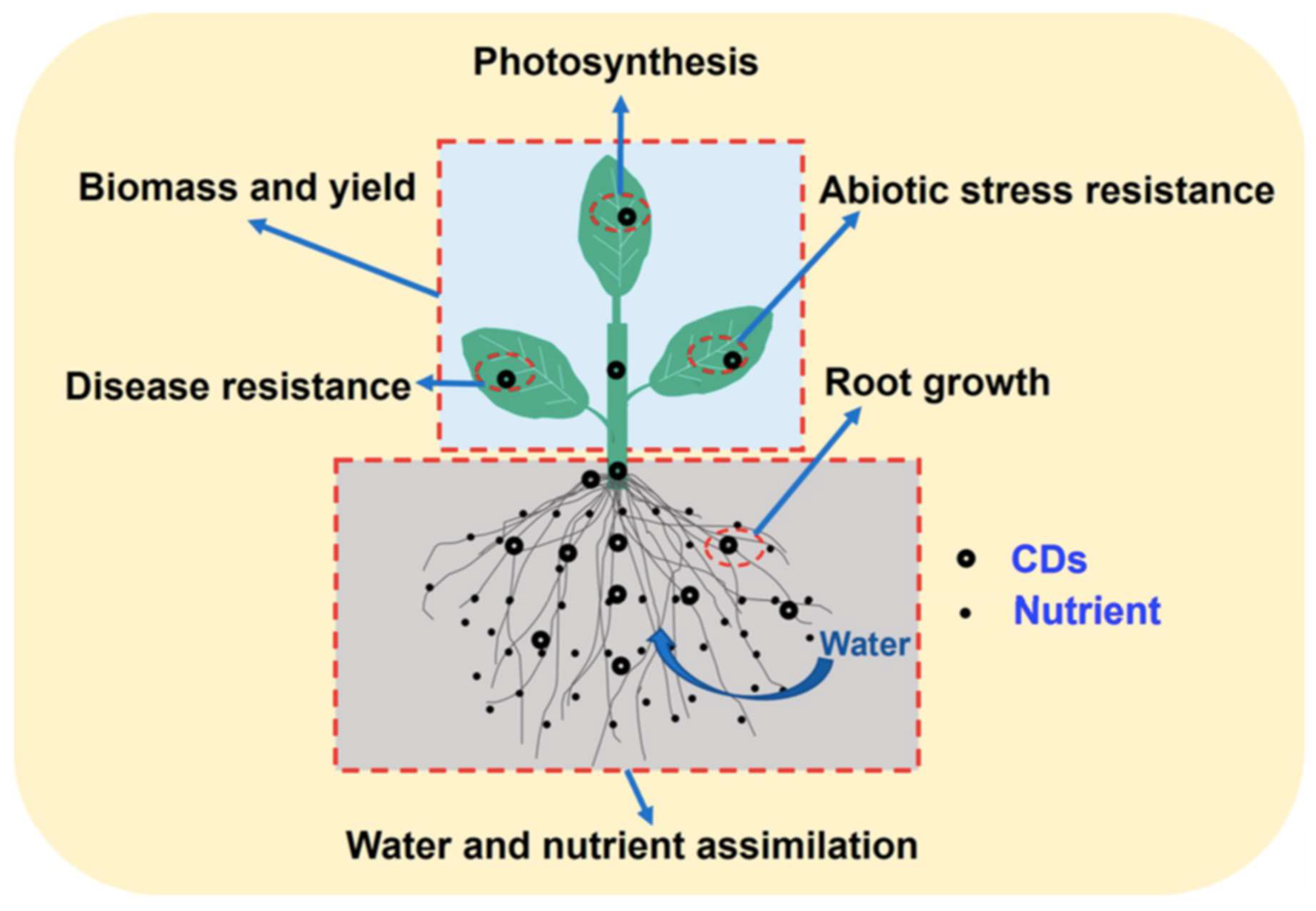
2.1. Carbon bssed nanomaterials
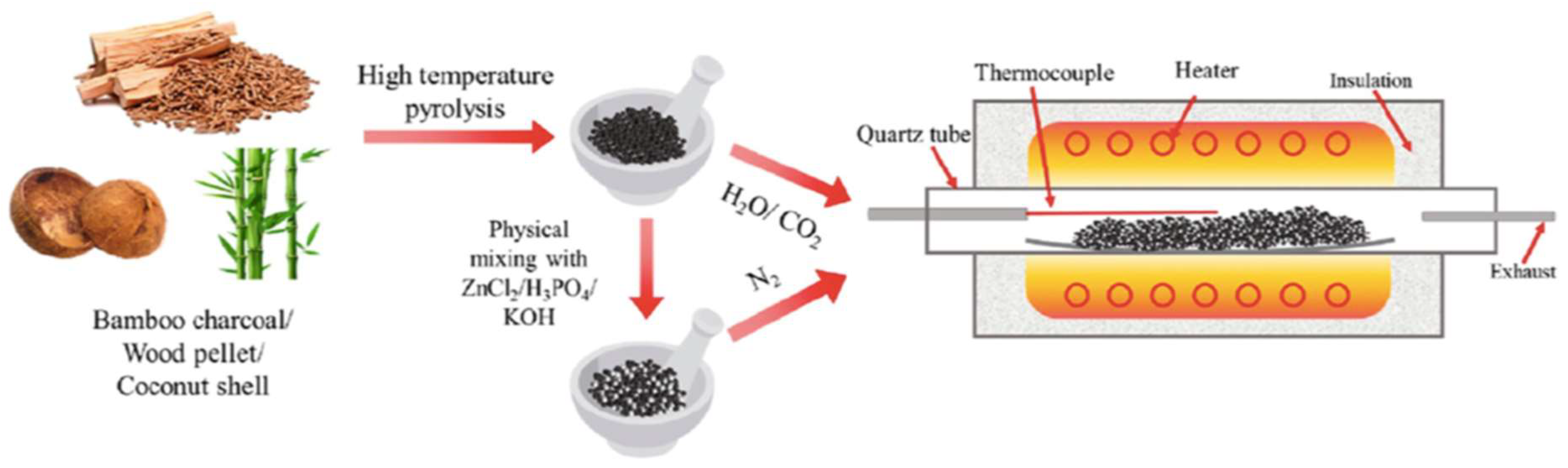
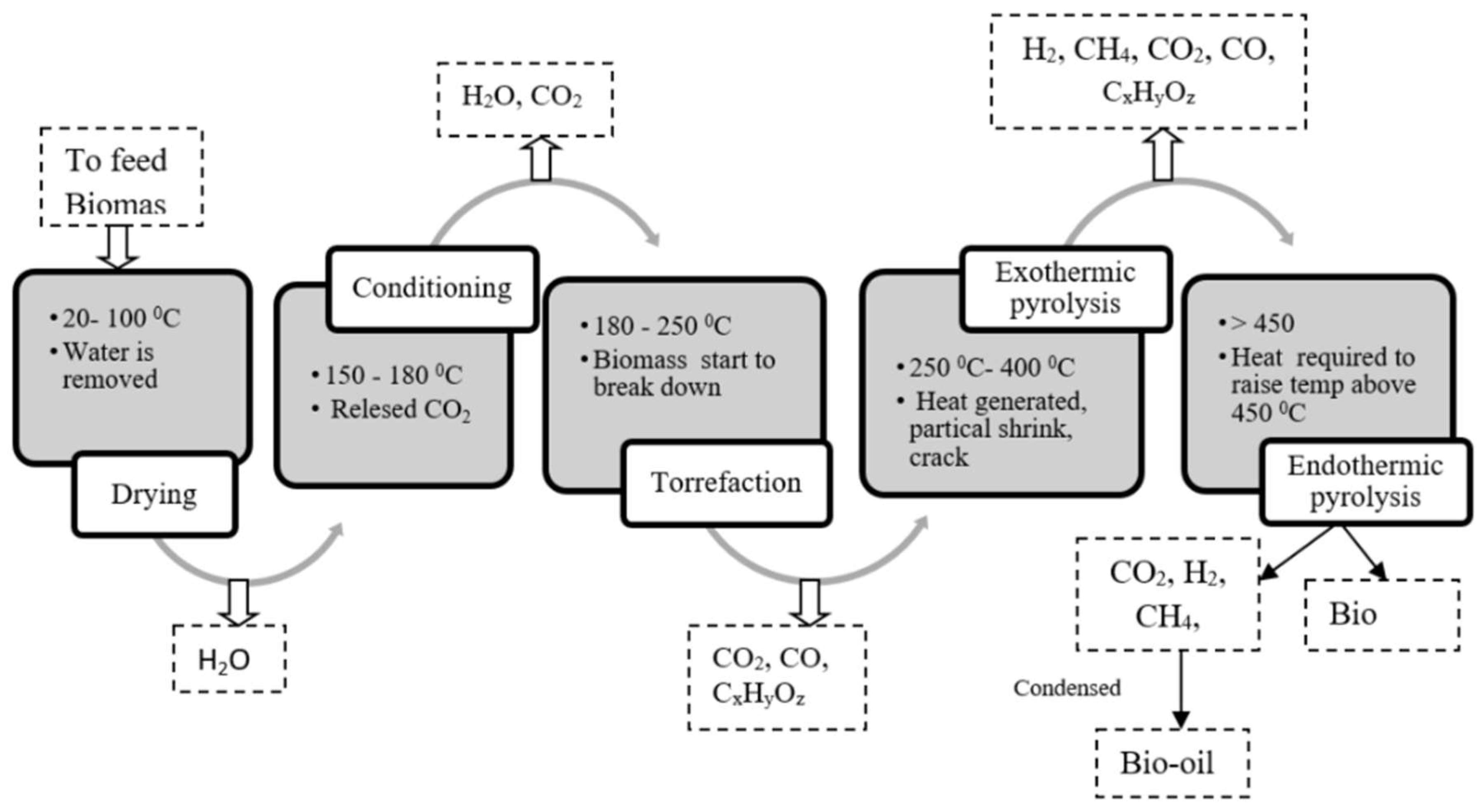
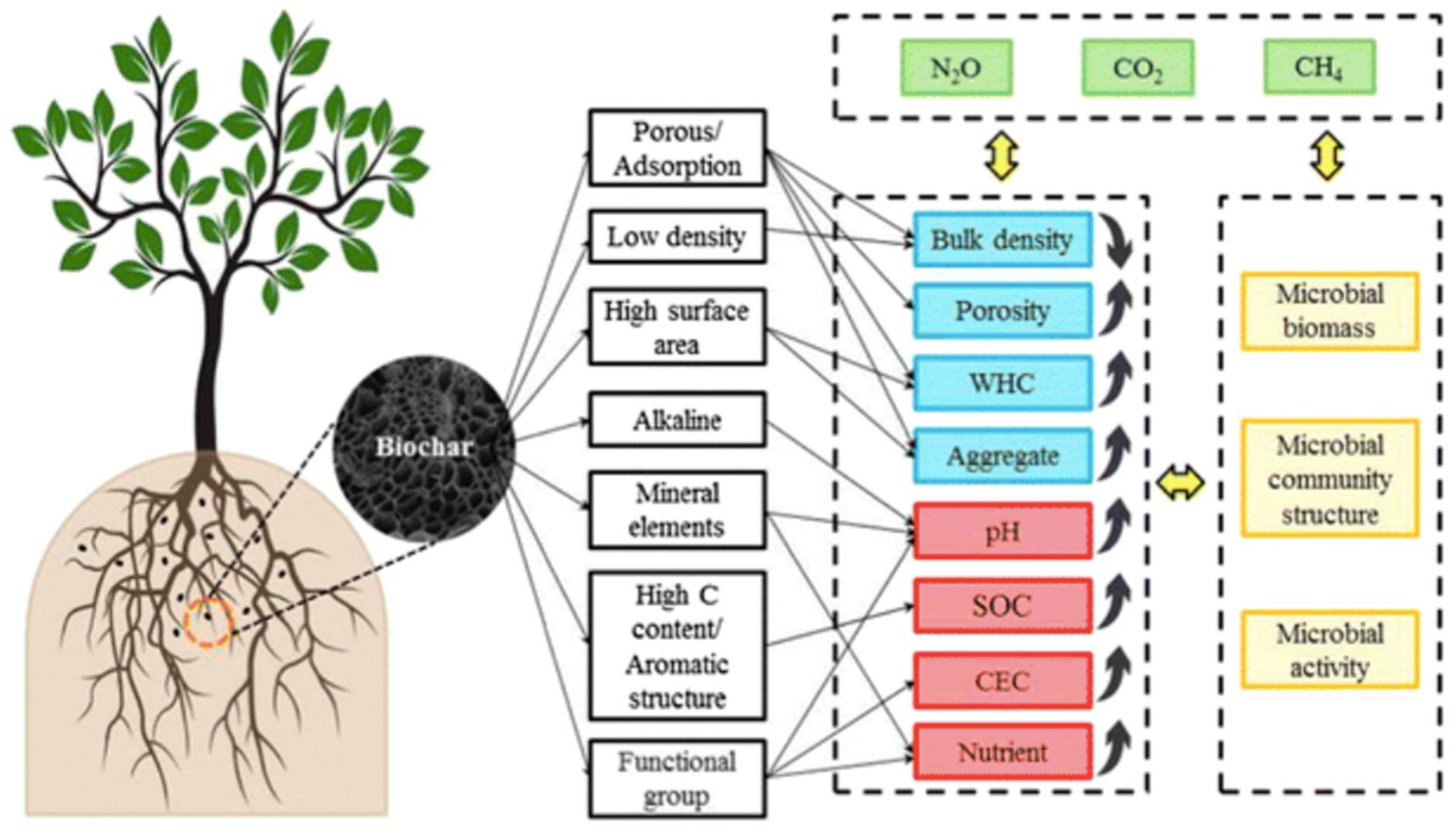
2.2. Carbon based materials – biochar
- (i)
- The pyrolysis of hardwood biomass results in a biochar with higher organic carbon. If biochar is made from animal manure it results in a higher NPK nutrient load and a higher CEC [26]. Different raw materials induce a nutrient-enriched biochar [30]: seaweed, potassium; manure, phosphorous; rice straw, silicon; bone, calcium; keratin, nitrogen.
- (ii)
- (iii)
3. Water treatments
4. Energy management
5. CO2 reduction and sequestration
6. Future perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bhattacharjee, T.; Konwar, A.; Boruah, J.; Chowdhury, D.; Majumdar, G. A sustainable approach for heavy metal remediation from water using carbon dot based composites: A review. Journal of Hazardous Materials Advances 2023, 10, 100295. [Google Scholar] [CrossRef]
- Hatimuria, M.; Phukan, P.; Bag, S.; Ghosh, J.; Gavvala, K.; Pabbathi, A.; Das, J. Green Carbon Dots: Applications in Development of Electrochemical Sensors, Assessment of Toxicity as Well as Anticancer Properties. Catalysts 2023, 13, 537. [Google Scholar] [CrossRef]
- De Oliveira Lima, L.; Souza Machado, W.; Schiavon, M. Carbon Dots: Chemical Synthesis, Properties and Applications – a review. Revista Virtual de Química 2023, 15, 1163–1178. [Google Scholar] [CrossRef]
- Jing, H.; Bardakci, F.; Akgöl, S.; Kusat, K.; Adnan, M.; Gupta, R.; Sahreen, S.; Chen, Y.; Gopinath, S.; Sasidharan, S. Green Carbon Dots: Synthesis, Characterization, Properties and Biomedical Applications. Journal of Functional Biomaterials 2023, 14, 27. [Google Scholar] [CrossRef]
- Aswathi, V.; Meera, S.; Maria, C.; Nidhin, M. Green synthesis of nanoparticles from biodegradable waste extracts and their applications: a critical review. Nanotechnology of Environmental Engineering 2023, 8, 377–397. [Google Scholar] [CrossRef]
- Bressi, V.; Balu, A.; Iannazzo, D.; Espro, C. Recent advances in the synthesis of carbon dots from renewable biomass by high-efficient hydrothermal and microwave green approaches. Current Opinion in Green and Sustainable Chemistry 2023, 40, 100742. [Google Scholar] [CrossRef]
