Submitted:
07 February 2024
Posted:
07 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Nanofibers Preparation
2.2. Scanning Electron Microscopy (SEM)
2.3. X-ray Diffraction and Raman Spectroscopy
2.4. Dielectric spectroscopy
2.5. Pyroelectric Measurements
2.6. Piezoelectric Measurements
3. Results and Discussion
3.1. Dielectrics
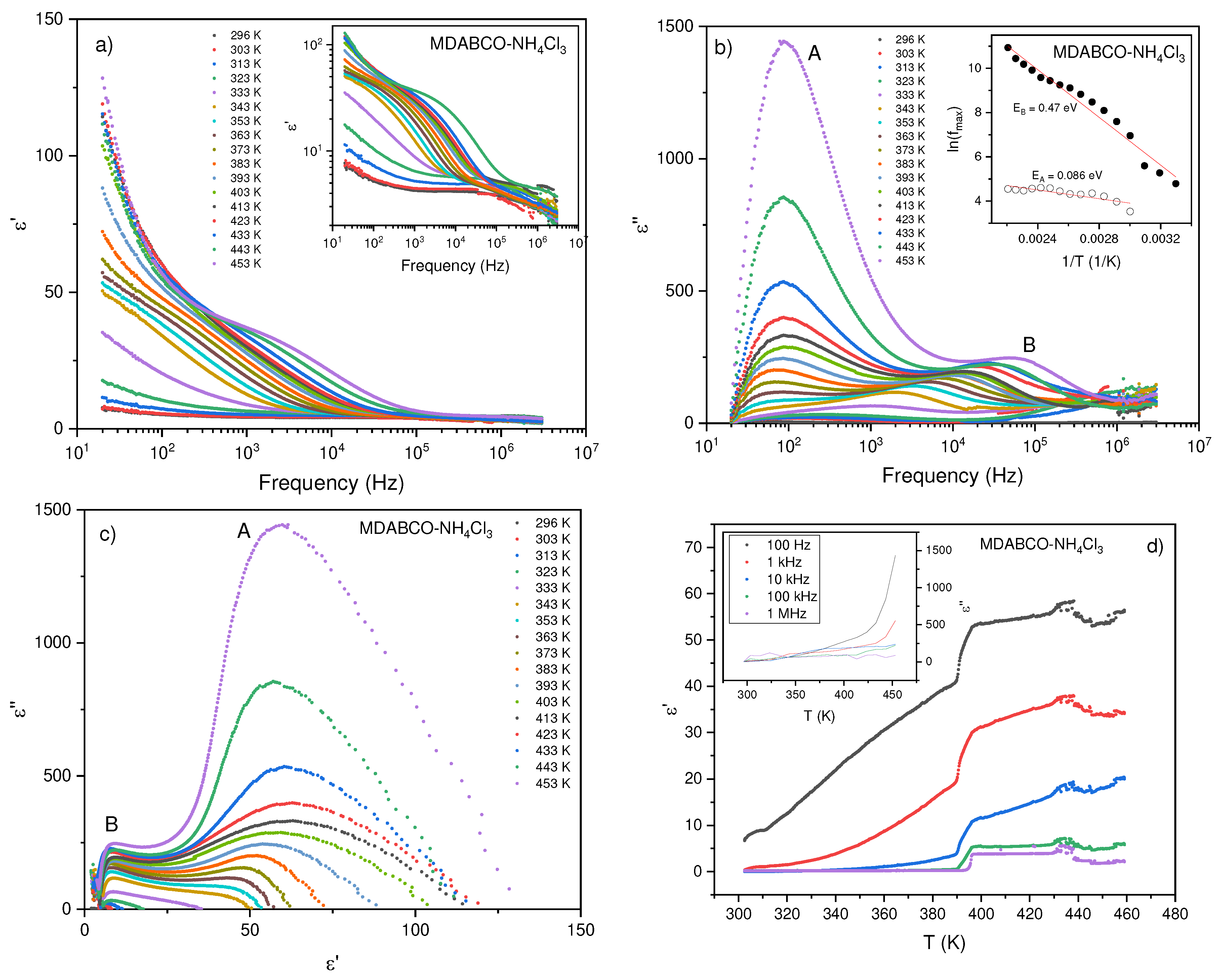
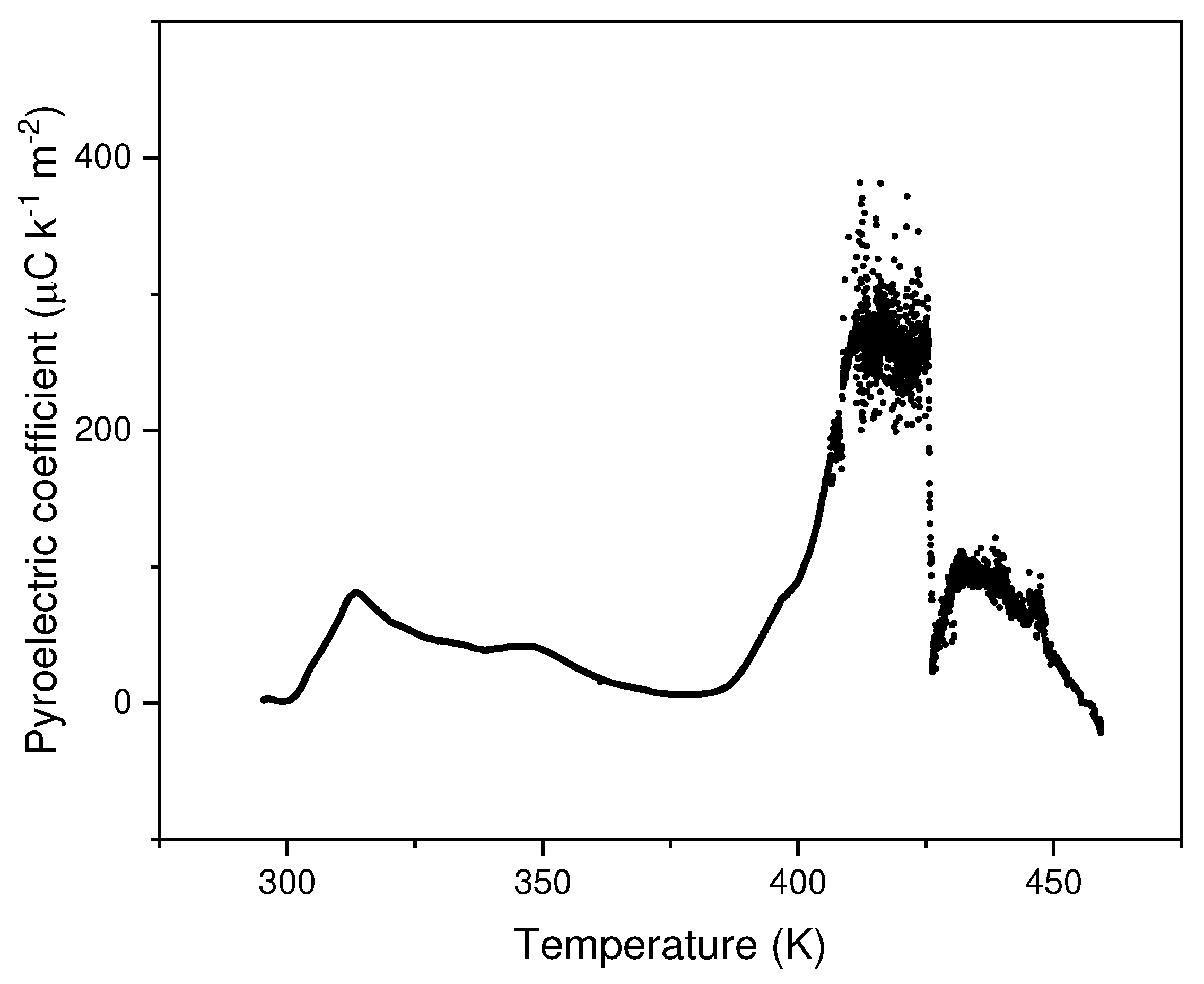
3.2. Pyroelectricity
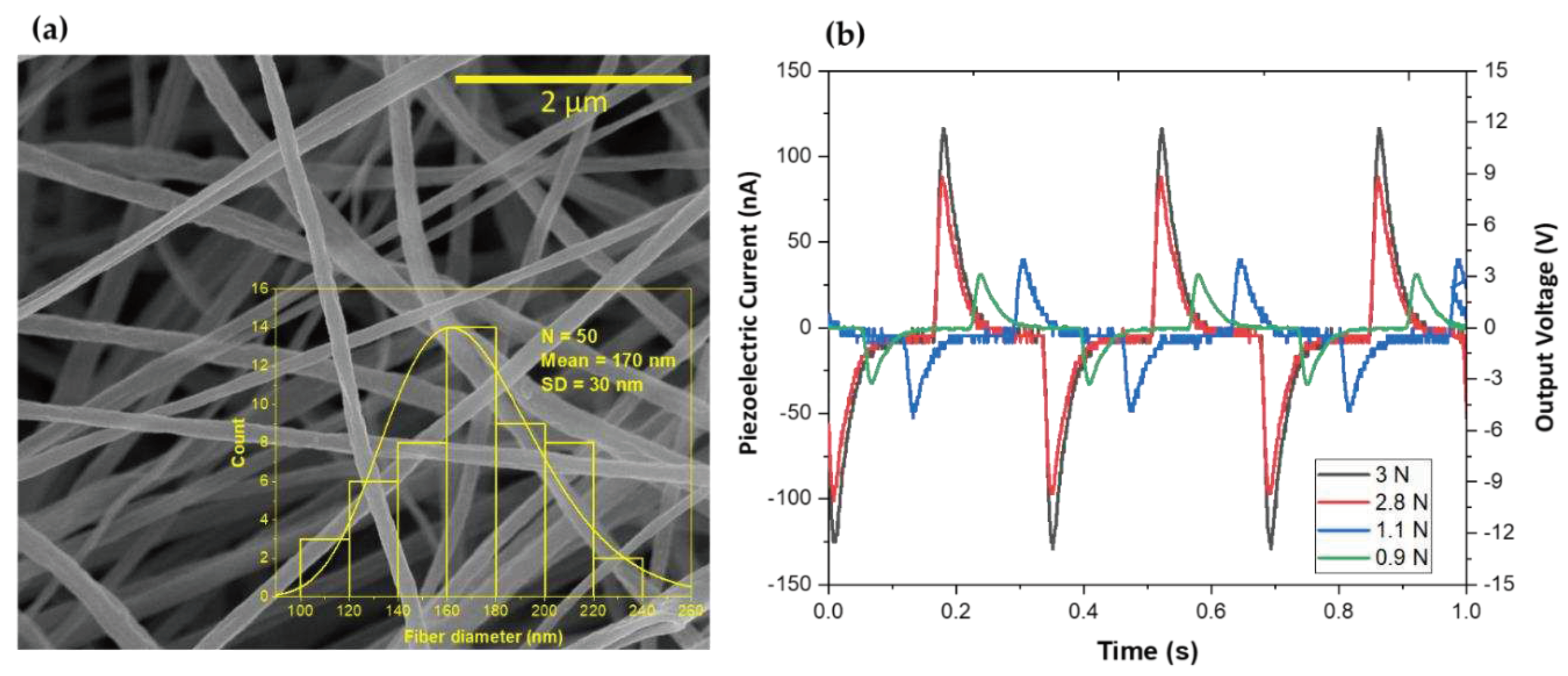
3.3. Piezoelectric Voltage and Current, and Effective Piezoelectric Coefficients in Fibers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lines, M.E.; Glass, A.M. Principles and applications of ferroelectrics and related materials; Oxford university press: 2001.
- Zhou, X.; Ke, Q.; Tang, S.; Luo, J.; Lu, Z. Ultraviolet photodetectors based on ferroelectric depolarization field. Journal of Energy Chemistry 2023, 77, 487–498. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Z.; Zhang, L.; Ke, Q. Wide-bandgap all-inorganic lead-free perovskites for ultraviolet photodetectors. Nano Energy 2023, 117, 108908. [Google Scholar] [CrossRef]
- Martínez-Goyeneche, L.; Gil-Escrig, L.; Susic, I.; Tordera, D.; Bolink, H.J.; Sessolo, M. Narrowband Monolithic Perovskite–Perovskite Tandem Photodetectors. Adv Opt Mater 2022, 10, 2201047. [Google Scholar] [CrossRef]
- Bowen, C.R.; Taylor, J.; LeBoulbar, E.; Zabek, D.; Chauhan, A.; Vaish, R. Pyroelectric materials and devices for energy harvesting applications. Energy & Environmental Science 2014, 7, 3836–3856. [Google Scholar] [CrossRef]
- Valasek, J. Properties of Rochelle Salt Related to the Piezo-electric Effect. Physical Review 1922, 20, 639–664. [Google Scholar] [CrossRef]
- Valasek, J. Piezo-Electric and Allied Phenomena in Rochelle Salt. Physical Review 1921, 17, 475–481. [Google Scholar] [CrossRef]
