Submitted:
06 February 2024
Posted:
07 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Definitions
2.3. Statistical Analysis
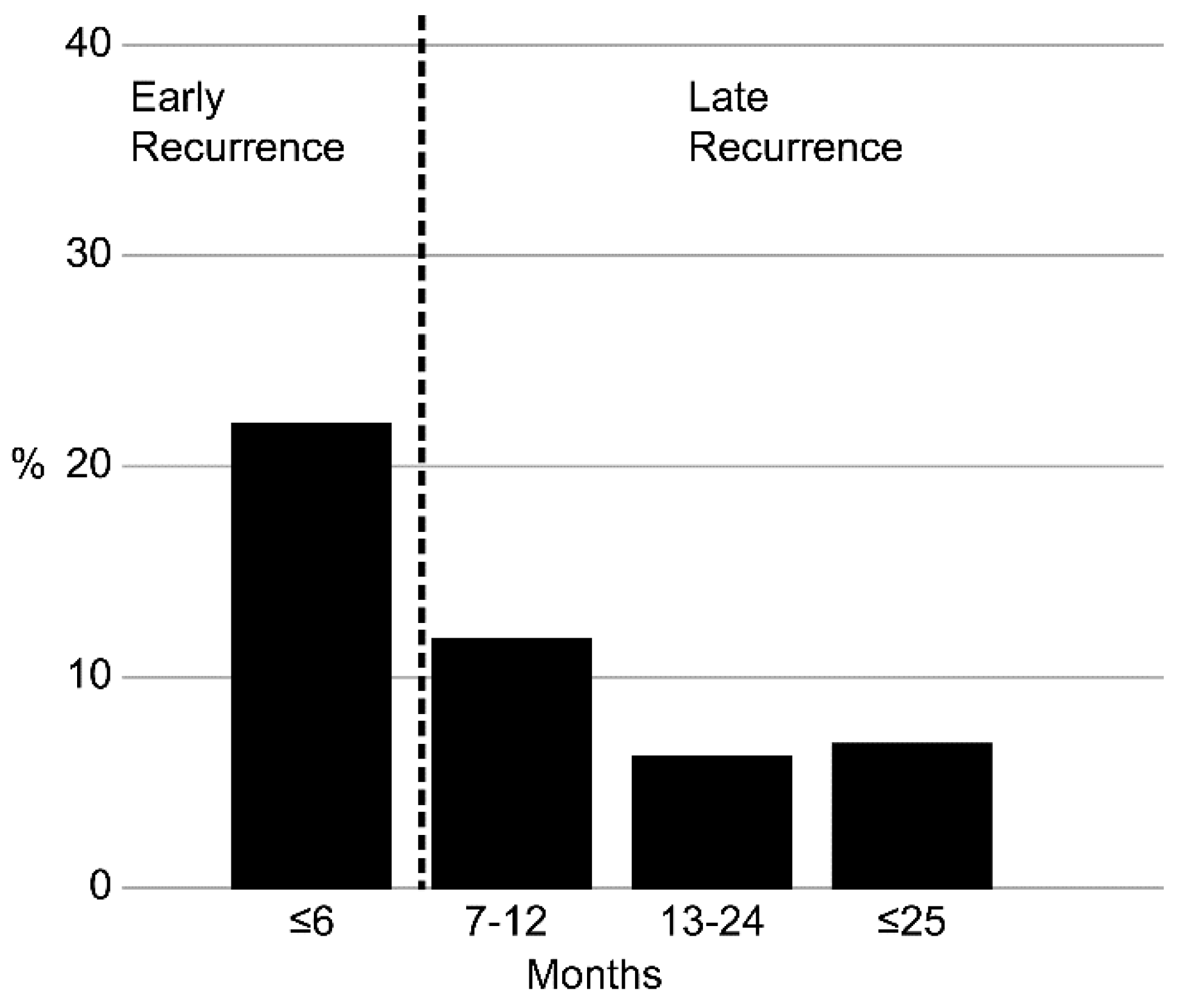
3. Results
3.1. Patient Characteristics
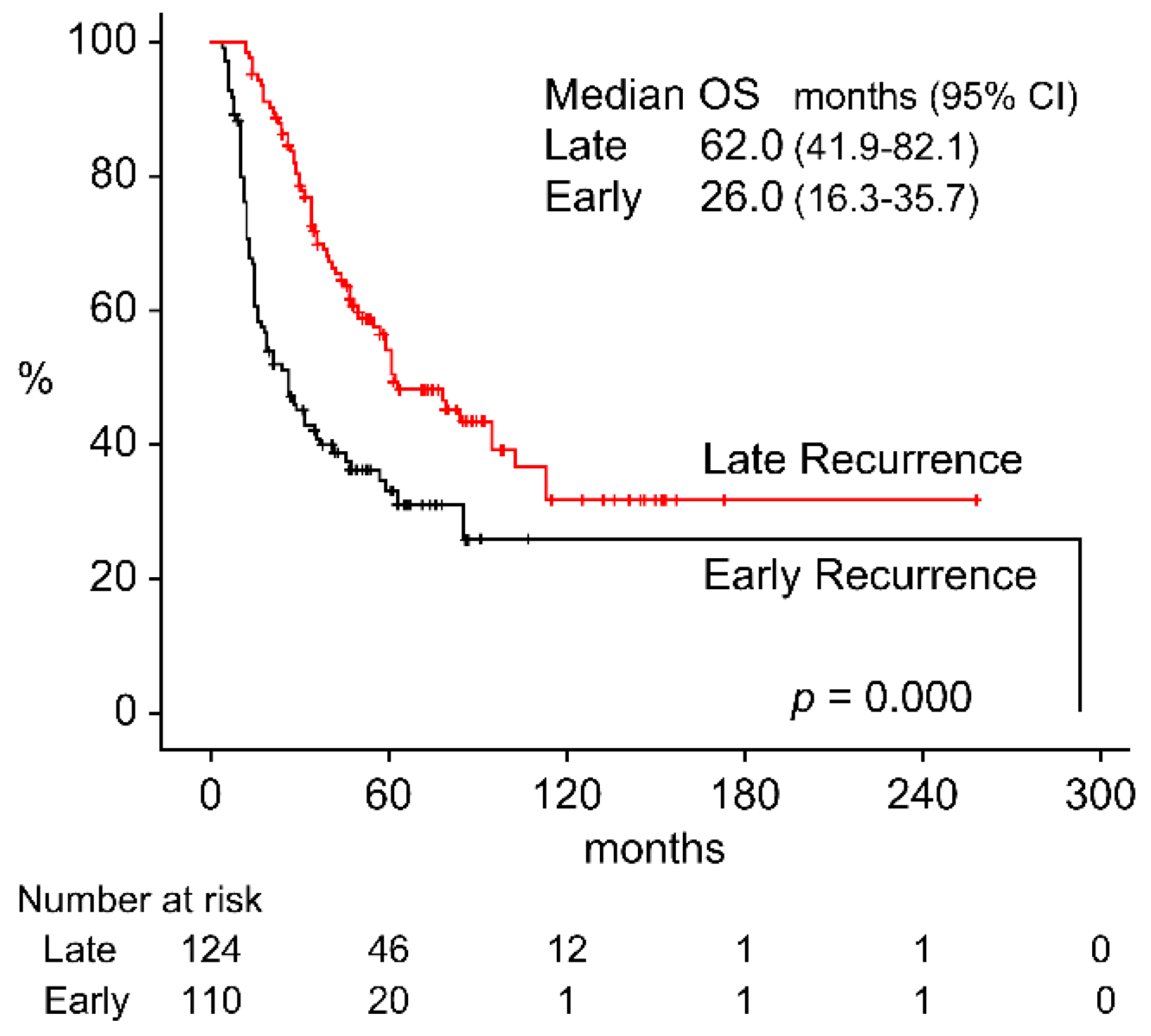
3.2. OS by Recurrence Status
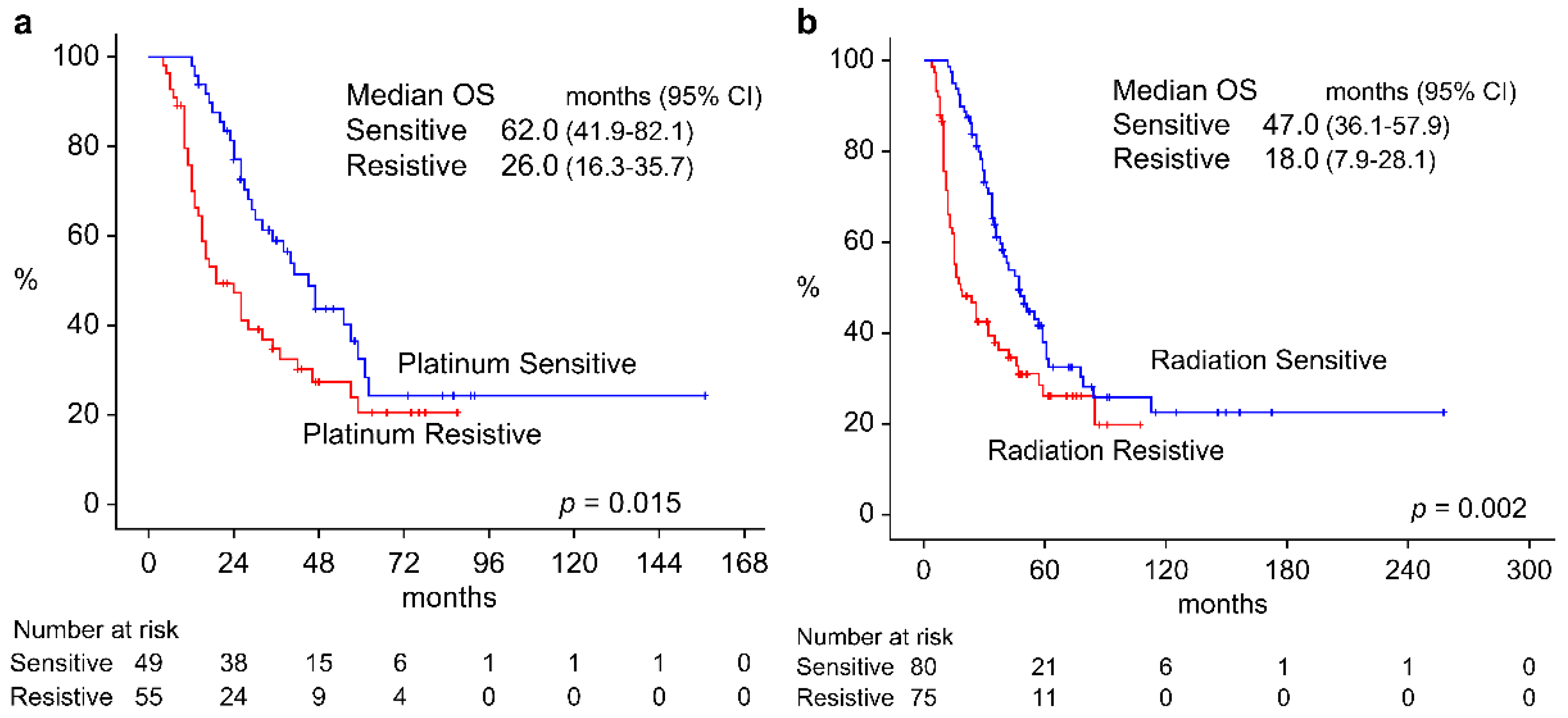
3.3. Survival Rate by Resistance to Platinum Preparation and Radiotherapy
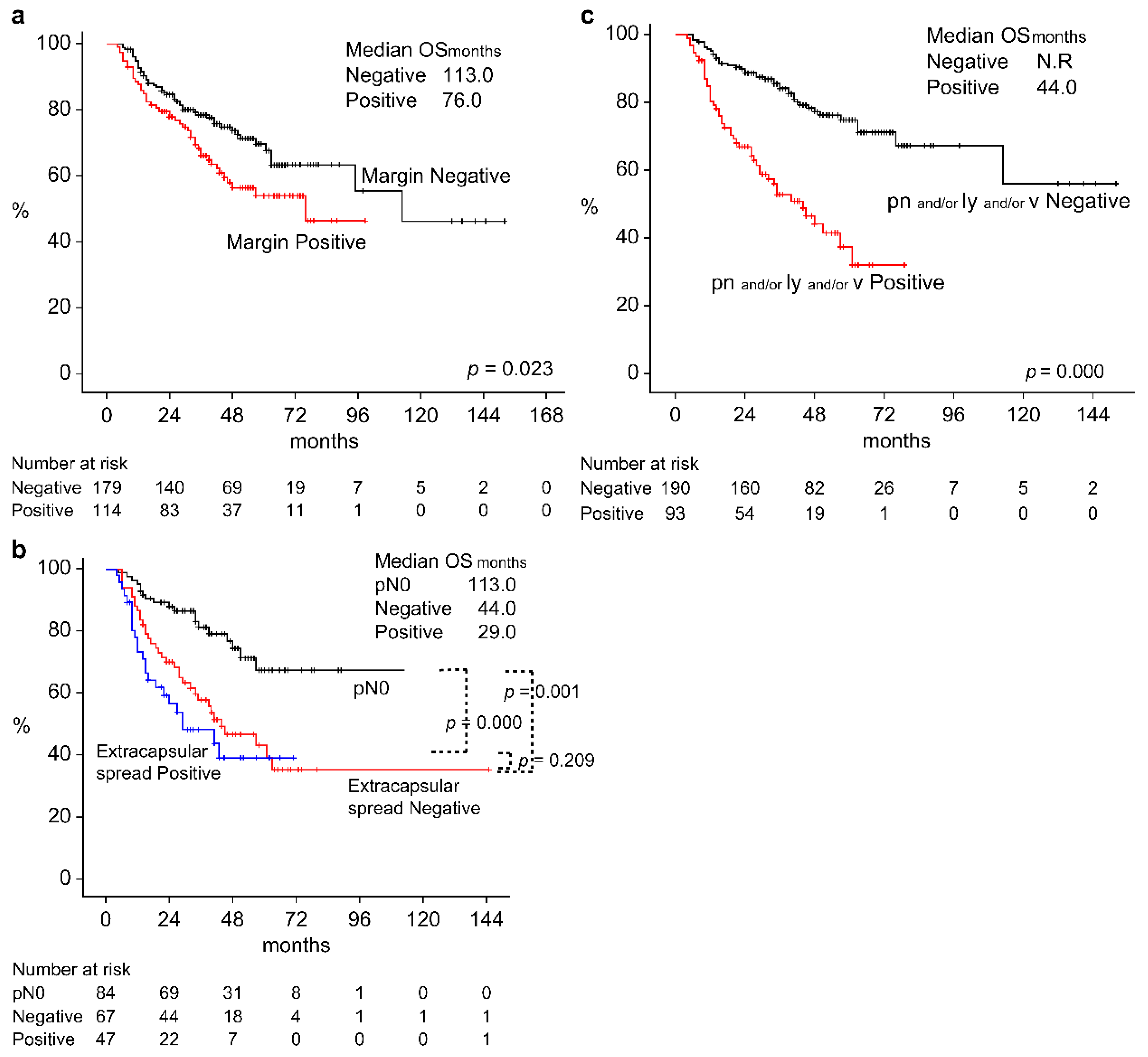
3.4. Survival Rate by Pathological Findings of Surgery Patients
3.5. Predictors of Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat Rev Dis Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Chow, L.Q.M. Head and Neck Cancer. N Engl J Med 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Sacco, AG; Cohen, EE. Current Treatment Options for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. J Clin Oncol 2015, 33, 3305–3313. [Google Scholar] [CrossRef] [PubMed]
- Borsetto, D.; Sethi, M.; Polesel, J.; Tomasoni, M.; Deganello, A.; Nicolai, P.; Bossi, P.; Fabbris, C.; Molteni, G.; Marchioni, D.; Tofanelli, M.; Cragnolini, F.; Tirelli, G.; Ciorba, A.; Pelucchi, S.; Corazzi, V.; Canzi, P.; Benazzo, M.; Lupato, V.; Giacomarra, V.; Cazzador, D.; Bandolin, L.; Menegaldo, A.; Spinato, G.; Obholzer, R.; Fussey, J.; Boscolo-Rizzo, P. The Risk of Recurrence in Surgically Treated Head and Neck Squamous Cell Carcinomas: A Conditional Probability Approach. Acta Oncol 2021, 60, 942–947. [Google Scholar] [CrossRef]
