Submitted:
08 February 2024
Posted:
08 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Isolation of Essential Oil
2.2.1. Isolation of Essential Oil for Phytochemical and Pharmacological Analysis
2.2.2. Isolation of Essential Oil for Cytotoxicity Studies
2.3. Preparation of Extracts
2.4. Phytochemical Analysis
2.4.1. Gas-Chromatographic Analysis of Essential Oil
2.4.2. Assay of Main Phenolics by Spectrophotometry
2.4.3. Analysis of Phenolic Compounds by UPLC-MS/MS
2.5. Molecular Docking of M. discoidea BAS
- lipoxygenase-5 (LOX-5) (PDB ID 6NCF) enzyme with a natural non-competitive inhibitor – pentacyclic triterpenoid acid (3α,8α,17α,18α-3-acetyloxy-11-oxours-12-en-23-oic acid – AKBA) in the active site [19];
- ionotropic glutamate NMDA receptors in conformation with a non-competitive antagonist of direct action - ketamine (7EU7) [22];
- the GABA receptor in conformation with the agonist phenobarbital (6X3W) [23].
2.6. Pharmacological Study
2.6.1. Cytotoxicity Studies
2.6.2. Analgesic Activity
2.6.3. Soporific Activity
2.7. Statistical Analysis
3. Results
3.1. Phytochemical Composition of Dry Extracts and Essential Oil
3.2. Optimization of a Dry Extract P2 Preparation
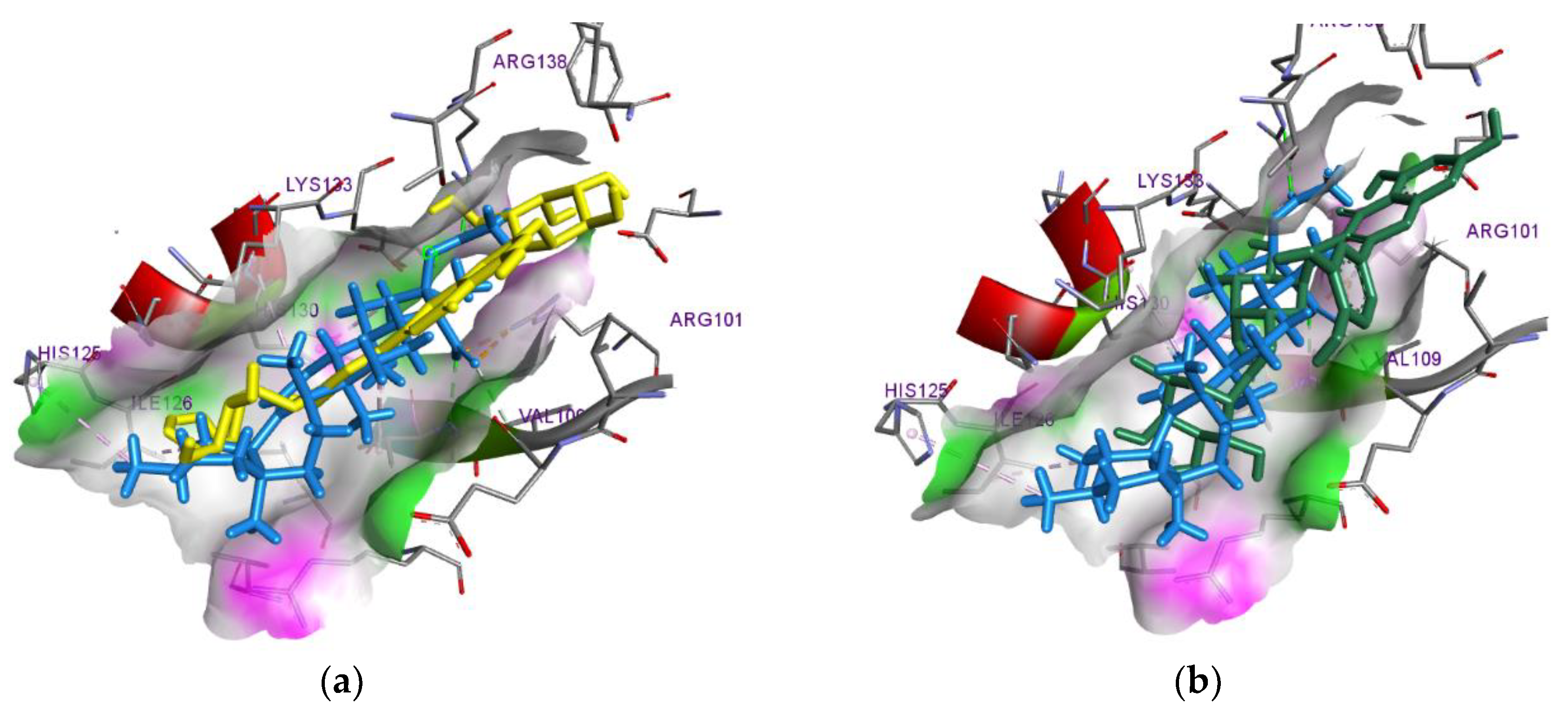
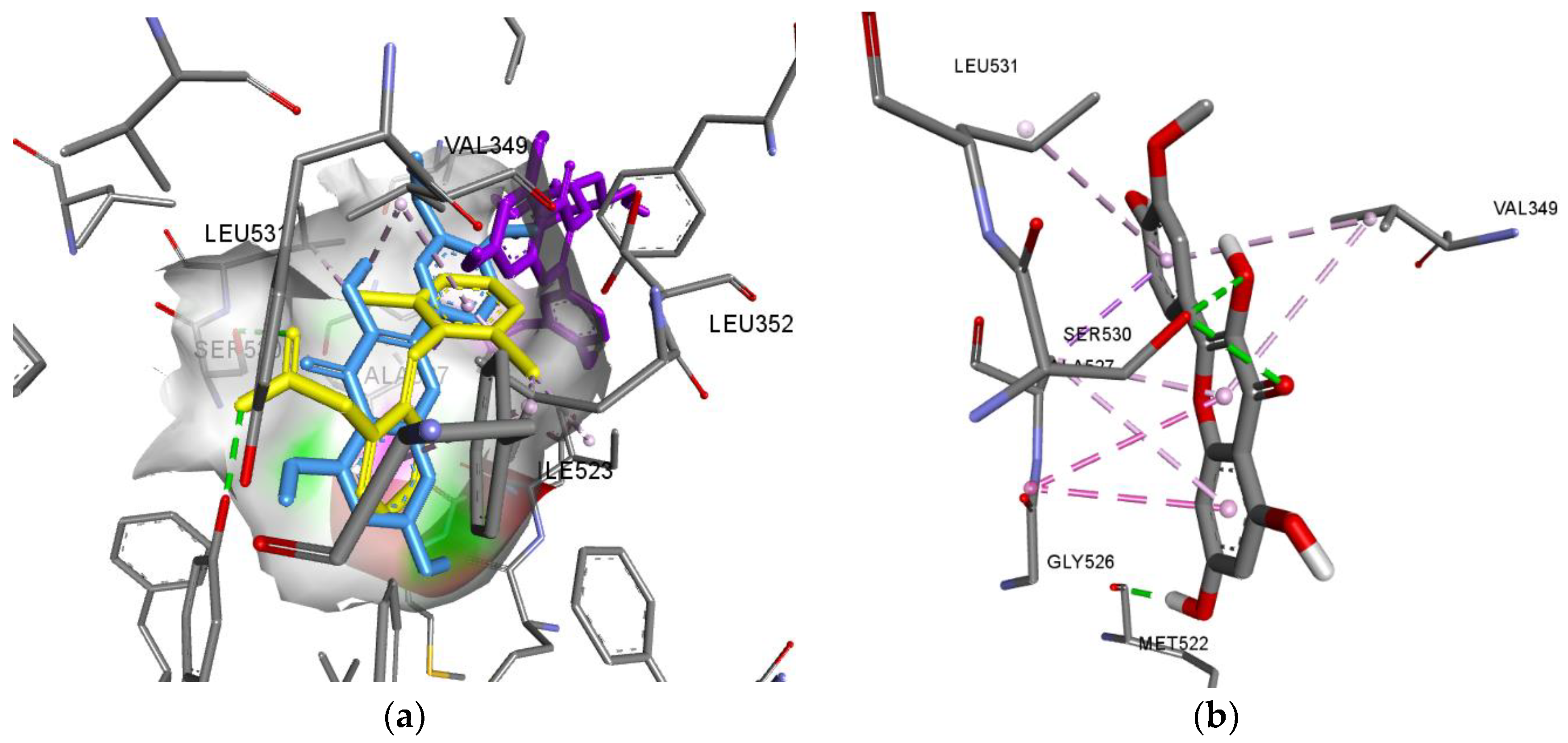
3.3. In-Silico Prediction of the Pharmacological Activity of M. discoidea BAS
3.4. Pharmacological Study
3.4.1. Cytotoxicity Study
3.4.2. Analgesic Activity
3.4.3. Soporific Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arak, E.; Raal, A.; Tammeorg, J. Aerial Parts of Matricaria Matricarioides: A Substitute for Matricaria Recutita Flowers. Farmatsiya 1986, 35, 19–22. [Google Scholar]
- U.S.S.R. Pharmacopoeia, 10th ed.; Meditsina: Moscow, 1968.
- Orav, A.; Sepp, J.; Kailas, T.; Müürisepp, M.; Arak, E.; Raal, A. Composition of Essential Oil of Aerial Parts of Chamomilla Suaveolens from Estonia. Natural Product Communications 2010, 5, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Püssa, T.; Sepp, J.; Malmiste, B.; Arak, E. Content of Phenolic Compounds in Aerial Parts of Chamomilla Suaveolens from Estonia. Nat Prod Commun 2011, 6, 1107–1110. [Google Scholar] [PubMed]
