1. Introduction
At present, the global population is expected to increase to nine billion by 2050 [
1]. It is generally believed that in the foreseeable future, agricultural production must be greatly increased to accommodate population growth and animal farming production for food and feed, and food security will become a key issue to be solved urgently. Insecticides are one of the most effective controls used in protecting plants against insects, animals and weeds in farming [
2,
3]. Pesticides, however, are an environmental exogenous organism that can enter the environment by leave spraying, seed sowing, fumigation or direct dressing, causing the accumulation of residual persistent pesticides, which can adversely affect human health and ecosystems [
4]. Soil can act as a ' reservoir ' and ' distribution center ' of pesticides in the environment, which may hold about 50% of the persistent pesticides [
5]. Although the residual pesticides in soil can be gradually dissipated through absorption by crops, evaporating and degrading, the rates are normally slower than the agricultural cycle[
6]. In addition, soil pesticides can also form bound residues, which can be absorbed by crop roots or gradually leached into groundwater. Since the pesticides can be transferred to crops while many areas use groundwater as drinking water source, the contamination of pesticides is threatening the soil ecological balance and human long term health [
7,
8].
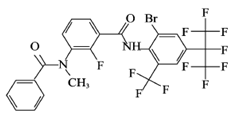
[3-benzamido-N-(4-(perfluoropropan-2-yl) phenyl) benzamide], known as Broflanilide (
Table 1), is a new class of organic halide pesticide containing diamide. It was jointly invented by Mitsui Chemical Agricultural Co., Ltd. and BASF.
Broflanilide shows potent insecticidal properties against various pests including beetles, termites, caterpillars, and other common insects found on cereals, leafy vegetables, and perennial plants [
9]. Research indicates that it produces desmethyl-broflanilide through metabolism. Broflanilide acts as a noncompetitive antagonist against the resistant-to-dieldrin γ-aminobutyric acid receptor. Interestingly, desmethyl-broflanilide has a different binding mechanism from the traditional noncompetitive antagonists, such as fipronil [
10]. Broflanilide formulations have received approval for use in several countries including Japan (2018), Canada (2020), Australia (2020), China (2020), and the United States (2021) [
11]. Consequently, broflanilide is poised to play a significant role in pest management, particularly against pests resistant to conventional noncompetitive antagonists [
10].
Pesticide multi-residue analysis methods include sample preparation, pesticide extraction and separation followed by quantitative analysis. The QuEChERS method was first proposed at the European Pesticide Residues Symposium (EPRW) in Rome in 2002. Anastassiades et al. published this method in 2003 [
12], which has since become widely adopted as a sample preparation technique in the analysis of pesticide residues. The QuEChERS method is suitable for a variety of pesticides, reduces the use of organic solvents and sample volume, and has a higher recovery rate[
13]. In instrumental analysis, UHPLC-QTOF-MS is recognized as an emerging technology for analyzing pesticide residues in food, mainly due to its precise mass measurement capabilities. It offers a balanced combination of high resolution, precision, sensitive full scan capabilities, and comprehensive mass spectrometry data. Thus, QTOF complements other mass spectrometers like quadrupole and ion trap instruments for both identification and quantification [
14,
15,
16]. In this study, we utilized ultra-high performance liquid chromatography-time of flight-mass spectrometry (UHPLC-QTOF-MS) in conjunction with a modified QuEChERS method to enhance the response and recovery of each analyte. We optimized the conditions and parameters of UHPLC-QTOF-MS and compared various solvents and adsorbents. Consequently, our research established an efficient and reliable method for detecting broflanilide residues in soil samples, confirming its potential for the analysis of broflanilide residues in soil.
2. Results and discussion
2.1. Optimization of separation conditions of UHPLC-QTOF-MS
2.1.1. Chromatographic column optimization
The choice of chromatographic column is critical in inferencing the retention behavior and peak shape of analytes [
17]. Specifically, selecting an appropriate chromatographic column can enhance the stability and efficiency of the analysis. In the determination of pesticide compounds, the most commonly used chromatographic column is octadecyl bonded C18 column[
18]. To reduce the running time and achieve optimal peak shapes, we employed two distinct C18 columns for optimizing the UHPLC conditions: the Zorbax Eclipse XDB-C18 column (4.6mm × 150mm × 5μm) and the Zorbax SB-C18 column (2.1mm × 100mm × 1.8μm). With identical mobile phases, the retention time of broflanilide on the XDB-C18 column is longer, suggesting a superior separation between broflanilide and impurity peaks. Consequently, the XDB-C18 column was selected for further study.
2.1.2. Mobile phase optimization
To enhance the performance of UHPLC, the impact of flow rate and mobile phase on broflanilide separation was investigated. The compared mobile phases are methanol-water, acetonitrile-water (with or without 0.1% formic acid). The acetonitrile-water phases produced faster separation with narrower peaks compared to the methanol-water phase. This is likely because acetonitrile exhibits stronger solvation tendencies towards solutes than methanol [
19,
20,
21], and acetonitrile-water mixtures demonstrate higher solvent permeability [
22], effectively removing impurities in the column.
Adding formic acid (0.1%) optimized peak shapes, thereby enhancing the sensitivity [
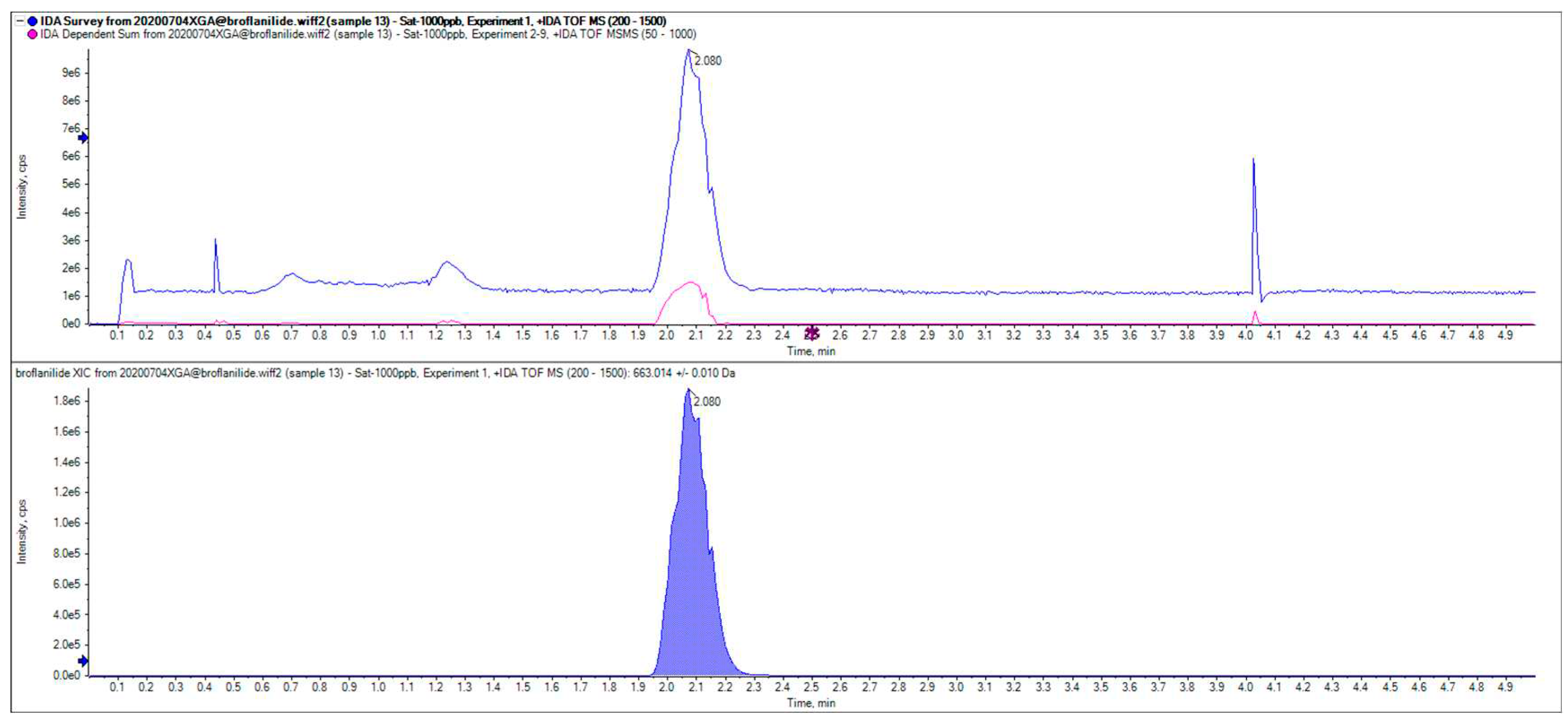
23]. However, adding formic acid (0.1%) in acetonitrile-water led to prolonged retention times due to the low acetonitrile ratio, delaying the detection of subsequent samples. Conversely, a high acetonitrile proportion resulted in shorter retention times, introducing impurity peak interference. When using acetonitrile-water with formic acid (0.1% ) at a 75:25 volume ratio at flow rate of 0.5 mL/min, clear separation was achieved between the broflanilide and the impurity peaks. Here, a symmetric peak was obtained together with a stable baseline, and a shortened retention time of 2.08 minutes. Hence, this mobile phase offers the optimal composition for analysis of broflanilide in HPLC.