- Lacas, B.; Carmel, A.; Landais, C.; Wong, S.J.; Licitra, L.; Tobias, J.S.; Burtness, B.; Ghi, M.G.; Cohen, E.E.W.; Grau, C.; Wolf, G.; Hitt, R.; Corvò, R.; Budach, V.; Kumar, S.; Laskar, S.G.; Mazeron, J.J.; Zhong, L.P.; Dobrowsky, W.; Ghadjar, P.; Fallai, C.; Zakotnik, B.; Sharma, A.; Bensadoun, R.J.; Ruo Redda, M.G.; Racadot, S.; Fountzilas, G.; Brizel, D.; Rovea, P.; Argiris, A.; Nagy, Z. T.; Lee, J.W.; Fortpied, C.; Harris, J.; Bourhis, J.; Aupérin, A.; Blanchard, P.; Pignon, J. P. ; MACH-NC Collaborative Group, Meta-analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update on 107 Randomized Trials and 19,805 Patients, On Behalf of MACH-NC Group. Radiother Oncol 2021, 156, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.W.; Liu, D.L.; Chen, J.X.; Lin, L.Z.; Zhuang, L.P.; Hou, X.H. Early and Late Recurrences in Lymph Node-Negative Gastric Cancer: A Retrospective Cohort Study. Ann Saudi Med 2021, 41, 336–349. [Google Scholar] [CrossRef]
- Yan, W.T.; Li, C.; Yao, L.Q.; Qiu, H.B.; Wang, M.D.; Xu, X.F.; Zhou, Y.-H.; Wang, H.; Chen, T.H.; Gu, W.M.; Zhong, J.H.; Wu, H.; Pawlik, T. M.; Lau, W. Y.; Shen, F.; Yang, T. Predictors and Long-Term Prognosis of Early and Late Recurrence for Patients Undergoing Hepatic Resection of Hepatocellular Carcinoma: A Large-Scale Multicenter Study. Hepatobiliary Surg Nutr 2023, 12, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Xu, S.; Gao, Y.X.; Zhao, Z.M.; Zhao, G.D.; Hu, M.G.; Tan, X.L.; Lau, W.Y.; Liu, R. Early and Late Recurrence Patterns of Pancreatic Ductal Adenocarcinoma After Pancreaticoduodenectomy: A Multicenter Study. Int J Surg 2023, 109, 785–793. [Google Scholar] [CrossRef]
