Submitted:
06 February 2024
Posted:
07 February 2024
You are already at the latest version
Abstract
Keywords:
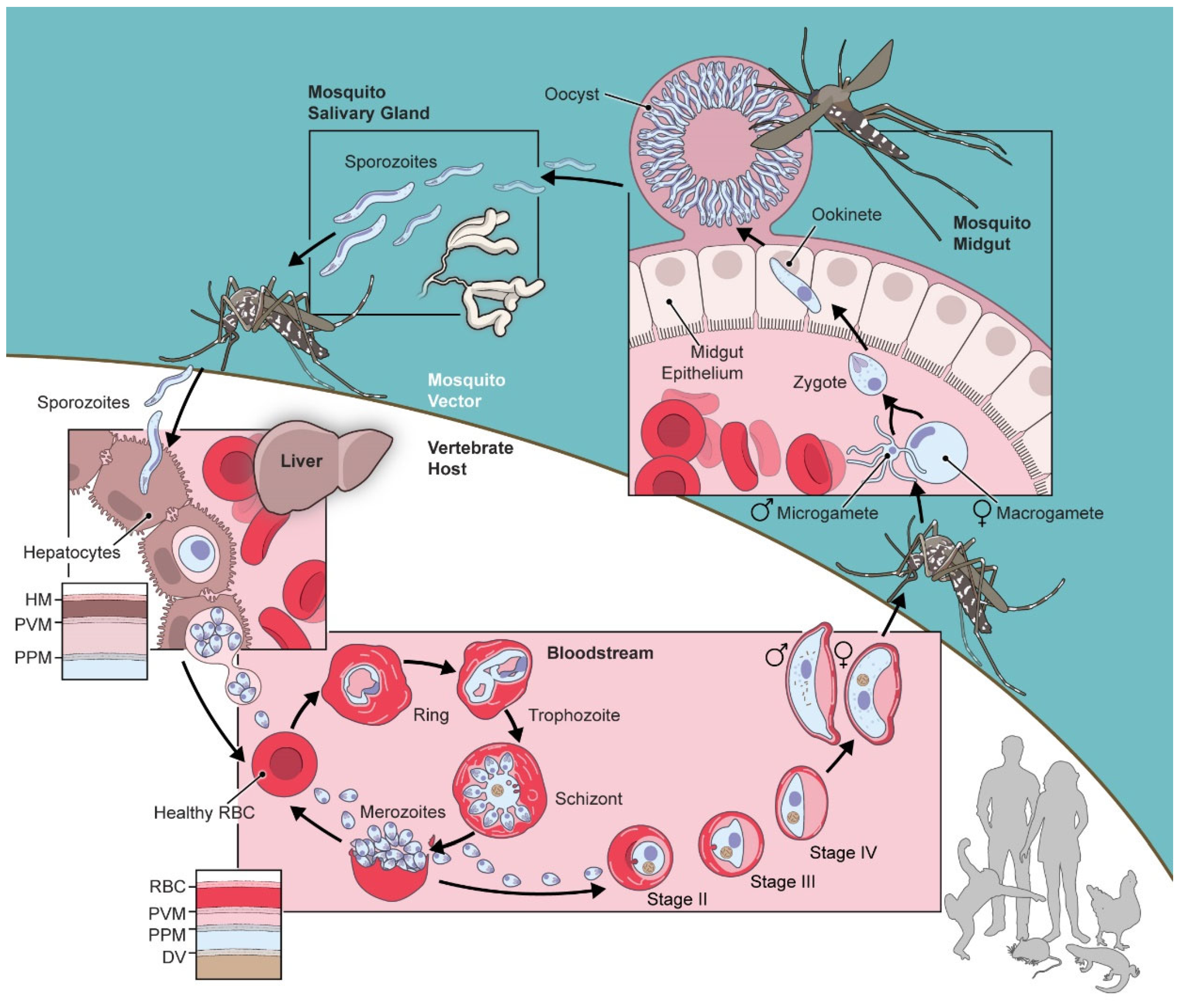
1. Introduction
| Gene | Annotation | # TMD | References |
|---|---|---|---|
| PF3D7_1227200 | potassium channel K1 | 8 | [18,19,20] |
| PF3D7_1465500 | potassium channel K2 | 8 | [19,21] |
| PF3D7_1107900 | small-conductance mechanosensitive ion channel (MscS) | 6 | [22] |
| PF3D7_1432100 | VDAC | 0 | [23] |
| PF3D7_1250200 | CSC1-like protein, putative | 11 | [24] |
| PF3D7_0810400 | aquaporin | 2 | [25] |
| PF3D7_1132800 | aquaglyceroporin | 6 | [26,27,28] |
| PF3D7_0408700 | perforin-like protein 1 | 0 | [29,30,31] |
| PF3D7_1216700 | perforin-like protein 2 | 0 | [29,32] |
| PF3D7_0923300 | perforin-like protein 3 | 0 | [33] |
| PF3D7_1473700 | nucleoporin NUP116/NSP116, putative | 0 | [34] |
2. The Plasmodial Surface Anion Channel (PSAC)
2.1. Background
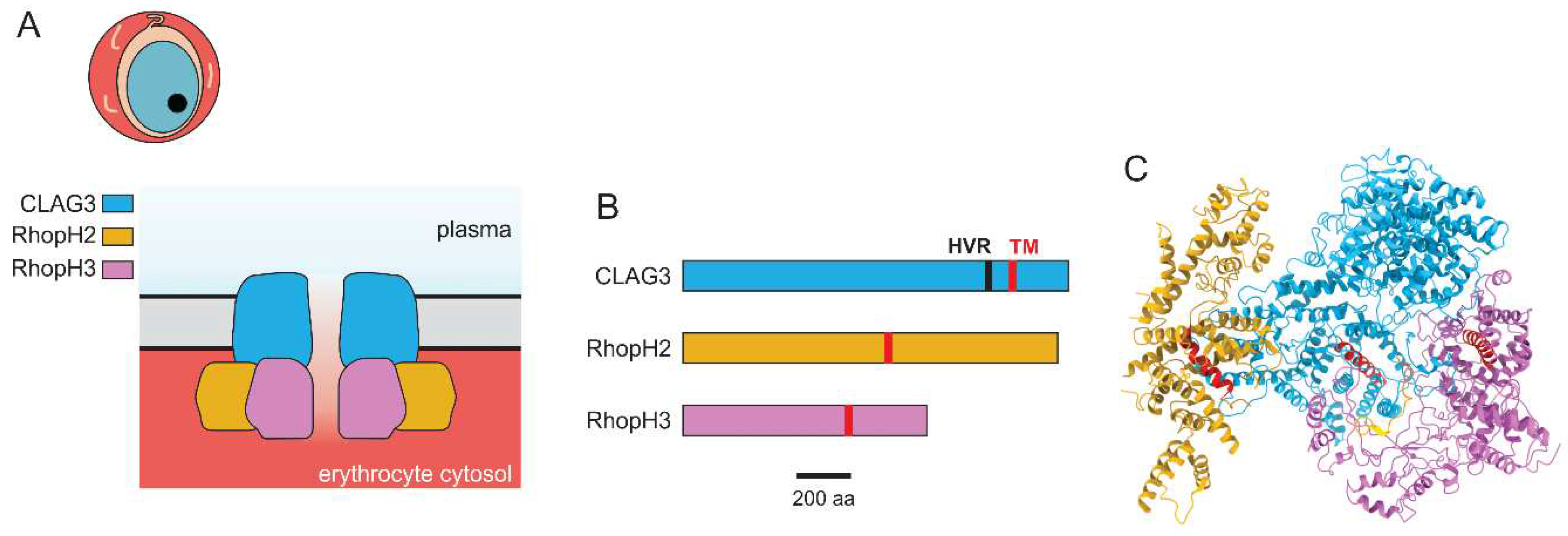
2.2. Identification of the rhoph genes as PSAC determinants
2.3. Unusual properties of the encoded channel
2.4. Essential role in nutrient uptake and a druggable target
2.5. Drug discovery and development targeting PSAC
3. The PVM channel and PTEX translocon
3.1. Background
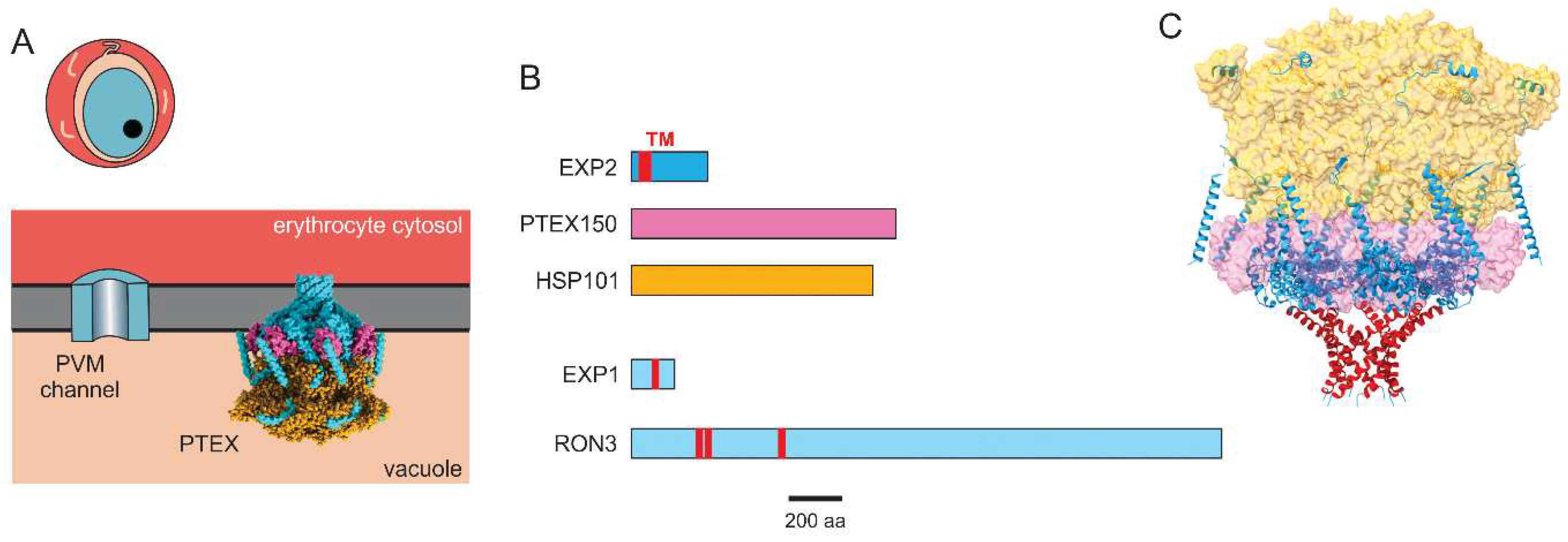
3.2. Identification of PTEX components and other proteins invovled in export
3.3. PTEX translocon structure
3.4. Transport studies linking the translocon to the PVM channel
4. Other channel activities that may be encoded by novel parasite-specific genes
Funding
Data Availability Statement
Conflicts of Interest
References
- Divo, A.A.; Geary, T.G.; Davis, N.L.; Jensen, J.B. Nutritional requirements of Plasmodium falciparum in culture. I. Exogenously supplied dialyzable components necessary for continuous growth. J. Protozool 1985, 32, 59–64. [Google Scholar] [CrossRef]
- Overman, R.R. Reversible cellular permeability alterations in disease. In vivo studies on sodium, potassium and chloride concentrations in erythrocytes of the malarious monkey. Am. J. Physiol 1948, 152, 113–121. [Google Scholar] [CrossRef]
- Ginsburg, H.; Kutner, S.; Krugliak, M.; Cabantchik, Z.I. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol. Biochem. Parasitol 1985, 14, 313–322. [Google Scholar] [CrossRef]
- Kirk, K.; Horner, H.A.; Elford, B.C.; Ellory, J.C.; Newbold, C.I. Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J. Biol. Chem 1994, 269, 3339–3347. [Google Scholar] [CrossRef]
- Gero, A.M.; Wood, A.M. New nucleoside transport pathways induced in the host erythrocyte membrane of malaria and Babesia infected cells. Adv. Exp. Med. Biol 1991, 309A, 169–172. [Google Scholar]
- Bokhari, A.A.; Solomon, T.; Desai, S.A. Two distinct mechanisms of transport through the plasmodial surface anion channel. J. Membr. Biol 2008, 226, 27–34. [Google Scholar] [CrossRef]
- Wasserman, M.; Alarcon, C.; Mendoza, P.M. Effects of Ca++ depletion on the asexual cell cycle of Plasmodium falciparum. Am. J. Trop. Med. Hyg 1982, 31, 711–717. [Google Scholar] [CrossRef]
- Tanabe, K.; Mikkelsen, R.B.; Wallach, D.F. Calcium transport of Plasmodium chabaudi-infected erythrocytes. J. Cell Biol 1982, 93, 680–684. [Google Scholar] [CrossRef]
- Zipprer, E.M.; Neggers, M.; Kushwaha, A.; Rayavara, K.; Desai, S.A. A kinetic fluorescence assay reveals unusual features of Ca++ uptake in Plasmodium falciparum-infected erythrocytes. Malar. J 2014, 13, 184. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Apolis, L.; Ito, D.; Desai, S.A. Increased Ca(++) uptake by erythrocytes infected with malaria parasites: evidence for exported proteins and novel inhibitors. Cell Microbiol 2018, 20, e12853. [Google Scholar] [CrossRef]
- Stauffer, T.P.; Guerini, D.; Carafoli, E. Tissue distribution of the four gene products of the plasma membrane Ca2+ pump. A study using specific antibodies. J. Biol. Chem 1995, 270, 12184–12190. [Google Scholar] [CrossRef]
- Cali, T.; Brini, M.; Carafoli, E. Regulation of cell calcium and role of plasma membrane calcium ATPases. Int Rev Cell Mol Biol 2017, 332, 259–296. [Google Scholar] [CrossRef]
- Kirk, K. Ion regulation in the malaria parasite. Annu Rev Microbiol 2015, 69, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Hall, N.; Fung, E.; White, O.; Berriman, M.; Hyman, R.W.; Carlton, J.M.; Pain, A.; Nelson, K.E.; Bowman, S.; et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 2002, 419, 498–511. [Google Scholar] [CrossRef]