Figure 1 depicts the UHPLC chromatogram of the standard broflanilide sample.
2.2. QuEChERS method optimization
2.2.1. Solvents optimization
The most time-consuming and intricate process in residual pesticide analysis involves the pretreatment of raw samples. The choice of solvent for extraction is dictated by the chemical behavior of the insecticides. Commonly employed solvents include acetone [
24], acetonitrile [
25], methanol [
26], ethyl acetate, and dichloromethane [
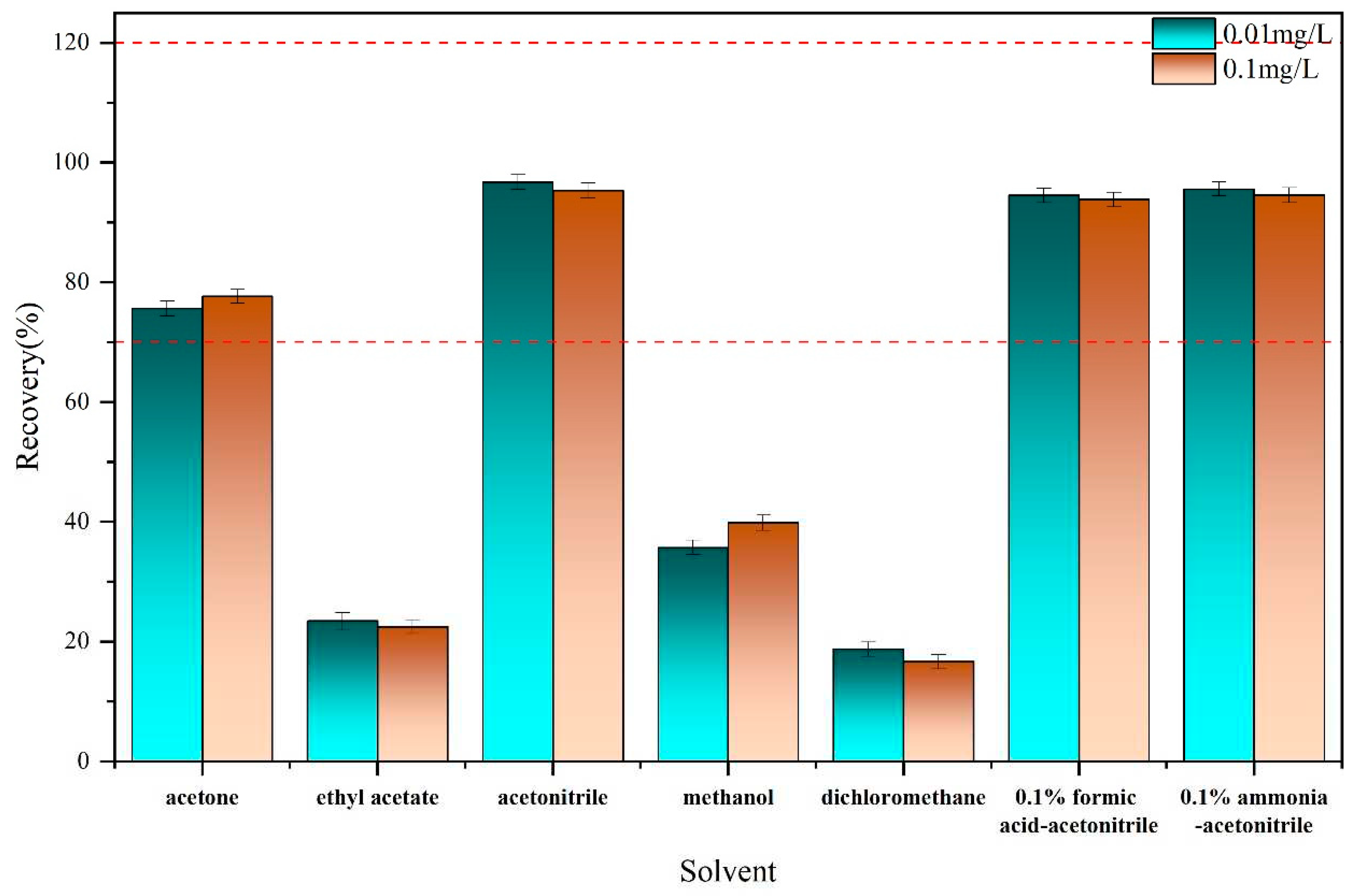
27]. Here, the recovery rates for the paddy soil samples using these solvents were compared. As shown in
Figure 2, it was found that the recovery rate of acetonitrile was the highest, reaching 96.73% at a spiked concentration of 0.01 mg/L, and 95.34% at a spiked concentration of 0.1 mg/L. In addition, compared with other solvents, acetonitrile offers several advantages in pesticide residue analysis. It is known for generating minimal interference from lipids and proteins, exhibiting high compatibility with UHPLC systems, and extracting fewer co-extracted matrix components [
28].
In order to determine whether formic acid and sodium hydroxide have an optimization effect on the solvent, formic acid and NaOH were mixed into acetonitrile at 0.1 % Vol, as shown in
Figure 2. Upon the addition of acid or alkali to acetonitrile, the recovery rates reached 94.53% and 95.60% at 0.01 mg/L spiked concentration, and 93.81% and 94.56% at a 0.1 mg/L spiked concentration, respectively. However, no optimization effect was observed for the broflanilide recovery rate in paddy soil. Consequently, unmodified acetonitrile was chosen for extraction.
2.2.2. Adsorbent optimization
Satisfactory extraction of broflanilide diamide was observed for the C18, GCB, and PSA adsorbents using the QuEChERS method. As an anion exchanger, PSA extracts carbohydrates and acids from the mixed sample [
29]. C18, on the other hand, is used to extract non-polar and medium-polar compounds since it is a non-polar material. GCB is an effective adsorbent for absorbing pigments, such as carotenoids and chlorophyll [
30]. Adding MgSO
4 and NaCl can improve partition and generate a better phase separation with high recovery rates [
31]. In this study, 50 mg of each specific adsorbent (0.1 mg/kg) was used for broflanilide extraction with the addition of 150 mg MgSO
4. As indicated in
Table 2, the average recovery rates of various adsorbents in rice soil ranged from 89.85% to 93.81%, satisfying the criteria of the Chinese national agricultural standard between 70%-120% [
32]. Notably, PSA demonstrated the highest recovery rate, reaching 93.81%.