- Lee, C.H.; Chung, J.; Kwak, C.; Jeong, C.W.; Il Seo, S.; Kang, M.; Hong, S.H.; Song, C.; Park, J.Y.; Hwang, E.C.; Lee, H.; Ku, J.Y.; Seo, W.I.; Choi, S.H.; Ha, H.K.; Korean Renal Cancer Study Group. Targeted therapy response in early versus late recurrence of renal cell carcinoma after surgical treatment: A propensity score-matched study using the Korean Renal Cancer Study Group database. Int J Urol 2021, 28, 417–423. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Zheng, A.; Yang, H.; Li, J.; Wu, S.; Zhao, J.; Meng, P.; Zhou, F. Definition and Risk Factors of Early Recurrence Based on Affecting Prognosis of Esophageal Squamous Cell Carcinoma Patients After Radical Resection. Transl Oncol 2021, 14, 101066. [Google Scholar] [CrossRef]
- Chang, J.H.; Wu, C.C.; Yuan, K.S.P.; Wu, A.T.H.; Wu, S.Y. Locoregionally Recurrent Head and Neck Squamous Cell Carcinoma: Incidence, Survival, Prognostic Factors, and Treatment Outcomes. Oncotarget 2017, 8, 55600–55612. [Google Scholar] [CrossRef] [PubMed]
- Weckx, A.; Riekert, M.; Grandoch, A.; Schick, V.; Zöller, J.E.; Kreppel, M. Time to Recurrence and Patient Survival in Recurrent Oral Squamous Cell Carcinoma. Oral Oncol 2019, 94, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Rettig, E.; Fakhry, C. Understanding the Impact