- Fan, J.; Kang, L.; Cheng, X.; Liu, D.; Zhang, S. Biomass-Derived Carbon Dots and Their Sensing Applications. Nanomaterials 2022, 12, 4473. [Google Scholar] [CrossRef] [PubMed]
- Wareing, T.; Gentile, P.; Phan, A. Biomass-Based Carbon Dots: Current Development and Future Perspectives. ACS Nano 2021, 15, 15471–15501. [Google Scholar] [CrossRef]
- Gan, J.; Chen, L.; Chen, Z.; Zhang, J.; Yu, W.; Huang, C.; Wu, Y.; Zhang, K. Lignocellulosic Biomass-Based Carbon Dots: Synthesis Processes, Properties, and Applications. Small 2023, 19, 2304066. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Iravani, S.; Varma, R. Biowaste-Derived Carbon Dots: A Perspective on Biomedical Potentials. Molecules 2022, 27, 6186. [Google Scholar] [CrossRef] [PubMed]
- Debnath, P.; Dutta, D.; Choudhury, B. A review on carbon dots produced from biomass waste-its development and bio-applications. International Journal of Pharmaceutical Science and Research 1 IJPSR 2023, 14, 1–12. [Google Scholar] [CrossRef]
- Qin, F.; Li, J.; Zhang, C.; Zeng, G.; Huang, D.; Tan, X.; Qin, D.; Tan, H. Biochar in the 21st century: A data-driven visualization of collaboration, frontier identification, and future trend. Science of the Total Environment 2022, 818, 151774. [Google Scholar] [CrossRef]
- Lefebvre, D.; Fawzy, S.; Aquije, C.A.; Osman, A.I.; Draper, K.T.; Trabold, T.A. Biomass residue to carbon dioxide removal: quantifying the global impact of biochar. Biochar 2023, 5, 65. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Wang, Y.; Wang, L.; Sun, Y.; Liu, L. Recent advances in biochar-based adsorbents for CO2 capture. Carbon Capture Science & Technology 2022, 4, 100059. [Google Scholar] [CrossRef]
- Zhang, C.; Ji, Y.; Li, C.; Zhang, Y.; Sun, S.; Xu, Y.; Jiang, L.; Wu, C. The Application of Biochar for CO2 Capture: Influence of Biochar Preparation and CO2 Capture Reactors. Ind. Eng. Chem. Res. 2023, 62, 17168–17181. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.C.; Nighojkar, A.; Kandasubramanian, B. Relevance of wood biochar on CO2 adsorption: A review. Hybrid Advances 2023, 3, 100056. [Google Scholar] [CrossRef]