- Lines, M.E.; Glass, A.M. Principles and Applications of Ferroelectrics and Related Materials; Oxford University Press: Oxford, 2001; p. 694. [Google Scholar]
- Scott James, F.; Paz de Araujo Carlos, A. Ferroelectric Memories. Science 1989, 246, 1400–1405. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, Y.; Dou, S.X.; Liang, J. Flexible nanogenerators for wearable electronic applications based on piezoelectric materials. Materials Today Energy 2021, 20, 100690. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. A comprehensive review on the state-of-the-art of piezoelectric energy harvesting. Nano Energy 2021, 80, 105567. [Google Scholar] [CrossRef]
- Cross, E. Lead-free at last. Nature 2004, 432, 24–25. [Google Scholar] [CrossRef]
- Saito, Y.; Takao, H.; Tani, T.; Nonoyama, T.; Takatori, K.; Homma, T.; Nagaya, T.; Nakamura, M. Lead-free piezoceramics. Nature 2004, 432, 84–87. [Google Scholar] [CrossRef]
- Chen, X.-G.; Song, X.-J.; Zhang, Z.-X.; Li, P.-F.; Ge, J.-Z.; Tang, Y.-Y.; Gao, J.-X.; Zhang, W.-Y.; Fu, D.-W.; You, Y.-M.; et al. Two-Dimensional Layered Perovskite Ferroelectric with Giant Piezoelectric Voltage Coefficient. J Am Chem Soc 2020, 142, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.-M.; Gong, Y.-J.; Li, Z.-G.; Liu, Y.-M.; Li, W.; Li, Z.-Y.; Bu, X.-H. A New Hybrid Lead-Free Metal Halide Piezoelectric for Energy Harvesting and Human Motion Sensing. Small 2022, 18, 2103829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Tang, Y.-Y.; Shi, P.-P.; Xiong, R.-G. Toward the Targeted Design of Molecular Ferroelectrics: Modifying Molecular Symmetries and Homochirality. Accounts Chem Res 2019, 52, 1928–1938. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Deschler, F.; Gao, S.; Friend, R.H.; Cheetham, A.K. Chemically diverse and multifunctional hybrid organic–inorganic perovskites. Nature Reviews Materials 2017, 2, 16099. [Google Scholar] [CrossRef]
