Submitted:
29 January 2024
Posted:
06 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
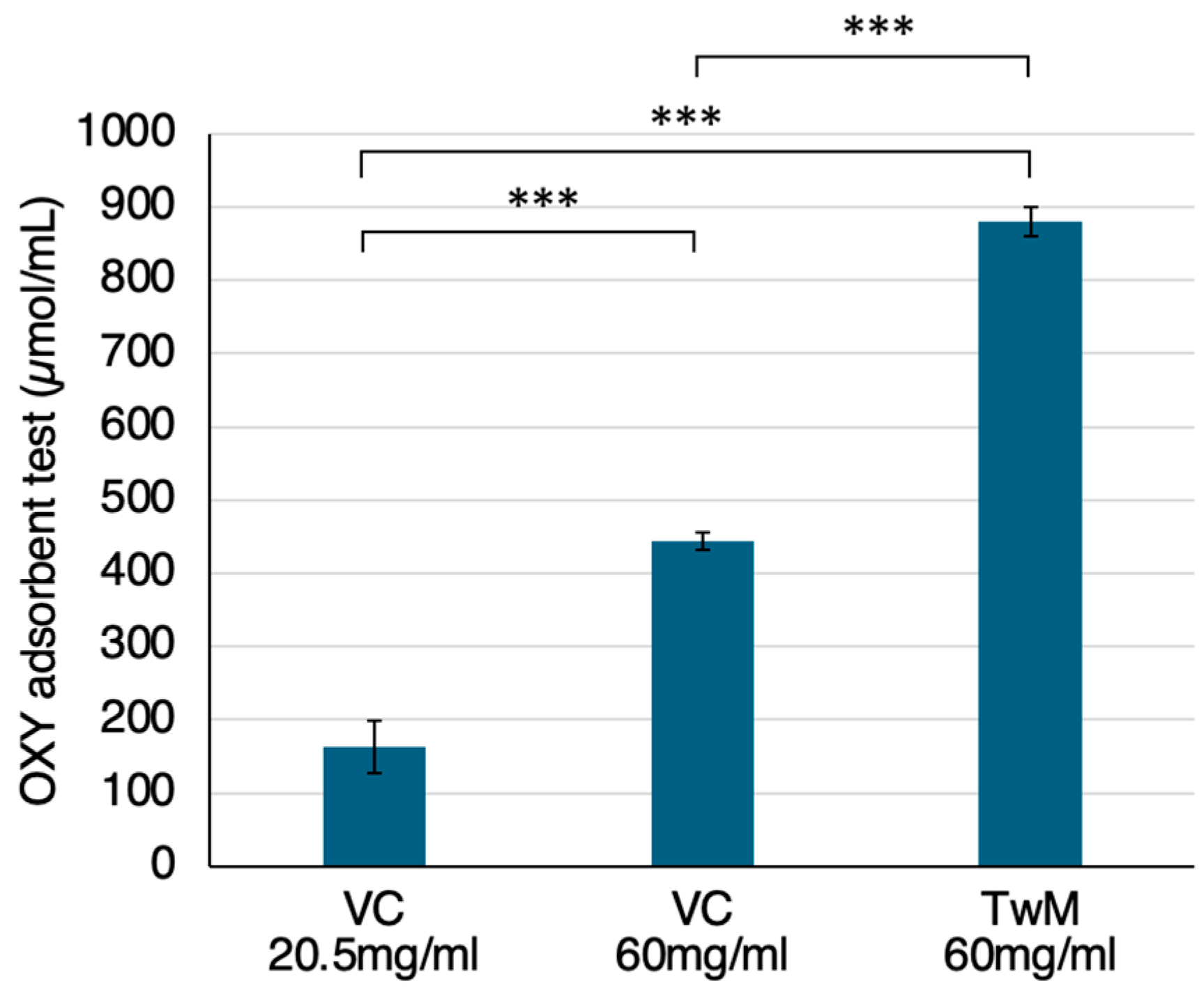
2.1. OS Scavenging Ability of TwM
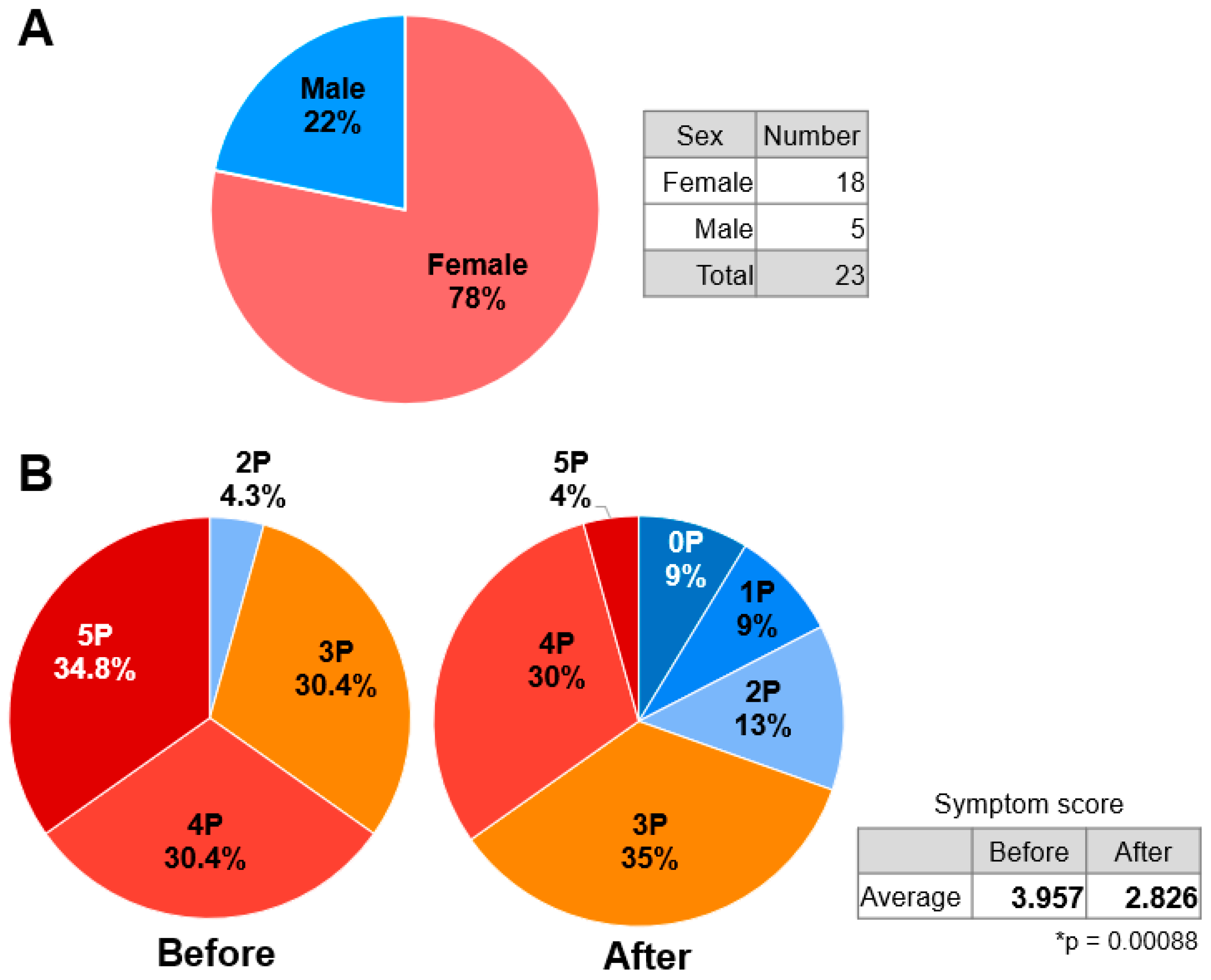
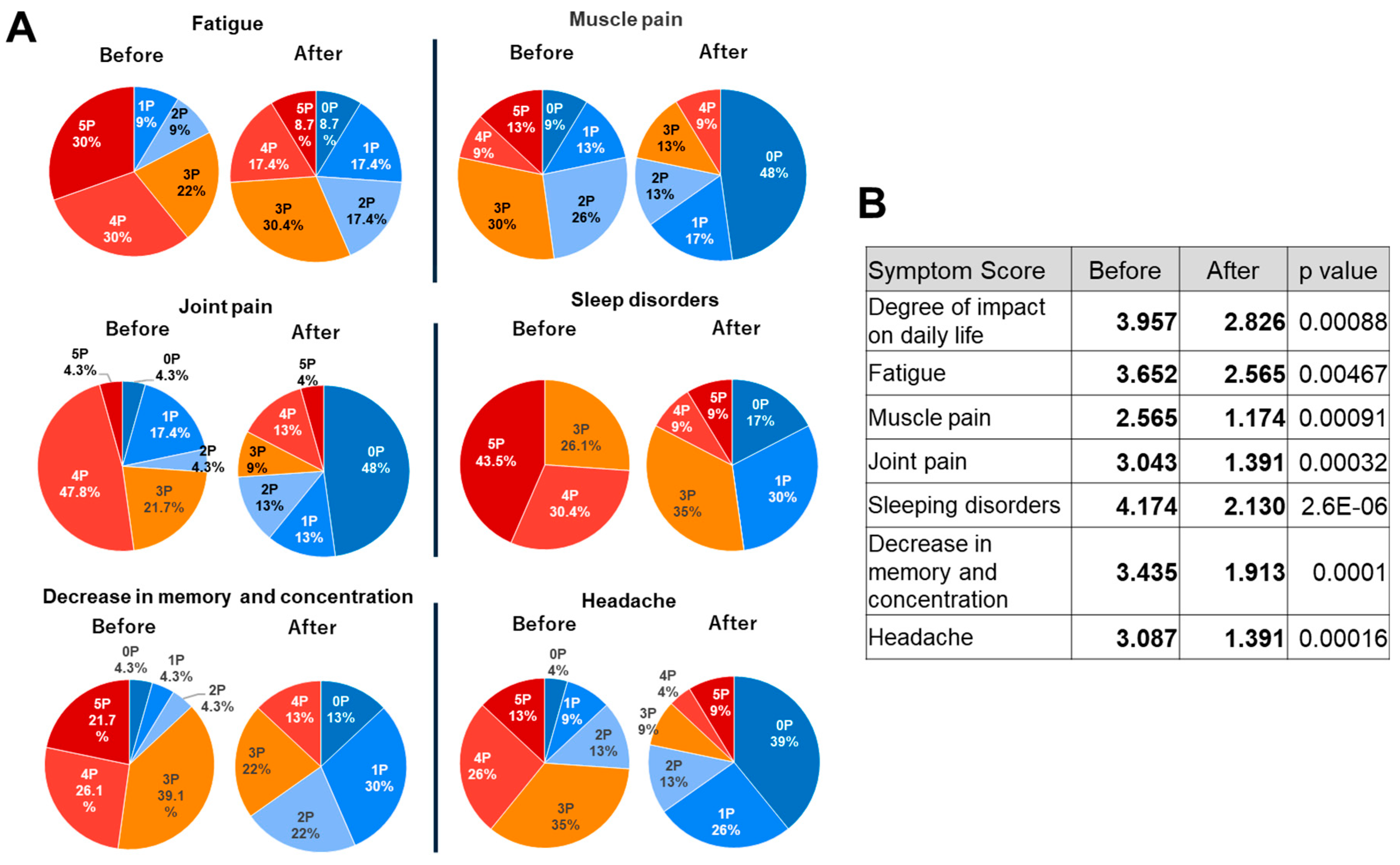
2.2. Effects of TwM on Various Symptoms in CFS
3. Materials and Methods
3.1. Materials
3.2. Antioxidant Measurement of Solutions
3.3. Questionnaire Design
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinardi, G.; Scarlato, G. La sindrome della "fatica cronica". Approccio multifattoriale e possibilità di trattamento [The chronic fatigue syndrome. A multifactorial approach and the treatment possibilities]. Recenti Prog Med. 1990, 81, 773–777. [Google Scholar]
- Komaroff, A.L.; Buchwald, D. Symptoms and signs of chronic fatigue syndrome. Rev Infect Dis. 1991, 13 Suppl 1, S8–11. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Pen, J.J.; Chirumbolo, S.; Aaseth, J. Chronic fatigue syndrome (CFS): Suggestions for a nutritional treatment in the therapeutic approach. Biomed Pharmacother. 2019, 109, 1000–1007. [Google Scholar] [CrossRef]
- You, F.; Tanaka, S.; Yoshikawa, T.; von Greiffenclau, M.M.; Inufusa, H. Effects of Antioxidant composition Twendee X on side effects of SARS -COV-2 mRNA vaccine. Brain Supplement. 2022, 4, 1–6. [Google Scholar]
- You, F.; Tanaka, S.; Yoshikawa, T.; von Greiffenclau, M.M.; Inufusa, H. Antioxidant composition Twendee X may improve long COVID symptoms. Brain Supplement. 2022, 4, 7–12. [Google Scholar]
- Theoharides, T.C.; Cholevas, C.; Polyzoidis, K.; Politis, A. Long-COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. 2021, 47, 232-241. [CrossRef]
- Hirano, S.I.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Molecular Hydrogen as a Medical Gas for the Trearment of Myalgic Ensephalomyelitis/Chronic Fatigue Syndrome: Possible Efficacy Based on Literture Review. Front Neurol. 2022, 13, 841310. [Google Scholar] [CrossRef]
- Nakatomi, Y.; Mizuno, K.; Ishii, A.; Wada, Y.; Tanaka, M.; Tazawa, S.; Onoe, K.; Fukuda, S.; Kawabe, J.; Takahashi, K.; et al. Neuroinflammation in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An 11C-(R)-PK1195 PET Study. J Nucl Med. 2014, 55, 945–950. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yamashita, T.; Shang, J.; Shi, X.; Morihara, R.; Huang, Y.; Sato, K.; Takemoto, M.; Hishikawa, N.; Ohta, Y.; et al. Clinical and Pathological Benefit of Twendee X in Alzheimer’s Disease Transgenic Mice with Chronic Cerebral Hypoperfusion. J. Stroke Cerebrovasc. Dis. 2019, 28, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020 Oct;143:110102. [CrossRef]
- Logan, A.C.; Wong, C. Chronic fatigue syndrome: oxidative stress and dietary modifications. Altern Med Rev. 2001, 6, 450–459. [Google Scholar]
- Morris, G.; Anderson, G.; Maes, M. Hypothalamic-pituitary-adrenal hypofunction in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) as a consequence of activated immune-inflammatory and oxidative and nitrosative pathways. Mol Neurobiol. 2017, 54, 6806–6819. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Stubbs, B.; Köhler, C.A.; Walder, K.; Slyepchenko, A.; Berk, M.; Carvalho, A.F. The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med Rev. 2018, 41, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Inufusa, H. Characterization of cell protection effects of Twendee X by oxidative stress. J. World Mitochondria Soc. 2016, 2, 42. [Google Scholar]
- Inufusa, H. Composition for protection against cytotoxic effects. TIMA Foundation. Patent No. 5777821, 2015-9-9. 9.