- Premchand, P.; Demichelis, F.; Chiaramonti, D.; Bensaid, S.; Fino, D. Biochar production from slow pyrolysis of biomass under CO2 atmosphere: A review on the effect of CO2 medium on biochar production, characterisation, and environmental applications. Journal of Environmental Chemical Engineering 2023, 11, 110009. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Jacinthe, P.A.; Lal, R.; Lorenz, K.; Singh, M.P.; Demyan, S.M.; Ren, W.; Lindsey, L.E. Biochar as a negative emission technology: A synthesis of field research on greenhouse gas emissions. J. Environ. Qual. 2023, 52, 769–798. [Google Scholar] [CrossRef] [PubMed]
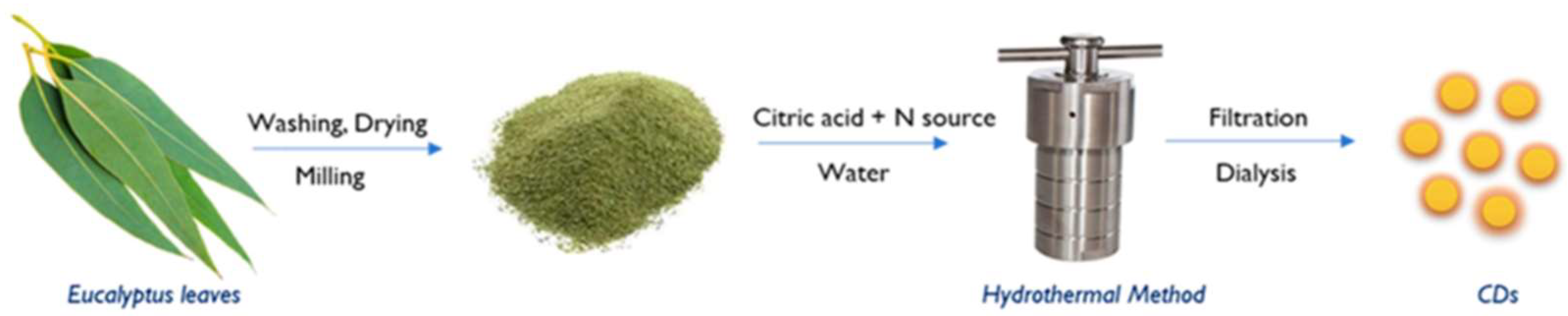
- Johny, A.; Pinto da Silva, L.; Pereira, C.; Esteves da Silva, J. Sustainability Assessment of Highly Fluorescent Carbon Dots Derived from Eucalyptus Leaves. Environments 2024, 6, 11. [Google Scholar] [CrossRef]
- Li, K.; Tan, H.; Li, J.; Li, Z.; Qin, F.; Luo, H.; Qin, D.; Weng, H.; Zhang, C. Unveiling the Effects of Carbon-Based Nanomaterials on Crop Growth: From Benefits to Detriments. J. Agric. Food Chem. 2023, 71, 11860–11874. [Google Scholar] [CrossRef]
- Chandel, M.; Kaur, K.; Sahu, B.K.S.; Sandeep Sharma, S.; Panneerselvam, R.; Shanmugam, V. Promise of nano-carbon to the next generation sustainable agriculture. Carbon 2022, 188, 461–481. [Google Scholar] [CrossRef]
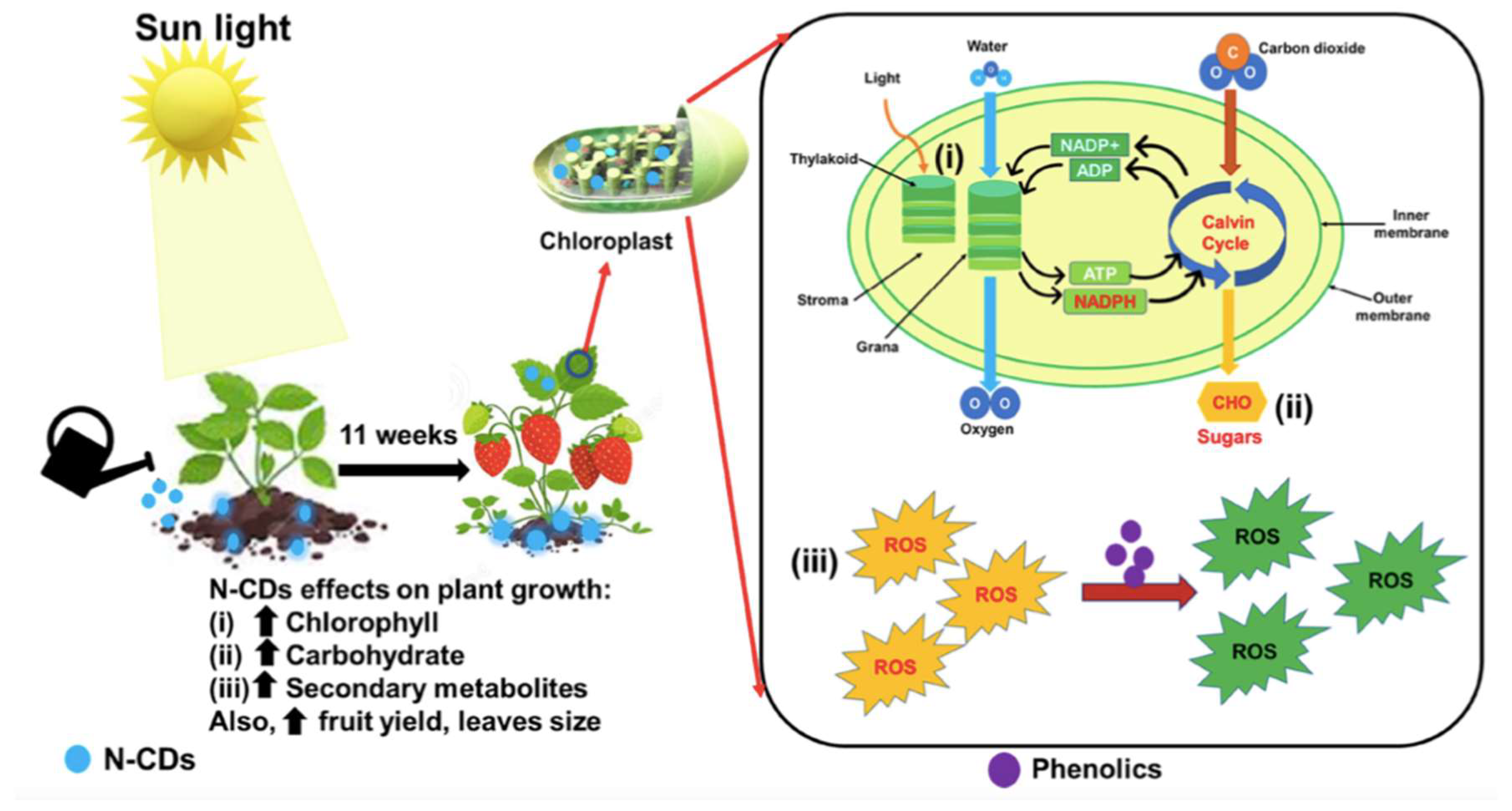
- Salha, A.B.; Saravanan, A.; Maruthapandi, M.; Perelshtein, I.; Gedanken, M. Plant-Derived Nitrogen-Doped Carbon Dots as an Effective Fertilizer for Enhanced Strawberry Growth and Yield. ACS EST Engineering 2023, 3, 1165–1175. [Google Scholar] [CrossRef]
- Kara, M.; Seçgin, Z.; Arslanoglu, S.F.; Dinç, S. Endogenous Food-Borne Sugar Beet Molasses Carbon Dots for Alleviating the Drought and Salt Stress in Tobacco Plant. Journal of Plant Growth Regulation 2023, 42, 4541–4556. [Google Scholar] [CrossRef]
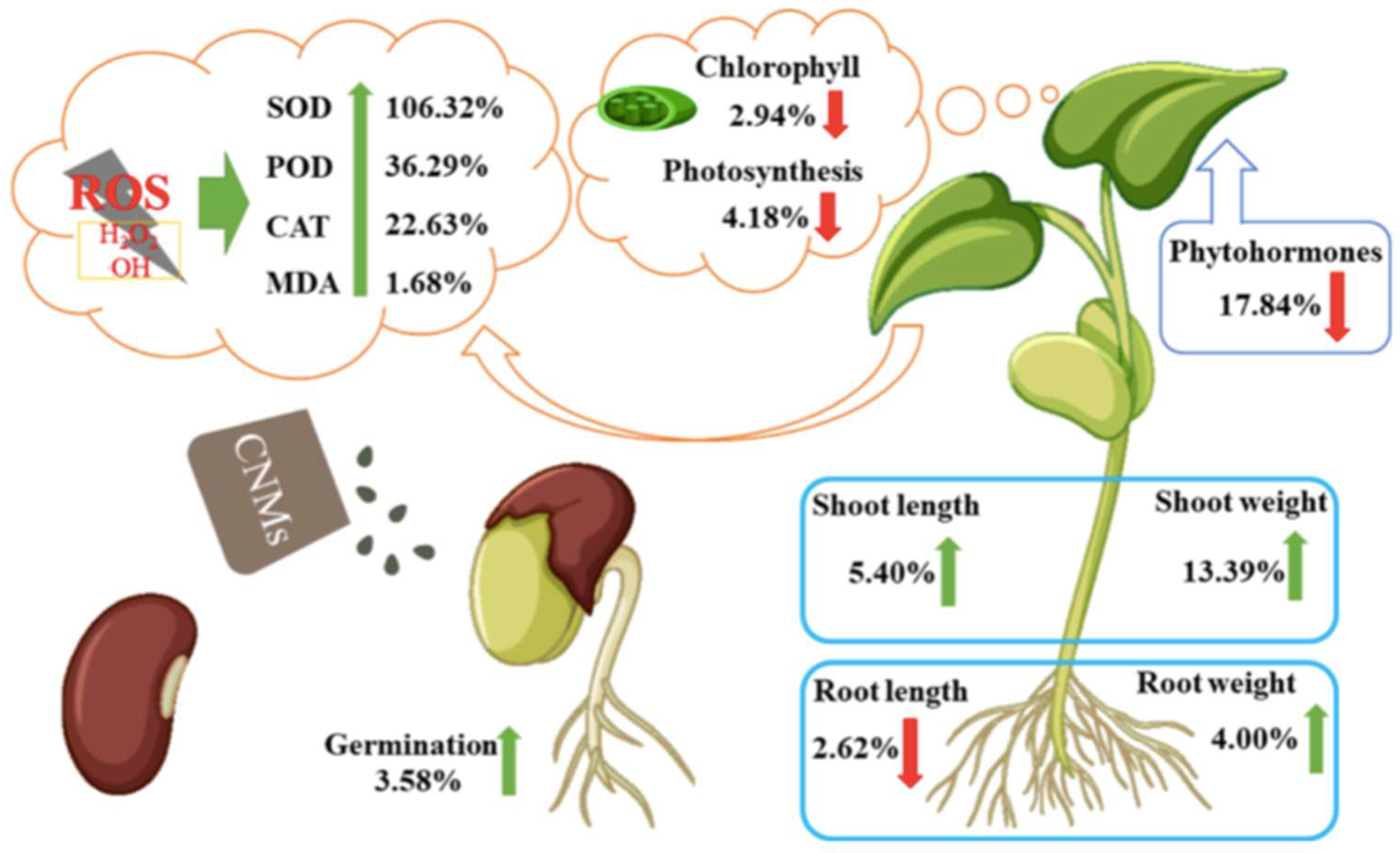
- Guerrero-Gonzalez, R.; Vázquez-Dávila, F.; Saucedo-Flores, E.; Ruelas, R.; Ceballos-Sánchez, O.; Pelayo, J. Green approach synthesis of carbon quantum dots from agave bagasse and their use to boost seed germination and plant growth. SN Applied Science 2023, 5, 204. [Google Scholar] [CrossRef]
