1. Opossum general characteristics and habitat
These unique marsupials, native to the Americas, exhibit a range of intriguing physical characteristics. With over 100 different species, opossums come in various sizes, from the pocket-sized pygmy opossum to the Virginia opossum, which is about the size of a domestic cat [
1,
2].
When we think of opossums, the common image that often comes to mind is that of the Southern or Black-eared opossum, also known as the Gambá. However, it is essential to note that the common opossum and the Virginia opossum are two distinct species. Common opossum
Didelphis marsupialis is found in regions such as the West Indies (including Trinidad and Tobago and the Windwards), the Guianas, Mexico, and the Amazon basin (including Bolivia, Brazil, Colombia, Ecuador, Peru, and Venezuela. Features include a cone-shaped nose with a pink tip, a long hairless tail, and fur that can be a mix of white, gray, and black [
3].
Virginia Opossum (
Didelphis virginiana): is the only marsupial naturally found in North America. Spans across the United States, Mexico, Central America, South America, and Canada. Similar cone-shaped nose and long tail, with fur variations [
3]. These adaptable creatures thrive in various habitats, although they show a preference for arboreal environments. They are often found in wet areas like marshes, swamps, and streams [
1]
Opossums are night owls, active during the dark hours while resting during the day. Although they don’t hibernate, their activity levels dip in winter. They seek refuge in burrows filled with dry leaves or shredded paper, relying on their fat reserves to stay warm. In summer, opossums cool down by licking themselves and covering their fur with saliva. As omnivores, they consume both plant-based and meat-based food. Their wild diet includes nuts, grass, fruit, insects, mice, wild birds, snakes, worms, and even chickens. In urban areas, opossums are resourceful scavengers, feasting on roadkill and garbage. Male opossums sport bifurcated penises, once thought to be for mating with the female’s nose. However, their reproductive process aligns with other mammals. After mating, the male departs, leaving the female (a jill) to give birth to up to 20 live young (joeys). These tiny joeys, akin to jelly beans in size, immediately crawl into their mother’s pouch for continued development [
1,
2,
3]
Opossums, particularly the medium-sized species within the Didelphis genus, play a crucial role in their ecosystems across the Americas. They contribute to seed dispersal and help control insect populations. However, their close proximity to human habitats exposes them to various pathogens [
4], which include:
Protozoa: Leishmania infantum, Trypanosoma cruzi, and Toxoplasma gondii.
Helminths: Ancylostoma caninum, Trichinella spiralis, Alaria marcianae, and Paragonimus spp.
Arthropods: Ticks and fleas.
These organisms can cause diseases in humans, pets, and livestock, posing a significant public health risk. The interaction between humans, domestic animals, and Didelphis spp. can lead to the spread of these zoonotic parasites and vector-borne pathogens [
4].
2. Immune system
The immune system of marsupials, including opossums, is remarkably intricate and comparable to that of eutherian mammals. In a study, researchers identified
23 key immune genes in the genome of the grey short-tailed opossum (
Monodelphis domestica), including IFN-γ, IL-2, IL-4, IL-6, IL-12, and IL-13.To pinpoint these genes, they employed gene prediction methods that incorporate techniques like BLAST, SYNTENY + BLAST, and HMMER [
5]
This discovery highlights the sophistication of marsupial immunity, challenging the notion that their immune system is primitive compared to placental mammals [
5]
Table 1 shows some information about these methods.
2.1. CD1 Protein and Its Evolution in Marsupials and Eutherians [9,10]:
CD1 is a protein found in the major histocompatibility complex (MHC) class I family. It is present in both eutherian mammals (placental mammals) and birds.
The primary role of CD1 is to present lipid antigens to T cells and natural killer (NK) T cells.
In eutherians, the CD1 gene has undergone duplication, resulting in the creation of multiple isoforms.
Researchers discovered a marsupial equivalent of CD1 in the thymus of the bandicoot species Isoodon macrourus.
Both I. macrourus and a distantly related marsupial, the opossum Monodelphis domestica, were found to have a single copy of the CD1 gene.
The opossum CD1 gene is located in a genomic region that shares conserved synteny with the chromosomal regions containing human and mouse CD1.
A phylogenetic analysis revealed that marsupial CD1 is not orthologous to the eutherian CD1 isoforms.
This suggests that the eutherian CD1 isoforms arose from gene duplication after marsupials and eutherians diverged approximately 170-180 million years ago.
In I. macrourus, the CD1 gene is actively transcribed and appears to encode a functional protein.