- Lauger, P. Kinetic properties of ion carriers and channels. J Membr Biol 1980, 57, 163–178. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. 2022. [Google Scholar] [CrossRef]
- Ellekvist, P.; Ricke, C.H.; Litman, T.; Salanti, A.; Colding, H.; Zeuthen, T.; Klaerke, D.A. Molecular cloning of a K+ channel from the malaria parasite Plasmodium falciparum. Biochem. Biophys. Res. Commun 2004, 318, 477–484. [Google Scholar] [CrossRef]
- Molbaek, K.; Tejada, M.; Ricke, C.H.; Scharff-Poulsen, P.; Ellekvist, P.; Helix-Nielsen, C.; Kumar, N.; Klaerke, D.A.; Pedersen, P.A. Purification and initial characterization of Plasmodium falciparum K+ channels, PfKch1 and PfKch2 produced in Saccharomyces cerevisiae. Microb Cell Fact 2020, 19, 183. [Google Scholar] [CrossRef]
- Waller, K.L.; McBride, S.M.; Kim, K.; McDonald, T.V. Characterization of two putative potassium channels in Plasmodium falciparum. Malar J 2008, 7, 19. [Google Scholar] [CrossRef]
- Ellekvist, P.; Mlambo, G.; Kumar, N.; Klaerke, D.A. Functional characterization of malaria parasites deficient in the K+ channel Kch2. Biochem Biophys Res Commun 2017, 493, 690–696. [Google Scholar] [CrossRef]
- Kenthirapalan, S.; Waters, A.P.; Matuschewski, K.; Kooij, T.W. Functional profiles of orphan membrane transporters in the life cycle of the malaria parasite. Nat Commun 2016, 7, 10519. [Google Scholar] [CrossRef]
- Anaguano, D.; Dedkhad, W.; Brooks, C.F.; Cobb, D.W.; Muralidharan, V. Time-resolved proximity biotinylation implicates a porin protein in export of transmembrane malaria parasite effectors. J Cell Sci 2023, 136. [Google Scholar] [CrossRef]
- Hou, C.; Tian, W.; Kleist, T.; He, K.; Garcia, V.; Bai, F.; Hao, Y.; Luan, S.; Li, L. DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res 2014, 24, 632–635. [Google Scholar] [CrossRef]
- Kenthirapalan, S.; Waters, A.P.; Matuschewski, K.; Kooij, T.W. Flow cytometry-assisted rapid isolation of recombinant Plasmodium berghei parasites exemplified by functional analysis of aquaglyceroporin. Int J Parasitol 2012, 42, 1185–1192. [Google Scholar] [CrossRef]
- Hansen, M.; Kun, J.F.; Schultz, J.E.; Beitz, E. A single, bi-functional aquaglyceroporin in blood-stage Plasmodium falciparum malaria parasites. J. Biol. Chem 2002, 277, 4874–4882. [Google Scholar] [CrossRef]
- Newby, Z.E.; O'Connell, J., 3rd; Robles-Colmenares, Y.; Khademi, S.; Miercke, L.J.; Stroud, R.M. Crystal structure of the aquaglyceroporin PfAQP from the malarial parasite Plasmodium falciparum. Nat Struct Mol Biol 2008, 15, 619–625. [Google Scholar] [CrossRef]
- Song, J.; Almasalmeh, A.; Krenc, D.; Beitz, E. Molar concentrations of sorbitol and polyethylene glycol inhibit the Plasmodium aquaglyceroporin but not that of E. coli: involvement of the channel vestibules. Biochim Biophys Acta 2012, 1818, 1218–1224. [Google Scholar] [CrossRef]
- Garg, S.; Agarwal, S.; Kumar, S.; Yazdani, S.S.; Chitnis, C.E.; Singh, S. Calcium-dependent permeabilization of erythrocytes by a perforin-like protein during egress of malaria parasites. Nat Commun 2013, 4, 1736. [Google Scholar] [CrossRef] [PubMed]
- Ishino, T.; Chinzei, Y.; Yuda, M. A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol 2005, 7, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Camargo, N.; Coppens, I.; Morrisey, J.M.; Vaidya, A.B.; Kappe, S.H. A member of a conserved Plasmodium protein family with membrane-attack complex/perforin (MACPF)-like domains localizes to the micronemes of sporozoites. Mol. Biochem. Parasitol 2004, 133, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wirth, C.C.; Glushakova, S.; Scheuermayer, M.; Repnik, U.; Garg, S.; Schaack, D.; Kachman, M.M.; Weissbach, T.; Zimmerberg, J.; Dandekar, T.; et al. Perforin-like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cell Microbiol 2014, 16, 709–733. [Google Scholar] [CrossRef]
- Kadota, K.; Ishino, T.; Matsuyama, T.; Chinzei, Y.; Yuda, M. Essential role of membrane-attack protein in malarial transmission to mosquito host. Proc Natl Acad Sci U S A 2004, 101, 16310–16315. [Google Scholar] [CrossRef]
- Guizetti, J.; Martins, R.M.; Guadagnini, S.; Claes, A.; Scherf, A. Nuclear pores and perinuclear expression sites of var and ribosomal DNA genes correspond to physically distinct regions in Plasmodium falciparum. Eukaryot Cell 2013, 12, 697–702. [Google Scholar] [CrossRef]