- Li, G.; Xu, J.; Xu, K. Physiological Functions of Carbon Dots and Their Applications in Agriculture: A Review. Nanomaterials 2023, 13, 2684. [Google Scholar] [CrossRef] [PubMed]
- Xiea, Y.; Wang, L.; Li, H.; Westholm, L.J.; Carvalho, L.; Thorin, E.; Yu, Z.; Yu, X.; Skreiberg, Ø. A critical review on production, modification and utilization of biochar. Journal of Analytical and Applied Pyrolysis 2022, 161, 105405. [Google Scholar] [CrossRef]
- Ahmad Bhat, S.; Kuriqi, A.; Dar, M.U.D.; Bhat, O.; Sammen, S.S.; Towfiqul Islam, A.R.M.; Elbeltagi, A.; Shah, O.; AI-Ansari, N.; Ali, R.; et al. Application of Biochar for Improving Physical, Chemical, and Hydrological Soil Properties: A Systematic Review. Sustainability 2022, 14, 11104. [Google Scholar] [CrossRef]
- Bo, X.; Zhang, Z.; Wang, J.; Guo, S.; Li, Z.; Lin, H.; Huang, Y.; Han, Z.; Kuzyakov, Y.; Zou, J. Benefits and limitations of biochar for climate-smart agriculture: a review and case study from China. Biochar 2023, 5, 77. [Google Scholar] [CrossRef]
- Jagnade, P.; Panwar, N.L.; Gupta, T.; Agrawal, C. Role of Biochar in Agriculture to Enhance Crop Productivity: An Overview. Biointerface Research in Applied Chemistry 2023, 13, 429. [Google Scholar]
- Chen, Z.; Liu, T.; Dong, J.; Chen, G.; Li, Z.; Zhou, J.; Zhang Chen, Z. Sustainable Application for Agriculture Using Biochar-Based Slow- Release Fertilizers: A Review. ACS Sustainable Chem. Eng. 2023, 11, 1–12. [Google Scholar] [CrossRef]
- Waller, A.; Swanson, T.; Wang, Z.; Pignatello, J.; Elmer, W.; Wang, Y.; Musante, C.; Parikh, S. Modified Biochars Reduce Leaching while Maintaining Bioavailability of Phosphate to Dragoon Lettuce (Lactuca sativa) in Potting Tests. ACS Agric. Sci. Technol. 2023, 3, 1103–1112. [Google Scholar] [CrossRef]
- Abiola, W.A.; Diogo, R.V.C.; Tovihoudji, P.G.; Mien, A.K.; Schalla, A. Research trends on biochar-based smart fertilizers as an option for the sustainable agricultural land management: Bibliometric analysis and review. Front. Soil Sci. 2023, 3, 1136327. [Google Scholar] [CrossRef]
- Hamidzadeh, Z.; Ghorbannezhad, P.; Ketabchi, M.R. Yeganeh, B. Biomass-derived biochar and its application in agriculture. Fuel 2023, 341, 127701. [Google Scholar] [CrossRef]