However, in M. domestica, no transcription of the CD1 gene was detected in any tissue, and the predicted CD1 gene sequence contains deletions that render it a pseudogene.
In summary, the evolution of CD1 in marsupials and eutherians provides insights into the diversification of immune-related genes over millions of years. The common opossum,
Didelphis marsupialis, is the only species of opossum that is found in the West Indies [
1].
The groundbreaking sequencing of the gray short-tailed opossum’s genome (
Monodelphis domestica) has provided scientists with a unique opportunity to explore the immunome of this marsupial. By analyzing its genetic makeup, researchers have pinpointed crucial immune-related genes, including chemokines, defensins, cathelicidins, and natural killer cell receptors. Interestingly, this study suggests that the complexity of mammalian immune system had already evolved significantly before the divergence of marsupials and eutherians (placental mammals) approximately 180 million years ago. It appears that the ancestral mammalian genomes likely harbored all the essential immune gene families. However, subsequent evolution on different continents, in the presence of diverse pathogens, led to lineage-specific expansions and contractions. As a result, we observe minor variations in gene numbers and compositions across different mammalian lineages. In essence, the opossum’s genome sheds light on the ancient origins and intricate adaptations of the immune system, revealing a fascinating evolutionary journey spanning millions of years [
11].
The diversity and abundance of antimicrobial peptide genes in opossums might be a result of their newborns’ survival strategy. Since opossums lack a fully developed adaptive immune system at birth, their genes have evolved to help them combat pathogens in their challenging environment [
12]. Researchers suggest that due to the genomic similarities between marsupials and eutherians (placental mammals), marsupials serve as excellent model organisms for studying developmental immunology. Their immune system architecture provides valuable insights into how immunity develops and functions [
13].
Researchers have discovered a VpreB surrogate light (SL) chain in the marsupial opossum (
Monodelphis domestica). This opossum VpreB is similar to VpreB3 found in eutherian (placental mammals) and avian species. VpreB3 associates with newly formed immunoglobulin chains in the endoplasmic reticulum but does not typically appear on the cell surface as part of the pre-B cell receptor. Interestingly, while eutherian mammals have other known SL chains like VpreB1, VpreB2, and λ5, these were not found in the opossum genome. Additionally, these