- Raal, A.; Püssa, T.; Sepp, J.; Malmiste, B.; Arak, E. Content of Phenolic Compounds in Aerial Parts of Chamomilla Suaveolens from Estonia. Natural Product Communications 2011, 6, 1107–1110. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Püssa, T.; Valner, C.; Malmiste, B.; Arak, E. Content of Essential Oil, Terpenoids and Polyphenols in Commercial Chamomile (Chamomilla Recutita L. Rauschert) Teas from Different Countries. Food Chemistry 2012, 131, 632–638. [Google Scholar] [CrossRef]
- Orav, A.; Raal, A.; Arak, E. Content and Composition of the Essential Oil of Chamomilla Recutita (L.) Rauschert from Some European Countries. Natural Product Research 2010, 24, 48–55. [Google Scholar] [CrossRef]
- Raal, A.; Kaur, H.; Orav, A.; Arak, E.; Kailas, T.; Müürisepp, M. Content and Composition of Essential Oils in Some Asteraceae Species. Proceedings of the Estonian Academy of Sciences 2011, 60, 55–63. [Google Scholar] [CrossRef]
- Martin, N.; Madrid-López, C.; Villalba-Méndez, G.; Talens-Peiró, L. New Techniques for Assessing Critical Raw Material Aspects in Energy and Other Technologies. Environ. Sci. Technol. 2022, 56, 17236–17245. [Google Scholar] [CrossRef]
- Koshovyi, O.; Vovk, G.; Akhmedov, Ey.; Komissarenko, AN. The Study of the Chemical Composition and Pharmacological Activity of Salvia Officinalis Leaves Extracts Getting by Complex Processing. Azerbaijan Pharmaceutical and Pharmacotherapy Journal 2015, 15, 30–34. [Google Scholar]
- Shanaida, M.; Hudz, N.; Jasicka-Misiak, I.; Wieczorek, P.P. Polyphenols and Pharmacological Screening of a Monarda Fistulosa L. Dry Extract Based on a Hydrodistilled Residue By-Product. Front. Pharmacol. 2021, 12, 563436. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 11th ed. Council of Europe: Strasbourg, 2022.
- Huzio, N.; Grytsyk, A.; Raal, A.; Grytsyk, L.; Koshovyi, O. Phytochemical and Pharmacological Research in Agrimonia Eupatoria L. Herb Extract with Anti-Inflammatory and Hepatoprotective Properties. Plants 2022, 11, 2371. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Jaama, M.; Utt, M.; Püssa, T.; Žvikas, V.; Jakštas, V.; Koshovyi, O.; Nguyen, K.V.; Thi Nguyen, H. The Phytochemical Profile and Anticancer Activity of Anthemis Tinctoria and Angelica Sylvestris Used in Estonian Ethnomedicine. Plants 2022, 11, 994. [Google Scholar] [CrossRef]
- Shinkovenko, I.L.; Kashpur, N.V.; Ilyina, T.V.; et al. The Immunomodulatory Activity of the Extracts and Complexes of Biologically Active Compounds of Galium Verum L. Herb. Ceska a Slovenska Farmacie, 67, 25–29.