- You, F.; Harakawa, Y.; Yoshikawa, T.; Inufusa, H. Why Does the Antioxidant Complex Twendee X® Prevent Dementia? Int. J. Mol. Sci. 2023, 24, 13018. [Google Scholar] [CrossRef]
- Tadokoro, K.; Morihara, R.; Ohta, Y.; Hishikawa, N.; Kawano, S.; Sasaki, R.; Matsumoto, N.; Nomura, E.; Nakano, Y.; Takahashi, Y.; et al. Clinical Benefits of Antioxidative Supplement Twendee X for Mild Cognitive Impairment: A Multicenter, Randomized, Double-Blind, and Placebo-Controlled Prospective Interventional Study. J. Alzheimers Dis. 2019, 71, 1063–1069. [Google Scholar] [CrossRef]
- You, F.; Harakawa, Y.; Yoshikawa, T.; Inufusa, H. Controlling Gut Microbiota by Twendee X® May Contribute to Dementia Prevention. Int J Mol Sci. 2023, 24, 16642. [Google Scholar] [CrossRef]
- Kusaki, M.; Ohta, Y.; Inufusa, H.; Yamashita, T.; Morihara, R.; Nakano, Y.; Liu, X.; Shang, J.; Tian, F.; Fukui, Y.; et al. Neuroprotective Effects of a Novel Antioxidant Mixture Twendee X in Mouse Stroke Model. J. Stroke Cerebrovasc. Dis. 2017, 26, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; You, F.; Kato, Y.; Kimura, M.; Harakawa, Y.; Yoshikawa, T.; Inufusa, H. Twendee X, a mixed antioxidant supplement, improves cognitive function, coordination, and neurotrophic factor expression in long-term vitamin E-deficient mice. J Clin Biochem Nutr. 2023, 72, 93–100. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Cadet, J.; Delatour, T.; Douki, T.; Gasparutto, D.; Pouget, J.P.; Ravanat, J.L.; Sauvaigo, S. Hydroxyl radicals and DNA base damage. Mutat. Res. 1999, 424, 9–21. [Google Scholar] [CrossRef]
- Morrell, C.N. Reactive oxygen species: finding the right balance. Circ Res. 2008, 103, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer's disease. Neurobiol Aging. 2021, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Yamashita, T.; Tsunoda, K.; Matsumoto, N.; Tadokoro, K.; Sasaki, R.; Abe, K. In Vitro Free Radical Scavenging Activities of Dietary Supplements by Electron Spin Resonance. Brain Suppl. 2020, 2, 1–12. [Google Scholar]
- Yamaguchi, F.; Yoshimura, Y.; Nakazawa, H.; Ariga, T. Free Radical Scavenging Activity of Grape Seed Extract and Antioxidants by Electron Spin Resonance Spectrometry in an H2O2/NaOH/DMSO System. J. Agric. Food Chem. 1999, 47, 2544–2548. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Inomata, T.; Nakazawa, H.; Kubo, H.; Yamaguchi, F.; Ariga, T. Evaluation of Free Radical Scavenging Activities of Antioxidants with an H2O2/NaOH/DMSO System by Electron Spin Resonance. J Agric Food Chem. 1999, 47, 4653–4656. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Iacob, E.; Light, A.R.; Donaldson, G.W.; Okifuji, A.; Hughen, R.W.; White, A.T.; Light, K.C. Gene expression factor analysis to differentiate pathways linked to fibromyalgia, chronic fatigue syndrome, and depression in a diverse patient sample. Arthritis Care Res (Hoboken). 2016, 68, 132–140. [Google Scholar] [CrossRef]
- Faro, M.; Sàez-Francás, N.; Castro-Marrero, J.; Aliste, L.; Fernández, de Sevilla, T.; Alegre, J. Gender differences in chronic fatigue syndrome Reumatol Clin. 2016, 12, 72-77. [CrossRef]
- Blomberg, J.; Gottfries, C.G.; Elfaitouri, A.; Rizwan, M.; Rosén, A. Infection elicited autoimmunity and myalgic encephalomyelitis/chronic fatigue syndrome: an explanatory model. Front Immunol. 2018, 9, 229. [Google Scholar] [CrossRef]
- de Vega, W.C.; Vernon, S.D.; McGowan, P.O. DNA methylation modifications associated with chronic fatigue syndrome. PLoS One. 2014, 9, e104757. [Google Scholar] [CrossRef]