Briefly, the appropriate broflanilide extraction and purification conditions via the QuEChERS procedure are identified to be 50 mg of PSA combined with 150 mg of MgSO4.
2.3. Verification of the method
2.3.1. Linearity, specificity, LOD, LOQ and matrix effects.
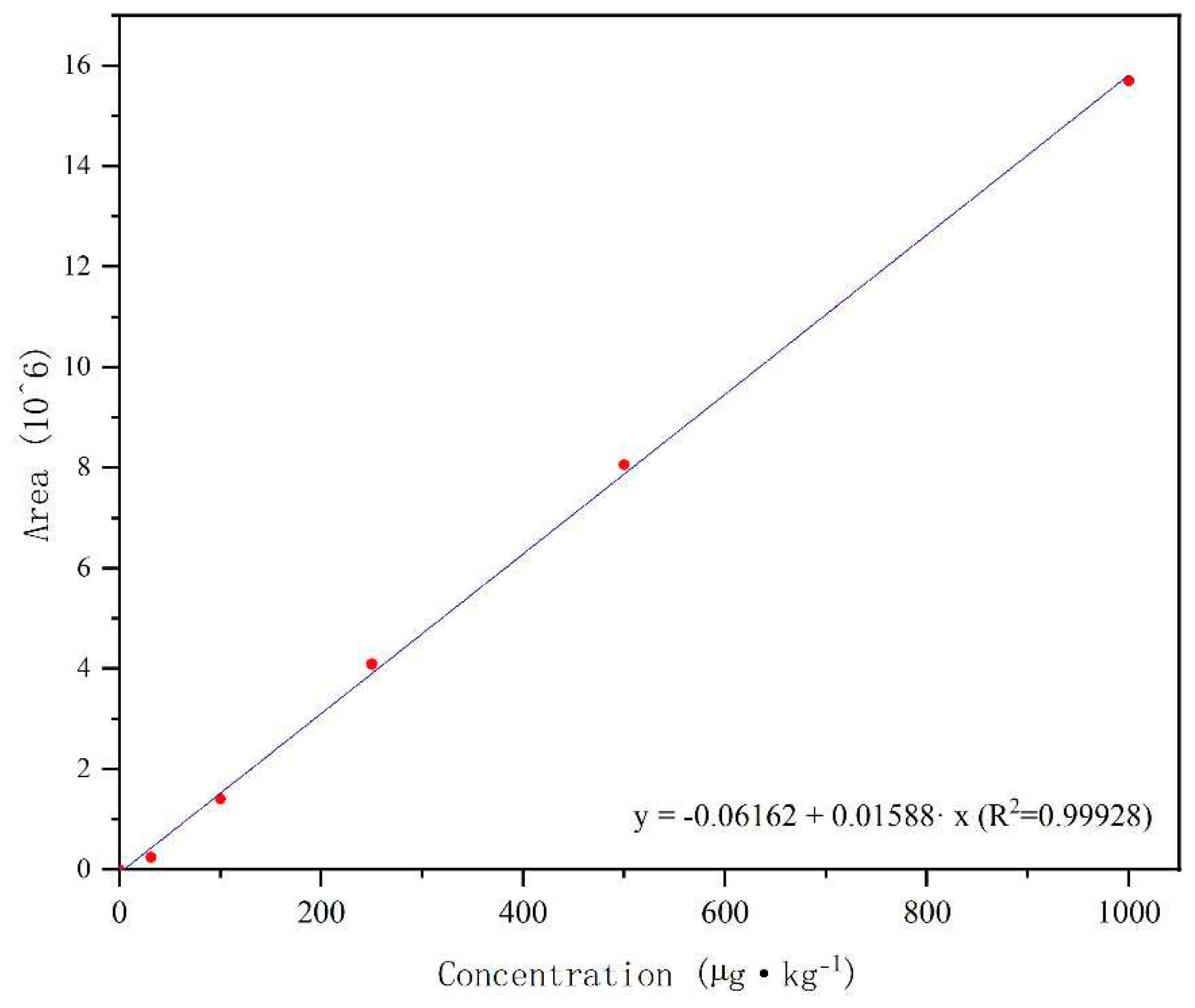
Throughout the retention time, no interference was observed in the blank with various matrices. indicating that the proposed method had high specificity for broflanilide. The 6-point calibration plots of broflanilide (31.25, 100, 250, 500 and 1000 μg/kg) were obtained by least squares linear regression, as shown in
Figure 3. All showed a good linear relationship in the matrix test (correlation coefficient > 0.999). The Quantification limit (LOQ) and detection limit (LOD) of broflanilide in soil samples were 5.94 and 1.25 μg/kg, respectively. The matrix effect value in the blank soil sample was -58 %, indicating matrix inhibition[
33]. The results showed that broflanilide had a signal inhibition effect in soil samples.
2.3.2. Accuracy and precision
Broflanilide was added into blank samples of soils at the concentration of 0.1, 0.5, and 1.0 mg/kg, and the recovery rate and the relative standard deviation (RSD) were determined, as presented in
Table 3. The average broflanilide recovery ranged from 87.7% to 92.91%, with RSD values between 5.49% and 7.51%. These results demonstrate the accuracy and precision of the established method.
2.4. Application in practical research
2.4.1. Applicability test
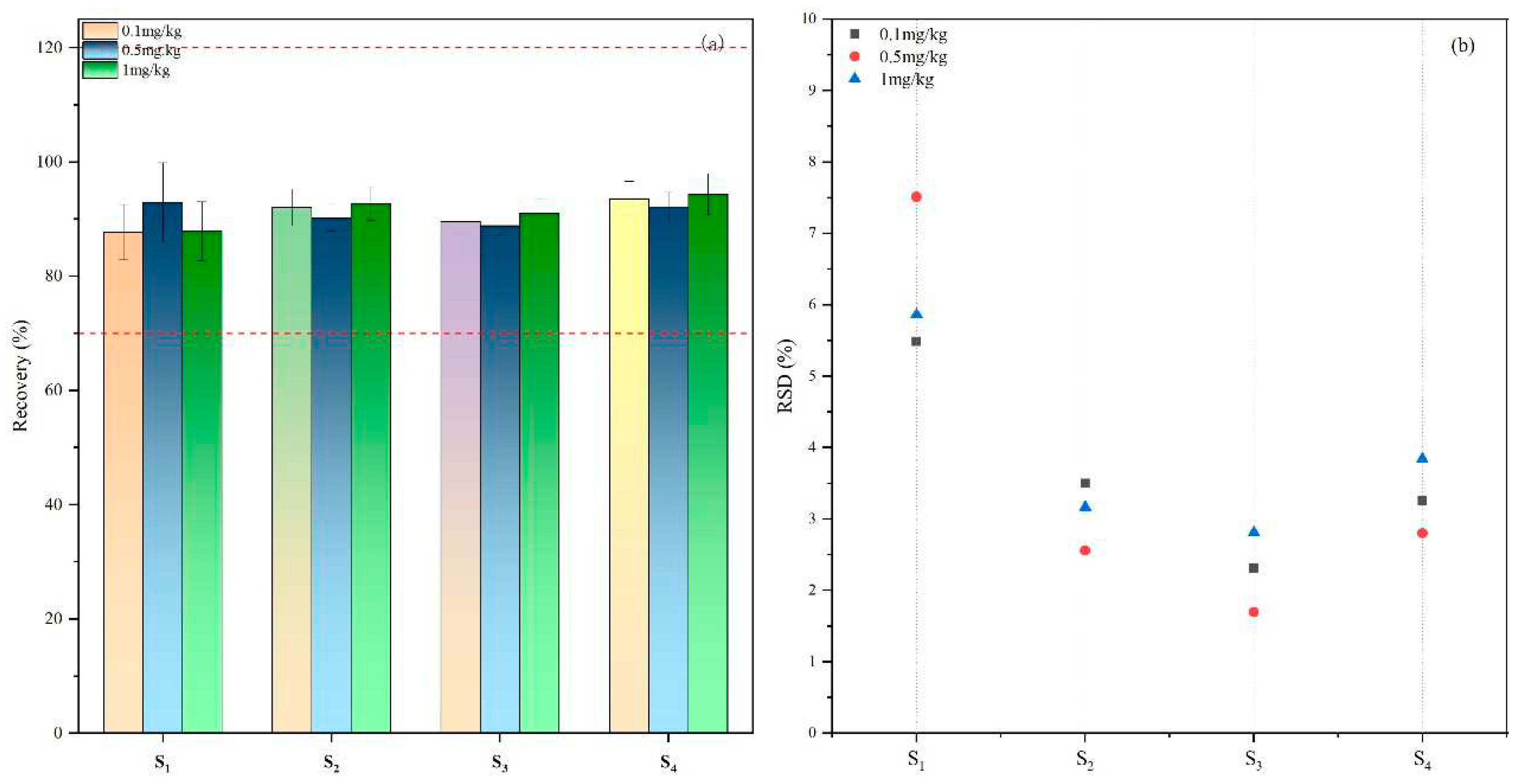
In order to verify the applicability and accuracy of the method, we added broflanilide to soil samples at the contents of 0.0, 0.1, 0.5 and 1.0 mg/kg before the adsorption-desorption experiment and leaching experiment, and used the improved method for testing. The results in
Figure 4 demonstrate the average broflanilide recovery rate was 87.70%-94.38% at different spiked concentrations. The RSD distribution map (
Figure 4 (b)) showed that the RSD value was 1.70% -7.53%. This shows that our method offers high quality accuracy and precision in different types of soils and can be used to analyze the concentration of broflanilide in different types of soils.
