Submitted:
07 February 2024
Posted:
07 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Exposure Solutions
2.2. Organisms Acquisition and Acclimation
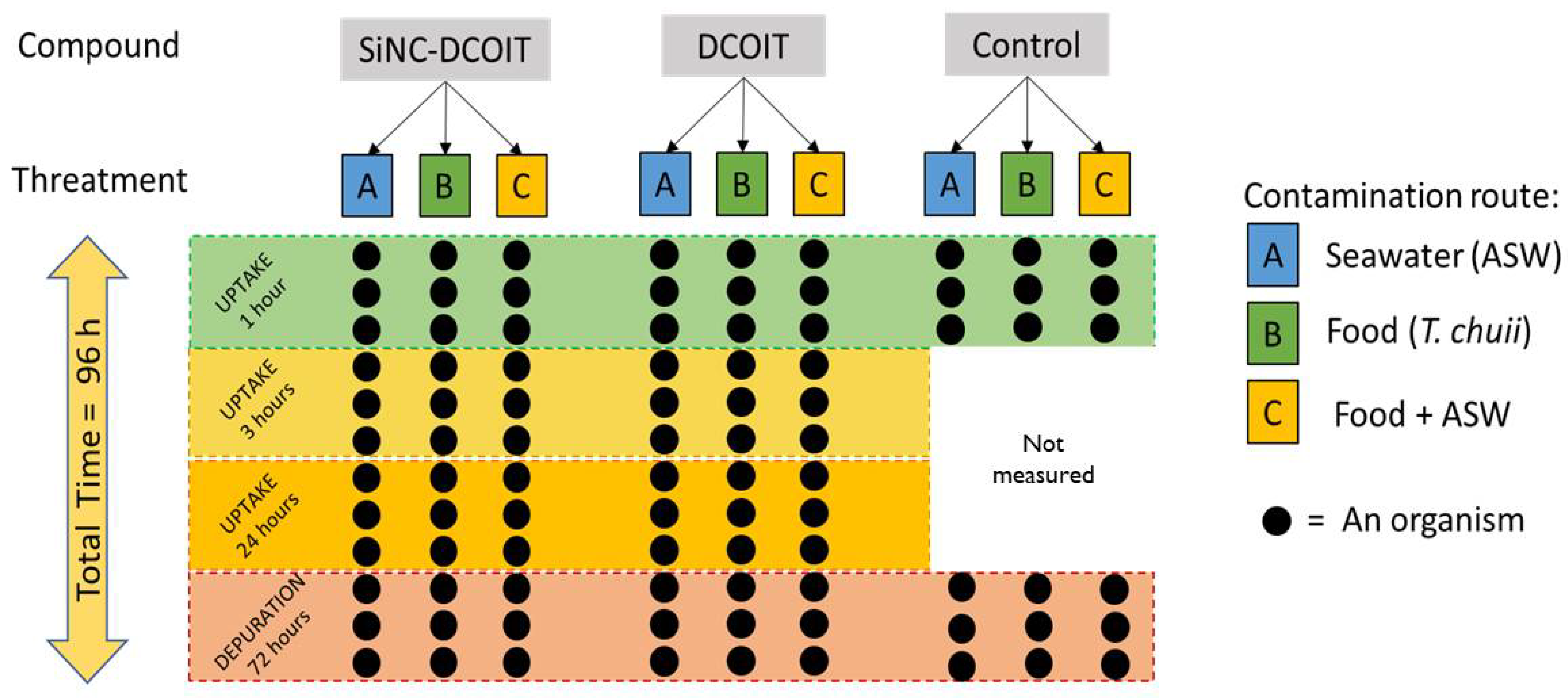
2.3. DCOIT and SiNC-DCOIT Bioaccumulation and Trophic Transfer Test
- Contamination through seawater (waterborne exposure): The tested concentration was 80 µg/L DCOIT, which was below the lethal concentration to 50% organisms (LC50) for M. galloprovincialis and above the detection limit for quantification. The bioconcentration factor (BCF) of M. galloprovincialis was assessed using this treatment.