- Kuratsune, H.; Yamaguti, K.; Lindh, G.; Evengård, B.; Hagberg, G.; Matsumura, K.; Iwase, M.; Onoe, H.; Takahashi, M.; Machii, T.; et al. Brain regions involved in fatigue sensation: reduced acetylcarnitine uptake into the brain. Neuroimage. 2002, 17, 1256–1265. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ouchi, Y.; Onoe, H.; Yoshikawa, E.; Tsukada, H.; Takahashi, H.; Iwase, M.; Yamaguti, K.; Kuratsune, H.; Watanabe, Y. Reduction of serotonin transporters of patients with chronic fatigue syndrome. Neuroreport. 2004, 15, 2571–2574. [Google Scholar] [CrossRef]
- Okada, T.; Tanaka, M.; Kuratsune, H.; Watanabe, Y.; Sadato, N. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol. 2004, 4, 14. [Google Scholar] [CrossRef]
- Natelson, B.H.; Weaver, S.A.; Tseng, C.L.; Ottenweller, J.E. Spinal fluid abnormalities in patients with chronic fatigue syndrome. Clin Diagn Lab Immunol. 2005, 12, 52–55. [Google Scholar] [CrossRef]
- Natelson, B.H.; Haghighi, M.H.; Ponzio, N.M. Evidence for the presence of immune dysfunction in chronic fatigue syndrome. Clin Diagn Lab Immunol. 2002, 9, 747–752. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. A neuro-immune model of myalgic encephalomyelitis/chronic fatigue syndrome. Metab Brain Dis. 2013, 28, 523–540. [Google Scholar] [CrossRef] [PubMed]
- de Lange, F.P.; Kalkman, J.S.; Bleijenberg, G.; Hagoort, P.; van der Werf, S.P.; van der Meer, J.W.; Toni, I. Neural correlates of the chronic fatigue syndrome: an fMRI study. Brain. 2004, 127, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; Gan, R.; Haier, J. Multiple co-infections (mycoplasma, chlamydia, human herpes virus-6) in blood of chronic fatigue syndrome patients: association with signs and symptoms. APMIS. 2003, 111, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, N.R.; Joseph, A.M.; Levin, D.G.; Gundermann, D.M.; Leeuwenburgh, C.; Sandesara, B.; Manini, T.M.; Adhihetty, P.J. Idiopathic chronic fatigue in older adults is linked to impaired mitochondrial content and biogenesis signaling in skeletal muscle. Oncotarget. 2016, 7, 52695–52709. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.W.; McGregor, N.R.; Lewis, D.P.; Butt, H.L.; Gooley, P.R. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics. 2015, 11, 1626–1639. [Google Scholar] [CrossRef]
- Jammes, Y.; Steinberg, J.G.; Mambrini, O.; Brégeon, F.; Delliaux, S. Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J Intern Med. 2005, 257, 299–310. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Khabour, O.F.; Rashid, B.A.; Damaj, I.M.; Salah, H.A. The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: the role of oxidative stress. Behav Brain Res. 2012, 226, 205–210. [Google Scholar] [CrossRef]
- Hill, V.M.; O’Connor, R.M.; Sissoko, G.B.; Irobunda, I.S.; Leong, S.; Canman, J.C.; Stavropoulos, N.; Shirasu-Hiza, M. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 2018, 16, e2005206. [Google Scholar] [CrossRef]
- Vaccaro, A.; Kaplan Dor, Y.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulja, D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell 2020, 181, 1307–1328. [Google Scholar] [CrossRef]
- Reimund, E. The free radical flux theory of sleep. Med. Hypotheses. 1994, 43, 231–233. [Google Scholar] [CrossRef]