- Ginsburg, H.; Stein, W.D. Biophysical analysis of a novel transport pathway induced in red blood cell membranes by the malaria parasite. Prog. Clin. Biol. Res 1988, 252, 317–322. [Google Scholar]
- Pouvelle, B.; Spiegel, R.; Hsiao, L.; Howard, R.J.; Morris, R.L.; Thomas, A.P.; Taraschi, T.F. Direct access to serum macromolecules by intraerythrocytic malaria parasites. Nature 1991, 353, 73–75. [Google Scholar] [CrossRef]
- Burns, E.R.; Pollack, S. P. falciparum infected erythrocytes are capable of endocytosis. In Vitro Cell Dev. Biol 1988, 24, 481–486. [Google Scholar] [CrossRef]
- Kutner, S.; Baruch, D.; Ginsburg, H.; Cabantchik, Z.I. Alterations in membrane permeability of malaria-infected human erythrocytes are related to the growth stage of the parasite. Biochim. Biophys. Acta 1982, 687, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.A.; Bezrukov, S.M.; Zimmerberg, J. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 2000, 406, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Lisk, G.; Desai, S.A. The plasmodial surface anion channel is functionally conserved in divergent malaria parasites. Eukaryot. Cell 2005, 4, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Gezelle, J.; Saggu, G.; Desai, S.A. Promises and pitfalls of parasite patch-clamp. Trends Parasitol 2021, 37, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Wanke, E. Channel noise in nerve membranes and lipid bilayers. Q. Rev. Biophys 1975, 8, 451–506. [Google Scholar] [CrossRef]
- Staines, H.M.; Alkhalil, A.; Allen, R.J.; De Jonge, H.R.; Derbyshire, E.; Egee, S.; Ginsburg, H.; Hill, D.A.; Huber, S.M.; Kirk, K.; et al. Electrophysiological studies of malaria parasite-infected erythrocytes: current status. Int. J Parasitol 2007, 37, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Egee, S.; Lapaix, F.; Decherf, G.; Staines, H.M.; Ellory, J.C.; Doerig, C.; Thomas, S.L. A stretch-activated anion channel is up-regulated by the malaria parasite Plasmodium falciparum. J. Physiol 2002, 542, 795–801. [Google Scholar] [CrossRef]
- Duranton, C.; Tanneur, V.; Lang, C.; Brand, V.B.; Koka, S.; Kasinathan, R.S.; Dorsch, M.; Hedrich, H.J.; Baumeister, S.; Lingelbach, K.; et al. A high specificity and affinity interaction with serum albumin stimulates an anion conductance in malaria-infected erythrocytes. Cell Physiol Biochem 2008, 22, 395–404. [Google Scholar] [CrossRef]
- Verloo, P.; Kocken, C.H.; Van der Wel, A.; Tilly, B.C.; Hogema, B.M.; Sinaasappel, M.; Thomas, A.W.; De Jonge, H.R. Plasmodium falciparum-activated chloride channels are defective in erythrocytes from cystic fibrosis patients. J. Biol. Chem 2004, 279, 10316–10322. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, G.; Egee, S.; Thomas, S.L. Three types of spontaneously active anionic channels in malaria-infected human red blood cells. Blood Cells Mol. Dis 2006, 36, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.M.; Duranton, C.; Henke, G.; Van De, S.C.; Heussler, V.; Shumilina, E.; Sandu, C.D.; Tanneur, V.; Brand, V.; Kasinathan, R.S.; et al. Plasmodium induces swelling-activated ClC-2 anion channels in the host erythrocyte. J. Biol. Chem 2004, 279, 41444–41452. [Google Scholar] [CrossRef]
- Ginsburg, H.; Stein, W.D. How many functional transport pathways does Plasmodium falciparum induce in the membrane of its host erythrocyte? Trends Parasitol 2005, 21, 118–121. [Google Scholar] [CrossRef]
- Winograd, E.; Sherman, I.W. Malaria infection induces a conformational change in erythrocyte band 3 protein. Mol. Biochem. Parasitol 2004, 138, 83–87. [Google Scholar] [CrossRef]
- Ancelin, M.L.; Parant, M.; Thuet, M.J.; Philippot, J.R.; Vial, H.J. Increased permeability to choline in simian erythrocytes after Plasmodium knowlesi infection. Biochem. J 1991, 273, 701–709. [Google Scholar] [CrossRef]
- Alkhalil, A.; Cohn, J.V.; Wagner, M.A.; Cabrera, J.S.; Rajapandi, T.; Desai, S.A. Plasmodium falciparum likely encodes the principal anion channel on infected human erythrocytes. Blood 2004, 104, 4279–4286. [Google Scholar] [CrossRef]
- Hill, D.A.; Pillai, A.D.; Nawaz, F.; Hayton, K.; Doan, L.; Lisk, G.; Desai, S.A. A blasticidin S-resistant Plasmodium falciparum mutant with a defective plasmodial surface anion channel. Proc Natl Acad Sci U S A 2007, 104, 1063–1068. [Google Scholar] [CrossRef]