SL chains have not been identified in nonmammals’ genomes. VpreB3 likely evolved independently from earlier gene duplication events that generated VpreB1 and VpreB2 in eutherians. The absence of VpreB1, VpreB2, and λ5 in marsupials suggests that the extracellular pre-B cell receptor, as defined in humans and mice, may be unique to eutherian mammals. However, the conservation of VpreB3 in marsupials and its presence in nonmammals aligns with the hypothesis that it plays a more fundamental role in B cell development across different species [
14].
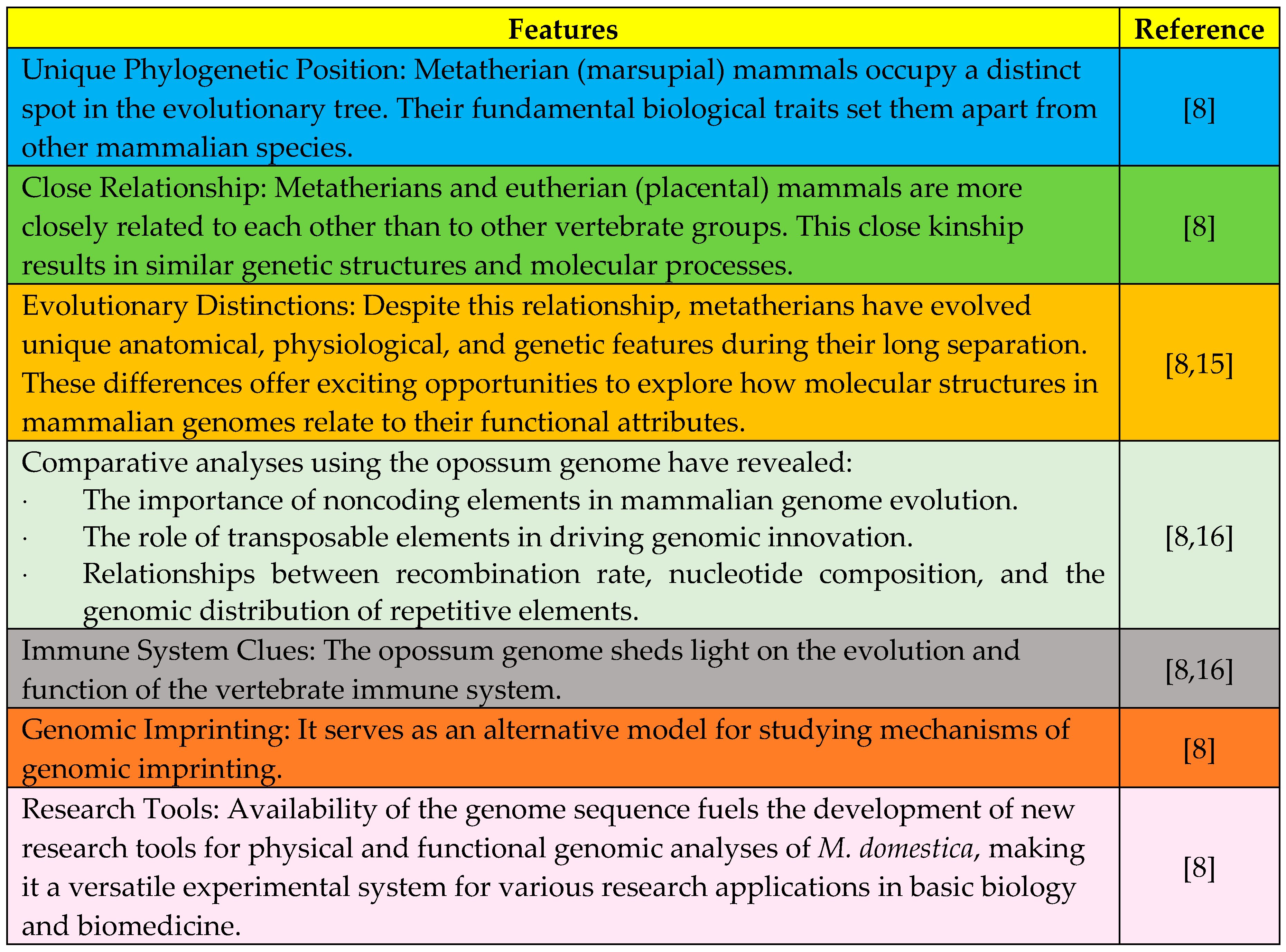
The genome sequence of the gray, short-tailed opossum (
Monodelphis domestica) holds strategic significance due to its unique phylogenetic position among metatherian (marsupial) mammals. While metatherians share a close relationship with eutherians (placental mammals), they have evolved distinct anatomical, physiological, and genetic features over millions of years. The opossum genome provides valuable insights into noncoding elements, transposable elements, immune system evolution, and serves as an alternative model for studying genomic imprinting, making it a versatile tool for research in basic biology and biomedicine [
8] as shown in
Figure 1.
The marsupial immuno-lymphatic system plays a crucial role in the day-to-day survival of marsupials. It includes lymphatic vessels and immune system tissues such as the adenoids, tonsils, lymph nodes, Peyer’s patches, thymus gland, and spleen. Additionally, there are tissues collectively referred to as the mucosa-associated lymphoid tissues (MALT) [
13]. The neonatal marsupial, being immature at birth (referred to as
altricial), undergoes organogenesis within the pouch. This distinctive feature makes pouch young an exceptional and accessible model for studying the development of the mammalian immuno-lymphatic system [
17].
In a groundbreaking study researchers have uncovered the first molecular evidence of molecular evidence of
Borrelia puertoricensis in opossums from Colombia. In 2017, 53 serum samples were collected from
Didelphis marsupialis in Colosó, Sucre, Colombia. Through real-time PCR, 18.8% of the samples tested positive for Borrelia. Subsequent next-generation sequencing confirmed the presence of
Borrelia puertoricensis. This discovery is particularly significant because it marks the first detection of this spirochete in a vertebrate host since its initial isolation from
Ornithodoros puertoricensis in Panama, underscoring the epidemiological importance of opossums as reservoirs for zoonotic diseases [
18]
Table 2.
Microorganisms that commonly infect
Didelphis marsupialis and Didelphis virginiana. Modified from [
19].
Table 2.