- Xiong, R.; Gunter, C.; Fleming, E.; Vernon, S.D.; Bateman, L.; Unutmaz, D.; Oh, J. Multi-'omics of gut microbiome-host interactions in short- and long-term myalgic encephalomyelitis/chronic fatigue syndrome patients. Cell Host Microbe. 2023, 31, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Zanatta, D.; Syeda, T.; Sánchez-Valle, V.; Irene-Fierro, M.; Torres-Aguilar, P.; Torres Ramos, M.A.; Shibayama-Salas, M.; Silva-Olivares, A.; Noriega, L.G.; Torres, N.; et al. Dietary Fiber Modulates the Release of Gut Bacterial Products Preventing Cognitive Decline in an Alzheimer's Mouse Model. Cell Mol Neurobiol. 2023, 43, 1595–1618. [Google Scholar] [CrossRef] [PubMed]
- Ricobaraza, A.; Cuadrado-Tejedor, M.; Marco, S.; Pérez-Otaño, I.; García-Osta, A. Phenylbutyrate rescues dendritic spine loss associated with memory deficits in a mouse model of Alzheimer disease. Hippocampus. 2012, 22, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory response in the CNS: Friend or foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Janssens, K.A.; Zijlema, W.L.; Joustra, M.L.; Rosmalen, J.G. Mood and anxiety disorders in chronic fatigue syndrome, fibromyalgia, and irritable bowel syndrome: results from the LifeLines cohort study. Psychosom Med. 2015, 77, 449–457. [Google Scholar] [CrossRef]
- Belleau, E.L.; Treadway, M.T.; Pizzagalli, D.A. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry. 2019, 85, 443–453. [Google Scholar] [CrossRef]
- Michel, T.M.; Frangou, S.; Thiemeyer, D.; Camara, S.; Jecel, J.; Nara, K.; Brunklaus, A.; Zoechling, R.; Riederer, P. Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder - a post-mortem study. Psychiatry Res. 2007, 151, 145–150. [Google Scholar] [CrossRef]
- Michel, T.M.; Thome, J.; Martin, D.; Nara, K.; Zwerina, S.; Tatschner, T.; Weijers, H.G.; Koutsilieri, E. Cu, Zn- and Mn-superoxide dismutase levels in brains of patients with schizophrenic psychosis. J Neural Transm (Vienna). 2004, 111, 1191–1201. [Google Scholar] [CrossRef]
- Michel, T.M.; Camara, S.; Tatschner, T.; Frangou, S.; Sheldrick, A.J.; Riederer, P.; Grünblatt, E. Increased xanthine oxidase in the thalamus and putamen in depression. World J Biol Psychiatry. 2010, 11, 314–20. [Google Scholar] [CrossRef]
- Quiroz, J.A.; Gray, N.A.; Kato, T.; Manji, H.K. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008, 33, 2551–2565. [Google Scholar] [CrossRef] [PubMed]
- Rezin, G.T.; Gonçalves, C.L.; Daufenbach, J.F.; Fraga, D.B.; Santos, P.M.; Ferreira, G.K.; Hermani, F.V.; Comim, C.M.; Quevedo, J.; Streck, E.L. Acute administration of ketamine reverses the inhibition of mitochondrial respiratory chain induced by chronic mild stress. Brain Res. Bull. 2009, 79, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Calingasan, N.Y.; Ho, D.J.; Wille, E.J.; Campagna, M.V.; Ruan, J.; Dumont, M.; Yang, L.; Shi, Q.; Gibson, G.E.; Beal, M.F. Influence of mitochondrial enzyme deficiency on adult neurogenesis in mouse models of neurodegenerative diseases. Neuroscience. 2008, 153, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Kirby, D.M.; Rennie, K.J.; Smulders-Srinivasan, T.K.; Acin-Perez, R.; Whittington, M.; Enriquez, J.A.; Trevelyan, A.J.; Turnbull, D.M.; Lightowlers, R.N. Transmitochondrial embryonic stem cells containing pathogenic mtDNA mutations are compromised in neuronal differentiation. Cell Prolif. 2009, 42, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Masini, S.; Carpeggiani, C.; L'Abbate, A.; Boni, C.; CarloZucchelli, G. In vivo total antioxidant capacity: comparison of two different analytical methods. Clin Chem Lab Med. 2004, 42, 84–89. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




