Submitted:
08 February 2024
Posted:
09 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
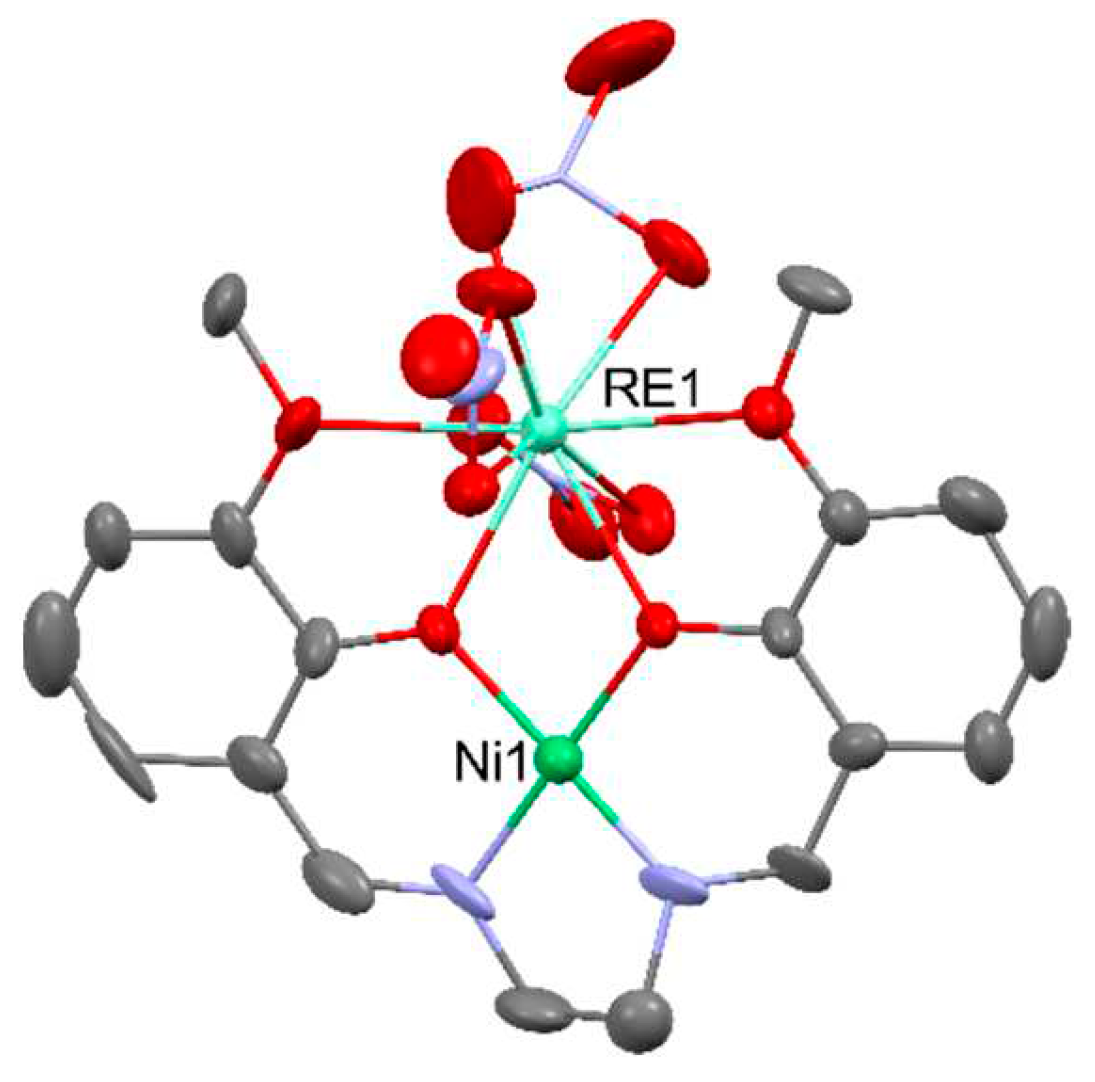
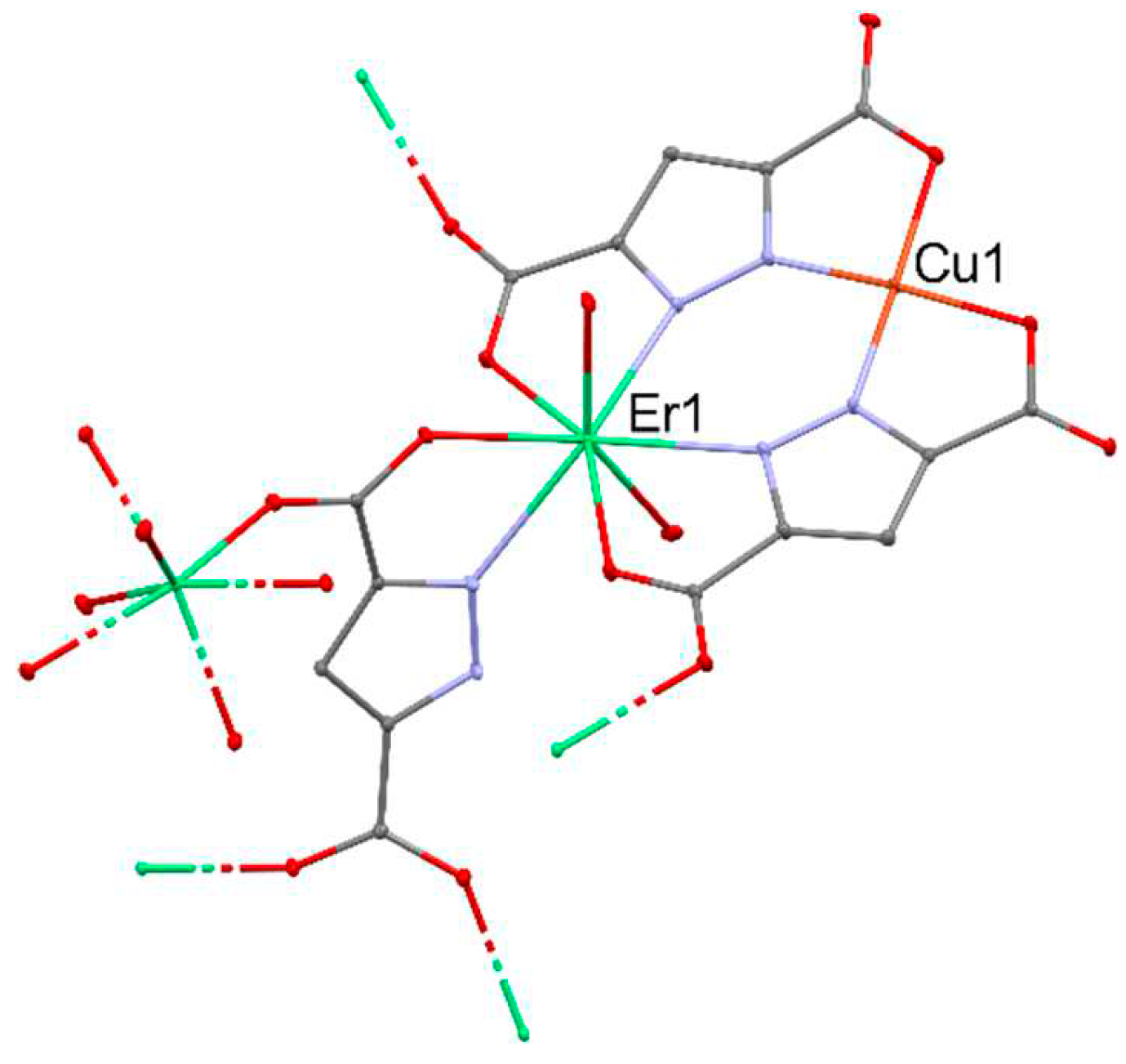