- Lisk, G.; Pain, M.; Gluzman, I.Y.; Kambhampati, S.; Furuya, T.; Su, X.Z.; Fay, M.P.; Goldberg, D.E.; Desai, S.A. Changes in the plasmodial surface anion channel reduce leupeptin uptake and can confer drug resistance in P. falciparum-infected erythrocytes. Antimicrob. Agents Chemother 2008, 52, 2346–2354. [Google Scholar] [CrossRef]
- Lisk, G.; Pain, M.; Sellers, M.; Gurnev, P.A.; Pillai, A.D.; Bezrukov, S.M.; Desai, S.A. Altered plasmodial surface anion channel activity and in vitro resistance to permeating antimalarial compounds. Biochim. Biophys. Acta 2010, 1798, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Nguitragool, W.; Bokhari, A.A.; Pillai, A.D.; Rayavara, K.; Sharma, P.; Turpin, B.; Aravind, L.; Desai, S.A. Malaria parasite clag3 genes determine channel-mediated nutrient uptake by infected red blood cells. Cell 2011, 145, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Nguitragool, W.; Rayavara, K.; Desai, S.A. Proteolysis at a specific extracellular residue implicates integral membrane CLAG3 in malaria parasite nutrient channels. PLoS. One 2014, 9, e93759. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bokhari, A.A.B.; Pillai, A.D.; Crater, A.K.; Gezelle, J.; Saggu, G.; Nasamu, A.S.; Ganesan, S.M.; Niles, J.C.; Desai, S.A. Complex nutrient channel phenotypes despite Mendelian inheritance in a Plasmodium falciparum genetic cross. PLoS Pathog 2020, 16, e1008363. [Google Scholar] [CrossRef] [PubMed]
- Alkhalil, A.; Pillai, A.D.; Bokhari, A.A.; Vaidya, A.B.; Desai, S.A. Complex inheritance of the plasmodial surface anion channel in a Plasmodium falciparum genetic cross. Mol Microbiol 2009, 72, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.S.; Kaewthamasorn, M.; Yahata, K.; Nakazawa, S.; Kaneko, O. Positive selection on the Plasmodium falciparum clag2 gene encoding a component of the erythrocyte-binding rhoptry protein complex. Trop. Med. Health 2011, 39, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.; Carret, C.; Kaneko, O.; Yim Lim, B.Y.; Ivens, A.; Holder, A.A. Epigenetic silencing of Plasmodium falciparum genes linked to erythrocyte invasion. PLoS. Pathog 2007, 3, e107. [Google Scholar] [CrossRef]
- Richards, J.S.; Arumugam, T.U.; Reiling, L.; Healer, J.; Hodder, A.N.; Fowkes, F.J.; Cross, N.; Langer, C.; Takeo, S.; Uboldi, A.D.; et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol 2013, 191, 795–809. [Google Scholar] [CrossRef]
- Crowley, V.M.; Rovira-Graells, N.; de Pouplana, L.R.; Cortes, A. Heterochromatin formation in bistable chromatin domains controls the epigenetic repression of clonally variant Plasmodium falciparum genes linked to erythrocyte invasion. Mol Microbiol 2011, 80, 391–406. [Google Scholar] [CrossRef]
- Rovira-Graells, N.; Crowley, V.M.; Bancells, C.; Mira-Martinez, S.; Ribas de, P.L.; Cortes, A. Deciphering the principles that govern mutually exclusive expression of Plasmodium falciparum clag3 genes. Nucleic Acids Res 2015, 43, 8243–8257. [Google Scholar] [CrossRef] [PubMed]
- Mira-Martinez, S.; Rovira-Graells, N.; Crowley, V.M.; Altenhofen, L.M.; Llinas, M.; Cortes, A. Epigenetic switches in clag3 genes mediate blasticidin S resistance in malaria parasites. Cell Microbiol 2013, 15, 1913–1923. [Google Scholar] [CrossRef]
- Desai, S.A. Epigenetics of malaria parasite nutrient uptake, but why? Trends Parasitol 2022, 38, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.D.; Nguitragool, W.; Lyko, B.; Dolinta, K.; Butler, M.M.; Nguyen, S.T.; Peet, N.P.; Bowlin, T.L.; Desai, S.A. Solute restriction reveals an essential role for clag3-associated channels in malaria parasite nutrient acquisition. Mol. Pharmacol 2012, 82, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Wollenberg, K.; Sellers, M.; Zainabadi, K.; Galinsky, K.; Moss, E.; Nguitragool, W.; Neafsey, D.; Desai, S.A. An epigenetic antimalarial resistance mechanism involving parasite genes linked to nutrient uptake. J. Biol. Chem 2013, 288, 19429–19440. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, O.; Yim Lim, B.Y.; Iriko, H.; Ling, I.T.; Otsuki, H.; Grainger, M.; Tsuboi, T.; Adams, J.H.; Mattei, D.; Holder, A.A.; Torii, M. Apical expression of three RhopH1/Clag proteins as components of the Plasmodium falciparum RhopH complex. Mol Biochem. Parasitol 2005, 143, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ling, I.T.; Florens, L.; Dluzewski, A.R.; Kaneko, O.; Grainger, M.; Yim Lim, B.Y.; Tsuboi, T.; Hopkins, J.M.; Johnson, J.R.; Torii, M.; et al. The Plasmodium falciparum clag9 gene encodes a rhoptry protein that is transferred to the host erythrocyte upon invasion. Mol Microbiol 2004, 52, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Vincensini, L.; Fall, G.; Berry, L.; Blisnick, T.; Braun, B.C. The RhopH complex is transferred to the host cell cytoplasm following red blood cell invasion by Plasmodium falciparum. Mol Biochem. Parasitol 2008, 160, 81–89. [Google Scholar] [CrossRef]