- Dietary exposure: 48 h before mussel exposure (previous experiments demonstrated that at 48 h, DCOIT was incorporated into the microalgae), two cultures of the microalgae T. chuii were contaminated with DCOIT and SiNC-DCOIT, respectively, at 5 µg/L DCOIT (10 times lower than the no-observed effect concentration (NOEC) for T. chuii). The mussels were fed 2.5 x 105 cells/mussel of T. chuii at the beginning of the experiment. Prior to mussel feeding, the microalgae were rinsed with uncontaminated ASW through a 0.045 µm filter to remove the culture media. The cell density was spectrophotometrically measured at fluorescence (λexc = 475 nm and λemi = 645 nm). The obtained results allowed for the calculation of the bioaccumulation factor (BAF) of DCOIT for T. chuii and the biomagnification factor (BMFTL) for M. galloprovincialis.
- Contamination through both water and food: In this treatment, mussels were exposed to DCOIT and SiNC-DCOIT concomitantly with water (80 µg/L DCOIT) and food (5 µg/L DCOIT), as described above (items 1 and 2). At the end of the experiment, the BAFs were calculated for mussels.
2.4. Chemical Quantification
2.4.1. Water Extraction
2.4.2. Mussel Extraction
2.4.3. Microalgae Extraction
2.4.4. DCOIT Quantification - Gas-Chromatography Analysis
2.5. Bioaccumulation, Bioconcentration and Biomagnification End-Points Calculation
3. Results and Discussion
3.1. DCOIT Bioaccumulation, Biomagnification, and Trophic Transfer
3.2. SiNC-DCOIT Bioaccumulation, Biomagnification, and Trophic Transfer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IMO [International Marine Organization]. International Convention on the Control of Harmful Antifouling Systems on Ships. 2001.
- Lam, N.H.; Jeong, H.; Kang, S.; Kim, D.J.; Ju, M.J.; Horiguchi, T.; Cho, H.S. Organotins and new antifouling biocides in water and sediments from three Korean Special Management Sea Areas following ten years of tributyltin regulation: Contamination profiles and risk assessment. Mar. Pollut. Bull. 2017, 121, 302–312. [Google Scholar] [CrossRef]
- Jacobson, A.H.; Willingham, G.L. 2000. Sea-nine antifoulant: An environmentally acceptable alternative to organotin antifoulants. Sci. Total Environ. 2000, 258, 103–110. [Google Scholar] [CrossRef]
- Chen, L.; Lam, J.C.W.W. SeaNine 211 as antifouling biocide: A coastal pollutant of emerging concern. J. Environ. Sci. 2017, 61, 68–79. [Google Scholar] [CrossRef]
- Campos, B.G.; Figueiredo, J.; Perina, F.; Abessa, D.M.S.; Loureiro, S.; Martins, R. Occurrence, effects and environmental risk of antifouling biocides (EU PT21): Are marine ecosystems threatened? Crit. Rev. Environ. Sci. Technol. 2022a, 52, 3179–3210. [Google Scholar] [CrossRef]
- Campos, B.G.; Fontes, M.K.; Gusso-Choueri, P.K.; Marinsek, G.P.; Nobre, C.R.; Moreno, B.B.; Abreu, F.E.L.; Fillmann, G.; Mari, R.B.; Abessa, D.M.S. A preliminary study on multi-level biomarkers response of the tropical oyster Crassostrea brasiliana to exposure to the antifouling biocide DCOIT. Mar. Pollut. Bull. 2022, 174. [Google Scholar] [CrossRef] [PubMed]
