Challenges Posed by a Hematological Cancer
Leukemia stands as a prevalent hematologic malignancy, casting a shadow on the lives of thousands globally. The National Cancer Institute predicts that there will be an estimated 59610 new cases of Leukemia and 23710 estimated deaths in 2023 [
1]. Leukemia poses a formidable challenge for treatment due to its complex nature and the heterogeneity of the disease. Its various subtypes, including acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia, and chronic lymphocytic leukemia (CLL), exhibit diverse genetic and molecular abnormalities. This diversity makes it challenging to develop a one-size-fits-all therapeutic approach. The aggressive growth of abnormal blood cells interferes with normal blood cell production, leading to compromised immune function and anemia. Furthermore, leukemia cells can infiltrate other organs, complicating treatment strategies, unlike solid tumor cancers, surgical resection of the tumor is not an option, limiting strategies to chemotherapy, immunotherapy and radiation therapy, which are sometimes nonspecific, leading to organ damage and toxicity. The etiology of leukemia is multifaceted, often involving genetic mutations, exposure to certain environmental factors, and a combination of genetic predisposition.
At its core, leukemia disrupts the hematopoietic processes. The pathophysiology of this malignancy unfolds within the bone marrow, where abnormal hematopoietic stem cells undergo unregulated differentiation, leading to an overproduction of immature and dysfunctional white blood cells [
1,
2,
3,
4]. This aberrant cellular proliferation infiltrates the bloodstream, compromising the immune system function and giving rise to a cascade of detrimental consequences [
5,
6]. The disease manifests in various forms, with acute and chronic subtypes, and can affect the myeloid of lymphoid cell lines, each presenting its own set of challenges in diagnosis and treatment. Despite advances in medical research and therapeutic interventions, the blood born nature of leukemia remains a challenge, necessitating continuous efforts to unravel its intricacies and develop more effective treatment strategies.
On a molecular level, leukemia often involves mutations in key genes responsible for regulating cell cycle progression, apoptosis, and DNA repair [
7,
8,
9,
10]. These genetic aberrations disrupt the balance that ensures the controlled division and differentiation of blood cells of the myeloid or lymphoid stem line. The resultant accumulation of malignant cells not only impairs the production of healthy blood cells but also leads to the infiltration of leukemic cells into vital organs, further exacerbating the disease’s impact on overall health.
From a clinical standpoint, leukemia manifests as a relentless battle for those affected. The compromised immune system leaves patients vulnerable to infections, anemia, and bleeding disorders [
11,
12,
13,
14,
15,
16,
17]. Despite advancements in therapeutic approaches, the intricate interplay between genetic mutations and the dysregulation of hematopoietic processes continues to pose challenges in selecting an optimum treatment strategy and achieving long-term remission
Leukemia Treatment Strategies and Their Complications
The goal of leukemia treatment is remission and ultimately cure, complete remission is defined as the patient’s bone marrow being free of malignant cells, which can be confirmed microscopically, post biopsy. In the management of leukemia, therapeutic interventions encompass a spectrum of modalities, including chemotherapy, targeted therapy, immunotherapy, radiation and stem cell transplantation, each, with distinct advantages and limitations. Chemotherapy, the primary strategy in leukemia treatment, exerts its efficacy through a systemic approach, facilitating its widespread impact on leukemia cells within diverse anatomical compartments. Often used as a combination of multiple drugs with different mechanisms of actions, its cytotoxic mechanism disrupts the cell cycle, impeding the rapid division of malignant cells. While chemotherapy’s versatility renders it applicable across various leukemia subtypes, its non-selectivity induces collateral damage to normal, rapidly dividing cells, culminating in adverse effects ranging from mild, such as nausea and fatigue, to severe such as tumor lysis syndrome, cardiotoxicity, suppressed fertility, and myelosuppression (suppression of bone marrow leading to anemia, thrombocytopenia and neutropenia, and susceptibility to hemorrhage and infection), with the major causes of treatment related mortality being hemorrhage and infection. Chemotherapy can either be used as a stand-alone therapy or in combination with other treatments such as targeted and radiation therapy, or as an adjuvant therapy, to shrink a tumor before surgical intervention.
In contrast, targeted therapies offer a precision-oriented paradigm (monoclonal antibodies to receptors on cancerous cells that either mark cancer cells for immune recognition, carry toxins to target cells or have cytostatic effects), selectively addressing leukemia cells harboring specific molecular or genetic aberrations, thereby mitigating off-target effects. Nonetheless, limitations include the potential development of resistance and applicability restricted to certain genetic subtypes.