of Survival and Human Papillomavirus Tumor Status on Timing of Recurrence in Oropharyngeal Squamous Cell Carcinoma. Oral Oncol 2016, 52, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ray, C.M.; Spira, A.I.; Trepel, J.B.; Carter, C.A.; Cottrill, H.M. A Brief Review of the Management of Platinum-Resistant-Platinum-Refractory Ovarian Cancer. Med Oncol 2017, 34, 103. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; Worden, F.; Saba, N.F.; Iglesias Docampo, L.C.; Haddad, R.; Rordorf, T.; Kiyota, N.; Tahara, M.; Monga, M.; Lynch, M.; Geese, W.J.; Kopit, J.; Shaw, J.W.; Gillison, M.L. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Tsukahara, K.; Okamoto, I.; Katsube, Y.; Shimizu, A.; Kondo, T.; Hanyu, K.; Fushimi, C.; Okada, T.; Miura, K. Clinical Outcomes of Platinum-Based Chemotherapy plus Cetuximab for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: Comparison Between Platinum-Sensitive and Platinum-Resistant Patients. Acta Otolaryngol 2019, 139, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Safi, A.F.; Kauke, M.; Grandoch, A.; Nickenig, H.J.; Zöller, J.E.; Kreppel, M. Analysis of Clinicopathological Risk Factors for Locoregional Recurrence of Oral Squamous Cell Carcinoma – Retrospective Analysis of 517 Patients. J Craniomaxillofac Surg 2017, 45, 1749–1753. [Google Scholar] [CrossRef]