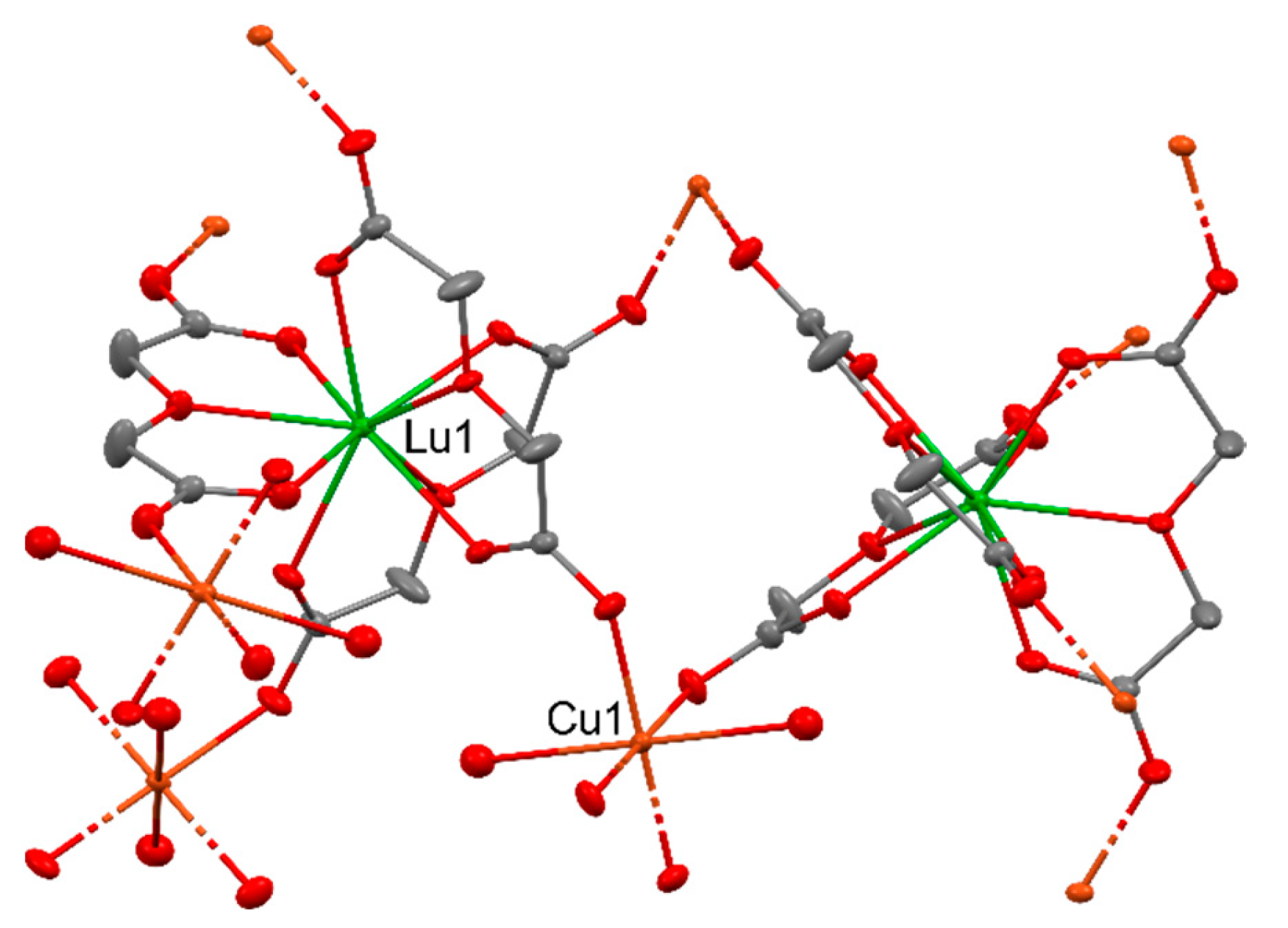
2. Heteromatallic rare-earth