Immunotherapy, an evolving frontier, capitalizes on the immune system’s intrinsic capacity to recognize and eliminate leukemia cells. While immunotherapy produces sustained responses, in some cases, its variable efficacy and potential for immune-related adverse events necessitate careful consideration. An example of immunotherapy is Chimeric Antigen Receptor CAR-T cell therapy, these cells are taken from the patient or a donor and genetically modified to recognize cancer cell antigens [
18]. Currently, CAR-T cell therapy has FDA approval for B-cell acute lymphoblastic leukemia, in both pediatrics and adults. However, despite the specificity, CAR-T cells have been shown to cause multiple adverse effects, including cytokine release syndrome, allergic reactions and dyspnea [
19,
20,
21]. Moreover, recent data has shown that CAR-T cell therapy has been linked to secondary malignancy development, emphasizing the need for both regulation and further investigation before determining that the benefits outweigh the risks in this cohort of patients.
Radiation therapy is used in combined treatment strategies, usually alongside chemotherapy or before a bone marrow transplantation, to destroy leukemia cells, alleviate discomfort caused by enlarged liver, spleen or lymph nodes or treat pain from bone damage. Total body irradiation (TBI) is usually the choice prechemotherapy or stem cell transplantation (suppresses the immune system to reduce probability of donor stem cell rejection). External bean radiation therapy (EBRT), is often used to reduce swelling in the liver, spleen or lymph nodes, while total bone marrow irradiation (TMI) is used for precise delivery of radiation to the bone marrow, commonly used for patients undergoing stem cell transplantation, however, radiation has not been shown to be effective as an individual treatment modality
Stem cell transplantation remains the optimum treatment for leukemias, as replenishment of bone marrow stem cells with healthy cells would result in remission or cure, however, it is a challenge to find a donor and even with a donor, allogeneic transplantation can lead to graft-versus-host disease (GVHD), which can range from mild to life threatening. In delineating the treatment landscape, the distinct attributes of each modality prompt an individualized approach, often involving combination therapies to optimize therapeutic outcomes while mitigating associated drawbacks. The treatment of choice is determined by overall health of the patient, possible metastasis of the cancer, age, and subtype of leukemia. Due to the frequency of complications with current treatment strategies and the immunosuppressive effects of therapeutic options. Underscoring the dynamic landscape of leukemia therapeutics, it is important for ongoing research to seek to refine existing strategies and develop diagnostic approaches for early detection of treatment complications to prevent mortality due to secondary causes.
Anthracycline-Induced Cardiotoxicity
Anthracyclines, also known as ANTs, and including doxorubicin and daunorubicin are a class of drugs that are developed from
Streptomyces species to treat many types of cancers, including leukemias. The structure of anthracyclines contains a planar anthracyclinone core with substituents of quinones, phenolic groups, and other groups. Of the four rings in this structure, one is saturated. While these molecules are water-insoluble, water solubility can be provided by mono- or multisaccharide substitution in the saturated ring. This substitution creates not only water solubility, but also stability for future DNA binding [
22].
These chemotherapy drugs have become widely used since their discovery fifty years ago and have drastically reduced the mortality rate of cancer for patients, becoming one of the leading treatment options for several different cancers. This class of drugs work in several different ways including their interaction with topoisomerase-II, inhibition of DNA and RNA synthesis, the formation of free radicals, and the formation of adducts with DNA to stop the replication [
23,
24,
25,
26].
Topoisomerase-II is an enzyme that is critical to the maintenance of DNA. This enzyme uses the hydrolysis of ATP to cut both strands of the DNA helix to wind and unwind DNA during replication and transcription. ANTs interact with Topoisomerase-II to form a complex that prevents the re-ligation of the cuts in the double stranded DNA. In this way, ANTs inhibit cell growth and promote cell death. Another way that ANTs inhibit DNA and RNA synthesis is by intercalating DNA, thereby inhibiting DNA and RNA synthesis. Finally, ANTs have been found to combine with DNA to form adducts, which induce cell death by blocking transcription factors. In addition to these functions that prohibit DNA and RNA synthesis, ANTs are also involved in the formation of free radicals and Reactive Oxygen Species (ROS), which can damage lipids, proteins, and nucleic acids. With this damage, cell death is induced [
23,
24,
27,
28].
This multifaceted cytotoxicity targets rapidly dividing cancer cells, including those in the bone marrow, making anthracyclines particularly effective against the proliferative nature of leukemia. Their role extends beyond induction therapy, as anthracyclines are integral components of consolidation and maintenance regimens, contributing to enhanced outcomes. Despite their efficacy, anthracycline use is tempered by the risk of cardiotoxicity, emphasizing the necessity for vigilant monitoring and judicious dosing. Ongoing research seeks to refine treatment protocols, minimize adverse effects, and uncover strategies to optimize the therapeutic benefit of anthracyclines in leukemia management.