- Ito, D.; Schureck, M.A.; Desai, S.A. An essential dual-function complex mediates erythrocyte invasion and channel-mediated nutrient uptake in malaria parasites. Elife 2017, 6, e23485. [Google Scholar] [CrossRef]
- Sherling, E.S.; Knuepfer, E.; Brzostowski, J.A.; Miller, L.H.; Blackman, M.J.; van, O.C. The Plasmodium falciparum rhoptry protein RhopH3 plays essential roles in host cell invasion and nutrient uptake. Elife 2017, 6. [Google Scholar] [CrossRef]
- Counihan, N.; Chisholm, S.A.; Bullen, H.E.; Srivastava, A.; Sanders, P.R.; Jonsdottir, T.K.; Weiss, G.E.; Ghosh, S.; Crabb, B.S.; Creek, D.J.; et al. Plasmodium falciparum parasites deploy RhopH2 into the host erythrocyte to obtain nutrients, grow and replicate. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Trenholme, K.R.; Gardiner, D.L.; Holt, D.C.; Thomas, E.A.; Cowman, A.F.; Kemp, D.J. clag9: A cytoadherence gene in Plasmodium falciparum essential for binding of parasitized erythrocytes to CD36. Proc. Natl. Acad. Sci. U. S. A 2000, 97, 4029–4033. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Valiyaveettil, M.; Achur, R.N.; Goyal, A.; Mattei, D.; Salanti, A.; Trenholme, K.R.; Gardiner, D.L.; Gowda, D.C. Dual stage synthesis and crucial role of cytoadherence-linked asexual gene 9 in the surface expression of malaria parasite var proteins. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 16643–16648. [Google Scholar] [CrossRef] [PubMed]
- Rungruang, T.; Kaneko, O.; Murakami, Y.; Tsuboi, T.; Hamamoto, H.; Akimitsu, N.; Sekimizu, K.; Kinoshita, T.; Torii, M. Erythrocyte surface glycosylphosphatidyl inositol anchored receptor for the malaria parasite. Mol Biochem. Parasitol 2005, 140, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Chugh, M.; Kumar, S.; Singh, S.; Kanodia, S.; Hossain, M.J.; Korde, R.; Grover, A.; Dhawan, S.; Chauhan, V.S.; et al. Proteome analysis reveals a large merozoite surface protein-1 associated complex on the Plasmodium falciparum merozoite surface. J. Proteome. Res 2011, 10, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Rayavara, K.; Ito, D.; Basore, K.; Desai, S.A. A CLAG3 mutation in an amphipathic transmembrane domain alters malaria parasite nutrient channels and confers leupeptin resistance. Infect. Immun 2015, 83, 2566–2574. [Google Scholar] [CrossRef] [PubMed]
- Schureck, M.A.; Darling, J.E.; Merk, A.; Shao, J.; Daggupati, G.; Srinivasan, P.; Olinares, P.D.B.; Rout, M.P.; Chait, B.T.; Wollenberg, K.; et al. Malaria parasites use a soluble RhopH complex for erythrocyte invasion and an integral form for nutrient uptake. Elife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Manzella-Lapeira, J.; Saggu, G.; Ito, D.; Brzostowski, J.A.; Desai, S.A. Live-cell FRET reveals that malaria nutrient channel proteins CLAG3 and RhopH2 remain associated throughout their tortuous trafficking. mBio 2020, 11. [Google Scholar] [CrossRef]
- Ho, C.M.; Jih, J.; Lai, M.; Li, X.; Goldberg, D.E.; Beck, J.R.; Zhou, Z.H. Native structure of the RhopH complex, a key determinant of malaria parasite nutrient acquisition. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef]
- Ho, C.M.; Li, X.; Lai, M.; Terwilliger, T.C.; Beck, J.R.; Wohlschlegel, J.; Goldberg, D.E.; Fitzpatrick, A.W.P.; Zhou, Z.H. Bottom-up structural proteomics: cryoEM of protein complexes enriched from the cellular milieu. Nat Methods 2020, 17, 79–85. [Google Scholar] [CrossRef]
- Desai, S.A. Unique properties of nutrient channels on Plasmodium-infected erythrocytes. Pathogens 2023, 12. [Google Scholar] [CrossRef]
- Cohn, J.V.; Alkhalil, A.; Wagner, M.A.; Rajapandi, T.; Desai, S.A. Extracellular lysines on the plasmodial surface anion channel involved in Na+ exclusion. Mol. Biochem. Parasitol 2003, 132, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Saliba, K.J.; Horner, H.A.; Kirk, K. Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J. Biol. Chem 1998, 273, 10190–10195. [Google Scholar] [CrossRef] [PubMed]