- Song, X.; Hodes, G.; Zhao, K.; Liu, S. Metal-Free Organic Halide Perovskite: A New Class for Next Optoelectronic Generation Devices. Adv Energy Mater 2021, 11, 2003331. [Google Scholar] [CrossRef]
- Chu, L.-L.; Zhang, T.; Zhang, W.-Y.; Shi, P.-P.; Gao, J.-X.; Ye, Q.; Fu, D.-W. Three-Dimensional Metal-Free Molecular Perovskite with a Thermally Induced Switchable Dielectric Response. The Journal of Physical Chemistry Letters 2020, 11, 1668–1674. [Google Scholar] [CrossRef]
- You, Y.-M.; Liao, W.-Q.; Zhao, D.; Ye, H.-Y.; Zhang, Y.; Zhou, Q.; Niu, X.; Wang, J.; Li, P.-F.; Fu, D.-W.; et al. An organic-inorganic perovskite ferroelectric with large piezoelectric response. Science 2017, 357, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Saparov, B.; Mitzi, D.B. Organic–Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chemical Reviews 2016, 116, 4558–4596. [Google Scholar] [CrossRef]
- Kamat, P.V.; Bisquert, J.; Buriak, J. Lead-Free Perovskite Solar Cells. ACS Energy Letters 2017, 2, 904–905. [Google Scholar] [CrossRef]
- Boix, P.P.; Agarwala, S.; Koh, T.M.; Mathews, N.; Mhaisalkar, S.G. Perovskite Solar Cells: Beyond Methylammonium Lead Iodide. The Journal of Physical Chemistry Letters 2015, 6, 898–907. [Google Scholar] [CrossRef]
- Ye, H.-Y.; Tang, Y.-Y.; Li, P.-F.; Liao, W.-Q.; Gao, J.-X.; Hua, X.-N.; Cai, H.; Shi, P.-P.; You, Y.-M.; Xiong, R.-G. Metal-free three-dimensional perovskite ferroelectrics. Science 2018, 361, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, H.; Zhang, Z.; Liu, Z.; Lv, Z.; Li, T.; Ju, W.; Li, H.; Cai, X.; Han, H. Large piezoelectric response in a family of metal-free perovskite ferroelectric compounds from first-principles calculations. npj Computational Materials 2019, 5, 17. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. Journal of Electrostatics 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: solving global issues. Materials Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Isakov, D.V.; de Matos Gomes, E.; Vieira, L.G.; Dekola, T.; Belsley, M.S.; Almeida, B.G. Oriented single-crystal-like molecular arrangement of optically nonlinear 2-methyl-4-nitroaniline in electrospun nanofibers. Acs Nano 2011, 5, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Richard-Lacroix, M.; Pellerin, C. Molecular Orientation in Electrospun Fibers: From Mats to Single Fibers. Macromolecules 2013, 46, 9473–9493. [Google Scholar] [CrossRef]
- Rørvik, P.M.; Grande, T.; Einarsrud, M.-A. One-Dimensional Nanostructures of Ferroelectric Perovskites. Adv Mater 2011, 23, 4007–4034. [Google Scholar] [CrossRef]
- Isakov, D.; de Matos Gomes, E.; Almeida, B.; Kholkin, A.L.; Zelenovskiy, P.; Neradovskiy, M.; Shur, V.Y. Energy harvesting from nanofibers of hybrid organic ferroelectric dabcoHReO4. Appl Phys Lett 2014, 104, 032907. [Google Scholar] [CrossRef]
- Sultana, A.; Ghosh, S.K.; Alam, M.M.; Sadhukhan, P.; Roy, K.; Xie, M.; Bowen, C.R.; Sarkar, S.; Das, S.; Middya, T.R.; et al. Methylammonium Lead Iodide Incorporated Poly(vinylidene fluoride) Nanofibers for Flexible Piezoelectric–Pyroelectric Nanogenerator. Acs Appl Mater Inter 2019, 11, 27279–27287. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. Chemsuschem 2015, 8, 513–523. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. Broadband dielectric spectroscopy; Springer Science & Business Media, 2003. [Google Scholar]
- Ghane-Motlagh, R.; Kroener, M.; Goldschmidtboeing, F.; Danilewsky, A.N.; Woias, P. A dynamic method for the measurement of pyroelectric properties of materials. Smart Mater Struct 2018, 27. [Google Scholar] [CrossRef]
- Bharti, V.; Kaura, T.; Nath, R. Improved piezoelectricity in solvent-cast PVC films. IEEE Transactions on Dielectrics and Electrical Insulation 1995, 2, 1106–1110. [Google Scholar] [CrossRef]
- Baptista, R.M.F.; Lopes, P.E.; Rodrigues, A.R.O.; Cerca, N.; Belsley, M.S.; de Matos Gomes, E. Self-assembly of Boc-p-nitro-l-phenylalanyl-p-nitro-l-phenylalanine and Boc-l-phenylalanyl-l-tyrosine in solution and into piezoelectric electrospun fibers. Materials Advances 2022, 3, 2934–2944. [Google Scholar] [CrossRef]