- Rex, P.; Mohammed Ismail, K.R.; Meenakshisundaram, N.; Barmavatu, P.; Sai Bharadwaj, A.V.S.L. Agricultural Biomass Waste to Biochar: A Review on Biochar Applications Using Machine Learning Approach and Circular Economy. ChemEngineering 2023, 7, 50. [Google Scholar] [CrossRef]
- Xia, L.; Cao, L.; Yang, Y.; Ti, C.; Liu, Y.; Smith, P.; van Groenigen, K.J.; Lehmann, J.; Lal, R.; Butterbach-Bahl, K.; Kiese, R.; Zhuang, M.; Lu, X.; Yan, X. Integrated biochar solutions can achieve carbon-neutral staple crop production. Nat. Food 2023, 4, 236–246. [Google Scholar] [CrossRef]
- Xue, N.; Anwar, S.; Shafiq, F.; Gul-e-Kainat; Ullah, K.; Zulqarnain, M.; Haider, I.; Ashraf, M. Nanobiochar Application in Combination with Mulching Improves Metabolites and Curd Quality Traits in Cauliflower. Horticulturae 2023, 9, 687. [Google Scholar] [CrossRef]
- Rashid, M.I.; Shah, G.A.; Iqbal, Z.; Ramzan, M.; Rehan, M.; Ali, N.; Shahzad, K.; Summan, A.; Ismail, I.M.I.; Ondrasek, G. Nanobiochar Associated Ammonia Emission Mitigation and Toxicity to Soil Microbial Biomass and Corn Nutrient Uptake from Farmyard Manure. Plants 2023, 12, 1740. [Google Scholar] [CrossRef]
- Thines, R.K; Mubarak, N.M.; Nizamuddin, S.; Sahu, J.N.; Abdullah, E.C. Application potential of carbon nanomaterials in water and wastewater treatment: A review. Journal of the Taiwan Instituto of Chemical Engineers 2017, 1876. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R. Carbon-based sustainable nanomaterials for water treatment: State-of-art and future perspectives. Chemosphere 2021, 263. [Google Scholar] [CrossRef]
- Homaeigohar, S. Water Treatment with New Nanomaterials. Water 2020, 12, 1507. [Google Scholar] [CrossRef]
- Cailoto, S.; Massari, D.; Gigli, M.; Campalani, C.; Bonini, M.; You, S.; Vomiero, A.; Selva, M.; Perosa, A.; Crestini, A. N-Doped Carbon Dot Hydrogels from Brewing Waste for Photocatalytic Wastewater Treatment. ACS Omega 2022, 7, 4052–4061. [Google Scholar] [CrossRef] [PubMed]
- Varshney, N.; Tariq, M.; Arshad, F.; Sk, Md P. Biomass-derived carbon dots for efficient clean of oil spills. Journal of Water Process Engineering 2022, 49, 103016. [Google Scholar] [CrossRef]