- Saliba, K.J.; Martin, R.E.; Broer, A.; Henry, R.I.; McCarthy, C.S.; Downie, M.J.; Allen, R.J.; Mullin, K.A.; McFadden, G.I.; Broer, S.; Kirk, K. Sodium-dependent uptake of inorganic phosphate by the intracellular malaria parasite. Nature 2006, 443, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Staines, H.M.; Ellory, J.C.; Kirk, K. Perturbation of the pump-leak balance for Na(+) and K(+) in malaria- infected erythrocytes. Am. J. Physiol Cell Physiol 2001, 280, C1576–C1587. [Google Scholar] [CrossRef] [PubMed]
- Lew, V.L.; Macdonald, L.; Ginsburg, H.; Krugliak, M.; Tiffert, T. Excess haemoglobin digestion by malaria parasites: a strategy to prevent premature host cell lysis. Blood Cells Mol Dis 2004, 32, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Kutner, S.; Breuer, W.V.; Ginsburg, H.; Cabantchik, Z.I. On the mode of action of phlorizin as an antimalarial agent in in vitro cultures of Plasmodium falciparum. Biochem. Pharmacol 1987, 36, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Staines, H.M.; Dee, B.C.; O'Brien, M.; Lang, H.J.; Englert, H.; Horner, H.A.; Ellory, J.C.; Kirk, K. Furosemide analogues as potent inhibitors of the new permeability pathways of Plasmodium falciparum-infected human erythrocytes. Mol. Biochem. Parasitol 2004, 133, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.A.; Alkhalil, A.; Kang, M.; Ashfaq, U.; Nguyen, M.L. PSAC-independent phloridzin resistance in Plasmodium falciparum. J. Biol. Chem 2005, 280, 16861–16867. [Google Scholar] [CrossRef]
- Mauritz, J.M.; Seear, R.; Esposito, A.; Kaminski, C.F.; Skepper, J.N.; Warley, A.; Lew, V.L.; Tiffert, T. X-ray microanalysis investigation of the changes in Na, K, and hemoglobin concentration in Plasmodium falciparum-infected red blood cells. Biophys. J 2011, 100, 1438–1445. [Google Scholar] [CrossRef]
- Jorgensen, P.L.; Hakansson, K.O.; Karlish, S.J. Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu. Rev. Physiol 2003, 65, 817–849. [Google Scholar] [CrossRef]
- Pillai, A.D.; Addo, R.; Sharma, P.; Nguitragool, W.; Srinivasan, P.; Desai, S.A. Malaria parasites tolerate a broad range of ionic environments and do not require host cation remodeling. Mol. Microbiol 2013, 88, 20–34. [Google Scholar] [CrossRef]
- Singh, S.; Alam, M.M.; Pal-Bhowmick, I.; Brzostowski, J.A.; Chitnis, C.E. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS. Pathog 2010, 6, e1000746. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.M.; Waidyarachchi, S.L.; Shao, J.; Nguyen, S.T.; Ding, X.; Cardinale, S.C.; Morin, L.R.; Kwasny, S.M.; Ito, M.; Gezelle, J.; et al. Optimized pyridazinone nutrient channel inhibitors are potent and specific antimalarial leads. Mol Pharmacol 2022, 102, 172–182. [Google Scholar] [CrossRef]
- Taylor, H.M.; McRobert, L.; Grainger, M.; Sicard, A.; Dluzewski, A.R.; Hopp, C.S.; Holder, A.A.; Baker, D.A. The malaria parasite cyclic GMP-dependent protein kinase plays a central role in blood-stage schizogony. Eukaryot Cell 2010, 9, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Dvorin, J.D.; Martyn, D.C.; Patel, S.D.; Grimley, J.S.; Collins, C.R.; Hopp, C.S.; Bright, A.T.; Westenberger, S.; Winzeler, E.; Blackman, M.J.; et al. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science 2010, 328, 910–912. [Google Scholar] [CrossRef]
- Cooper, R.A.; Lane, K.D.; Deng, B.; Mu, J.; Patel, J.J.; Wellems, T.E.; Su, X.; Ferdig, M.T. Mutations in transmembrane domains 1, 4 and 9 of the Plasmodium falciparum chloroquine resistance transporter alter susceptibility to chloroquine, quinine and quinidine. Mol Microbiol 2007, 64, 1139. [Google Scholar] [CrossRef]
- Wicht, K.J.; Mok, S.; Fidock, D.A. Molecular mechanisms of drug resistance in Plasmodium falciparum malaria. Annu Rev Microbiol 2020, 74, 431–454. [Google Scholar] [CrossRef] [PubMed]
- Dluzewski, A.R.; Mitchell, G.H.; Fryer, P.R.; Griffiths, S.; Wilson, R.J.; Gratzer, W.B. Origins of the parasitophorous vacuole membrane of the malaria parasite, Plasmodium falciparum, in human red blood cells. J Cell Sci 1992, 102, 527–532. [Google Scholar] [CrossRef]