2.4.2. Application of soil adsorption-desorption
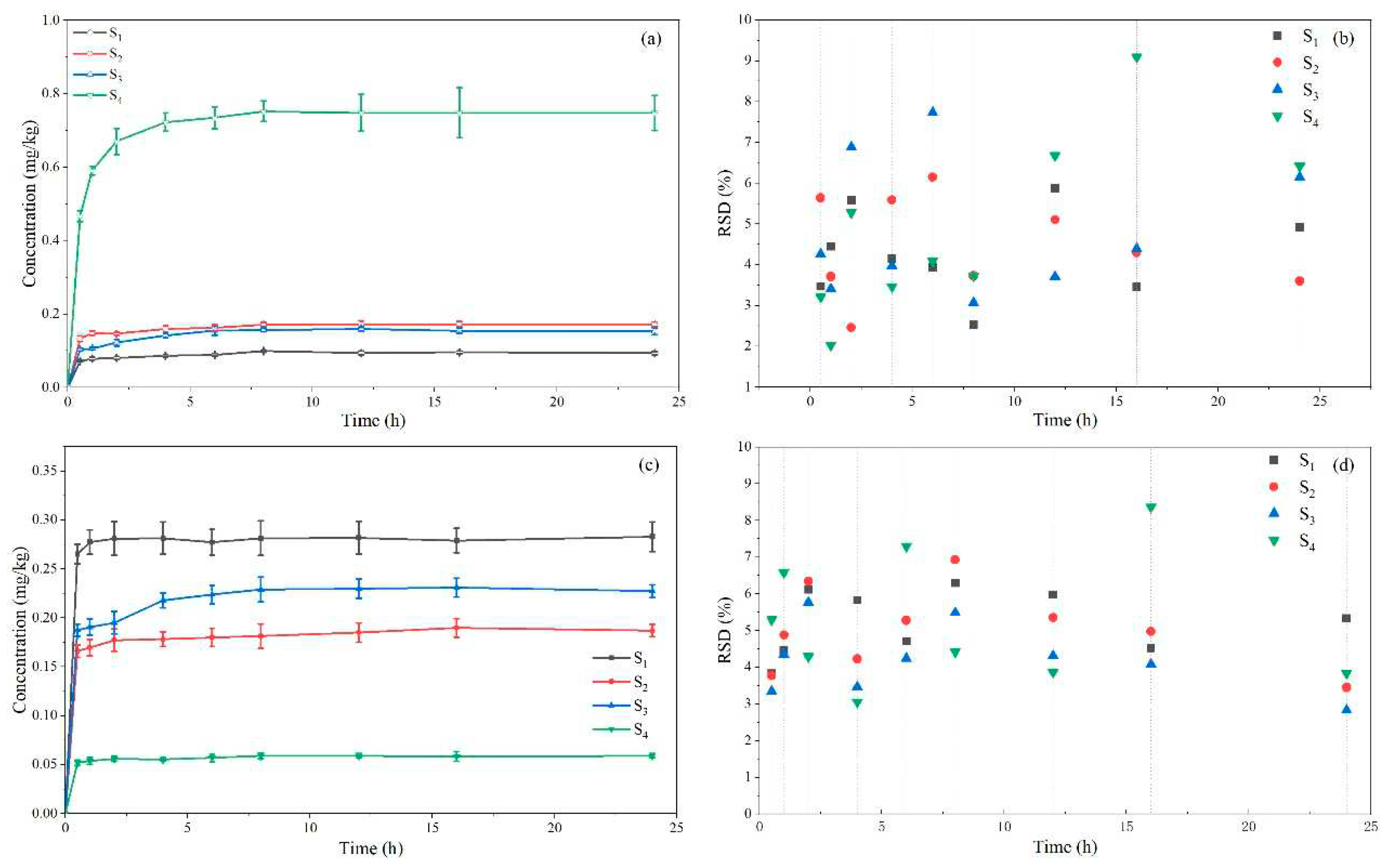
After measuring the soil in the adsorption-desorption process, the results are displayed in
Figure 5 to reveal the law of the adsorption-desorption process of the four soils. Phaeozems can better adsorb broflanilide, and it is less likely to be desorbed, determined by its large organic content. From literature, the adsorption of pesticide in soil is clearly affected by the concentrations of organic composition in soil [
34,
35,
36]. According to the RSD distribution map of the test results, it can be seen that the RSD is lower than 9.2%, so the method discussed in this paper has good stability and can be well applied to soil adsorption-desorption research and detection of broflanilide.