Chemical agents that induce cardiotoxicity are classified as either Type-I or Type-II agents. Type-I agents are those that cause irreversible cardiac damage and are dose dependent while Type-II agents are those that cause reversible cardiac damage and are not dose dependent. Patients may develop a variety of cardiac conditions such as arrhythmia, ischemia, pericarditis, systolic dysfunction, diastolic dysfunction, and congestive heart failure. Anthracyclines are considered a Type-I agent, thus limiting their usage as a treatment for cancer due to their high correlation with these adverse cardiac effects. Currently, there are thought to be 2 million individuals in the United States that are at risk for delayed anthracycline toxicity. With this in mind, it is imperative to understand the mechanism by which ANTs act to reduce the cardiotoxicity risk for patients [
29]
Anthracycline-induced cardiotoxicity can be defined as acute (after one dose or a single course presenting within 14 days), early onset chronic (presenting within a year),late onset chronic (more than a year) with the latter two being irreversible [
30]. The irreversibility of cardiomyocyte damage is a recognized concern in the realm of cancer treatment, particularly following the administration of anthracycline chemotherapy for leukemia and other malignancies. This cardiotoxicity stems from a complex interplay of mechanisms, including the generation of free radicals, mitochondrial dysfunction, cardiomyocyte apoptosis, inflammatory responses, disruption of calcium homeostasis, and the promotion of fibrosis and scarring. Anthracyclines’ capacity to induce oxidative stress and trigger apoptotic pathways in cardiomyocytes contributes to structural and functional changes in the heart, furthermore, these drugs may impact blood vessels, causing endothelial dysfunction and compromising overall cardiac function. Anthracycline toxicity may also present up to 20 years after exposure to anthracyclines, complicating its prevention and diagnosis [
22].
Chf as a Manifestation of Anthracycline Induced Cardiotoxicity
Congestive heart failure, also referred to as CHF, is a cardiac syndrome in which the affected individual has insufficient blood supply to the body as a result of inefficient myocardial performance. This syndrome can be a result of a variety of conditions but is characterized by decreased ventricular filling or ventricular ejection to circulation. CHF significantly decreases the quality of life of those that are affected by it and increases the risk of cardiovascular associated mortality [
31].
The incidence of anthracycline induced cardiotoxicity is dose dependent, ranging between 3–5% with 400 mg/m
2, but can be as high as 18-48 percent in patients who receive high dose regimens of anthracyclines (700 mg/m
2) [
32,
33]. Studies suggest that the potency of these drugs leads to cardiac injury at first drug administration, as supported by increased troponin release from myocardiocytes. While not yet fully understood, the pathophysiological progression of anthracycline toxicity is preceded by cardiomyocyte injury that eventually manifests as decrease in left ventricular ejection fraction (LVEF), and if not diagnosed and treated on time can eventually lead to congestive heart failure. Patients usually present with a dilated, hypokinetic cardiomyopathy, with a decreased LVEF. As a diagnostic criterion has been outlined as a decline in LVEF >10% points, with a final value<53%, however by the time patients present with these changes in cardiac function indexes in a chronic setting, the dilated cardiomyopathy is irreversible, and patients progress to congestive heart failure [
34]. Moreover, the American society of clinical oncology does not recommend monitoring for cardiac toxicity in low-risk patients, mainly due to burden of cost on patients, thus early progression may go unnoticed.
Diagnosis of Anthrocycline Induced Cardiotoxicity
Early detection is paramount to managing the risk and addressing complications associated with anthracycline-induced cardiotoxicity in clinical settings. This can be achieved by screening through cost efficient methods to determine which patients need vigilant monitoring, cardiac imaging (including ECG monitoring, and echocardiogram) and potential cardioprotective interventions. Previous studies have illustrated that Troponin I, being the gold standard for detecting cardiomyocyte injury would be a highly sensitive tool for determining whether there is cardiotoxicity and damage to cardiomyocytes [
35,
36,
37].
The use and evidence for the efficacy of other biomarkers beyond troponin-1 is very limited. Many issues are present with using troponin as a biomarker for cardiotoxicity including the need for specialist consultation, repeated testing, and lack of evidence for the timing of troponin testing. Subsequently, recent studies have investigated the use of biomarkers such as C-reactive protein (CRP) and galectin-3 (Gal-3) to monitor signs of CHF. CRP is a common marker of inflammation in the body, and gal-3 is a marker of fibrosis as well as inflammation, levels of both these inflammatory markers are usually low at baseline and increase with blood post cellular injury. Although we might expect CRP to increase with anthracycline use in cancer patients, given the increase of cellular damage studies are failing to find an association between CRP levels and cardiotoxicity, with one study showing that baseline levels compared to 3 months after anthracycline administration showing no significant differences in levels [
38].
Moreover, in both longer-term and shorter-term studies following anthracycline therapy, researchers fail to reveal any association between gal-3 and LVEF. Overall, research has not been able to reveal associations between these biomarkers and cardiovascular outcomes. Nonetheless, research in this area is not all negative. Increased levels of brain natriuretic peptide (BNP), for example, has been linked to lowered LVEF. Recent studies are looking into BNP as an early predictor of cardiotoxicity in patients undergoing anthracycline toxicity. In a study of cytotoxicity in patients undergoing anthracycline therapies, researchers found in all 11 patients which underwent a cardiac event in their study, all of them had at least 1 BNP value over 100 pg/mL (BNP study), however, there is need for confirmation of these data on a larger cohort of patients [
39,
40].