- Velmurugan, P.; Kumar, R.; Sivakumar, S.; Ravi, A. Fabrication of blue fluorescent carbon quantum dots using green carbon precursor Psidium guajava leaf extract and its application in water treatment. Carbon Letters 2022, 32, 119–129. [Google Scholar] [CrossRef]
- Palanimuthu, K.; Subbiah, U.; Sundharam, S.; Munusamy, C. Spirulina carbon dots: a promising biomaterial for photocatalytic textile industry Reactive Red M8B dye degradation. Environmental Science and Pollution Research 2023, 30, 52073–52086. [Google Scholar] [CrossRef]
- Kowsalya, P.; Bharathi, S.; Chamundeeswari, M. Photocatalytic treatment of textile effluents by biosynthesized photo-smart catalyst: an eco-friendly and cost-effective approach. Environment Development and Sustainability 2023. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Zh. Bekmyrza, K.; Haque, M.N.; Islam, S.N.; Hossain, M.A.; Hassan, M.; Roy, H.; et al. Advanced Applications of Carbonaceous Materials in Sustainable Water Treatment, Energy Storage, and CO2 Capture: A Comprehensive Review. Sustainability 2023, 15, 8815. [Google Scholar] [CrossRef]
- Rajkishore, S.K.; Devadharshini, K.P.; Sathya Moorthy, P.; Reddy Kiran Kalyan, V.S.; Sunitha, R.; Prasanthrajan, M.; Maheswari, M.; Subramanian, K.S.; Sakthivel, N.; Sakrabani, R. Novel Synthesis of Carbon Dots from Coconut Wastes and Its Potential as Water Disinfectant. Sustainability 2023, 15, 10924. [Google Scholar] [CrossRef]
- Adeola, A.; Duarte, M.; Naccache, R. Microwave-assisted synthesis of carbon-based nanomaterials from biobased resources for water treatment applications: emerging trends and prospects. Frontiers in Carbon 2023, 2. [Google Scholar] [CrossRef]
- Chen, F.; Sun, Y.; Liang, C.; Yang, T.; Mi, S.; Dai, Y.; Yu, M.; Yao, Q. Adsorption characteristics and mechanisms of Cd2+ from aqueous solution by biochar derived from corn stove. Scientific Reports 2022, 12, 17714. [Google Scholar] [CrossRef]
- Anand, A.; Gautam, S.; Ram, L.C. Feedstock and pyrolysis conditions affect suitability of biochar for various sustainable energy and environmental applications. Journal of Analytical and Applied Pyrolysis 2023, 170, 105881. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Elbehiry, F.; Almashad, A.A.; Khalifa, A.M.; Khalil, A.M.; El-Ramady, H.; Brevik, E.C. Contaminate Remediation with Biochar and Nanobiochar Focusing on Food Waste Biochar: A Review. Egyptian Journal of Soil Science 2023, 63, 641–658. [Google Scholar] [CrossRef]