- Baptista, R.M.F.; de Matos Gomes, E.; Raposo, M.M.M.; Costa, S.P.G.; Lopes, P.E.; Almeida, B.; Belsley, M.S. Self-assembly of dipeptide Boc-diphenylalanine nanotubes inside electrospun polymeric fibers with strong piezoelectric response. Nanoscale Advances 2019, 1, 4339–4346. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, C.R.; Baptista, R.M.F.; de Matos Gomes, E.; Lopes, P.E.; Raposo, M.M.M.; Costa, S.P.G.; Belsley, M.S. Anisotropic PCL nanofibers embedded with nonlinear nanocrystals as strong generators of polarized second harmonic light and piezoelectric currents. Nanoscale Advances 2020, 2, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-S.; Wei, S.-M.; Chen, S.-W.; Pan, H.-C.; Pan, W.-P.; Huang, S.-M.; Tsai, M.-L.; Yang, P.-K. Metal-Free Perovskite Piezoelectric Nanogenerators for Human–Machine Interfaces and Self-Powered Electrical Stimulation Applications. Adv Sci 2022, 9, 2105974. [Google Scholar] [CrossRef]
- Li, M.; Wondergem, H.J.; Spijkman, M.-J.; Asadi, K.; Katsouras, I.; Blom, P.W.M.; de Leeuw, D.M. Revisiting the δ-phase of poly(vinylidene fluoride) for solution-processed ferroelectric thin films. Nat Mater 2013, 12, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Wondergem, H.J.; Spijkman, M.J.; Asadi, K.; Katsouras, I.; Blom, P.W.M.; de Leeuw, D.M. Revisiting the delta-phase of poly(vinylidene fluoride) for solution-processed ferroelectric thin films. Nat Mater 2013, 12, 433–438. [Google Scholar] [CrossRef]
- Baptista, R.M.F.; Moreira, G.; Silva, B.; Oliveira, J.; Almeida, B.; Castro, C.; Rodrigues, P.V.; Machado, A.; Belsley, M.; de Matos Gomes, E. Lead-Free MDABCO-NH4I3 Perovskite Crystals Embedded in Electrospun Nanofibers. Materials 2022, 15, 8397. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