Microorganisms that commonly infect
Didelphis marsupialis and Didelphis virginiana. Modified from [
19].
| Microorganisms |
Didelphis marsupialis |
Didelphis virginiana |
| Helminths |
Schistosoma haematobium (Trematoda) [20,21]
Paragonimus caliensis (Trematoda) [22]
Paragominus mexicanus (Trematoda) [23] |
Spirometra mansonoides (Cestoda) [24]
Toxocara canis (Nematoda) [25]
Trichinella spiralis (Nematoda) [26]
Angiostrongylus cantonensis (Nematoda) [27,28]
Angiostrongylus costaricensis (Nematoda) [29]
Paragonimus kellicotti (Trematoda) [22]
Paragominus mexicanus (Trematoda) [22,23]
Alaria marcianae (Trematoda) [30]
|
| Protozoa |
Leishmania amazonensis [31]
Leishmania guyanensis [31]
Leishmania mexicana [31]
Leishmania panamensis [31]
Leishmania braziliensis [32]
Leishmania infantum [33]
Toxoplasma gondii [34]
Trypanosoma cruzi [35] |
Toxoplasma gondii [36]
Trypanosoma cruzi [37,38,39] |
Case study Presentation
In the verdant outskirts of the University of the West Indies, near the quaint locale known as the French Village, a male West Indian opossum (Didelphis marsupialis) was discovered. This particular opossum, estimated to be between 2 to 3 years old, was living freely in its natural habitat. The creature was gently captured and brought to a laboratory for a non-invasive procedure. A small sample of blood, approximately 5 milliliters, was carefully drawn from its tail. This procedure was carried out with the utmost care to ensure the opossum’s well-being, and it was subsequently released back into the wild, unharmed.
The collected blood sample underwent a series of comprehensive tests. These included a white blood cell (WBC) count, which is a common procedure to assess the overall health and immune status of an animal. Additionally, a blood chemistry test was performed to evaluate the opossum’s metabolic health, providing insights into the functioning of its vital organs. Furthermore, a microbiological culture was grown from the faeces swab sample. This allowed for the identification of any bacteria present in the opossum’s gut. An antibiotic sensitivity test was also conducted on the cultured bacteria. This test is crucial in determining the most effective antibiotic treatment, should the opossum require any medical intervention in the future.
This meticulous examination of the West Indian opossum (Didelphis marsupialis) not only contributes to our understanding of this specific individual’s health but also adds to the broader knowledge of the species’ physiology and adaptability in the wild. It underscores the importance of co-existing with wildlife and the value of ongoing research in preserving and protecting these fascinating creatures in their natural habitats. The laboratory results for the West Indian opossum (Didelphis marsupialis) revealed several abnormalities in its blood profile. The white blood cell (WBC) count was lower than normal, with a count of 3.20 x 109/L. Segmented neutrophils (segs) were also decreased, with a count of 0.93 x 109/L. Lymphocytes were found to be under the normal limit, at 2.11 x 109/L. Eosinophils and monocytes were recorded at 0.06 x 109/L and 0.09 x 109/L respectively.
The red blood cells (RBCs) were not counted, but the blood smear showed the presence of rouleaux formation (stacking of RBCs, indicated as 1+), poikilocytosis (abnormal RBC shapes, indicated as 1+), and a few polychromatophilic cells (immature RBCs). The reticulocyte count (young RBCs) was at 1.3%, suggesting some level of RBC production in response to anemia. The body’s production of reticulocytes in response to anemia suggests that it is trying to compensate for the reduced number of mature RBCs. The hematocrit (Hct), which measures the volume percentage of RBCs in blood, was decreased at 0.20 L/L. This is a clear indication of anemia, which could be due to various reasons including nutritional deficiencies, blood loss, or an underlying disease condition. These findings suggest that the opossum may be experiencing some health issues that could be affecting its immune system and blood health. It is important to note that these results should be interpreted in the context of the animal’s overall health status, environmental factors, and species-specific reference ranges [
40]
The results indicate several conditions in the opossum:
Hypokalemia: This refers to low levels of potassium in the blood [
5]. In humans, symptoms of hypokalemia are usually reversible after the correction of the condition. It can lead to cardiac arrhythmias, particularly in individuals with underlying heart disease [
5]. However, specific information about hypokalemia in opossums is not readily available.
Hypoglycemia: This is a condition characterized by abnormally low blood sugar levels. It’s often associated with malnutrition or metabolic disorders. In severe cases, it can lead to weakness, seizures, and even loss of consciousness.
Elevated CK (Creatine Kinase): High levels of CK usually indicate muscle damage. It could be due to physical trauma, inflammation, or diseases like rhabdomyolysis [
41]. CK levels increase quickly after muscle injury and return to normal within 24-48 hours.
High levels of albumin and globulins in an opossum could indicate various health conditions.