Collectively, results and density of research in this field is very much lacking. In efforts to reduce costs, increase access, improve predictability, and improve outcomes, further research is needed into examining potential biomarkers that may detect signs of early cardiotoxicity and/or CHF.
Challenges in Diagnosing Chf Post Antracycline Induced Cardiotoxicity
Studies have found links between anthracycline dose, chest radiation, presence of standard cardiovascular risk factors, age at initial cancer diagnosis, sex and the risk of cardiotoxicity developing into CHF. However, to date there is no established risk assessment criterion or scoring system for the development of anthracycline induced CHF. While Troponin I would be ideal for early screening post anthracycline administration, there is a need for follow-up evaluation in patients who have increased levels of Troponin I, since early detection of progression to congestive heart failure will allow for preventative measures before it becomes irreversible.
The use of a second screening tool that is predictive for CHF development would be a more practical and cost-efficient approach. In pediatric cohorts, studies have suggested that elevated cardiac troponins and natriuretic peptide levels during anthracycline exposure can be associated with the development of cardiac dysfunction in the first 3 years after treatment, but the association between these early findings and subsequent CHF is unclear [
41,
42]. Moreover, there can often be a latency period between the initial cardiotoxicity and onset of CHF, often leading to patients presenting at the hospital at the stage of clinical onset of symptoms (reduced LVEF and cardiac output).
Ideally this evaluation would be follow-up echocardiograms since it is sensitive to changes in heart contractility and LVEF. Children’s Oncology Group has developed the Long-Term Follow-Up Guidelines to screen Survivors of Childhood, Adolescent, and Young Adult Cancer, which are to: regularly monitor individuals at high risk for congestive heart failure (CHF) through echocardiography (high-risk criteria include exposure to anthracyclines of 250 mg/m2 or more, chest radiotherapy at 35 Gy or more, or a combination of lower-dose anthracyclines (≥ 100 mg/m2) and chest radiotherapy. They also recommend initiate screening within 2 years of anthracycline exposure, repeating it at least every 5 years thereafter and to consider more frequent screening based on risk level and clinical suspicion [
42,
43,
44]. Similarly, in adult cancer patients, the screening mechanism for CHF development post anthracycline toxicity is echocardiography [
45]. From a global health perspective, this type of screening requires specialist care, needing patients to be attended to by cardiologists, which is not cost efficient, specifically for those who lack access to health insurance and cannot afford the cost of echocardiography or cardiologist consultation fees. A more conducive and cost-efficient approach would be to screen for a protein marker associated with CHF that can be done in a laboratory setting.
Platelets as Dynamic Body Sensors
Platelets are dynamic biomarkers. Well known for their role in hemostasis [
46], platelets have more recently been recognized as important mediators of the inflammatory response [
47,
48]. Platelets are constantly being produced and have a 7–10-day life cycle in the circulating plasma. Numerous physiological processes can alter the number of platelets that are produced, the circulation lifetime for differentiated platelets, and the chemical signatures of platelets and their extracellular products. As such, platelets provide a window to our state of health, which we can peer through by monitoring the numbers, functions and products of platelets [
46].
Platelets are anuclear and are considered by many to be cellular fragments rather than true cells, however they maintain a discoid cellular shape and have mitochondria, RNA, and protein production machinery [
46]. Over time, platelet aging is believed to lead to a reduction of available RNAs, but their mitochondrial content is thought to remain constant, such that the ratio of RNA to mitochondria should give insights to platelet age [
49]. Platelet cellular functions are intact, including pinocytosis, so they are constantly sampling the plasma, offering protection for substances such as proteins or DNA that would normally be destroyed as naked entities in the plasma.
As platelets are constantly produced, they are also destroyed. Aged and senescent platelets lose sialic acid from their surface, exposing chemical signals that are recognized by Ashwell-Morell receptors on hepatocytes, leading to their clearance [
50]. Platelets are also activated for reasons not only related to hemostasis, but also thrombosis, inflammation and from signals released by cancerous cells. When platelets are activated appropriately or erroneously, they release markers of activation, such as CD154 [
51], P-selectin, and the triggering receptor expressed in myeloid cells (TREM) like transcript (TLT)-1 [
52,
53]. Platelet activation also leads to fragmentation and generation of platelet derived microparticles [
54,
55,
56].
As a result of these roles in sampling plasma and mediating inflammation, hemostasis, and thrombosis, and because both their production and survival are intricately tied to these processes, platelets carry signatures of health and disease. One example of platelet utility as a marker is there use in liquid biopsies and the detection of cancer. Many tumors release substances into the blood that are picked up by platelets [
57]. Moreover, many tumors express tissue factor, which initiates the coagulation cascade interaction with factor VII, and increases thrombin generation and platelet activation [
58]. Additionally, chemotherapeutic treatments can lead to platelet activation and release of platelet markers.