- Martínez, K.; Barceló, D. Determination of antifouling pesticides and their degradation products in marine sediments by means of ultrasonic extraction and HPLC-APCI-MS. Fresenius J. Anal. Chem., 2001, 370, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.N.; Kim, U.J.; Lee, I.S.; Choi, M.; Oh, J.E. Assessment of organotin and tin-free antifouling paints contamination in the Korean coastal area. Mar. Pollut. Bull. 2015, 99, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.; Oliveira, T.; Ferreira, V.; Sushkova, A.; Silva, S.; Carneiro, D.; Cardoso, D.N.; Gonçalves, S.F.; Maia, F.; Rocha, C.; Tedim, J.; Loureiro, S.; Martins, R. Toxicity of innovative anti-fouling nano-based solutions in marine species. Environ. Sci. Nano, 2019, 5. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.; Sun, J.; Wong, Y.H.; Han, Z.; Au, D.W.T.; Bajic, V.B.; Qian, P.Y. Proteomic changes in brain tissues of marine medaka (Oryzias melastigma) after chronic exposure to two antifouling compounds: Butenolide and 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT). Aquat. Toxicol., 2014, 157, 47–56. [Google Scholar] [CrossRef]
- Cima, F.; Bragadin, M.; Ballarin, L. Toxic effects of new antifouling compounds on tunicate haemocytes. I. Sea-Nine 211TM and chlorothalonil. Aquat. Toxicol., 2008, 86, 299–312. [Google Scholar] [CrossRef]
- Figueiredo, J.; Loureiro, S.; Martins, R. Hazard of novel anti-fouling nanomaterials and biocides DCOIT and silver to marine organisms. Environ. Sci.: Nano, 2020, 7, 1670–1680. [Google Scholar] [CrossRef]
- Jesus, É.P.S.; Figueirêdo, L.P.; Maia, F.; Martins, R.; Nilin, J. Acute and chronic effects of innovative antifouling nanostructured biocides on a tropical marine microcrustacean. Mar. Pollut. Bull., 2021, 164, 111970. [Google Scholar] [CrossRef]
- Santos, J.V.N.; Martins, R.; Fontes, M.K.; Galvao, B.; Silva, M.B.M.P.; Maia, F.; Abessa, D.M.S.; Perina, F.C. Can encapsulation of the biocide DCOIT affect the anti-fouling efficacy and toxicity on tropical bivalves? Appl. Sci. (Switzerland), 2020, 10, 8579. [Google Scholar] [CrossRef]
- Perina, F.; Ottoni, C.; Santos, J.; Santos, V.; Silva, M.; Campos, B.; Fontes, M.; Santana, D.; Maia, F.; Abessa, D.; Martins, R. Marine hazard assessment of soluble and nanostructured forms of the booster biocide DCOIT in tropical waters. Water, 2023, 15, 1185. [Google Scholar] [CrossRef]
- Eom, H.; Haque, N.; Nam, S.; Lee, D.; Rhee, J. Effects of sublethal concentrations of the antifouling biocide Sea-Nine on biochemical parameters of the marine polychaete Perinereis aibuhitensis. Comp. Biochem. Physiol. C Toxicol. Pharmacol., 2019, 222, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Gabe, H.B.; Guerreiro, A.S.; Sandrini, J.Z. Molecular and biochemical effects of the antifouling DCOIT in the mussel Perna perna. Comp. Biochem. Physiol. C Toxicol. Pharmacol., 2021, 239, 108870. [Google Scholar] [CrossRef]
- Cima, F.; Ferrari, G.; Ferreira, N.G.C.; Rocha, R.J.M.; Serôdio, J.; Loureiro, S.; Calado, R. Preliminary evaluation of the toxic effects of the antifouling biocide Sea-Nine 211 in the soft coral Sarcophyton cf. glaucum (Octocorallia, Alcyonacea) based on PAM fluorometry and biomarkers. Mar. Environ. Res., 2013, 83, 16–22. [Google Scholar] [CrossRef]
- Campos, B.G.; Silva, M.B.M.P.; Avelelas, F.; Maia, F.; Loureiro, S.; Perina, F.; Abessa, D.M.S.; Martins, R. 2022c. Toxicity of innovative antifouling additives on an early life stage of the oyster Crassostrea gigas: short and long-term exposure effects. Environ. Sci. Pollut. Res. 2022, 29, 27534–27547. [Google Scholar] [CrossRef]
- Ito, M.; Mochida, K.; Ito, K.; Onduka, T.; Fujii, K. Induction of apoptosis in testis of the marine teleost mummichog Fundulus heteroclitus after in vivo exposure to the antifouling biocide 4,5-dichloro-2-n-octyl-3(2H)-isothiazolone (Sea-Nine 211). Chemosphere, 2013, 90, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Do, J.W.; Haque, N.; Lim, H.; Hwa, B.; Lee, D. Constant exposure to environmental concentrations of the antifouling biocide Sea-Nine retards growth and reduces acetylcholinesterase activity in a marine mysid. Aquat. Toxicol., 2018, 205, 165–173. [Google Scholar] [CrossRef]