2.4.3. Application in Soil Leaching Experiment
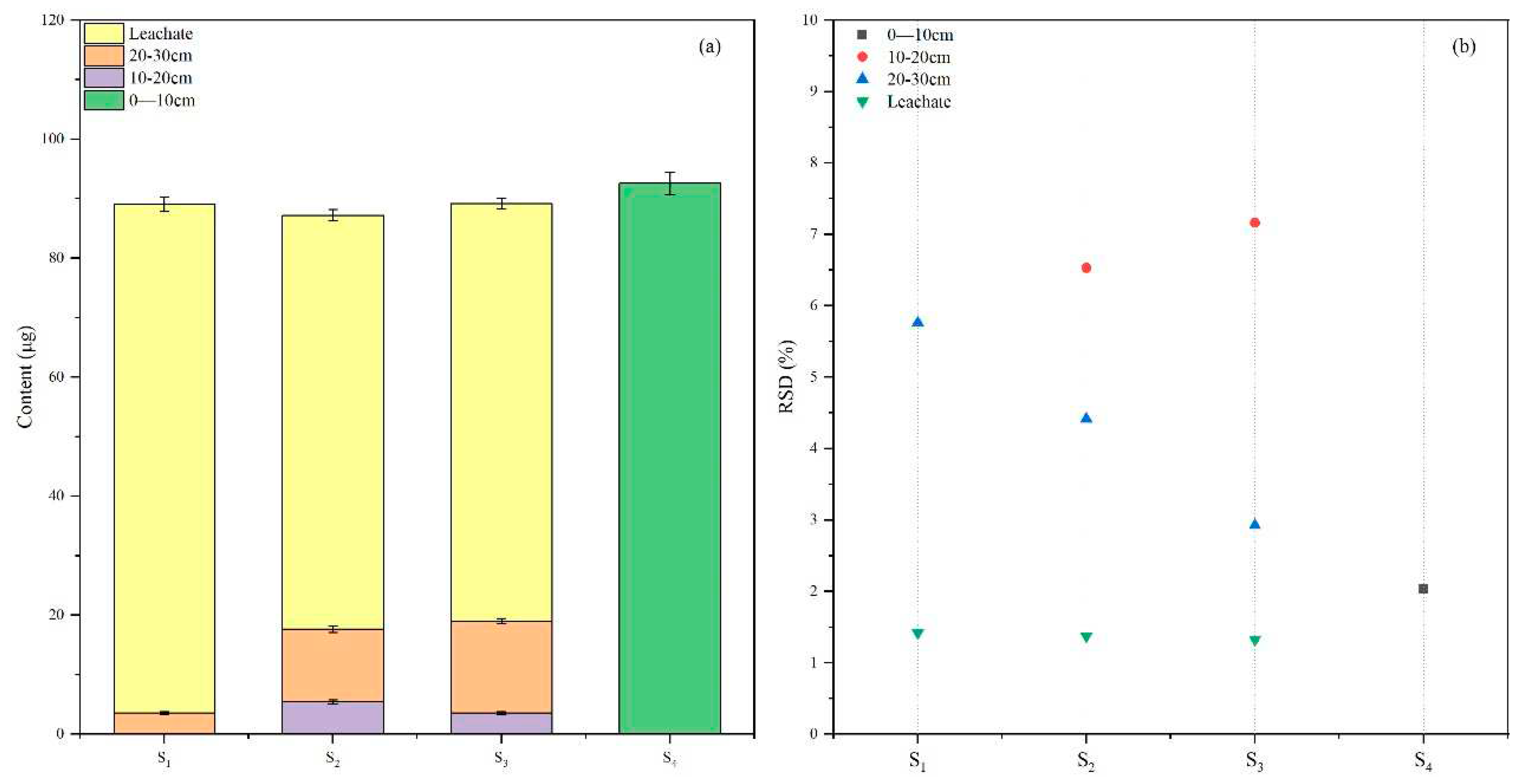
Leaching refers to the vertical downward movement of pesticides when aquatic solutions penetrate into the soil. This process involves the comprehensive behavior of pesticide distribution, desorption, and adsorption in conjunction with soil and water interactions [
37]. Utilizing the soil column leaching method, we simulated the migration process of broflanilide in natural soil and obtained leaching data, as depicted in
Figure 6. The concentration of broflanilide in different soil layers after leaching is related to its adsorption performance, which also determines the mobility of pesticides in soil[
38]. Corresponding to the results of adsorption-desorption experiments, Phaeozems had better stability and could stabilize the added broflanilide in the surface layer, while other soils did not have this ability. Most of the broflanilide in soil entered the leachate during the leaching process. The RSD of the leaching test results was 1.32% -7.16%, indicating that our method offers good applicability and stability for the detection of the leaching of broflanilide in soils.
3. Materials and methods
3.1. Chemicals
Broflanilide (≥99.6%) and its solution (5%) were supplied by Mitsui Chemicals Agro Inc. (Tokyo, Japan). GCB (60 μm) was purchased from Nanjing XFNANO Materials Tech Co., Ltd. (China). HCOOH (≥ 88 %, chromatographic grade) was supplied by Tianjin Komio Chemical Reagent Co., Ltd. (China). CH3OH, CH3CN, ethyl acetate, (CH3)2CO and CH2Cl2 with chromatographic grade were provided by Shantou Xilong Tech. Co., Ltd. (China), who also supplied NaOH, NaN3, CaCl2, NaCl and anhydrous MgSO4. C18 (40 μm) and N-propyl ethylenediamine (PSA) (40 μm) were purchased from Tianjin Bonna-Agela Technologies (China). Deionized water was produced using a Milli-Q water purification system.
101.4 mg Broflanilide was ultrasonically dissolved in 85 mL acetonitrile in a volumetric flask (100 mL) for 20 min before being stood for 40 min at room temperature (RT). The solution (1 mg/mL) was well shaken before being stored at 3 °C. To prepare the working solution, the broflanilide solution was diluted in acetonitrile to the concentrations of 31.25, 100, 250, 500, and 1000 μg/kg. The matrix matching standard solution was prepared by adding the blank soil extract to the working solutions. The prepared solutions were refrigerated at − 20 ° C without light.
3.2. Instrument and conditions
The broflanilide concentration was determined using ultra-high pressure liquid chromatography (UHPLC) coupled with a quadrupole time-of-flight mass spectrometer AB SCIEX X500R. An isocratic elution process was employed using 40% solvent for 6 minutes at 0.3 mL/min. 10 μL sample was injected and kept at 277.15 K.
The spray voltage of the electrospray ion source was 5500 V at 473.15 K. The ion transport tube was maintained at 823.15 K. The pressure of ion source was 10 bar, while the pressures of the ion source 2, and curtain gas were both set at 2 bar. The instrument operated in high-resolution multiple reaction monitoring (HR MRM) scanning mode measuring the cations, covering a mass range of 100 to 1400 Da. The resolutions first and second mass were greater than 26000 and 25000 full widths at half maximum, respectively. The collision energy was maintained at 35 ± 15 eV.
3.3. Sample collection and pretreatment
The blank sample was obtained from a paddy field plot measuring 10 m × 6 m in Zengjia Village (E 115.1, N 28.3), Jiangxi, China. Samples were collected during the rice harvesting period. 1 kg Paddy soil was collected from each of the five random points at a depth of 0-20 cm and thoroughly mixed before being air-dried at RT. The soil samples were sieved using a sieve (20-mesh), and a 500 g sub-sample was prepared through four-fold extraction [
39].
Following the ISO 10400-206 standard, fresh soils were collected from the surface layer (0-15 cm) of farmland without pesticides, fertilizers, and biological additives in April 2023 from 4 different sampling locations detailed in
Table 4. Soil samples were collected and transported in sealed vessels to maintain their initial properties. In the lab, all samples were air-dried at RT, lightly crushed, and passing through a 2mm mesh sieve to get rid of large stones and debris. The left-over soils were stored at low temperature (<276.15 K). The used samples were collected and processed by Huagen Environmental Group Co., Ltd. to prevent pollution.
The physical and chemical characteristics of the soils were determined according to the World Soil Resource Reference (WRB) [
40] and the standard protocol [
41], as outlined in
Table 4. Based on the soil classification system developed by the United Nations Food and Agriculture Organization [
42], the four soils (S1 to S4) were classified as Luvisols, Anthrosols, Gleysols, and Phaeozems.
3.4. Validation of method and data analysis
The QuEChERS method proposed here was validated following the guidelines specified by the International Pesticide Analysis Cooperation Committee. Various qualities including linearity, specificity, accuracy, LOD, LOQ, precision and matrix effect, were comprehensively evaluated across variety matrices [
43,
44].
To assess specificity, the blank samples were analyzed. The lack of interference confirmed its specificity to broflanilide. The least squares regression was used to determine the R
2, gradient, and intercept based on the measurement from various broflanilide concentrations of 31.25, 100, 250, 500, and 1000 μg/kg. Acceptance criteria for R
2 was set at ≥0.99 which the goodness of fit was set ≤20% [
45]. Additionally, the LOQ and LOD values were calculated based on SN ratios of 10:1 and 3:1 for the spiked samples, respectively [
46].