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic Fractions from Vaccinium Vitis-Idaea L. and Their Antioxidant and Anticancer Activities Assessment. Antioxidants 2020, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational Protein–Ligand Docking and Virtual Drug Screening with the AutoDock Suite. Nat Protoc 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Protein Data Bank.
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and Mechanistic Insights into 5-Lipoxygenase Inhibition by Natural Products. Nat Chem Biol 2020, 16, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.S.; Lee, J.Y.; Yuan, C.; Smith, W.L. Comparison of Cyclooxygenase-1 Crystal Structures: Cross-Talk between Monomers Comprising Cyclooxygenase-1 Homodimers, Biochemistry 2010, 49, 7069–7079. [Google Scholar] [CrossRef]
- Wang, J.L.; Limburg, D.; Graneto, M.J.; Springer, J.; Hamper, J.R.B.; Liao, S.; Pawlitz, J.L.; Kurumbail, R.G.; Maziasz, T.; Talley, J.J.; et al. The Novel Benzopyran Class of Selective Cyclooxygenase-2 Inhibitors. Part 2: The Second Clinical Candidate Having a Shorter and Favorable Human Half-Life. Bioorganic & Medicinal Chemistry Letters 2010, 20, 7159–7163. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, F.; Zhang, T.; Lv, S.; Zhou, L.; Du, D.; Lin, H.; Guo, F.; Luo, C.; Zhu, S. Structural Basis of Ketamine Action on Human NMDA Receptors. Nature 2021, 596, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Gharpure, A.; Teng, J.; Zhuang, Y.; Howard, R.J.; Zhu, S.; Noviello, C.M.; Walsh, R.M.; Lindahl, E.; Hibbs, R.E. Shared Structural Mechanisms of General Anaesthetics and Benzodiazepines. Nature 2020, 585, 303–308. [Google Scholar] [CrossRef]
- European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes; 1986; Vol. Official Journal L 222, p. P 0031-0037.
- Council Directive 2010/63/EU of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; 2010; pp. 33–79.
- On the Protection of Animals from Cruel Treatment, 2009.
- On Approval of the Procedure for Preclinical Study of Medicinal Products and Examination of Materials of Preclinical Study of Medicinal Products; 2009.
- Regulating the Application of Principles of Good Laboratory Practice and the Verification of Their Applications for Tests on Chemical Substances; 1986; Vol. 1, pp. 145–146.
- Stefanov, O.V. Preclinical Studies of Drugs; Avicenna: Kyiv, Ukraine, 2001. [Google Scholar]
- Masocha, W.; Kombian, S.B.; Edafiogho, I.O. Evaluation of the Antinociceptive Activities of Enaminone Compounds on the Formalin and Hot Plate Tests in Mice. Sci Rep 2016, 6, 21582. [Google Scholar] [CrossRef]
- Inaltekin, A.; Kivrak, Y. Evaluation of the Effect of Vortioxetine on Pain Threshold by Hot-Plate Test in Mice. Archives of Neuropsychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Md.F.; Talukder, B.; Rana, M.N.; Tasnim, R.; Nipun, T.S.; Uddin, S.M.N.; Hossen, S.M.M. In Vivo Sedative Activity of Methanolic Extract of Stericulia Villosa Roxb. Leaves. BMC Complement Altern Med 2016, 16, 398. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Ghorbani, A.; Hosseini, M.; Rakhshandeh, H. Hydroalcoholic Extract of Needles of Pinus Eldarica Enhances Pentobarbital-Induced Sleep: Possible Involvement of GABAergic System. Avicenna J Phytomed 2016, 6, 449–457. [Google Scholar] [PubMed]
- Sepp, J.; Koshovyi, O.; Jakstas, V.; Žvikas, V.; Botsula, I.; Kireyev, I.; Tsemenko, K.; Kukhtenko, O.; Kogermann, K.; Heinämäki, J.; et al. Phytochemical, Technological and Pharmacological Study on the Galenic Dry Extracts Prepared from German Chamomile (Matricaria Chamomilla L.) Flowers; Medicine and Pharmacology. 2023. [Google Scholar]
- Kafarov, V.V. Methods of Cybernetics in Chemistry and Chemical Technology; Chemistry.; M., 1976.
- Marzullo, L.; Ochkur, O.; Orlandini, S.; Renai, L.; Gotti, R.; Koshovyi, O.; Furlanetto, S.; Del Bubba, M. Quality by Design in Optimizing the Extraction of (Poly)Phenolic Compounds from Vaccinium Myrtillus Berries. Journal of Chromatography A 2022, 1677, 463329. [Google Scholar] [CrossRef] [PubMed]
- Koshovyi, O.M.; Kukhtenko, O.S.; Kovalova, A.M.; Komissarenko, A.M.; Vinnyk, O.V.; Sholom, Yu.G. Optimization of the Biologically Active Substances Extraction Process from Eucalypt Leaves: Multiplicity of Extraction. Current issues of pharma-ceutical and medical science and practice, 2010; XIII, 47–49. [Google Scholar]
- Vyshnevska, L.; Severina, H.I.; Prokopenko, Y.; Shmalko, A. Molecular Docking Investigation of Anti-Inflammatory Herbal Compounds as Potential LOX-5 and COX-2 Inhibitors. PHAR 2022, 69, 733–744. [Google Scholar] [CrossRef]
- Gupta Chamomile: A Herbal Medicine of the Past with a Bright Future (Review). Mol Med Rep 2010, 3. [CrossRef] [PubMed]
- Chang, S.; Chen, C. Effects of an Intervention with Drinking Chamomile Tea on Sleep Quality and Depression in Sleep Disturbed Postnatal Women: A Randomized Controlled Trial. Journal of Advanced Nursing 2016, 72, 306–315. [Google Scholar] [CrossRef]
- Arak, E.H. Results of Essential Oil Analysis of Pineapple Weed and Wild Chamomile by Gas Chromatographic Method.; Tallinn, 1981; p. 79.