- Bragadin, M.A.B.; Avoni, B.R.P.; Cutari, G.U.S.; Anente, S.A.M. An in vitro study of the interaction of Sea-Nine with rat liver mitochondria. Environ. Toxicol. Chem., 2005, 24, 1074–1078. [Google Scholar] [CrossRef]
- Hilvarsson, A.; Ohlauson, C.; Blanck, H.; Granmo, Å. Bioaccumulation of the new antifoulant medetomidine in marine organisms. Mar. Environ. Res., 2009, 68, 19–24. [Google Scholar] [CrossRef]
- European Commission [EC]. 2003. Technical Guidance Document on Risk Assessment In support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances, Commission Regulation (EC) No 1488/94.
- Maia, F.; Silva, A.P.; Fernandes, S.; Cunha, A.; Almeida, A.; Tedim, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Incorporation of biocides in nanocapsules for protective coatings used in maritime applications. Chem. Eng. J., 2015, 270, 150–157. [Google Scholar] [CrossRef]
- Reybuck, S.E.; Schwartz, C. Blends of encapsulated biocides (Patent No. US7377968B2). 2008. https://patentimages.storage.googleapis.com/d5/65/82/a5d45426162c23/US7377968.pdf.
- Vieira, A.A.; Caldas, S.S.; Kupski, L.; Tavella, R.A.; Primel, E.G. Extraction of chlorothalonil, dichlofluanid, DCOIT, and TCMTB from fish tissues employing the vortex assisted matrix solid-phase dispersion. Microchem. J., 2018, 143, 92–98. [Google Scholar] [CrossRef]
- González-Barreiro, O.; Rioboo, C.; Herrero, C.; Cid, A. Removal of triazine herbicides from freshwater systems using photosynthetic microorganisms. Environ. Pollut., 2006, 144, 266–271. [Google Scholar] [CrossRef]
- Arnot, J.A.; Gobas, F.A.P.C. A review of bioconcentration factor (BCF) and bioaccumulation factor ( BAF ) assessments for organic chemicals in aquatic organisms. Environ. Rev., 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Willingham, G.L.; Jacobson, A.H. Designing an environmentally safe marine antifoulant. ACS Symposium Series, 1996, 640, 224–233. [Google Scholar] [CrossRef]
- Campos, B.G.; Moreira, L.B.; Pauly, G.F.E.; Cruz, A.C.F.; Perina, F.C.; Abreu, F.; Fillmann, G.; Abessa, D.M.S. Water and sediment toxicity and hazard assessment of DCOIT towards neotropical marine organisms. Environ. Pollut.. 2023, 330. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.B.; Guerreiro, A.S.; Vargas, M.A.; Sandrini, J.Z. Effects of DCOIT (4,5-dichloro-2-octyl-4-isothiazolin-3-one) to the haemocytes of mussels Perna perna. Comp. Biochem. Physiol. C Toxicol. Pharmacol., 2020, 232, 108737. [Google Scholar] [CrossRef]
- Kookana, R.S.; Shareef, A.; Fernandes, M.B.; Hoare, S.; Gaylard, S.; Kumar, A. Bioconcentration of triclosan and methyl-triclosan in marine mussels (Mytilus galloprovincialis) under laboratory conditions and in metropolitan waters of Gulf St Vincent, South Australia. Mar. Pollut. Bull., 2013, 74, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Sheehan, P.; Kotzias, D.; Freitag, D.; Korte, F. Prediction of ecotoxicological behaviour of chemicals: Relationship between physico-chemical properties and bioaccumulation of organic chemicals in the mussel Mytilus edulis. Chemosphere, 1982, 11, 1121–1134. [Google Scholar] [CrossRef]
- Jacobson, A.; Mazza, L.S.; Lawrence, L.J.; Lawrence, B.; Jackson, S.; Kesterson, A. Fate of an Antifoulant in an Aquatic Environment. In: Racke, K.D., Leslie, A.R. (Eds.) Pesticides in Urban Environment. American Chemical Society Symp. Series 522, ACS, Washington, 1993. Chapter 12, pp. 127-138. https://pubs.acs.org/doi/abs/10.1021/bk-1993-0522.