Discussion
The laboratory results for the West Indian opossum (
Didelphis marsupialis) revealed that its plasma protein levels were elevated at 97g/L, which could be indicative of an inflammatory response, dehydration, or other conditions [
42]. Hemolysis was also observed, which is the breakdown of red blood cells and can be caused by various genetic disorders. Furthermore, the platelet count was found to be decreased, which could be due to inherited thrombocytopenia caused by mutations in genes such as MYH9 [
43]. It is important to note that these results should be interpreted in the context of the animal’s overall health status. Please note that while these genes are known to be involved in these conditions in humans and some other animals, their role in opossums specifically would need further research. It is also possible that other genes not mentioned here could be involved. Cytokines are a large, diverse family of small proteins or glycoproteins that play a crucial role in cell signaling, particularly within the immune system. They are produced by a variety of cells, including helper T cells and macrophages. The effects of cytokines are manifested in changes in gene transcription and protein expression [
44].
Several genes are known to be involved in the pathology of cytokines. For instance, the TNF alpha gene plays a significant role due to the activation of NF-kappaB signaling pathways, which are pro-inflammatory, and facilitate apoptosis and other forms of cell death [
45]. Interferons (IFNs) are a multigene family of inducible cytokines with antiviral, antiproliferative, and immunomodulatory function. The IFN genes are involved in the production of these cytokines [
44].
Pathogenesis-related (PR) proteins are produced in plants in response to a pathogen attack. Infections activate genes that produce PR proteins [
46]. In terms of protein pathology, the TIMELESS and CDK1 genes have been associated with melanoma and pancreatic cancer, respectively [
47]. Please note that while these genes are known to be involved in these conditions, their role in opossums specifically would need further research. It is also possible that other genes not mentioned here could be involved.
It is important to note that the pathology of proteins and cytokines is a complex field, and the genes mentioned above are just a few examples. Many other genes could potentially be involved, and the exact mechanisms can vary depending on the specific condition and individual. Further research is needed to fully understand these processes and their implications for health and disease [
44].
Certainly, let us delve deeper into the health status of the West Indian opossum (Didelphis marsupialis) based on the
Proteus mirabilis and
Escherichia coli laboratory results and microbial tests. The presence of
Proteus mirabilis and
Escherichia coli bacteria in the opossum’s system is noteworthy. Both are common bacteria that can be found in various environments, including the gastrointestinal tract of animals, where they were found. However, their presence in the gut and the presence of immunodeficiency could indicate a systemic infection; however, blood culture was not performed and indeed we cannot assure it. The resistance of these bacteria to multiple antibiotics is a concern. Antibiotic resistance can complicate treatment efforts and is a growing problem in both human and veterinary medicine. The resistance of
Proteus mirabilis to doxycycline and tetracycline, and
Escherichia coli’s resistance to ampicillin, cephalexin, doxycycline, and tetracycline, limits the options for effective antibiotic therapy [
48]
The opossum’s immunodeficiency disorder affecting both T and B cells is another significant finding. T cells are responsible for cell-mediated immunity, while B cells produce antibodies for humoral immunity. A deficiency in both these cells suggests a severe compromise of the opossum’s immune system, making it vulnerable to infections and diseases. The absence of viral and fungal infections, as indicated by the lack of viral studies and the absence of fungal growth, is a positive sign. However, it doesn’t rule out the possibility of other types of infections or health issues. Immunodeficiency diseases, including those affecting T and B cells, are typically characterized by a predisposition to infections. They can be primary (congenital) or secondary (acquired). Primary immunodeficiencies usually develop in very young animals and are often the result of genetic mutations. Secondary immunodeficiencies, on the other hand, tend to occur in adult animals and can result from various factors such as viral infections, malnutrition, stress, old age, or toxins. In the context of marsupials or other small mammals, specific information about T and B cell immunodeficiencies is limited. However, It is important to note that many of the principles of immunodeficiency diseases in animals would apply. For instance, defects in the innate and antibody-mediated immune systems often result in uncontrollable bacterial infections, whereas defects in the cell-mediated immune system (which includes T cells) tend to result in overwhelming viral and fungal infections [
49]
The elevated plasma protein levels could be indicative of an inflammatory response, dehydration, or other conditions. Hemolysis, or the breakdown of red blood cells, was also observed, which can be caused by various genetic disorders. The decreased platelet count could be due to inherited thrombocytopenia caused by mutations in certain genes. These findings underscore the need for further diagnostic tests and veterinary consultation to determine the exact cause of these abnormalities and devise an appropriate treatment plan. A member of the species Didelphis marsupialis was unable to receive treatment at the veterinary hospital due to the lack of facilities for treating such small animals, unlike domestic animals like dogs and cats. However, in the future, conditions could be established to medically assist marsupials. Additionally, these animals are carriers of zoonosis diseases, so caution should be exercised when admitting such animals to hospitals.