- Cooper, J.S.; Zhang, Q.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; Lustig, R.; Ensley, J.F.; Thorstad, W.; Schultz, C.J.; Yom, S.S.; Ang, K.K. Long-Term Follow-Up of the RTOG 9501/Intergroup Phase III Trial: Postoperative Concurrent Radiation Therapy and Chemotherapy in High-Risk Squamous Cell Carcinoma of the Head and Neck. Int J Radiat Oncol Biol Phys 2012, 84, 1198–1205. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; Cognetti, F.; Bourhis, J.; Kirkpatrick, A.; van Glabbeke, M.; European Organization for Research and Treatment of Cancer Trial 22931. Postoperative Irradiation with or Without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N Engl J Med 2004, 350, 1945–1952. [Google Scholar] [CrossRef]
- Haring, C.T.; Kana, L.A.; Dermody, S.M; Brummel, C.; McHugh, J.B.; Casper, K.A.; Chinn, S.B.; Malloy, K.M.; Mierzwa, M.; Prince, M.E.P.; Rosko, A.J.; Shah, J.; Stucken, C.L.; Shuman, A.G.; Brenner, J.C.; Spector, M.E.; Worden, F.P.; Swiecicki, P.L. Patterns of recurrence in head and neck squamous cell carcinoma to inform personalized surveillance protocols. Cancer 2023, 129, 2817–2827. [Google Scholar] [CrossRef]
- Liao, C.T.; Chang, J.T.; Wang, H.M.; Ng, S.H.; Hsueh, C.; Lee, L.Y.; Lin, C.H.; Chen, I.H.; Huang, S.F.; Cheng, A.J.; Yen, T.C. Salvage Therapy in Relapsed Squamous Cell Carcinoma of the Oral Cavity: How and When? Cancer 2008, 112, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Li, Z.; Liu, S.; Li, H.; Xu, Z. Benefit of Salvage Total Pharyngolaryngoesophagectomy for Recurrent Locally Advanced Head and Neck Cancer After Radiotherapy. Radiat Oncol 