- Harino, H. Occurrence and degradation of representative TBT free-antifouling biocides in aquatic environment. Coast. Mar. Sci., 2004, 29, 28–39. [Google Scholar]
- Silva, V.; Silva, C.; Soares, P.; Garrido, E.M.; Borges, F.; Garrido, J. Isothiazolinone biocides: chemistry, biological, and toxicity profiles. Molecules, 2020, 25, 991. [Google Scholar] [CrossRef]
- Bollmann, U.E.; Fernández-Calviño, D.; Brandt, K.K.; Storgaard, M.S.; Sanderson, H.; Bester, K. Biocide runoff from building facades: degradation kinetics in soil. Environ. Sci. Technol., 2017, 51, 3694–3702. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Lu, G.; Yang, C. Rapid degradation of two antifouling agents in seawater as affected by plankton and dissolved oxygen. Research Square. 2022. [Google Scholar] [CrossRef]
- Zhu, Y.; Xue, J.; Cao, J.; Xiao, H. A potential mechanism for degradation of 4,5-dichloro-2-(n-octyl)-3[2H]-isothiazolone (DCOIT) by brown-rot fungus Gloeophyllum trabeum. J. Hazard. Mater., 2017, 337, 72–79. [Google Scholar] [CrossRef] [PubMed]
| Compound | Exposure | Replicate | Time (h) | Muss.Conc. | ASW.Conc. | BCF |
|---|---|---|---|---|---|---|
| DCOIT | Water | 1 | 1 | <DL | 29.6 | n.a |
| 2 | 19971.9 | 79.6 | 251 | |||
| 3 | 20887.4 | 31.5 | 663 | |||
| 1 | 3 | 29450.0 | 0.5 | 58119 | ||
| 2 | 4231.7 | 57.6 | 74 | |||
| 3 | 214.2 | 30.1 | 7 | |||
| 1 | 24 | <DL | <DL | n.a | ||
| 2 | <DL | <DL | n.a | |||
| 3 | <DL | 29.7 | n.a | |||
| 1 | 96 | <DL | <DL | n.a | ||
| 2 | <DL | <DL | n.a | |||
| 3 | <DL | <DL | n.a |
| Compound | Exposure | Replicate | Time (h) | Muss.Conc. | ASW.Conc | BMFTL |
|---|---|---|---|---|---|---|
| DCOIT | Food | 1 | 1 | <DL | 31.1 | n.a |
| 2 | 19721.8 | 38.3 | 70 | |||
| 3 | <DL | 34.9 | n.a | |||
| 1 | 3 | <DL | <DL | n.a | ||
| 2 | 78671.6 | 33.1 | 282 | |||
| 3 | <DL | <DL | n.a | |||
| 1 | 24 | <DL | 0 | n.a | ||
| 2 | 19723.6 | 30.9 | 71 | |||
| 3 | <DL | <DL | n.a | |||
| 1 | 96 | <DL | <DL | n.a | ||
| 2 | 3259.5 | 34.2 | 12 | |||
| 3 | <DL | <DL | n.a |