- Sonowal, S.; Koch, N.; Sarma, H.; Prasad, K.; Prasad, R. A Review on Magnetic Nanobiochar with Their Use in Environmental Remediation and High-Value Applications. Journal of Nanomaterials 2023, 48819522. [Google Scholar] [CrossRef]
- Bhandari, G.; Gangola, S.; Dhasmana, A.; Rajput, V.; Gupta, S.; Malik, S.; Slama, P. Nano- biochar: recent progress, challenges, and opportunities for sustainable environmental remediation. Front. Microbiol. 2023, 14, 1214870. [Google Scholar] [CrossRef]
- Jiang, M.; He, L.; Niazi, N.K.; Wang, H.; Gustave, W.; Vithanage, M.; Geng, K.; Shang, H.; Zhang, X.; Wang, Z. Nanobiochar for the remediation of contaminated soil and water: challenges and opportunities. Biochar 2023, 5, 2. [Google Scholar] [CrossRef]
- Li, R.; Reza Kamali, A. Carbonization of Corn Leaf Waste for Na-Ion Storage Application Using Water-Soluble Carboxymethyl Cellulose Binder. Gels 2023, 701. [Google Scholar] [CrossRef] [PubMed]
- Pathaare, Y.; Reddy, A.; Sangrulkar, P.; Kandasubramanian, B.; Satapathy, A. Carbon hybrid nano-architectures as an efficient electrode material for supercapacitor applications. Hybrid Advances 2023, 3, 100041. [Google Scholar] [CrossRef]
- Raja, S.; da Silva, G.; Anbu, S.; Ribeiro, C.; Luiz, C.; Mattoso, C. Cellulosic biomass-derived carbon quantum dots: "On-off-on" nanosensor for rapid detection of multi-metal ions and green photocatalytic CO2 reduction in water. Biomass Conversions and Biorefinery 2023. [Google Scholar] [CrossRef]
- Layek, J.; Narzari, R.; Hazarika, S.; Das, A.; Rangappa, K.; Devi, S.; Balusamy, A.; Saha, S.; Mandal, S.; Idapuganti, R.G.; et al. Prospects of Biochar for Sustainable Agriculture and Carbon Sequestration: An Overview for Eastern Himalayas. Sustainability 2022, 14, 6684. [Google Scholar] [CrossRef]
- Azad, H.; Bhat, J.; Shameem, S. Potential of Biochar to Sequester Carbon and Mitigate Greenhouse Gas Emissions. Current Journal of Applied Science and Technology 2023, 42, 4. [Google Scholar] [CrossRef]