complexes, synthesis and applications
2.1. Synthesis routes
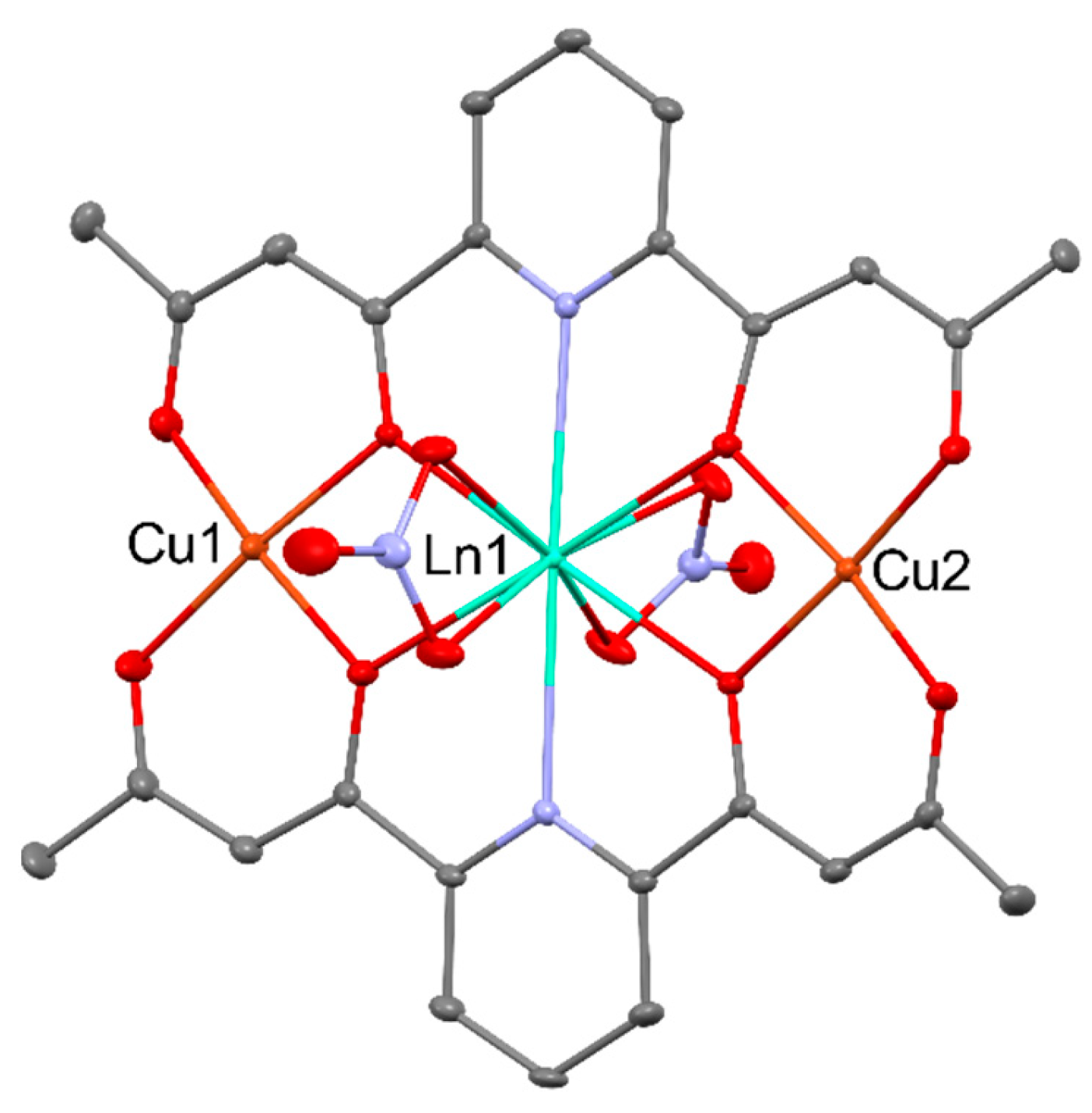
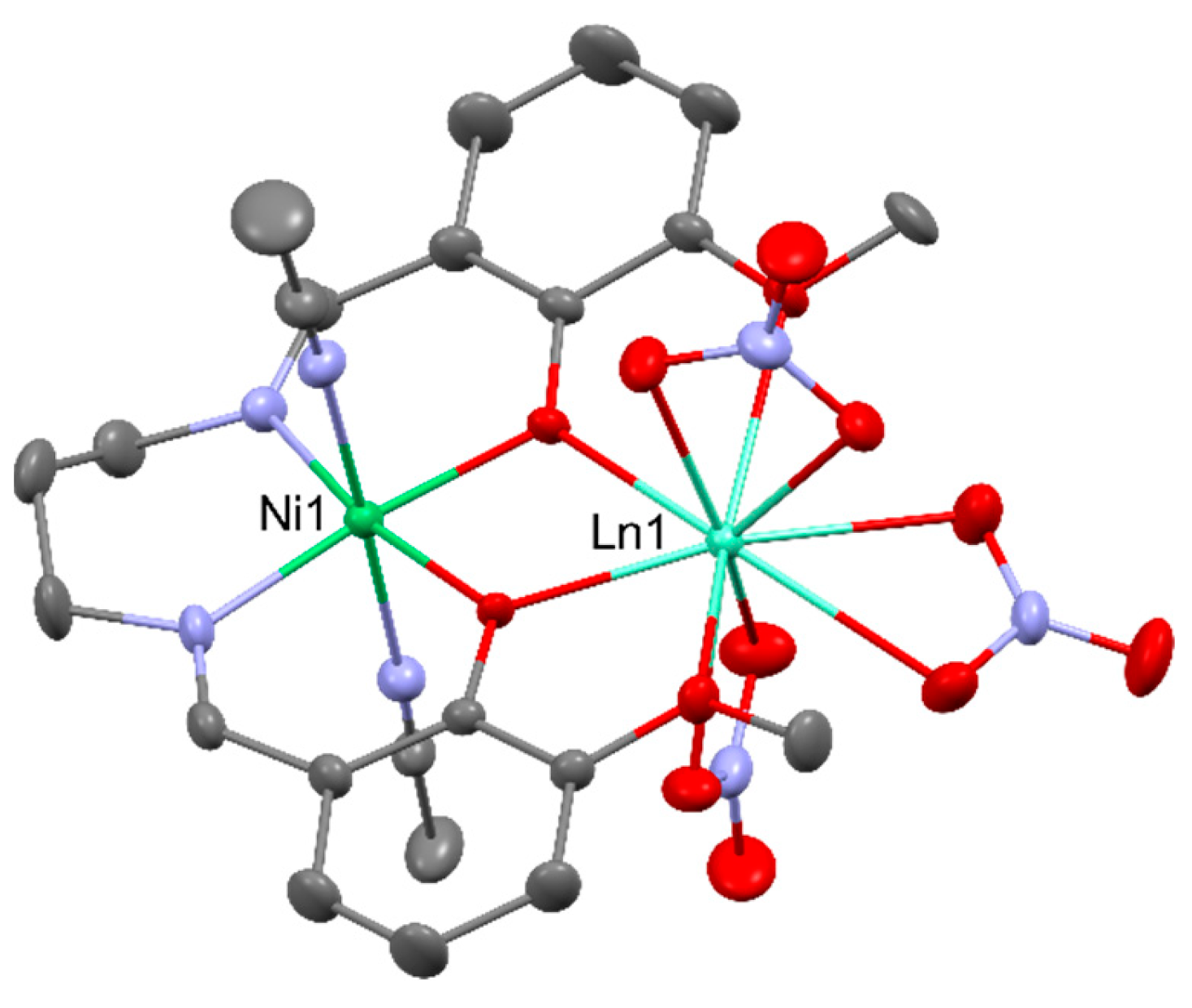