The effect of matrix was assessed using pure CH
3CN as the blank. Broflanilide standard solutions were prepared with a range of concentrations to produce a standard calibration curve in different matrix. The matrix effect constant (ME) was determined below:[
47]:
where s and m are the slopes of the calibration curve in the solvent and the matrix, respectively. ME>10% represents the matrix enhancing effect, while ME<-10% suggests the matrix inhibiting effect. If the ME values are with ±10%, the matrix effect is negligible.
Accuracy and precision were assessed through recovery experiments. To determine accuracy, broflanilide solutions (0.1, 0.5, and 1.0 mg/kg (n=5)) were added to blank soil samples on three separate occasions. The broflanilide recovery rate (%) was determined against the calibration curve. Acceptable recovery rates fell within the range of 70% to 120% [
48]. Precision was determined by the RSD of the broflanilide recovery rate. A value of less than 20% indicated high precision [
49].
3.5. Method application
3.5.1. Applicability experiment
According to the above recovery test method, the recovery experiments with spiked concentrations of 0.1,0.5 and 1 mg/kg were carried out on four different soils on three dates, and the recovery rate and relative standard deviation of broflanilide were calculated and obtained.
3.5.2. Adsorption-desorption experiment
The broflanilide adsorption-desorption in soil was investigated using the equilibrium oscillating method suggested by several reputable organizations, including the U.S. Environmental Protection Agency [
50] and the Organization for Economic Co-operation and Development (OECD) [
51], as well as a treatment method reported by Jiangxi Agricultural University [
39,
52]. To prepare the standard solution, 100 mg of NaN
3 was dissolved in a 0.01 mol/L CaCl
2 solution. To enhance phase separation and inhibit microbial degradation of broflanilide in soils, CaCl
2 solution and NaN
3 solution, which mimic the ionic strength of natural soil solution, were used respectively. The initial concentration of broflanilide was set to 1 mg/L. A 1:5 soil-water (w/v ratio) was maintained. In a centrifuge tube (50 mL), 2.0 g soil was added into 10 mL of the NaN
3 and CaCl
2 solution. The tubes were then oscillated at 180 rpm using a thermostatic oscillator at RT for varying durations of 0.5, 1, 2, 4, 6, 8, 12, 16, and 24 hours to achieve equilibrium. The supernatant was separated, and the soil portion was collected. The soil was then extracted using the method described above and analyzed using UHPLC-QTOF-MS.
After the adsorption experiment, the supernatant was discarded. In addition, CaCl2 injected the same amount of solution into the tube without pesticides. Afterwards, the oscillator was used to oscillate the test tube at an ambient temperature of (298.15±1 K). The subsequent operation was the same as the above adsorption experiment operation, and the desorption experiment was repeated 3 times.
3.5.3. Leaching experiment
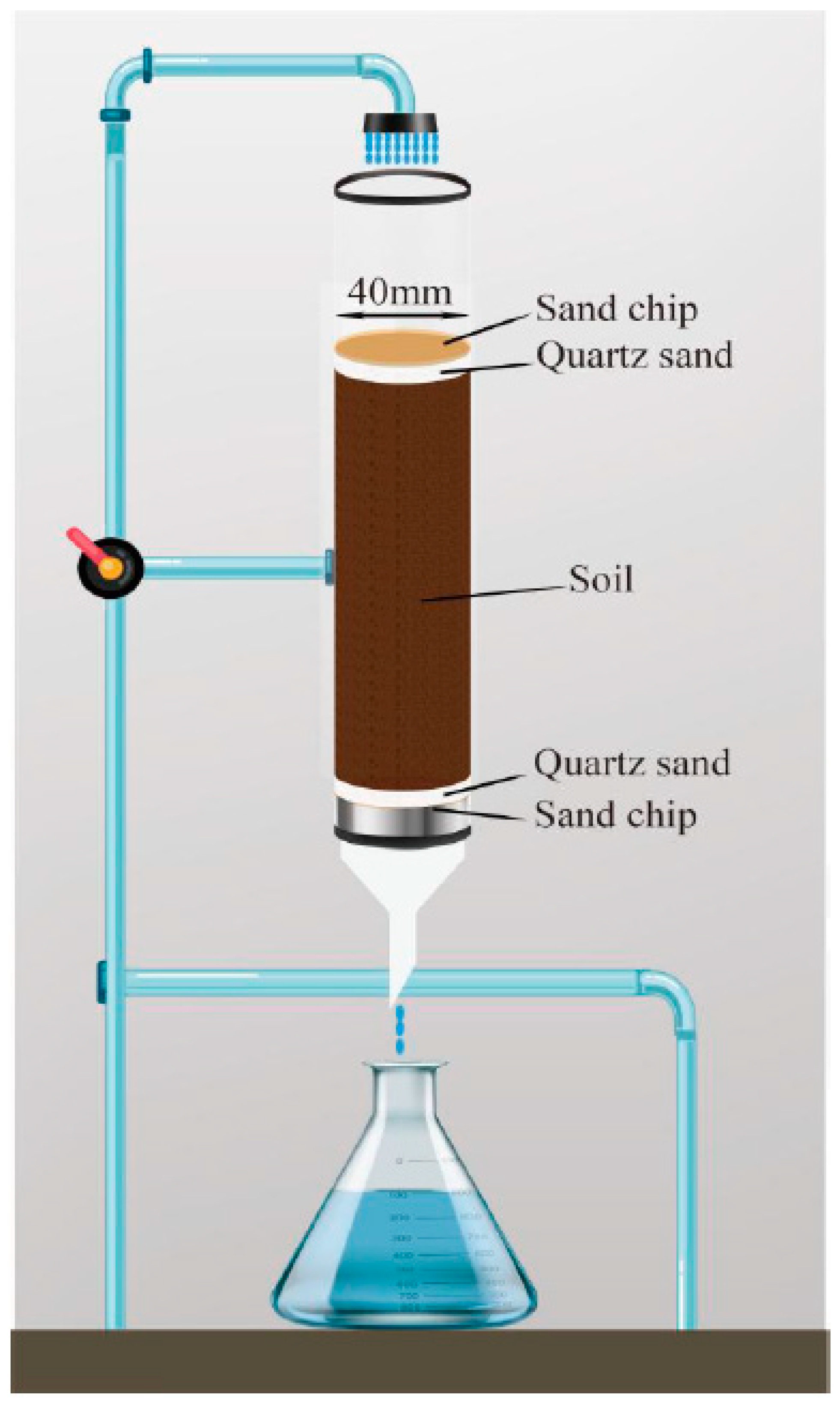
Under saturated flow conditions, the leaching behavior of broflanilide was studied in a filled soil column made of polyethylene plastic pipe (inner diameter 4 cm, height 30 cm), as shown in
Figure 7. Two samples fortified with 250 and 500 μg were chosen following the OECD guidelines [
51]. Before the start of the leaching test, quartz sand (1cm) and a layer of sand (G1 hole) were added to the bottom and top of the soil column to avoid disturbance of soil. To eliminate any trapped air, the soil column was tightly packed with 500 to 700 g of soil. Subsequently, the reverse osmosis method utilizing a 0.01 mol/L CaCl
2 solution was employed [
53]. The remaining water was eliminated using a blow dryer. Then, 700 μL of a 100 mg/L broflanilide solution was infiltrated onto the soil surface. Thes oil sample was soaked for 24 hours to reach adsorption equilibrium. For simulating rainfall leaching, 2000 mL of 0.01 mol/L CaCl
2 solution was fed through the soil samples with a peristaltic pump operating in 96 hours. The leachate was collected every 8 hours in conical flasks. After completion of the leaching experiment, the soil column was removed, and cut into three sections in 10 cm long. The broflanilide was then extracted using the QuEChERS method, and the residue was quantified using UHPLC-QTOF-MS to determine the broflanilide content in both the leachate and soil components.