In conclusion, while the laboratory results provide valuable insights into the health status of the West Indian opossum species, they also raise several questions. Further studies, including more comprehensive diagnostic tests and veterinary consultations, would be necessary to fully understand the health issues affecting this opossum and to devise an appropriate treatment plan. This case underscores the complexity of wildlife health and the importance of ongoing research in this field.
Author Contributions
The manuscript was conceptualized by A.J.-V. and planning and discussion were conducted by all authors. A.J.-V. wrote the initial draft of the manuscript. A.J.-V. and D.G. reviewed the manuscript. All authors investigated. All authors have read and agreed to the published version of the manuscript.
Funding
This study did not receive any external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bradford, A. Facts About the Common Opossum. Available online: https://www.livescience.com/56182-opossum-facts.html (accessed on 4 February 2024).
- SciELO Books. Available online: https://books.scielo.org/id/sfwtj/24 (accessed on 4 February 2024).
- Gardner, A.L. Mammals of South America, Volume 1: Marsupials, Xenarthrans, Shrews, and Bats; Gardner, A.L., Ed.; University of Chicago Press: Chicago, IL, 2007; ISBN 9786611956844. [Google Scholar]
- Carreira, J.C.A.; da Silva, A.V.M.; de Pita Pereira, D.; Brazil, R.P. Natural Infection of Didelphis Aurita (Mammalia: Marsupialia) with Leishmania Infantum in Brazil. Parasit. Vectors 2012, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.S.; Young, L.J.; Papenfuss, A.T.; Belov, K. In Silico Identification of Opossum Cytokine Genes Suggests the Complexity of the Marsupial Immune System Rivals that of Eutherian Mammals. Immunome Res. 2006, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Irimia, M. Irimia, M., Tena, J.J., Alexis, M.S., et al.} Conservation of Retinoic Acid Signaling in Vertebrate Lineage. In Proc. Natl. Acad. Sci. U.S.A; 2013.
- Samollow, P.B. The Opossum Genome: Insights and Opportunities from an Alternative Mammal. Genome Res. 2008, 18, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.L.; Miller, R.D. Evolution of Mammalian CD1: Marsupial CD1 Is Not Orthologous to the Eutherian Isoforms and Is a Pseudogene in the Opossum Monodelphis Domestica. Immunology 2007, 121, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Zuviría, B.; Mínguez-Toral, M.; Díaz-Perales, A.; Garrido-Arandia, M.; Pacios, L.F. Structural Dynamics of the Lipid Antigen-Binding Site of CD1d Protein. Biomolecules 2020, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Belov, K.; Sanderson, C.E.; Deakin, J.E.; Wong, E.S.W.; Assange, D.; McColl, K.A.; Gout, A.; de Bono, B.; Barrow, A.D.; Speed, T.P.; et al. Characterization of the Opossum Immune Genome Provides Insights into the Evolution of the Mammalian Immune System. Genome Res. 2007, 17, 982–991. [Google Scholar] [CrossRef]
- Wong, E.S.W.; Papenfuss, A.T.; Belov, K. Immunome Database for Marsupials and Monotremes. BMC Immunol. 2011, 12, 48. [Google Scholar] [CrossRef]
- Cisternas, P.; Armati, P.J. The Immunolymphatic System. In Marsupials; Cambridge University Press, 2006; pp. 186–198. [Google Scholar]
- Wang, X.; Parra, Z.E.; Miller, R.D. A VpreB3 Homologue in a Marsupial, the Gray Short-Tailed Opossum, Monodelphis Domestica. Immunogenetics 2012, 64, 647–652. [Google Scholar] [CrossRef]
- Franchini, L.F.; Pollard, K.S. Human Evolution: The Non-Coding Revolution. BMC Biol. 2017, 15, 89. [Google Scholar] [CrossRef]
- Klein, S.J.; O’Neill, R.J. Transposable Elements: Genome Innovation, Chromosome Diversity, and Centromere Conflict. Chromosome Res. 2018, 26, 5–23. [Google Scholar] [CrossRef]