- Orav, A.; Kailas, T.; Kann, J. Volatile Constituents of Matricaria Matricariodes (Less.) Port. Journal of Essential Oil Research, 1999; 243–245. [Google Scholar]
- Raal, A.; Kaur, H.; Orav, A.; Arak, E.; Kailas, T.; Müürisepp, M. Content and Composition of Essential Oils in Some Asteraceae Species. PROCEEDINGS OF THE ESTONIAN ACADEMY OF SCIENCES 2011, 60, 55–63. [Google Scholar] [CrossRef]
- Jain, T.C.; Karchesy, J.J. Concerning the Chemical Constituents of Matricaria Marticarioides. Phytochemistry 1971, 10, 2825–2826. [Google Scholar] [CrossRef]
- Lawrence, B.M.; Terhune, S.J.; Hogg, J.W. Volatile Constituents of Matricaria Matricarioides. Phytochemistry 1971, 10, 2827–2827. [Google Scholar] [CrossRef]
- Oleshko, G.I.; Prosovskyi, M.A. Dynamics of the Contents of Essential Oil and Its Main Components in Matricaria Discoidea DC. Rastitel’nye Resursy 1986, 22, 377–382. [Google Scholar]
- Lopes, D.; Kolodziejczyk, P.P. Essential Oil Composition of Pineapple-Weed (Matricaria Discoidea DC.) Grown in Canada. Journal of Essential Oil Bearing Plants 2005, 8, 178–182. [Google Scholar] [CrossRef]
- Ma, C.; Winsor, L.; Daneshtalab, M. Quantification of Spiroether Isomers and Herniarin of Different Parts of Matricaria Matricarioides and Flowers of Chamaemelum Nobile. Phytochemical Analysis 2007, 18, 42–49. [Google Scholar] [CrossRef]
- Orav, A.; Sepp, J.; Kailas, T.; Müürisepp, M.; Arak, E.; Raal, A. Composition of Essential Oil of Aerial Parts of Chamomilla Suaveolens from Estonia. Nat Prod Commun 2010, 5, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Chaves, P.F.P.; Hocayen, P.D.A.S.; Dallazen, J.L.; De Paula Werner, M.F.; Iacomini, M.; Andreatini, R.; Cordeiro, L.M.C. Chamomile Tea: Source of a Glucuronoxylan with Antinociceptive, Sedative and Anxiolytic-like Effects. International Journal of Biological Macromolecules 2020, 164, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Du, K.; Li, N.; Zheng, Z.; Qin, Y.; Liu, J.; Sun, R.; Su, Y. Evaluation of Anti-Nociceptive and Anti-Inflammatory Effect of Luteolin in Mice. J Environ Pathol Toxicol Oncol 2018, 37, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal Medicine for