4. Conclusion
In this study, an efficient, rapid and sensitive method for the detection and analysis of the content of broflanilide in soil was established. The parameters and conditions in the QuEChERS-UHPLC-ESI-QTOF-MS method were optimized, and the specificity, linearity, accuracy and precision of the method were successfully verified. At different spiked concentrations, the average recovery rate (87.7% -94.38%) was obtained in different types of soil. In the adsorption-desorption and leaching experiments applied to the soil, the concentration of the soil during the experiment was accurately determined, and the RSD of the test results was less than 9.2%, showing excellent stability. The results showed that the method was suitable for the determination of the content of broflanilide in different types of soil and the experimental study of adsorption-desorption and leaching.
Author Contributions
GX conceived and designed the experiments. GX and NX carried out experiments. AY and HL analyzed data. GX drafted the manuscript. AY and XN project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by Comprehensive survey of ecological restoration in key areas of Wuyi Mountain (No.DD20230479) ; Investigation of surface matrix in typical red soil area of Nanping City (No.DD20220865); Jiangxi Forestry Bureau Forestry Science and Technology Innovation Project (No.2022-12); Doctoral Initiation Project, Jiangxi Academy of Forestry (No.2022522501); Youth Talent Training Project, Jiangxi Academy of Forestry(No.2022512501); Special Project of Basic Research and Talent Research, Jiangxi Academy of Forestry (No.2024512502); Camphor tree research project of Jiangxi Forestry Bureau (No.2020-07); The Geological Survey commissioned by Changsha Natural Resources Comprehensive Survey Center, China Geological Survey (No.2023-179); the Training Program for Academic and Technical Leaders of Major Discipline in Jiangxi Province-Young Talent Project (No. 20212BCJ23013); The Key R&D Program of Jiangxi Science and Technology Department (No.20212BBF63046). The authors would like to express their gratitude to EditSprings (
https://www.editsprings.com/) for the expert linguistic services provided.
Institutional Review Board Statement
Not applicable.
Data Availability Statements
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Ethical approval
The research did not involve human participants and/or animals.
References
- Godfray, H. C. J.; Beddington, J. R.; Crute, I. R.; Haddad, L.; Lawrence, D.; Muir, J. F.; Pretty, J.; Robinson, S.; Thomas, S. M.; Toulmin, C., Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327 (5967), 812-818. [CrossRef]
- Sparks, T. C.; Hahn, D. R.; Garizi, N. V., Natural products, their derivatives, mimics and synthetic equivalents: role in agrochemical discovery. Pest Manage. Sci. 2017, 73 (4), 700-715. [CrossRef]
- Oerke, E. C.; Dehne, H. W., Safeguarding production—losses in major crops and the role of crop protection. Crop Protect. 2004, 23 (4), 275-285. [CrossRef]
- van der Werf, H. M., Assessing the impact of pesticides on the environment. Agric., Ecosyst. Environ. 1996, 60 (2-3), 81-96. [CrossRef]
- Pimentel, D.; Burgess, M., Small amounts of pesticides reaching target insects. Springer: 2012; Vol. 14, pp 1-2. [CrossRef]
- Navarro, S.; Vela, N.; Navarro, G., An overview on the environmental behaviour of pesticide residues in soils. Spanish journal of agricultural research 2007, 5 (3), 357-375. [CrossRef]
- Calderbank, A., The occurrence and significance of bound pesticide residues in soil. Reviews of environmental contamination and toxicology 1989, 71-103. [CrossRef]
- Chiou, C. T.; Sheng, G.; Manes, M., A partition-limited model for the plant uptake of organic contaminants from soil and water. Environ. Sci. Technol. 2001, 35 (7), 1437-1444. [CrossRef]
- Qi, H.; Cui, L.; Wang, Q.; Liu, F.; Rui, C., Toxicity of broflanilide to Plutella xylostella and its influence on the activities of related enzymes in P. xylostella. Plant Prot 2017, 43 (1), 112-116.
- Nakao, T.; Banba, S., Broflanilide: A meta-diamide insecticide with a novel mode of action. Biorg. Med. Chem. 2016, 24 (3), 372-377. [CrossRef]
- Cui, Y.; Wang, S.; Mao, X.; Gao, X.; Ge, H.; Qu, S.; Qiao, X.; Jiang, X.; Wang, J.; Li, G., Hydrolytic Behavior of Novel Pesticide Broflanilide and Its Dissipative Properties in Different Types of Soils. Bull. Environ. Contam. Toxicol. 2023, 111 (1), 8. [CrossRef]
- Anastassiades, M.; Lehotay, S. J.; Štajnbaher, D.; Schenck, F. J., Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86 (2), 412-431. [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J. A. M.; Silva, C.; Medina, S.; Câmara, J. S., QuEChERS - Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1-28. [CrossRef]
- Wang, J.; Chow, W.; Leung, D., Applications of LC/ESI-MS/MS and UHPLC QqTOF MS for the determination of 148 pesticides in fruits and vegetables. Anal. Bioanal. Chem. 2010, 396 (4), 1513-1538. [CrossRef]
- Ribeiro Begnini Konatu, F.; Sales Fontes Jardim, I. C., Development and validation of an analytical method for multiresidue determination of pesticides in lettuce using QuEChERS–UHPLC–MS/MS. J. Sep. Sci. 2018, 41 (8), 1726-1733. [CrossRef]
- Chen, Y.; Guo, M.; Liu, X.; Xu, J.; Dong, F.; Wu, X.; Li, B.; Zheng, Y., Determination and dissipation of afidopyropen and its metabolite in wheat and soil using QuEChERS–UHPLC–MS/MS. J. Sep. Sci. 2018, 41 (7), 1674-1681. [CrossRef]
- Xie, G.; Zhou, W.; Jin, M.; Yu, A.; Rao, L.; Jia, H.; Luo, J.; He, Y.; Li, B., Residues Analysis and Dissipation Dynamics of Broflanilide in Rice and Its Related Environmental Samples. International Journal of Analytical Chemistry 2020, 2020, 8845387. [CrossRef]
- Žuvela, P.; Skoczylas, M.; Jay Liu, J.; Ba̧czek, T.; Kaliszan, R.; Wong, M. W.; Buszewski, B., Column Characterization and Selection Systems in Reversed-Phase High-Performance Liquid Chromatography. Chem. Rev. 2019, 119 (6), 3674-3729. [CrossRef]
- Carr, P. W.; Doherty, R. M.; Kamlet, M. J.; Taft, R. W.; Melander, W.; Horvath, C., Study of temperature and mobile-phase effects in reversed-phase high-performance liquid chromatography by the use of the solvatochromic comparison method. Anal. Chem. 1986, 58 (13), 2674-2680. [CrossRef]
- Stalcup, A. M.; Martire, D. E.; Wise, S. A., Thermodynamic comparison of monomeric and polymeric C18 bonded phases using aqueous methanol and acetonitrile mobile phases. Journal of Chromatography A 1988, 442, 1-14. [CrossRef]
- Alvarez-Zepeda, A.; Barman, B. N.; Martire, D. E., Thermodynamic study of the marked differences between acetonitrile/water and methanol/water mobile-phase systems in reversed-phase liquid chromatography. Anal. Chem. 1992, 64 (17), 1978-1984. [CrossRef]