- Deane, E.M.; Cooper, D.W. Immunological Development of Pouch Young Marsupials. In The Developing Marsupial: Models for Biomedical Research; Tyndale-Biscoe, C.H., Janssens, P.A., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 1988; pp. 190–199. ISBN 9783642884023. [Google Scholar]
- López, Y.; Faccini-Martínez, Á.A.; Muñoz-Leal, S.; Contreras, V.; Calderón, A.; Rivero, R.; Muñoz, M.; Ramírez, J.D.; Mattar, S. Borrelia Puertoricensis in Opossums (Didelphis Marsupialis) from Colombia. Parasit. Vectors 2023, 16, 448. [Google Scholar] [CrossRef]
- Bezerra-Santos, M.A.; Ramos, R.A.N.; Campos, A.K.; Dantas-Torres, F.; Otranto, D. Didelphis Spp. Opossums and Their Parasites in the Americas: A One Health Perspective. Parasitol. Res. 2021, 120, 4091–4111. [Google Scholar] [CrossRef]
- Kuntz, R.E.; Myers, B.J.; Cheever, A.W. Schistosoma Haematobium Infection in the Opossum (Didelphis Marsupialis): Involvement of the Urogenital System. Bull. World Health Organ. 1971, 45, 21–25. [Google Scholar] [PubMed]
- Kuntz, R.E.; Myers, B.J.; Moore, J.A.; Huang, T.C. Parasitological Aspects of Schistosoma Haemotobium (Iran) Infection in the American Opossum (Didelphis Marsupialis L.). Int. J. Parasitol. 1975, 5, 21–26. [Google Scholar] [CrossRef]
- Blair, D.; Xu, Z.B.; Agatsuma, T. Paragonimiasis and the Genus Paragonimus. Adv. Parasitol. 1999, 42, 113–222. [Google Scholar] [PubMed]
- López-Caballero, J.; Oceguera-Figueroa, A.; León-Règagnon, V. Detection of Multiple Species of Human Paragonimus from Mexico Using Morphological Data and Molecular Barcodes. Mol. Ecol. Resour. 2013, 13, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Corkum, K.C. Sparganosis in Some Vertebrates of Louisiana and Observations on a Human Infection. J. Parasitol. 1966, 52, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, E.M.; Kirkland, G.L. THE BIOLOGY OF THE OPOSSUM, DIDELPHIS VIRGINIANA IN SOUTHCENTRAL PENNSYLVANIA. Proceedings of the Pennsylvania Academy of Science 1976, 50, 81–85. [Google Scholar]
- Leiby, D.A.; Schad, G.A.; Duffy, C.H.; Murrell, K.D. Trichinella Spiralis in an Agricultural Ecosystem. III. Epidemiological Investigations of Trichinella Spiralis in Resident Wild and Feral Animals. J. Wildl. Dis. 1988, 24, 606–609. [Google Scholar] [CrossRef]
- Kim, D.Y.; Stewart, T.B.; Bauer, R.W.; Mitchell, M. Parastrongylus (=Angiostrongylus) Cantonensis Now Endemic in Louisiana Wildlife. J. Parasitol. 2002, 88, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Dalton, M.F.; Fenton, H.; Cleveland, C.A.; Elsmo, E.J.; Yabsley, M.J. Eosinophilic Meningoencephalitis Associated with Rat Lungworm (Angiostrongylus Cantonensis) Migration in Two Nine-Banded Armadillos (Dasypus Novemcinctus) and an Opossum (Didelphis Virginiana) in the Southeastern United States. Int. J. Parasitol. Parasites Wildl. 2017, 6, 131–134. [Google Scholar] [CrossRef]
- Miller, C.L.; Kinsella, J.M.; Garner, M.M.; Evans, S.; Gullett, P.A.; Schmidt, R.E. Endemic Infections of Parastrongylus (=Angiostrongylus) Costaricensis in Two Species of Nonhuman Primates, Raccoons, and an Opossum from Miami, Florida. J. Parasitol. 2006, 92, 406–408. [Google Scholar] [CrossRef]
- Shoop, W.L.; Corkum, K.C. Epidemiology of Alaria Marcianae Mesocercariae in Louisiana. J. Parasitol. 1981, 67, 928–931. [Google Scholar] [CrossRef]
- Bruschi, F.; Gradoni, L. The Leishmaniases: Old Neglected Tropical Diseases; Springer, 2018; ISBN 9783319723860. [Google Scholar]
- Schallig, H.D.F.H.; da Silva, E.S.; van der Meide, W.F.; Schoone, G.J.; Gontijo, C.M.F. Didelphis Marsupialis (common Opossum): A Potential Reservoir Host for Zoonotic Leishmaniasis in the Metropolitan Region of Belo Horizonte (Minas Gerais, Brazil). Vector Borne Zoonotic Dis. 2007, 7, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Travi, B.L.; Jaramillo, C.; Montoya, J.; Segura, I.; Zea, A.; Goncalves, A.; Velez, I.D. Didelphis Marsupialis, an Important Reservoir of Trypanosoma (Schizotrypanum) Cruzi and Leishmania (Leishmania) Chagasi in Colombia. Am. J. Trop. Med. Hyg. 1994, 50, 557–565. [Google Scholar] [CrossRef]