Platelets that have been exposed to the tumor microenvironment are considered to be educated by tumors and are called tumor-educated platelets [
59]. One example of tumors educating platelets was demonstrated by evaluating circular RNAs from individuals with gastroenteropancreatic tumors. It has been demonstrated that the platelets had significant changes in 4983 circular RNAs showing a unique tumor signature and the potential to be predictive [
60]. The interaction between the tumor microenvironment also changes platelet mRNA. mRNA sequencing of platelets from 283 patients allowed the discernment of patients with tumors that were localized, or metastasized, from those individuals that were healthy with incredible precision [
61]. Monitoring platelets in patients can help us understand cancer stages and direction of progression for a more precise treatment regimen.
Trem Like Transcript-1, a Platelet Specific Receptor, for Chf Screening
The utilization of biomarker or protein screening (that can be done in a laboratory setting) for the early detection and treatment of congestive heart failure (CHF) in leukemia patients represents a pivotal advancement in cardiovascular care. A cornerstone feature of the pathophysiological progression of congestive heart failure is platelet dysfunction [
62,
63]. Patients with congestive heart failure are at increased risk of thromboembolisms, arrythmias and sudden cardiac death. Studies dated all the way back to 1970 have demonstrated greater platelet aggregation in patients with CHF. The altered hemodynamics in CHF, including stasis of blood flow, can lead to elevated platelet activation and aggregation. Several studies have demonstrated platelets have baseline activation during CHF, as shown by increased levels of procoagulant factors (tissue factor), platelet activation markers (s-P-Sel and s-TLT-1), plasma proteins that facilitate clot formation (fibrinogen) [
64,
65,
66,
67]. These data introduce the potential for a diagnostic screening criterion, where Troponin I detection indicate that there is cardiotoxicity, prompting a screening for a platelet activation marker (which would be sensitive to CHF), as a gold standard for early detection of cardiotoxicity and progression to congestive heart failure, respectively. Early diagnosis would allow for preventative treatment with medications such as B-Blockers, which have been shown to decrease incidence of LVEF dysfunction, heart failure and death [
68].
A study by Bayrón-Marrero et al. underscores the potential of incorporating a biomarker and an early detection screening protein, specifically soluble Trem-Like Transcript-1 (s-TLT-1) into clinical practice. Trem-Like Transcript -1 is a platelet-specific receptor that is stored in the platelet alpha granules and released onto the platelet surface upon platelet activation [
69]. Several studies have demonstrated that plasma levels of s-TLT-1 is a highly sensitive marker of platelet activation, even more so that s-P-sel, the most used marker of platelet activation [70]. Bayrón-Marrero et al observed correlation between lower plasma s-TLT-1 levels and left ventricular dysfunction in patients with cardiovascular disease suggests its potential as a sensitive indicator of early cardiac impairment. Remarkably, patients with s-TLT-1 levels exceeding 544 pg/mL exhibited a significant association with congestive heart failure, emphasizing the potential use of s-TLT-1 levels above 544 pg/mL as a warning signal for progression to congestive heart failure. [
69]. Early identification of cardiac dysfunction through s-TLT-1 measurement could enable timely intervention and tailored treatment strategies, thus mitigating the risk of CHF progression in leukemia patients. Integrating such a diagnostic screening criterion into routine monitoring protocols holds promise for enhancing patient outcomes, offering a proactive approach to CHF management in this vulnerable population. Further research and validation of s-TLT-1 and other diagnostic screening methods may usher in a new era of precision medicine, transforming the landscape of CHF detection and treatment in leukemia patients.