- Rafferty, J. L.; Siepmann, J. I.; Schure, M. R., Mobile phase effects in reversed-phase liquid chromatography: A comparison of acetonitrile/water and methanol/water solvents as studied by molecular simulation. Journal of Chromatography A 2011, 1218 (16), 2203-2213. [CrossRef]
- Sun, H.; Dong, X.; Lu, X.; Wang, H.; Han, J., Separation and determination of fluoroquinolones with a molecularly imprinted polymer. Chinese Journal of Chromatography 2003, 21 (3), 233-238. [CrossRef]
- Payá, P.; Anastassiades, M.; Mack, D.; Sigalova, I.; Tasdelen, B.; Oliva, J.; Barba, A., Analysis of pesticide residues using the Quick Easy Cheap Effective Rugged and Safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and tandem mass spectrometric detection. Anal. Bioanal. Chem. 2007, 389, 1697-1714. [CrossRef]
- Hajjo, R.; Afifi, F.; Battah, A., Multiresidue pesticide analysis of the medicinal plant Origanum syriacum. Food Addit. Contam. 2007, 24 (3), 274-279. [CrossRef]
- Riaz, M.; Bilal Butt, S., Gamma radiolytic degradation of the endrin insecticide in methanol and monitoring of radiolytic degradation products by HPLC. J. Radioanal. Nucl. Chem. 2010, 285 (3), 697-701. [CrossRef]
- Nordmeyer, K.; Thier, H.-P., Solid-phase extraction for replacing dichloromethane partitioning in pesticide multiresidue analysis. Zeitschrift für Lebensmitteluntersuchung und-Forschung A 1999, 208, 259-263. [CrossRef]
- Chen, G.; Cao, P.; Liu, R., A multi-residue method for fast determination of pesticides in tea by ultra performance liquid chromatography–electrospray tandem mass spectrometry combined with modified QuEChERS sample preparation procedure. Food Chem. 2011, 125 (4), 1406-1411. [CrossRef]
- Li, S.; Liu, X.; Dong, F.; Xu, J.; Xu, H.; Hu, M.; Zheng, Y., Chemometric-assisted QuEChERS extraction method for the residual analysis of thiacloprid, spirotetramat and spirotetramat’s four metabolites in pepper: Application of their dissipation patterns. Food Chem. 2016, 192, 893-899. [CrossRef]
- Lehotay, S. J., Quick, easy, cheap, effective, rugged, and safe approach for determining pesticide residues. Pesticide protocols 2006, 239-261. [CrossRef]
- Brondi, S. H.; De Macedo, A. N.; de Souza, G. B.; Nogueira, A. R., Application of QuEChERS method and gas chromatography-mass spectrometry for the analysis of cypermethrin residue in milk. Journal of Environmental Science and Health, Part B 2011, 46 (8), 671-677.
- Guideline for the testing of pesticide resudues in crops. Ministy of Agriculture and Rural Affairs of the Peopel's Republic of China: China, 2018.
- Wang, Y.-Q.; Ye, D.-Q.; Zhu, B.-Q.; Wu, G.-F.; Duan, C.-Q., Rapid HPLC analysis of amino acids and biogenic amines in wines during fermentation and evaluation of matrix effect. Food Chem. 2014, 163, 6-15. [CrossRef]
- Zhang, Y.; Li, W.; Zhou, W.; Jia, H.; Li, B., Adsorption-desorption characteristics of pyraclonil in eight agricultural soils. J. Soils Sed. 2020, 20, 1404-1412. [CrossRef]
- Plakas, K. V.; Karabelas, A. J., Membrane retention of herbicides from single and multi-solute media: The effect of ionic environment. J. Membr. Sci. 2008, 320 (1-2), 325-334. [CrossRef]
- Zhang, J.; Zhaojun, L.; Gaofei, G.; Wanchun, S.; Liang, Y.; Laosheng, W., Impacts of soil organic matter, pH and exogenous copper on sorption behavior of norfloxacin in three soils. Journal of Environmental Sciences 2009, 21 (5), 632-640. [CrossRef]
- Xu, X.; Song, W.; Wang, M., Adsorption-desorption and leaching characteristics of fluazinam in soils. China Environ. Sci. 2013, 33 (4), 669-673.
- Xie, G.; Li, B.; Tang, L.; Rao, L.; Dong, Z., Adsorption-desorption and leaching behaviors of broflanilide in four texturally different agricultural soils from China. J. Soils Sed. 2021, 21 (2), 724-735. [CrossRef]
- Li, W.; Zhang, Y.; Jia, H.; Zhou, W.; Li, B.; Huang, H., Residue analysis of tetraniliprole in rice and related environmental samples by HPLC/MS. Microchem. J. 2019, 150, 104168. [CrossRef]
- L'Huillier, L.; Dupont, S.; Dubus, I.; Becquer, T.; Bourdon, E.; Laubreaux, P.; Bonzon, B., Carence et fixation du phosphore dans les sols ferrallitiques ferritiques de Nouvelle-Calédonie. XVIe Congrès Mondial de Science du Sol 1998, 20-26.
- Piper, C. S., Soil and plant analysis. Scientific Publishers: 2019.
- Deckers, J.; Driessen, P.; Nachtergaele, F.; Spaargaren, O., World reference base for soil resources. In Encyclopedia of soil science, Marcel Dekker: 2002; pp 1446-1451.
- Fanigliulo, A.; De Filippis, P.; Curcuruto, O.; Repeto, P.; Roveda, D.; Hartenstein, M.; Adams, E.; Cabooter, D., Development and validation of a stability indicating method for s-carboxymethyl-l-cysteine and related degradation products in oral syrup formulation. J. Pharm. Biomed. Anal. 2015, 115, 39-47. [CrossRef]
- Seccia, S.; Albrizio, S.; Fidente, P.; Montesano, D., Development and validation of a solid-phase extraction method coupled to high-performance liquid chromatography with ultraviolet-diode array detection for the determination of sulfonylurea herbicide residues in bovine milk samples. Journal of Chromatography A 2011, 1218 (9), 1253-1259. [CrossRef]
- Kuang, Y.; Qiu, F.; Kong, W.; Luo, J.; Cheng, H.; Yang, M., Simultaneous quantification of mycotoxins and pesticide residues in ginseng with one-step extraction using ultra-high performance liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. B 2013, 939, 98-107. [CrossRef]
- Guideline, I. H. T., Validation of analytical procedures: text and methodology. Q2 (R1) 2005, 1 (20), 05.
- Zhu, Y.; Liu, X.; Xu, J.; Dong, F.; Liang, X.; Li, M.; Duan, L.; Zheng, Y., Simultaneous determination of spirotetramat and its four metabolites in fruits and vegetables using a modified quick, easy, cheap, effective, rugged, and safe method and liquid chromatography/tandem mass spectrometry. Journal of Chromatography A 2013, 1299, 71-77. [CrossRef]
- Garrido Frenich, A.; González-Rodríguez, M. J.; Arrebola, F. J.; Martínez Vidal, J. L., Potentiality of gas chromatography− triple quadrupole mass spectrometry in vanguard and rearguard methods of pesticide residues in vegetables. Anal. Chem. 2005, 77 (14), 4640-4648. [CrossRef]
- Zapata, M.; Rodríguez, F.; Garrido, J. L., Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29-45. [CrossRef]
- Prevention, P., United States Environmental Protection Agency. Hospital 2020, 3862, 104.
- Guideline, P.-B. T., OECD guideline for the testing of chemicals. The Hershberger 2001, 601, 858.
- Rao, L.; Luo, J.; Zhou, W.; Zou, Z.; Tang, L.; Li, B., Adsorption–desorption behavior of benzobicyclon hydrolysate in different agricultural soils in China. Ecotoxicol. Environ. Saf. 2020, 202, 110915. [CrossRef]
- Gupta, V. K.; Ali, I., Water treatment by reverse osmosis method. Environmental Water 2013, 117-134. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).