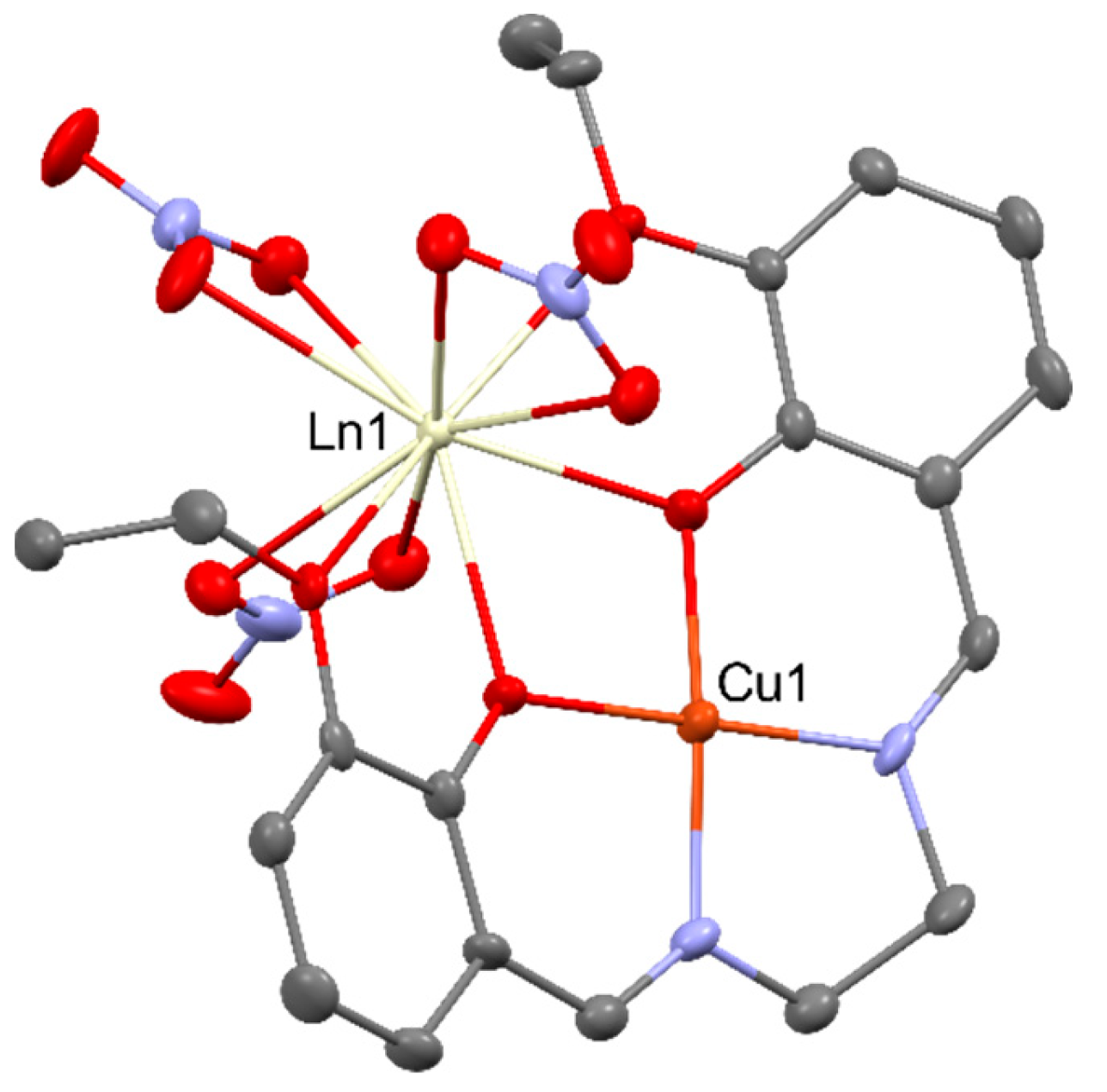
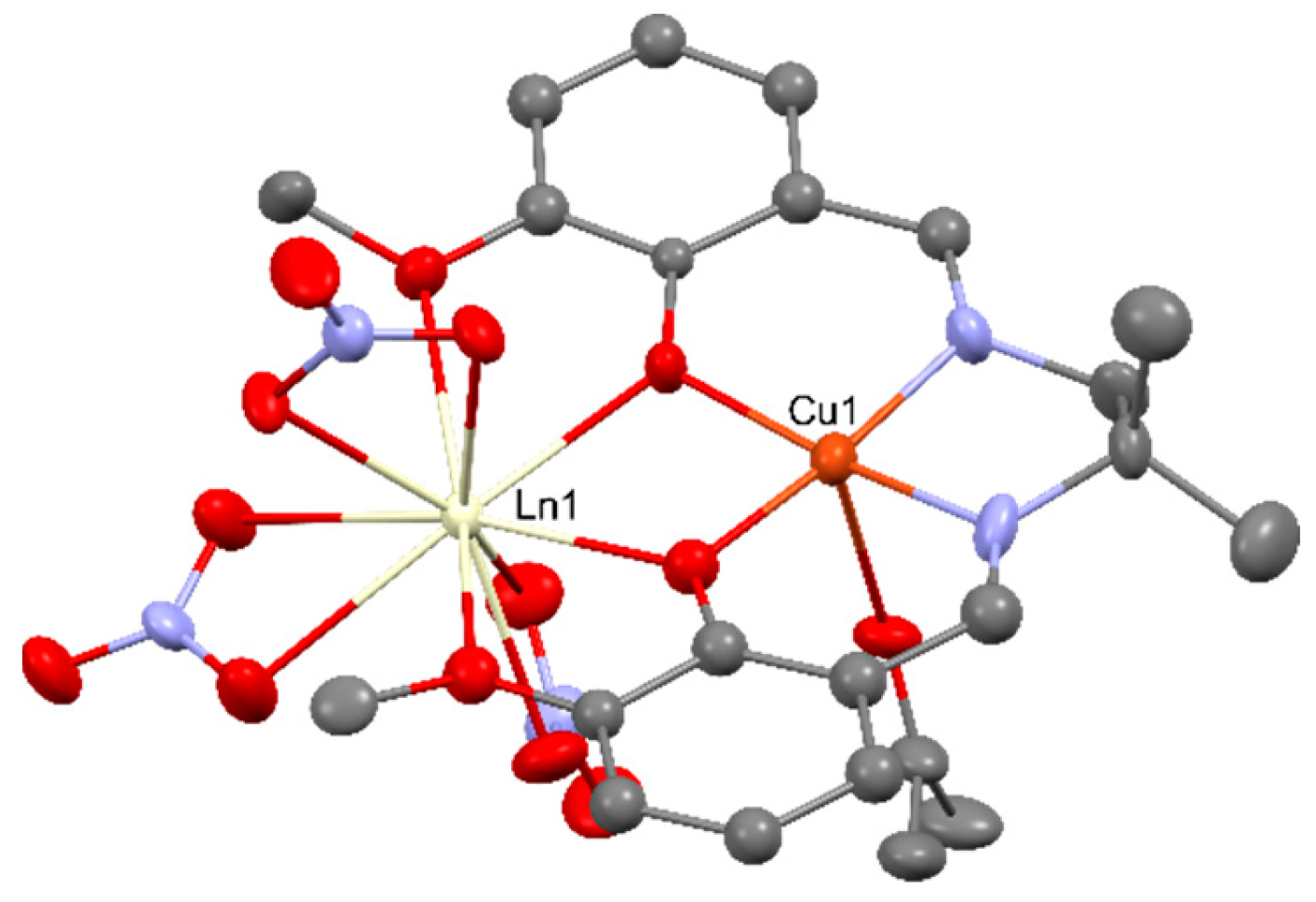
2.2. Magnetism