2017, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Heft Neal, M.E.; Brennan, J.; Haring, C.T.; Brenner, J.C.; Worden, F.; Swiecicki, P.; Mierzwa, M.; Casper, K.A.; Malloy, K.M.; Stucken, C.L.; McLean, S.A.; Prince, M.E.; Bradford, C.R.; Wolf, G.T.; Shuman, A.G.; Chinn, S.B.; Chepeha, D.B.; Rosko, A.J.; Spector, M.E. Predictors of Survival in Patients Undergoing Oropharyngeal Surgery for Cancer Recurrence After Radiation Therapy. Eur Arch Otorhinolaryngol 2020, 277, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Lupato, V.; Giacomarra, V.; Alfieri, S.; Fanetti, G.; Polesel, J. Prognostic Factors in Salvage Surgery for Recurrent Head and Neck Cancer: A Systematic Review and Meta-analysis. Crit Rev Oncol Hematol 2022, 169, 103550. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Abraham, J.; Ross, E.; Ridge, J.A.; Lango, M.N.; Liu, J.C.; Bauman, J.R.; Avkshtol, V.; Galloway, T. J. Rapid Recurrence in Head and Neck Cancer: Underappreciated Problem with Poor Outcome. Head Neck 2021, 43, 212–222. [Google Scholar] [CrossRef]
- Salehi, A.; Wang, L.; Coates, P. J.; Norberg Spaak, L.; Gu, X.; Sgaramella, N.; Nylander, K. Reiterative Modeling of Combined Transcriptomic and Proteomic Features Refines and Improves the Prediction of Early Recurrence in Squamous Cell Carcinoma of Head and Neck. Comput Biol Med 2022, 149, 105991. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Head and Neck Cancers, version 1.2024. http://www.nccn.org/Home.