Depression, Anxiety and Insomnia: A Review of Psychopharmacology and Clinical Evidence. European Neuropsychopharmacology 2011, 21, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.S.L.D.S.; Pardo, P.E.; Oba, E.; Kronka, S.D.N.; Frazatti-Gallina, N.M. Matricaria Chamomilla CH 12 Decreases Handling Stress in Nelore Calves. J Vet Sci 2006, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Shinomiya, K.; Inoue, T.; Utsu, Y.; Tokunaga, S.; Masuoka, T.; Ohmori, A.; Kamei, C. Hypnotic Activities of Chamomile and Passiflora Extracts in Sleep-Disturbed Rats. Biological & Pharmaceutical Bulletin 2005, 28, 808–810. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Li, Y.; Soeller, I.; Rockwell, K.; Mao, J.J.; Shults, J. A Randomized, Double-Blind, Placebo-Controlled Trial of Oral Matricaria Recutita (Chamomile) Extract Therapy for Generalized Anxiety Disorder. Journal of Clinical Psychopharmacology 2009, 29, 378–382. [Google Scholar] [CrossRef]
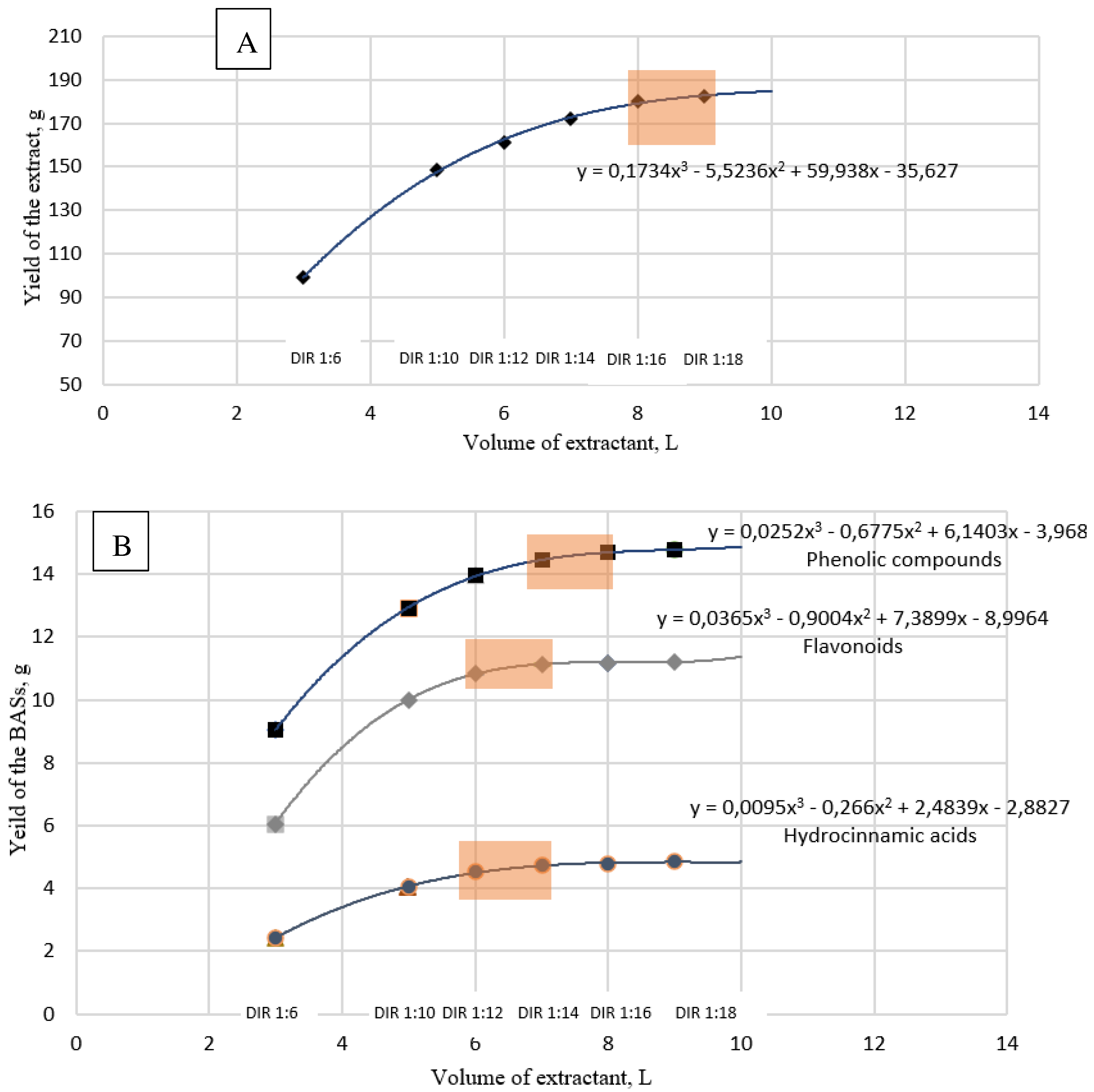
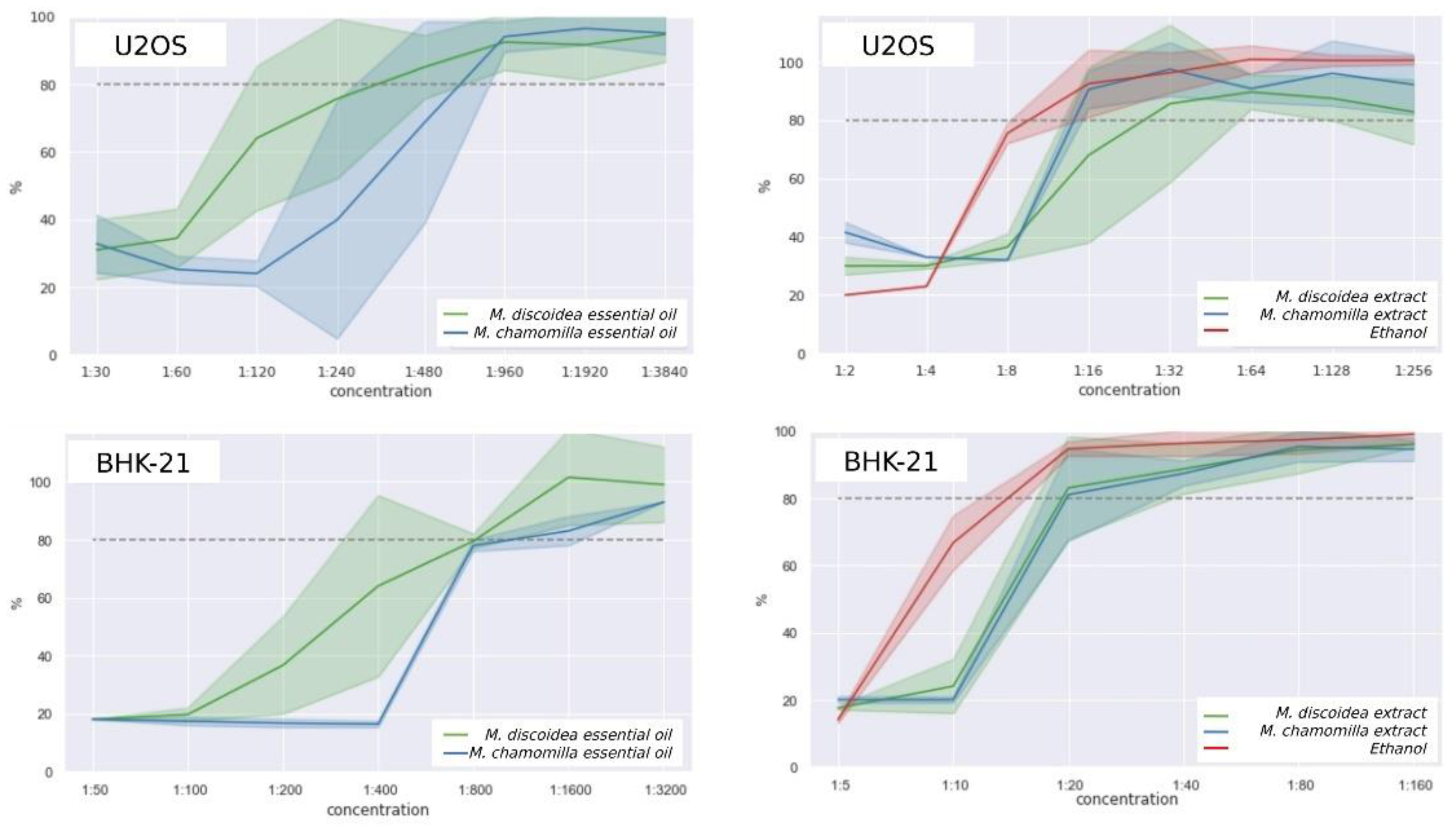
| RI (DB-5) | Compound | Content in the oil, % | |
|---|---|---|---|
| M. chamomilla* | M. discoidea | ||
| 987 | Myrcene | <0.01 | 7.99 |
| 1455 | (E)-ß-Farnesene | 24.72 | 42.51 |
| 1472 | Germacrene D | 1.01 | 1.23 |
| 1570 | Spathulenol | 2.39 | 1.12 |
| 1609 | Geranyl isovalerate | <0.01 | 29.50 |
| 1649 | α-Bisabolol oxide B | 22.27 | 1.06 |
| 1673 | α-Bisabolone oxide A | 10.40 | 2.11 |
| 1715 | Chamazulene | 7.89 | - |
| 1740 | α-Bisabolol oxide A | 21.78 | 1.48 |
| 1874 | (Z)-Enyne-bicycloether | 8.26 | 8.86 |
| In total | 98.72 | 95.86 | |
| Substance | Content in the extract | ||
|---|---|---|---|
| P1 | P2 | P3 | |
| UPLC-MS/MS, µg/g of dry extract | |||
| Neochlorogenic acid | 2109.57 ± 70.12 | 474.21 ± 4.02 | 805.71± 32.49 |
| Luteolin | 271.53 ± 24.12 | 1927.41 ± 70.51 | 114.13 ± 25.62 |
| Cryptochlorogenic acid | 19.81 ± 2.66 | 228.8 ± 17.58 | 23.44 ± 3.11 |
| Luteolin-4-O-glucoside | 6.93 ± 1.07 | 9.27 ± 1.98 | 0 |
| Chlorogenic acid | 3148.29 ± 143.312 | 10836.74 ± 203.23 | 2202.01 ± 20.64 |
| Isorhamnetin-3-glucoside | 49.65 ± 3.11 | 40.52 ± 7.19 | 18.79 ± 1.86 |