- Bannister, L.H.; Hopkins, J.M.; Fowler, R.E.; Krishna, S.; Mitchell, G.H. A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol Today 2000, 16, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E.; Zimmerberg, J. Hardly vacuous: the parasitophorous vacuolar membrane of malaria parasites. Trends Parasitol 2020, 36, 138–146. [Google Scholar] [CrossRef]
- Desai, S.A.; Krogstad, D.J.; McCleskey, E.W. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature 1993, 362, 643–646. [Google Scholar] [CrossRef]
- Desai, S.A.; Rosenberg, R.L. Pore size of the malaria parasite's nutrient channel. Proc. Natl. Acad. Sci. U. S. A 1997, 94, 2045–2049. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.C.; Beckers, C.J.; Joiner, K.A. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. U. S. A 1994, 91, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Werner-Meier, R.; Entzeroth, R. Diffusion of microinjected markers across the parasitophorous vacuole membrane in cells infected with Eimeria nieschulzi (Coccidia, Apicomplexa). Parasitol Res 1997, 83, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Hviid, L.; Jensen, A.T. PfEMP1 - A parasite protein family of key importance in Plasmodium falciparum malaria immunity and pathogenesis. Adv. Parasitol 2015, 88, 51–84. [Google Scholar] [CrossRef] [PubMed]
- Kyes, S.A.; Rowe, J.A.; Kriek, N.; Newbold, C.I. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A 1999, 96, 9333–9338. [Google Scholar] [CrossRef]
- Glenister, F.K.; Fernandez, K.M.; Kats, L.M.; Hanssen, E.; Mohandas, N.; Coppel, R.L.; Cooke, B.M. Functional alteration of red blood cells by a megadalton protein of Plasmodium falciparum. Blood 2009, 113, 919–928. [Google Scholar] [CrossRef]
- Marti, M.; Good, R.T.; Rug, M.; Knuepfer, E.; Cowman, A.F. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 2004, 306, 1930–1933. [Google Scholar] [CrossRef]
- Hiller, N.L.; Bhattacharjee, S.; van, O.C.; Liolios, K.; Harrison, T.; Lopez-Estrano, C.; Haldar, K. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 2004, 306, 1934–1937. [Google Scholar] [CrossRef]
- Gehde, N.; Hinrichs, C.; Montilla, I.; Charpian, S.; Lingelbach, K.; Przyborski, J.M. Protein unfolding is an essential requirement for transport across the parasitophorous vacuolar membrane of Plasmodium falciparum. Mol Microbiol 2009, 71, 613–628. [Google Scholar] [CrossRef]
- Ansorge, I.; Benting, J.; Bhakdi, S.; Lingelbach, K. Protein sorting in Plasmodium falciparum-infected red blood cells permeabilized with the pore-forming protein streptolysin O. Biochem J 1996, 315, 307–314. [Google Scholar] [CrossRef] [PubMed]
- de Koning-Ward, T.F.; Gilson, P.R.; Boddey, J.A.; Rug, M.; Smith, B.J.; Papenfuss, A.T.; Sanders, P.R.; Lundie, R.J.; Maier, A.G.; Cowman, A.F.; Crabb, B.S. A newly discovered protein export machine in malaria parasites. Nature 2009, 459, 945–949. [Google Scholar] [CrossRef]
- Beck, J.R.; Muralidharan, V.; Oksman, A.; Goldberg, D.E. PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature 2014, 511, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, B.; Matthews, K.; Nie, C.Q.; Kalanon, M.; Charnaud, S.C.; Sanders, P.R.; Chisholm, S.A.; Counihan, N.A.; Shaw, P.J.; Pino, P.; et al. PTEX is an essential nexus for protein export in malaria parasites. Nature 2014, 511, 587–591. [Google Scholar] [CrossRef]
- Matthews, K.; Kalanon, M.; Chisholm, S.A.; Sturm, A.; Goodman, C.D.; Dixon, M.W.; Sanders, P.R.; Nebl, T.; Fraser, F.; Haase, S.; et al. The Plasmodium translocon of exported proteins (PTEX) component thioredoxin-2 is important for maintaining normal blood-stage growth. Mol Microbiol 2013, 89, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Matz, J.M.; Matuschewski, K.; Kooij, T.W. Two putative protein export regulators promote Plasmodium blood stage development in vivo. Mol Biochem Parasitol 2013, 191, 44–52. [Google Scholar] [CrossRef]
- Matz, J.M.; Ingmundson, A.; Costa Nunes, J.; Stenzel, W.; Matuschewski, K.; Kooij, T.W. In vivo function of PTEX88 in malaria parasite sequestration and virulence. Eukaryot Cell 2015, 14, 528–534. [Google Scholar] [CrossRef]
- Chisholm, S.A.; McHugh, E.; Lundie, R.; Dixon, M.W.; Ghosh, S.; O'Keefe, M.; Tilley, L.; Kalanon, M.; de Koning-Ward, T.F. Contrasting inducible knockdown of the auxiliary PTEX component PTEX88 in P. falciparum and P. berghei unmasks a role in parasite virulence. PLoS One 2016, 11, e0149296. [Google Scholar] [CrossRef]