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; Fuereder, T.; Hughes, B.G.M.; Mesía, R.; Ngamphaiboon, N.; Rordorf, T.; Wan Ishak, W.Z.; Hong, R.; González Mendoza, R.; Roy, A.; Zhang, Y.; Gumuscu, B.; Cheng, J.D.; Jin, F.; Rischin, D.; Lerzo, G.; Tatangelo, M.; Varela, M.; Zarba, J.J.; Boyer, M.; Gan, H.; Gao, B.; Hughes, B.; Mallesara, G.; Rischin, D.; Taylor, A.; Burian, M.; Fuereder, T.; Greil, R.; Barrios, C.H.; de Castro Junior, D.O.; Castro, G.; Franke, F.A.; Girotto, G.; Lima, I.P.F.; Nicolau, U.R.; Pinto, G.D.J.; Santos, L.; Victorino, A.; Chua, N.; Couture, F.; Gregg, R.; Hansen, A.; Hilton, J.; McCarthy, J.; Soulieres, D.; Ascui, R.; Gonzalez, P.; Villanueva, L.; Torregroza, M.; Zambrano, A.; Holeckova, P.; Kral, Z.; Melichar, B.; Prausova, J.; Vosmik, M.; Andersen, M.; Gyldenkerne, N.; Jurgens, H.; Putnik, K.; Reinikainen, P.; Gruenwald, V.; Laban, S.; Aravantinos, G.; Boukovinas, I.; Georgoulias, V.; Psyrri, A.; Kwong, D.; Al-Farhat, Y.; Csoszi, T.; Erfan, J.; Horvai, G.; Landherr, L.; Remenar, E.; Ruzsa, A.; Szota, J.; Billan, S.; Gluck, I.; Gutfeld, O.; Popovtzer, A.; Benasso, M.; Bui, S.; Ferrari, V.; Licitra, L.; Nole, F.; Fujii, T.; Fujimoto, Y.; Hanai, N.; Hara, H.; Matsumoto, K.; Mitsugi, K.; Monden, N.; Nakayama, M.; Okami, K.; Oridate, N.; Shiga, K.; Shimizu, Y.; Sugasawa, M.; Tahara, M.; Takahashi, M.; Takahashi, S.; Tanaka, K.; Ueda, T.; Yamaguchi, H.; Yamazaki, T.; Yasumatsu, R.; Yokota, T.; Yoshizaki, T.; Kudaba, I.; Stara, Z.; Wan Ishak, W.Z.; Cheah, S.K.; Aguilar Ponce, J.; Gonzalez Mendoza, R.; Hernandez Hernandez, C.; Medina Soto, F.; Buter, J.; Hoeben, A.; Oosting, S.; Suijkerbuijk, K.; Bratland, A.; Brydoey, M.; Alvarez, R.; Mas, L.; Caguioa, P.; Querol, J.; Regala, E.E.; Tamayo, M.B.; Villegas, E.M.; Kawecki, A.; Karpenko, A.; Klochikhin, A.; Smolin, A.; Zarubenkov, O.; Goh, B.C.; Cohen, G.; du Toit, J.; Jordaan, C.; Landers, G.; Ruff, P.; Szpak, W.; Tabane, N.; Brana, I.; Iglesias Docampo, L.; Lavernia, J.; Mesia, R.; Abel, E.; Muratidu, V.; Nielsen, N.; Cristina, V.; Rordorf, T.; Rothschild, S.; Hong, R.; Wang, H.; Yang, M.; Yeh, S.; Yen, C.; Ngamphaiboon, N.; Soparattanapaisarn, N.; Sriuranpong, V.; Aksoy, S.; Cicin, I.; Ekenel, M.; Harputluoglu, H.; Ozyilkan, O.; Harrington, K.; Agarwala, S.; Ali, H.; Alter, R.; Anderson, D.; Bruce, J.; Burtness, B.; Campbell, N.; Conde, M.; Deeken, J.; Edenfield, W.; Feldman, L.; Gaughan, E.; Goueli, B.; Halmos, B.; Hegde, U.; Hunis, B.; Jotte, R.; Karnad, A.; Khan, S.; Laudi, N.; Laux, D.; Martincic, D.; McCune, S.; McGaughey, D.; Misiukiewicz, K.; Mulford, D.; Nadler, E.; Neupane, P.; Nunnink, J.; Ohr, J.; O’Malley, M.; Patson, B.; Paul, D.; Popa, E.; Powell, S.; Redman, R.; Rella, V.; Rocha Lima, C.; Sivapiragasam, A.; Su, Y.; Sukari, A.; Wong, S.; Yilmaz, E.; Yorio, J. Pembrolizumab Alone or with Chemotherapy Versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Ciuleanu, T.E.; Tsekov, H.; Shparyk, Y.; Cuceviá, B.; Juhasz, G.; Thatcher, N.; Ross, G.A.; Dane, G.C.; Crofts, T. Phase III Trial Comparing Supportive Care Alone with Supportive Care with Oral Topotecan in Patients with Relapsed Small-Cell Lung Cancer. J Clin Oncol 2006, 24, 5441–5447. [Google Scholar] [CrossRef]