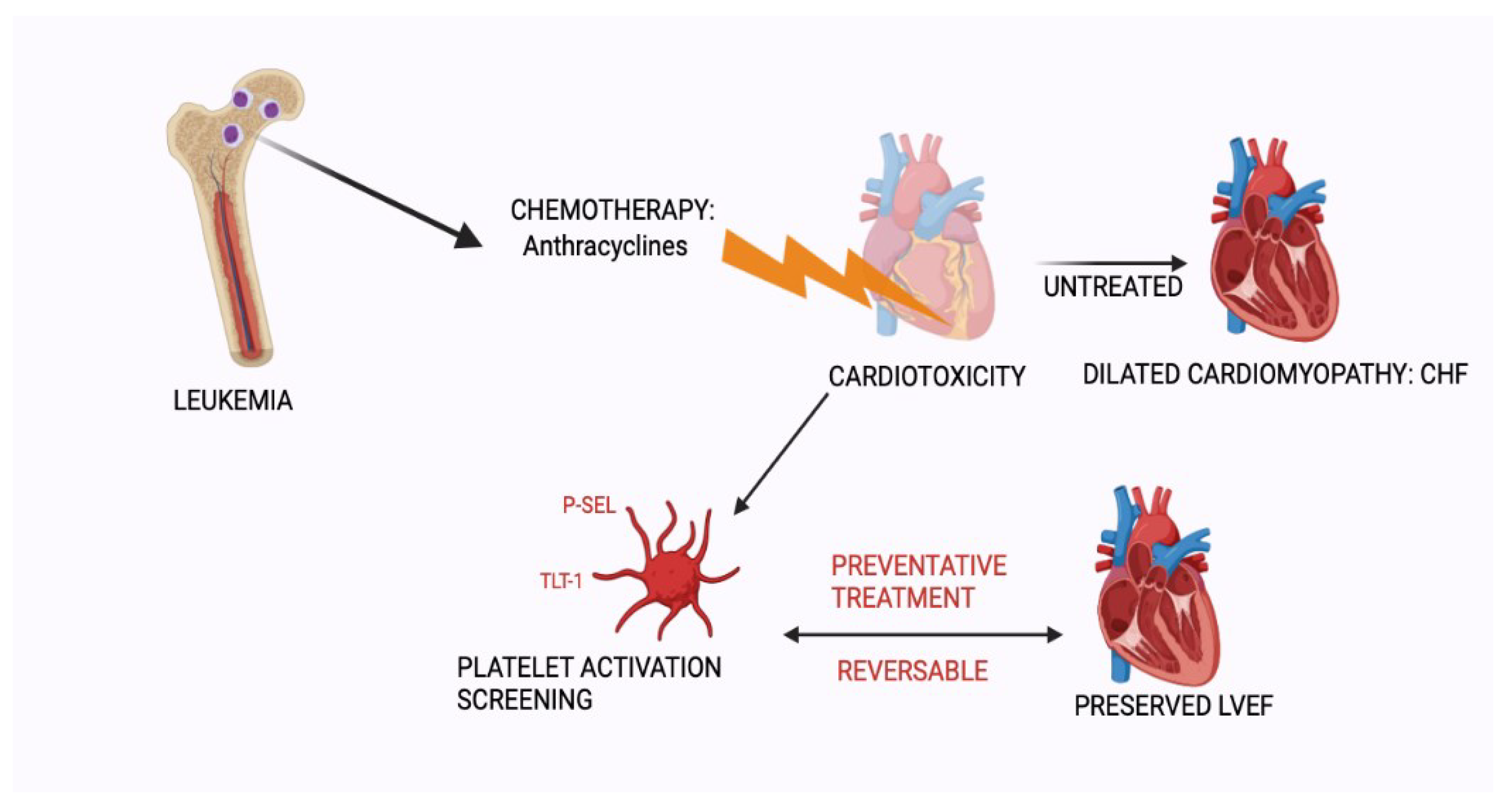
Figure 1.
Schematic representation of anthracycline induced cardiotoxicity. Early screening for cardiomyopathy would allow for preventative treatment and preservation of left ventricular function. “Created with BioRender.com”.
Figure 1.
Schematic representation of anthracycline induced cardiotoxicity. Early screening for cardiomyopathy would allow for preventative treatment and preservation of left ventricular function. “Created with BioRender.com”.
Author Contributions
Conceptualization and design of this review was done by Siobhan Branfield and A V Washington. Siobhan Branfield, Susan LaGrand, Ian Cleary, Angela Gibson and A Valance Washington contributed to the writing and editing of this review, the final draft was put together by Siobhan Branfield and A V Washington. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Institutes of Health: NHBLI HL140268.
Institutional Review Board Statement
Not applicable since all data was collected from published data for this review.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created, all data that contributed towards this study was obtained from peer reviewed articles which are referenced below.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leukemia — Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/leuks.html.
- Leukemia - Hematology.org. https://www.hematology.org/education/patients/blood-cancers/leukemia.
- Leukemia - Diagnosis and treatment - Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/leukemia/diagnosis-treatment/drc-20374378.
- Chennamadhavuni, A., Lyengar, V., Mukkamalla, S. K. R. & Shimanovsky, A. Leukemia. (2023).
- Chennamadhavuni, A., Lyengar, V., Mukkamalla, S. K. R. & Shimanovsky, A. Leukemia. (2023).
- Leukemia Diagnosis, Symptoms and Treatments | DKMS. https://www.dkms.org/learn-more/blood-cancer/leukemia#:~:text=Chronic%20lymphocytic%20leukemia%20occurs%20when,changed%20lymphocytes%20%2D%20white%20blood%20cells.
- Tiso, F.; et al. Genetic diversity within leukemia-associated immunophenotype-defined subclones in AML. Ann Hematol 2022, 101, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Alhmoud, J.F.; Mustafa, A.G.; Malki, M.I. Targeting DNA Repair Pathways in Hematological Malignancies. Int J Mol Sci 2020, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Puiggros, A.; Blanco, G.; Espinet, B. Genetic abnormalities in chronic lymphocytic leukemia: Where we are and where we go. Biomed Res Int 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Xiao, W.; Abdel-Wahab, O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood 2017, 130, 410–423. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; et al. Autoimmune cytopenias in chronic lymphocytic leukemia. Clin Dev Immunol 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Acute myeloid leukaemia - Complications - NHS. https://www.nhs.uk/conditions/acute-myeloid-leukaemia/complications/.