| Luteolin-3,7-diglucoside | 117.36 ± 5.927 | 157.59 ± 2.80 | 21.69 ± 2.3 |
| Vanillic acid | 23.87 ± 2.87 | 22.45 ± 1.19 | 14.25 ± 1.18 |
| Caffeic acid | 37.32 ± 3.81 | 32.33± 3.26 | 51.82 ± 5.66 |
| 3,4-Dihydroxyphenylacetic acid | 335.69 ± 9.49 | 117.88 ± 7.33 | 146.11 ± 7.11 |
| Isorhamnetin | 6.6 ± 0.39 | 26.96 ± 2.32 | 8. 4 ± 1.29 |
| Hyperoside | 139.61 ± 1.91 | 194.14 ± 17.13 | 51.95 ± 0.93 |
| Luteolin-7-O-glucoside | 2844.8± 212.97 | 8101.17 ± 1237.03 | 766.53 ± 188.39 |
| 4,5-Dicaffeoylquinic acid | 3339.61 ± 52.33 | 3049.98 ± 143.4 | 925.79 ± 48.57 |
| 3,5-Dicaffeoylquinic acid | 1708.29 ± 26.77 | 1578.86 ± 99.56 | 471.56 ± 26.17 |
| 3,4-Dicaffeoylquinic acid | 3502.78 ± 54.88 | 3233.96 ± 208.24 | 967.68 ± 54.97 |
| Spectrophotometry, % | |||
| Phenolic compounds | 5.62 ± 0.06 | 10.74 ± 0.39 | 3.17 ± 0.08 |
| Hydrocinnamic acids | 1.55 ± 0.28 | 3.31 ± 0.25 | 0.98 ± 0.31 |
| Flavonoids | 2.37 ± 0.13 | 8.09 ± 0.54 | 0.28 ± 0.06 |
| Extraction stage | Dry residue, % | Content (%) in the dry residue | ||
|---|---|---|---|---|
| Phenolic compounds |
Hydrocinnamic acids |
Flavonoids | ||
| 1 | 3.57 ± 0.88 | 12.11 ± 0.22 | 2.73 ± 0.07 | 8.75 ± 0.38 |
| 2 | 1.77 ± 0.52 | 10.74 ± 0.16 | 3.91 ± 0.13 | 9.47 ± 0.11 |
| 3 | 1.13 ± 0.09 | 8.96 ± 0.25 | 3.73 ± 0.26 | 5.13 ± 0.15 |
| 4 | 0,.67 ± 0.25 | 7.53 ± 0.53 | 2.59 ± 0.39 | 2.88 ± 0.04 |
| 5 | 0.3 | 7.00 ± 0.15 | 1.55 ± 0.19 | 2.25 ± 0.16 |
| 6 | 0.2 | 4.84 ± 0.18 | 1.01 ± 0.12 | 1.29 ± 0.03 |
| Ligand | Biotargets | ||||
|---|---|---|---|---|---|
| LOX-5 (6NCF) |
COX-1 (3N8Y) |
СОХ-2 (3LN1) |
NMDA (7EU7) |
ГАМКА (6X3W) |
|
| AKBA | -10,0 | – | – | – | – |
| Diclofenac | – | -8.5 | -8.4 | – | – |
| Celecoxib | – | – | -12.2 | – | – |
| Ketamine | – | – | – | -5.6 | – |
| Phenobarbital | – | – | – | – | -7.3 |
| Neochlorogenic acid | -7.9 | -7.1 | -7.5 | -7.1 | -6.8 |
| Chlorogenic acid | -7.8 | -7.1 | -7.5 | -6.9 | -6.8 |
| Cryptochlorogenic acid | -7.8 | -6.6 | -7.9 | -7.0 | -6.4 |
| Luteolin | -8.1 | -8.1 | -9.8 | -7.4 | -6.6 |
| Luteolin-4-O-glucoside | -9.0 | -5.6 | -8.6 | -8.1 | -6.0 |
| Luteolin-7-O-glucoside | -9.6 | -5.4 | -6.2 | -8.4 | -6.5 |
| Luteolin-3,7-diglucoside | -9.7 | -5.3 | -6.8 | -7.9 | -6.5 |