- Horita, N.; Yamamoto, M.; Sato, T.; Tsukahara, T.; Nagakura, H.; Tashiro, K.; Shibata, Y.; Watanabe, H.; Nagai, K.; Nakashima, K.; Ushio, R.; Ikeda, M.; Kobayashi, N.; Shinkai, M.; Kudo, M.; Kaneko, T. Amrubicin for Relapsed Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of 803 Patients. Sci Rep 2016, 6, 18999. [Google Scholar] [CrossRef]
| All (n = 500) |
Recurrence (n = 234) |
p value | |||
| ER (n = 110) |
LR (n = 124) |
||||
| Gender | Male Female |
377 123 |
80 30 |
96 28 |
0.450 (F) |
| Age (years), mean (SD) |
Median |
20–92 (67) |
33–92 (65) |
20–86 (66) |
0.995 (M) |
| Area | Oral cavity Nasopharynx Oropharynx Hypopharynx Larynx Nasal sinus External auditory canal |
145 21 79 113 64 41 37 |
51 2 15 20 7 9 6 |
33 7 16 32 20 12 4 |
0.014 (F) |
| T classification | T1 T2 T3 T4 |
84 162 112 142 |
9 32 20 49 |
23 41 26 34 |
0.003 (M) |
| N classification | N0 N1 N2 N3 |
252 74 155 19 |
44 16 43 7 |
59 12 47 6 |
0.370 (M) |
| P16 | Positive Negative Unmeasured |
48 43 409 |
7 12 91 |
7 10 107 |
0.736 (F) |
| Initial treatment | Surgery alone + chemoradiotherapy (platinum+) + radiotherapy (platinum-) + neoadjuvant chemotherapy (platinum+) Radiotherapy alone chemoradiotherapy (platinum+) chemoradiotherapy (platinum-) |
157 87 39 19 17 147 34 |
30 29 12 5 5 21 8 |
41 17 11 3 4 29 19 |
0.103 (F) |
| Postoperative pathological findings of the surgical therapy group |
Non-surgery group Surgery group Margin Positive Negative Unknown Extracapsular spread Yes No p0 pn, ly, vn Yes No Unknown |
198 302 115 182 5 47 67 88 94 194 10 |
34 76 35 39 2 24 21 15 42 29 4 |
52 72 30 38 3 8 20 13 19 45 4 |
0.103 (F) 0.795 (F) 0.091 (F) 0.002 (F) |
| Multiplicity cancer in other organs | No Yes |
394 106 |
93 17 |
98 26 |
0.313 (F) |
| Variables | OS univariate analysis | OS multivariate analysis | |
| HR (95% CI), p value | HR (95% CI), p value | ||
| Recurrence time | ER: Recurrence in two to six months after treatment (n = 110) LR: Recurrence in more than seven months after treatment (n = 124) |
2.126 (1.509–2.995), p = 0.000 Ref |
3.200(1.570–6.521) p = 0.001 Ref |
| Radiotherapy |
Recurrence in two to six months after radiotherapy (n = 75) Recurrence in more than seven months after treatment (n = 80) Recurrence without treatment (n = 79) |
1.824 (1.237–2.690), p = 0.002 Ref 0.593(0.382–0.923), p = 0.021 |
0.567 (0.232–1.390) p = 0.215 Ref 0.374 (0.191–0.733) p = 0.004 |
| Platinum preparation |
Platinum-resistive recurrence (n = 52) Platinum-sensitive recurrence (n = 54) Recurrence without platinum administration (n = 128) |
1.721 (1.078–2.747), p = 0.023 Ref 0.737(0.488–1.114), p = 0.148 |
0.998(0.501–1.988), p = 0.995 Ref 0.881 (0.535–1.452) p = 0.619 |
| Multiplicity cancer in other organs | No (n = 191) Yes (n = 43) |
Ref 1.387 (0.929, 2.071), p = 0.110 |
- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