- Lan, G.; Yang, J.; Ye, R.; Boyjoo, Y.; Liang, J.; Liu, X.; Li, Y.; Liu, J.; Qian, K. Sustainable Carbon Materials toward Emerging Applications. Small Methods 2021, 5. [Google Scholar] [CrossRef]
- Goswami, A.; Trivedi, D.; Jadhav, N.; Pinjari, D. Sustainable and green synthesis of carbon nanomaterials: A review. Journal of Environmental Chemical Engineering 2021, 9, 5. [Google Scholar] [CrossRef]
- Su, D.; Centi, G. A perspective on carbon materials for future energy application. Journal of Energy Chemistry 2013, 22, 2. [Google Scholar] [CrossRef]
- Ravi, S.; Vadukumpully, S. Sustainable carbon nanomaterials: Recent advances and its applications in energy and environmental remediation. Journal of Environmental Chemical Engineering 2016, 4, 1. [Google Scholar] [CrossRef]
- Gao, M.; Shih, C.; Pan, S.; Chueh, C.; Chen, W. Advances and challenges of green materials for electronics and energy storage applications: from design to end-of-life recovery. Journal of Materials Chemistry A 2018, 42, 6. [Google Scholar] [CrossRef]
- Pribat, D. A quick overview of carbon nanotubes and graphene applications for future electronics. International SoC Design Conference 2011. [Google Scholar] [CrossRef]
- Yusof, N.; Rahman, S.; Muhammad, A. Carbon Nanotubes and Graphene for Sensor Technology. Synthesis, Technology and Applications of Carbon Nanomaterials 2019. [Google Scholar] [CrossRef]
- Gaur, M.; Misra, C.; Yadav, A.; Swaroop, S.; Maolmhuaidh, F.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 20. [Google Scholar] [CrossRef]
- Burdanova, M.; Kharlamova, M.; Kramberger, C.; Nikitin, M. Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine. Nanomaterials 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.; Sree, R.; Aishwarya, S.; Kounaina, S.; Patil, A.; Hudeda, P.; More, S.; Muthucheliyan, K.; Kumar, T.; Raghu, A.; Reddy, K.; Zameer, F. Graphene-based functional nanomaterials for biomedical and bioanalysis applications. FlatChem 2020, 23. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Wang, J.; James, D.; Narkhede, P.; Singh, S.; Jassby, D.; Tour, J.; Arnusch, C. Laser-induced graphene and carbon nanotubes as conductive carbon-based materials in environmental technology. Materials Today 2020, 34. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