| Isorhamnetin-3-glucoside | -7.8 | -1.8 | -8.8 | -7.9 | -6.5 |
| Vanillic acid | -6.7 | -6.2 | -6.4 | -4.9 | -5.1 |
| Caffeic acid | -6.0 | -6.5 | -7.4 | -5.1 | -5.0 |
| 3,4-Dihydroxyphenylacetic acid | -6.7 | -6.1 | -6.6 | -5.2 | -4.9 |
| Isorhamnetin | -7.9 | -7.8 | -9.6 | -7.3 | -6.4 |
| Rutin | -8.9 | -0.6 | -3.7 | -9.1 | -6.2 |
| Hyperoside | -8.6 | -2.1 | -8.2 | -7.7 | -6.6 |
| 4,5-Dicaffeoylquinic acid | -8.8 | -6.1 | -9.1 | -8.1 | -7.0 |
| 3,5-Dicaffeoylquinic acid | -9.0 | -6.8 | -8.5 | -7.9 | -7.5 |
| 3,4-Dicaffeoylquinic acid | -8.8 | -6.0 | -8.8 | -7.9 | -7.3 |
| LOX-5 (6NCF) |
Luteolin- 3,7-diglucoside |
a: Thr104, His130, Leu163, Glu134, Pro164; b: Thr137, Val107(3); c: Arg101(Pi-Cation) |
|---|---|---|
| Rutin | a: Arg68, Arg101, Glu134, His130, Thr137; b: Lys133, Val107(3) |
|
| 3,5-Dicaffeoylquinic acid | a: Arg68, Arg101, Val110, His130, Asp166, Glu108; b: His130, Leu66, Val107 |
|
| СОХ-1 | Luteolin | a: Ser530(2), TYR385; b: Ala527(4), Gly526(2), Val349(2), Leu531 |
| Isorhamnetin | a: Ser530(2), Met522, Ala527; b: Gly526(2), Ala527(4),Val349(2), Leu531 |
|
| Chlorogenic acid | a: Tyr385, Ser530, Tyr385, Met522; b: Val349,Leu359, Ala527, Leu531 |
|
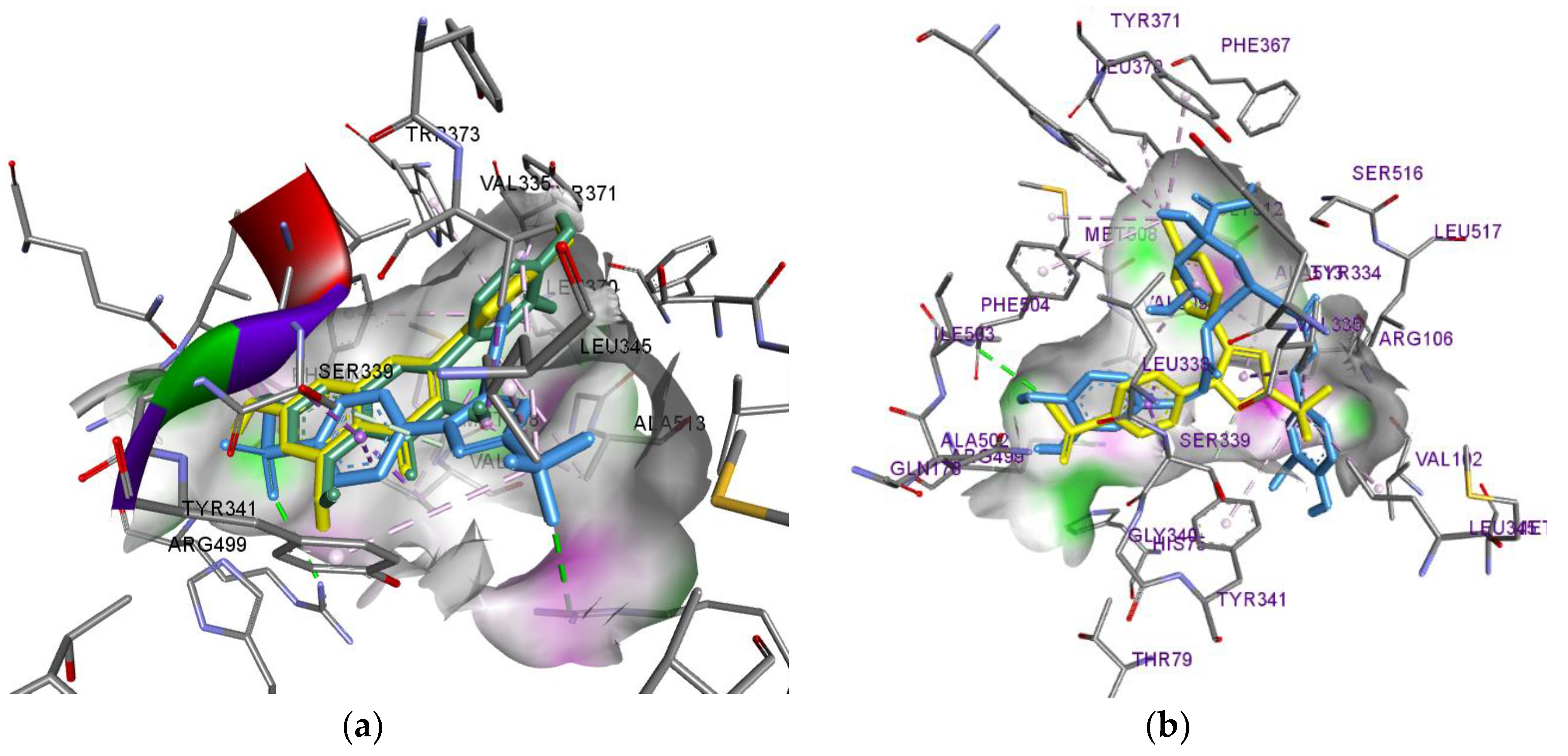
| СОХ-2 | Isorhamnetin | a: Tyr341, Ser516, Ser339, Tyr371; Leu338(2); b:Val509, Val335 |
| Luteolin | a: Tyr341, Ser516, Ile503, Phe504, Tyr371; b: Leu338, Val509(2), Leu338, Val335 |
|
| 4,5-Dicaffeoylquinic acid | a: Arg106, Tyr371, Gly512 b: Val509(3), Tyr341, Val102, Leu345, Ala502 |
|
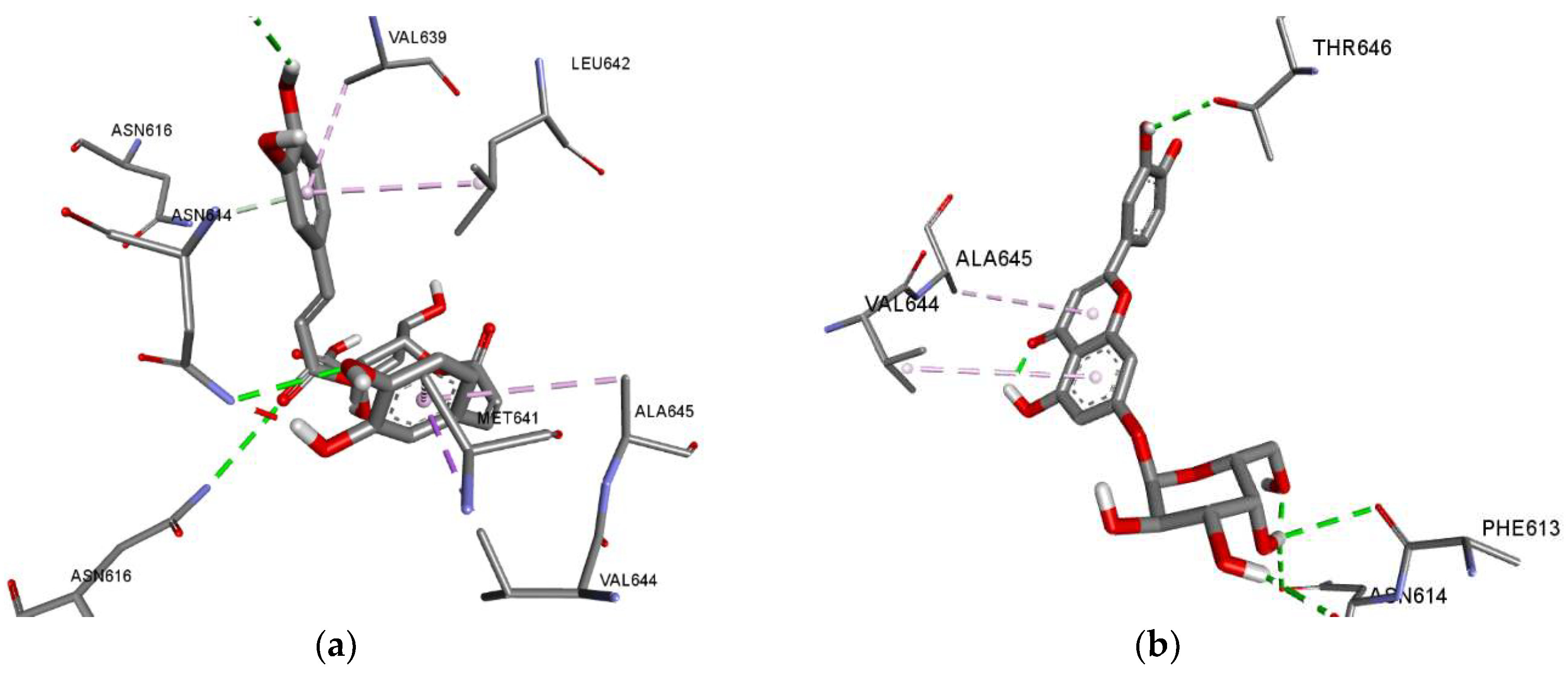
| NMDA | 4,5-Dicaffeoylquinic acid | a: Asn616(2), Asn614, Leu611, Asn616; b: Val644, Val639, Leu642, Met641, Ala645 |
| Luteolin | a: Phe613, Leu611(2), Asn615(2), b: Val644(2), Val639, Leu642 |
| Agent | Group | Dose, mg/kg | The time of discomfort occurrence (seconds) / Analgesic activity (%) in relation to [control] and (reference drug) | |||||
|---|---|---|---|---|---|---|---|---|
| after administration in | ||||||||
| 30 min | 60 min | 120 min | 180 min | 240 min | ||||
| Intact animals | 1 | 7.10±0.32 | 7.00±0.50 | 7.05±0.28 | 6.98±0,52 | 6.40±0.63 | ||