- Morrison, V.A. Infectious complications of chronic lymphocytic leukaemia: Pathogenesis, spectrum of infection, preventive approaches. Best Pract Res Clin Haematol 2010, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; et al. Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): A cohort study of newly diagnosed cases compared to controls. Leukemia 2013, 27, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Acute myeloid leukaemia - Complications - NHS. https://www.nhs.uk/conditions/acute-myeloid-leukaemia/complications/.
- Why People with Cancer Are More Likely to Get Infections | American Cancer Society. https://www.cancer.org/cancer/managing-cancer/side-effects/low-blood-counts/infections/why-people-with-cancer-are-at-risk.html.
- Forconi, F.; Moss, P. Perturbation of the normal immune system in patients with CLL. Blood 2015, 126, 573–581. [Google Scholar] [CrossRef] [PubMed]
- 6 Innovative Leukemia Treatment Options | MD Anderson Cancer Center. https://www.mdanderson.org/cancer-types/leukemia/leukemia-treatment.html.
- CAR T-cell Therapy and Its Side Effects | American Cancer Society. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/car-t-cell1.html.
- What is CAR T Cell Therapy? Know Before Treatment | MD Anderson Cancer Center. https://www.mdanderson.org/treatment-options/car-t-cell-therapy.html.
- FDA investigating cancer risk linked to CAR-T cell therapy | BioPharma Dive. https://www.biopharmadive.com/news/fda-car-t-cancer-risk-investigation-lymphoma/700874/#:~:text=Both%20drugmakers%20and%20the%20FDA,can%20also%20cause%20secondary%20malignancies.
- Bachur, N.R. Anthracyclines. Encyclopedia of Cancer 2002, 57–61. [Google Scholar] [CrossRef]
- Octavia, Y.; et al. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 2012, 52, 1213–1225. [Google Scholar] [CrossRef]
- Zhang, S.; et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 2012, 18, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; et al. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin 2016, 66, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Rayner, D.M.; Cutts, S.M. Anthracyclines. Side Effects of Drugs Annual 2023, 36, 683–694. [Google Scholar]
- Curigliano, G.; et al. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin 2016, 66, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Cipolla, C.M. Chemotherapy-induced cardiotoxicity: importance of early detection. Expert Rev Cardiovasc Ther 2016, 14, 1297–1299. [Google Scholar] [CrossRef]
- Dhingra, R.; Margulets, V.; Kirshenbaum, L.A. Molecular Mechanisms Underlying Anthracycline Cardiotoxicity: Challenges in Cardio-Oncology. Cardio-Oncology: Principles, Prevention and Management 2017, 25–34. [Google Scholar] [CrossRef]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front Cardiovasc Med 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Brito, D.; Vaqar, S.; Chhabra, L. Congestive Heart Failure. StatPearls (2023).
- Curigliano, G.; et al. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin 2016, 66, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Melendez, G.; Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Frontiers in Cardiovascular Medicine | www.frontiersin.org 7, 26 (2020). [CrossRef]
- Plana, J.C.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014, 15, 1063–1093. [Google Scholar] [CrossRef]
- Cardinale, D.; Biasillo, G.; Salvatici, M.; Sandri, M.T.; Cipolla, C.M. Using biomarkers to predict and to prevent cardiotoxicity of cancer therapy. Expert Rev Mol Diagn 2017, 17, 245–256. [Google Scholar] [CrossRef]
- Cardinale, D.; et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 2000, 36, 517–522. [Google Scholar] [CrossRef]
- O’Brien, P.J. Cardiac troponin is the most effective translational safety biomarker for myocardial injury in cardiotoxicity. Toxicology 2008, 245, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; et al. Early Increases in Multiple Biomarkers Predict Subsequent Cardiotoxicity in Patients With Breast Cancer Treated With Doxorubicin, Taxanes, and Trastuzumab. J Am Coll Cardiol 2014, 63, 809. [Google Scholar] [CrossRef]
- Lenihan, D.J.; et al. The Utility of Point-of-Care Biomarkers to Detect Cardiotoxicity During Anthracycline Chemotherapy: A Feasibility Study. J Card Fail 2016, 22, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.; Cunningham, J. The Role of Brain Natriuretic Peptide as a Prognostic Marker for Sepsis. Cureus 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 1997, 96, 2641–2648. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; et al. Changes in Cardiac Biomarkers During Doxorubicin Treatment of Pediatric Patients with High-Risk Acute Lymphoblastic Leukemia: Associations With Long-Term Echocardiographic Outcomes. J Clin Oncol 2012, 30, 1042–1049. [Google Scholar] [CrossRef]
- Mulder, R.L.; et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Review Lancet Oncol 2015, 16, 123–159. [Google Scholar] [CrossRef]
- Children’s Oncology Group. http://www.survivorshipguidelines.org/.