| Compound | Exposure | Replicate | Time (h) | Muss.Conc. | ASW.Conc. | BAF |
|---|---|---|---|---|---|---|
| DCOIT | ASW + Food | 1 | 1 | 24743.3 | 30.8 | 146 |
| 2 | 2223.9 | 29.7 | 13 | |||
| 3 | 27134.9 | 30.4 | 160 | |||
| 1 | 3 | 43323.7 | 0.9 | 309 | ||
| 2 | 3332.6 | 30.4 | 19 | |||
| 3 | 19832.2 | 31.0 | 116 | |||
| 1 | 24 | <DL | 0.5 | n.a | ||
| 2 | 39771.7 | 0.7 | 284 | |||
| 3 | <DL | 29.9 | n.a | |||
| 1 | 96 | <DL | 29.4 | n.a | ||
| 2 | <DL | 30.1 | n.a | |||
| 3 | <DL | 29.8 | n.a |
| Compound | Exposure | Replicate | Time (h) | Muss.Conc. | ASW.Conc | BCF |
|---|---|---|---|---|---|---|
| SiNC-DCOIT | Water | 1 | 1 | <DL | 47.1 | n.a |
| 2 | 31116.5 | 163.3 | 191 | |||
| 3 | <DL | 40.6 | n.a | |||
| 1 | 3 | 1378.2 | 0.8 | 1827 | ||
| 2 | 398.0 | 31.1 | 13 | |||
| 3 | 1995.4 | 109.5 | 18 | |||
| 1 | 24 | <DL | 0.5 | n.a | ||
| 2 | <DL | 30.0 | n.a | |||
| 3 | <DL | <DL | n.a | |||
| 1 | 96 | <DL | <DL | n.a | ||
| 2 | <DL | 30.7 | n.a | |||
| 3 | <DL | <DL | n.a |
| Compound | Exposure | Replicate | Time (h) | Muss.Conc. | ASW.Conc | BMFTL |
|---|---|---|---|---|---|---|
| SiNC-DCOIT | Food | 1 | 1 | <DL | <DL | n.a |
| 2 | <DL | 30.3 | n.a | |||
| 3 | 19955.3 | 30.0 | 85 | |||
| 1 | 3 | <DL | <DL | n.a | ||
| 2 | <DL | 30.9 | n.a | |||
| 3 | <DL | 30.0 | n.a | |||
| 1 | 24 | <DL | <DL | n.a | ||
| 2 | 28959.6 | 30.1 | 124 | |||
| 3 | <DL | <DL | n.a | |||
| 1 | 96 | <DL | <DL | n.a | ||
| 2 | <DL | 30.5 | n.a | |||
| 3 | <DL | <DL | n.a |
| Compound | Exposure | Replicate | Time (h) | Muss.Conc. | ASW.Conc | BAF |
|---|---|---|---|---|---|---|
| SiNC-DCOIT | ASW + Food | 1 | 1 | 21033.6 | <DL | 180 |
| 2 | 61169.8 | 38.2 | 394 | |||
| 3 | 24316.8 | 59.8 | 137 | |||
| 1 | 3 | 6122.2 | 31.6 | 41 | ||
| 2 | 19733.8 | 31.4 | 133 | |||
| 3 | 3981.8 | 30.6 | 27 | |||
| 1 | 24 | <DL | <DL | n.a | ||
| 2 | <DL | 41.4 | n.a | |||
| 3 | <DL | 30.3 | n.a | |||
| 1 | 96 | 2835.0 | <DL | 24 | ||
| 2 | <DL | 31.8 | n.a | |||
| 3 | <DL | 30.2 | n.a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