| Extract P1 | 2 | 25 | 8.85±0.69 / [25%] (-15%) |
9.13±0.77 / [30%] * (-12%) |
10.67±0.49/ [51%] * (1%) |
10.40±0.55/ [49%] * (9%) |
9.12±0.51/ [42%] * (9%) |
|
| 3 | 50 | 10.15±1.49/ [43] (-3%) |
10.30±1.01 / [47%] * (-1%) |
12.15±0.39/ [72%] * (15%) |
11.07±0,54/ [58%] * (16%) |
9.65±0.28/ [51%] * (15%) # |
||
| 4 | 100 | 10.67±2.79 / [50%] (2%) |
12.07±2.40/ [72%] (16%) |
11.12±1.27/ [58%] * (5%) |
10.57±1.19/ [50%] * (11%) |
8.87±1.27/ [39%] (6%) |
||
| Extract P2 | 5 | 25 | 10.63±1.01 / [50%] * (2%) |
10.42±0.88 / [49%] * (0%) |
10.72±0.62/ [52%] * (1%) |
10.47±0.67/ [50%] * (10%) |
9.48±0.92 / [48%] * (13%) |
|
| 6 | 50 | 10.98±0.58 / [55%] * (5%) |
11.67±0.53 / [67%] * (12%) |
12.78±1.87/ [81%] * (21%) |
11.72±1.76/ [68%] * (23%) |
10.10±1.20/ [58%] * (20%) |
||
| 7 | 100 | 11.65±1.46 / [64%] (12%) |
12.72±1.58 / [82%] * (22%) |
12.55±1.53/ [78%] * (19%) |
10.30±0.94/ [47%] * (8%) |
9.93±1.01/ [55%] * (18%) |
||
| Extract P3 | 8 | 25 | 8.97±0.83 / [26%] (-14%) |
9.42±1.31 / [35%] (-10%) |
9.93±1.11 / [41%] * (-6%) |
9.57±0.74 / [37%] * (1%) |
9.00±0.79 / [41%] * (7%) |
|
| 9 | 50 | 7.98±0.47 / [12%] (-24%) # |
9.85±1.17 / [41%] (-6%) |
10.37±1.21/ [47%] (-2%) |
10.08±0.99/ [44%] (6%) |
8.12±1.02 / [27%] (-3%) |
||
| 10 | 100 | 9.07±0.77 / [28%] (-13%) |
10.33±0.65 / [48%] * (-1%) |
11.03±0.75/ [57%] * (4%) |
10.25±1.10/ [47%] * (8%) |
8.75±0.60 / [37%] * (4%) |
||
| Acetaminophen | 11 | 50 | 10.45±0.73 [45%] * |
10.43±0.59 [49%] * |
10.57±0.71 [50%] * |
9.50±0.57 [36%] * |
8.38±0.33 [31%] * |
|
| Agent | Group | Dose, mg/kg | Average duration of a sleep, min | Soporific effect, % |
|---|---|---|---|---|
| Control group | 1 | 40 | 87.33±11.56 | 100.0% |
| Extract P1 | 2 | 25 | 180.17±11.37* | 206.3% |
| 3 | 50 | 171.67±2.87* | 196.6% | |
| 4 | 100 | 170.00±9.27* | 195.8% | |
| Extract P2 | 5 | 25 | 243.00±8.07*# | 278.2% |
| 6 | 50 | 215.50±10.57*# | 246.8% | |
| 7 | 100 | 248.67±6.10*# | 284.7% | |
| Extract P3 | 8 | 25 | 165.67±12.26* | 189.7% |
| 9 | 50 | 156.17±10.81*# | 178.8% | |
| 10 | 100 | 167.67±10.11* | 192.0% | |
| Valerian extract | 11 | 2.15 | 185.33±5.42* | 212.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