- Yai, L.E.O.; Cañon-Franco, W.A.; Geraldi, V.C.; Summa, M.E.L.; Camargo, M.C.G.O.; Dubey, J.P.; Gennari, S.M. Seroprevalence of Neospora Caninum and Toxoplasma Gondii Antibodies in the South American Opossum (Didelphis Marsupialis) from the City of São Paulo, Brazil. J. Parasitol. 2003, 89, 870–871. [Google Scholar] [CrossRef] [PubMed]
- Galaviz-Silva, L.; Mercado-Hernández, R.; Zárate-Ramos, J.J.; Molina-Garza, Z.J. Prevalence of Trypanosoma Cruzi Infection in Dogs and Small Mammals in Nuevo León, Mexico. Rev. Argent. Microbiol. 2017, 49, 216–223. [Google Scholar] [CrossRef]
- Torres-Castro, M.; Noh-Pech, H.; Puerto-Hernández, R.; Reyes-Hernández, B.; Panti-May, A.; Hernández-Betancourt, S.; Yeh-Gorocica, A.; González-Herrera, L.; Zavala-Castro, J.; Puerto, F.I. First Molecular Evidence of Toxoplasma Gondii in Opossums (Didelphis Virginiana) from Yucatan, Mexico. Open Vet J 2016, 6, 57–61. [Google Scholar] [CrossRef]
- Parada-López, J.; Hernández-Betancourt, S.F.; Ruiz-Piña, H.A.; Escobedo-Ortegón, F.J.; Medina-Peralta, S.; Panti-May, J.A. Trypanosoma Cruzi Infection in Didelphis Virginiana in Relation to Population Parameters and Variables Associated with Presence in Rural Community Dwellings in Yucatan, Mexico. Ecohealth 2013, 10, 31–35. [Google Scholar] [CrossRef]
- Cantillo-Barraza, O.; Garcés, E.; Gómez-Palacio, A.; Cortés, L.A.; Pereira, A.; Marcet, P.L.; Jansen, A.M.; Triana-Chávez, O. Eco-Epidemiological Study of an Endemic Chagas Disease Region in Northern Colombia Reveals the Importance of Triatoma Maculata (Hemiptera: Reduviidae), Dogs and Didelphis Marsupialis in Trypanosoma Cruzi Maintenance. Parasit. Vectors 2015, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pina, H.A.; Cruz-Reyes, A. The Opossum Didelphis Virginiana as a Synanthropic Reservoir of Trypanosoma Cruzi in Dzidzilché, Yucatán, México. Mem. Inst. Oswaldo Cruz 2002, 97, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Opossum (Didelphis Virginiana) Blood Normals. Available online: https://opossumsocietyus.org/opossum-blood-normals/ (accessed on 4 February 2024).
- Lehmann, J.; Stadler, P.F.; Prohaska, S.J. SynBlast: Assisting the Analysis of Conserved Synteny Information. BMC Bioinformatics 2008, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, N. Inherited Hemolytic Anemia: A Possessive Beginner’s Guide. Hematology Am. Soc. Hematol. Educ. Program 2018, 2018, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Carly Werner, R.D. Hereditary Thrombocytopenia: Common Types and FAQs Available online:. Available online: https://www.medicalnewstoday.com/articles/hereditary-thrombocytopenia (accessed on 4 February 2024).
- Justiz Vaillant, A.A.; Qurie, A. Interleukin; StatPearls Publishing, 2022. [Google Scholar]
- Lange, A.; Lange, J.; Jaskuła, E. Cytokine Overproduction and Immune System Dysregulation in alloHSCT and COVID-19 Patients. Front. Immunol. 2021, 12, 658896. [Google Scholar] [CrossRef]
- Jain, D.; Khurana, J.P. Role of Pathogenesis-Related (PR) Proteins in Plant Defense Mechanism. In Molecular Aspects of Plant-Pathogen Interaction; Singh, A., Singh, I.K., Eds.; Springer Singapore: Singapore, 2018; pp. 265–281. ISBN 9789811073717. [Google Scholar]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A Pathology Atlas of the Human Cancer Transcriptome. Science 2017, 357. [Google Scholar] [CrossRef]
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. Link Between Antibiotic Persistence and Antibiotic Resistance in Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2022, 12, 900848. [Google Scholar] [CrossRef]
- Merck; Co.; Inc. Staff The Merck Veterinary Manual; Merck, Incorporated, 2003; ISBN 9780911910803.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).