- Tan, T.C.; Scherrer-Crosbie, M. Assessing the Cardiac Toxicity of Chemotherapeutic Agents: Role of Echocardiography. Curr Cardiovasc Imaging Rep 2012, 5, 403. [Google Scholar] [CrossRef]
- Desborough, M.J.R.; Smethurst, P.A.; Estcourt, L.J.; Stanworth, S.J. Alternatives to allogeneic platelet transfusion. Br J Haematol 2016, 175, 381–392. [Google Scholar] [CrossRef]
- Ferrer-Acosta, Y.; González, M.; Fernández, M.; Valance, W.A. Emerging Roles for Platelets in Inflammation and Disease. J Infect Dis Ther 2014, 2. [Google Scholar]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the immune continuum. Nat Rev Immunol 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Supernat, A.; et al. Transcriptomic landscape of blood platelets in healthy donors. Sci Rep 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Grozovsky, R.; et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat Med 2015, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; et al. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Front Immunol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.W.; et al. TREM-like transcript 1: a more sensitive marker of platelet activation than P-selectin in humans and mice. Blood Adv 2018, 2, 2072. [Google Scholar] [CrossRef] [PubMed]
- Washington, A.V.; et al. A TREM family member, TLT-1, is found exclusively in the alpha-granules of megakaryocytes and platelets. Blood 2004, 104, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Lundström, A.; et al. Prognostic Value of Circulating Microvesicle Subpopulations in Ischemic Stroke and TIA. Transl Stroke Res 2020, 11, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Duchez, A.C.; Brisson, A. The diversity of platelet microparticles. Curr Opin Hematol 2015, 22, 437–444. [Google Scholar] [CrossRef]
- Panzer, S.; et al. Plasma levels of P-selectin are determined by platelet turn-over and the P-selectin Thr715Pro polymorphism. Thromb Res 2008, 121, 573–579. [Google Scholar] [CrossRef]
- Klement, G.L.; et al. Platelets actively sequester angiogenesis regulators. Blood 2009, 113, 2835–2842. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Mackman, N. Tissue Factor and Cancer: Regulation, Tumor Growth, and Metastasis. Semin Thromb Hemost 2019, 45, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Calverley, D.C.; et al. Significant downregulation of platelet gene expression in metastatic lung cancer. Clin Transl Sci 2010, 3, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.; Lip, G.Y.H. Platelets and heart failure. Eur Heart J 2006, 27, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.; Choudhury, A.; Lip, G.Y.H. Platelet activation in acute, decompensated congestive heart failure. Thromb Res 2007, 120, 709–713. [Google Scholar] [CrossRef]
- Stumpf, C.; et al. Enhanced levels of CD154 (CD40 ligand) on platelets in patients with chronic heart failure. Eur J Heart Fail 2003, 5, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Chin, B.S.P.; et al. Prognostic value of interleukin-6, plasma viscosity, fibrinogen, von Willebrand factor, tissue factor and vascular endothelial growth factor levels in congestive heart failure. Eur J Clin Invest 2003, 33, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, C.R.; Blann, A.D.; Watson, R.D.S.; Lip, G.Y.H. Abnormalities of hemorheological, endothelial, and platelet function in patients with chronic heart failure in sinus rhythm: Effects of angiotensin-converting enzyme inhibitor and β-blocker therapy. Circulation 2001, 103, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Varo, N.; et al. Soluble CD40L. Circulation 2003, 108, 1049–1052. [Google Scholar] [CrossRef]
- Bosch, X.; et al. Enalapril and Carvedilol for Preventing Chemotherapy-Induced Left Ventricular Systolic Dysfunction in Patients with Malignant Hemopathies: The OVERCOME Trial (prevention of left Ventricular dysfunction with Enalapril and carvedilol in patients submitted to intensive Chemo-therapy for the treatment of Malignant hemopathies). J Am Coll Cardiol 2013, 61, 2355–2362. [Google Scholar] [CrossRef]
- Branfield, S.; Washington, A.V. The enigmatic nature of the triggering receptor expressed in myeloid cells -1 (TLT- 1). Platelets 2021, 32, 753. [Google Scholar] [CrossRef] [PubMed]
- TREM-like transcript 1: a more sensitive marker of platelet activation than P-selectin in humans and mice - PMC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6113608/#:~:text=In%20conclusion%2C%20TLT%2D1%20is,shell%20of%20thrombi%20in%20vivo.
- Bayrón-Marrero, Z.; et al. The Characterization and Evaluation of the Soluble Triggering Receptor Expressed on Myeloid Cells-like Transcript-1 in Stable Coronary Artery Disease. Int J Mol Sci 2023, 24, 13632. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).