- Low, L.M.; Azasi, Y.; Sherling, E.S.; Garten, M.; Zimmerberg, J.; Tsuboi, T.; Brzostowski, J.; Mu, J.; Blackman, M.J.; Miller, L.H. Deletion of Plasmodium falciparum protein RON3 affects the functional translocation of exported proteins and glucose uptake. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Kondo, Y.; Takashima, E.; Iriko, H.; Thongkukiatkul, A.; Torii, M.; Otsuki, H. Roles of the RON3 C-terminal fragment in erythrocyte invasion and blood-stage parasite proliferation in Plasmodium falciparum. Front Cell Infect Microbiol 2023, 13, 1197126. [Google Scholar] [CrossRef] [PubMed]
- Mesen-Ramirez, P.; Bergmann, B.; Tran, T.T.; Garten, M.; Stacker, J.; Naranjo-Prado, I.; Hohn, K.; Zimmerberg, J.; Spielmann, T. EXP1 is critical for nutrient uptake across the parasitophorous vacuole membrane of malaria parasites. PLoS Biol 2019, 17, e3000473. [Google Scholar] [CrossRef]
- Nessel, T.; Beck, J.M.; Rayatpisheh, S.; Jami-Alahmadi, Y.; Wohlschlegel, J.A.; Goldberg, D.E.; Beck, J.R. EXP1 is required for organisation of EXP2 in the intraerythrocytic malaria parasite vacuole. Cell Microbiol 2020, 22, e13168. [Google Scholar] [CrossRef] [PubMed]
- Mesen-Ramirez, P.; Bergmann, B.; Elhabiri, M.; Zhu, L.; von Thien, H.; Castro-Pena, C.; Gilberger, T.W.; Davioud-Charvet, E.; Bozdech, Z.; Bachmann, A.; Spielmann, T. The parasitophorous vacuole nutrient channel is critical for drug access in malaria parasites and modulates the artemisinin resistance fitness cost. Cell Host Microbe 2021, 29, 1774–1787. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Beck, J.R.; Lai, M.; Cui, Y.; Goldberg, D.E.; Egea, P.F.; Zhou, Z.H. Malaria parasite translocon structure and mechanism of effector export. Nature 2018, 561, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Garten, M.; Nasamu, A.S.; Niles, J.C.; Zimmerberg, J.; Goldberg, D.E.; Beck, J.R. EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX. Nat Microbiol 2018, 3, 1090–1098. [Google Scholar] [CrossRef]
- Gold, D.A.; Kaplan, A.D.; Lis, A.; Bett, G.C.; Rosowski, E.E.; Cirelli, K.M.; Bougdour, A.; Sidik, S.M.; Beck, J.R.; Lourido, S.; et al. The Toxoplasma dense granule proteins GRA17 and GRA23 mediate the movement of small molecules between the host and the parasitophorous vacuole. Cell Host Microbe 2015, 17, 642–652. [Google Scholar] [CrossRef]
- Pitman, E.L.; Counihan, N.A.; Modak, J.K.; Chowdury, M.; Gilson, P.R.; Webb, C.T.; de Koning-Ward, T.F. Dissecting EXP2 sequence requirements for protein export in malaria parasites. Front Cell Infect Microbiol 2023, 13, 1332146. [Google Scholar] [CrossRef]
- Hodder, A.N.; Sleebs, B.E.; Czabotar, P.E.; Gazdik, M.; Xu, Y.; O'Neill, M.T.; Lopaticki, S.; Nebl, T.; Triglia, T.; Smith, B.J.; et al. Structural basis for plasmepsin V inhibition that blocks export of malaria proteins to human erythrocytes. Nat Struct Mol Biol 2015, 22, 590–596. [Google Scholar] [CrossRef]
- Kramer, R.; Ginsburg, H. Calcium transport and compartment analysis of free and exchangeable calcium in Plasmodium falciparum-infected red blood cells. J. Protozool 1991, 38, 594–601. [Google Scholar] [PubMed]
- Rohrbach, P.; Friedrich, O.; Hentschel, J.; Plattner, H.; Fink, R.H.; Lanzer, M. Quantitative calcium measurements in subcellular compartments of Plasmodium falciparum-infected erythrocytes. J. Biol. Chem 2005, 280, 27960–27969. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.A.; McCleskey, E.W.; Schlesinger, P.H.; Krogstad, D.J. A novel pathway for Ca++ entry into Plasmodium falciparum-infected blood cells. Am. J. Trop. Med. Hyg 1996, 54, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Tiffert, T.; Lew, V.L. Kinetics of inhibition of the plasma membrane calcium pump by vanadate in intact human red cells. Cell Calcium 2001, 30, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.A.; Schlesinger, P.H.; Krogstad, D.J. Physiologic rate of carrier-mediated Ca2+ entry matches active extrusion in human erythrocytes. J. Gen. Physiol 1991, 98, 349–364. [Google Scholar] [CrossRef]
- Matz, J.M. Plasmodium's bottomless pit: properties and functions of the malaria parasite's digestive vacuole. Trends Parasitol 2022, 38, 525–543. [Google Scholar] [CrossRef]
- Kloehn, J.; Lacour, C.E.; Soldati-Favre, D. The metabolic pathways and transporters of the plastid organelle in Apicomplexa. Curr Opin Microbiol 2021, 63, 250–258. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