2.3. Catalysis, ring-opening polymerization and copolymerization of cyclic esters
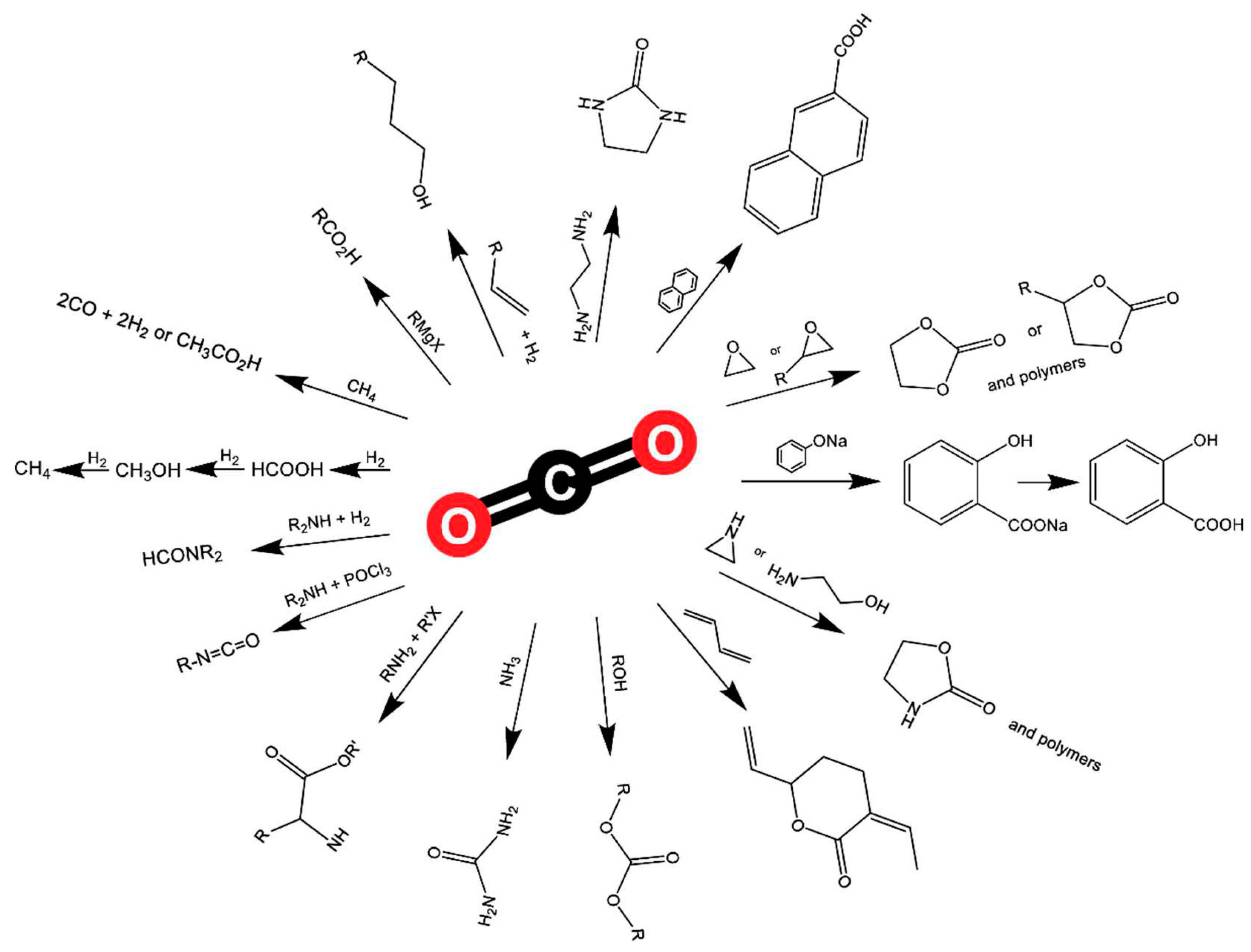
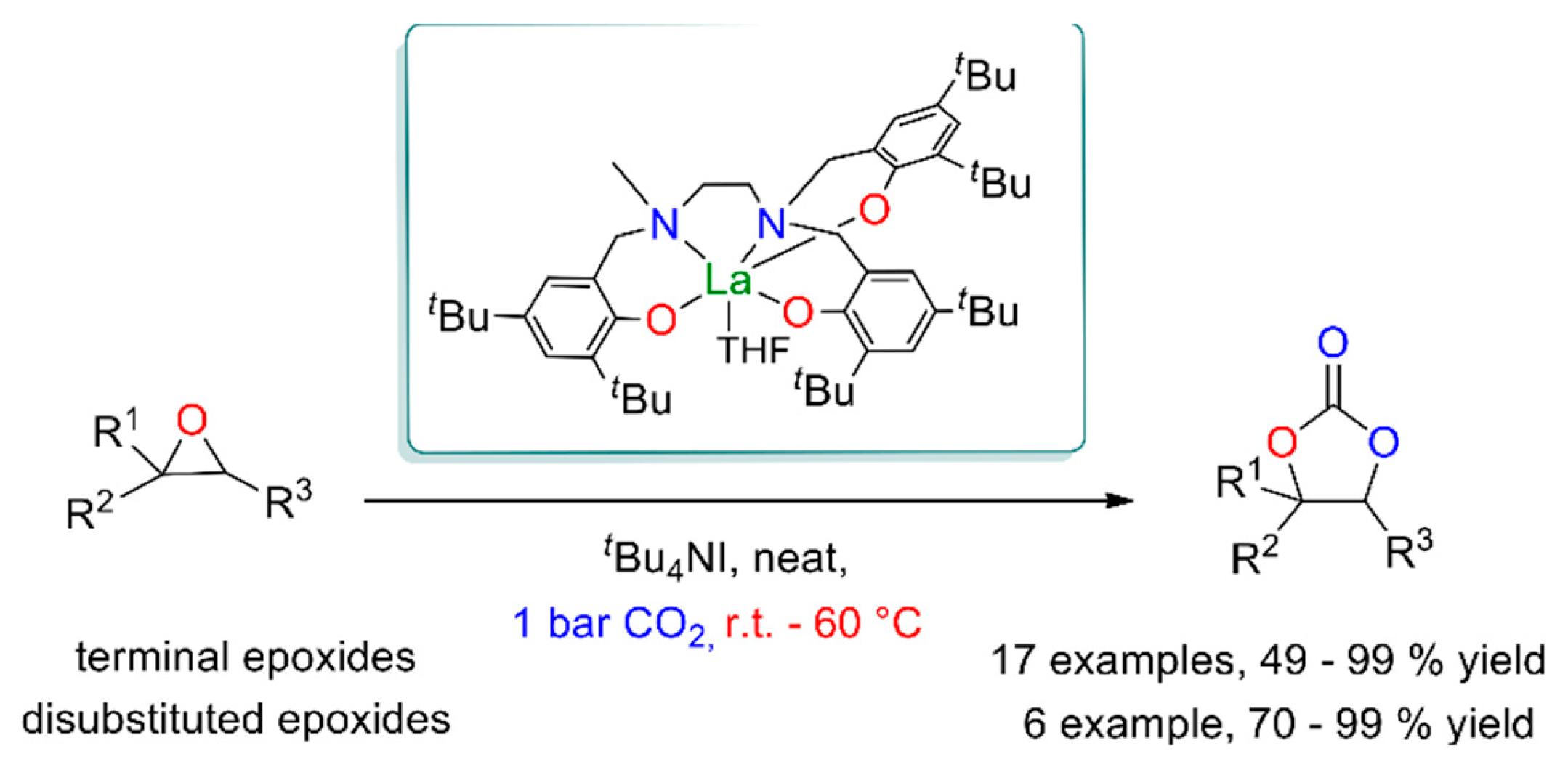
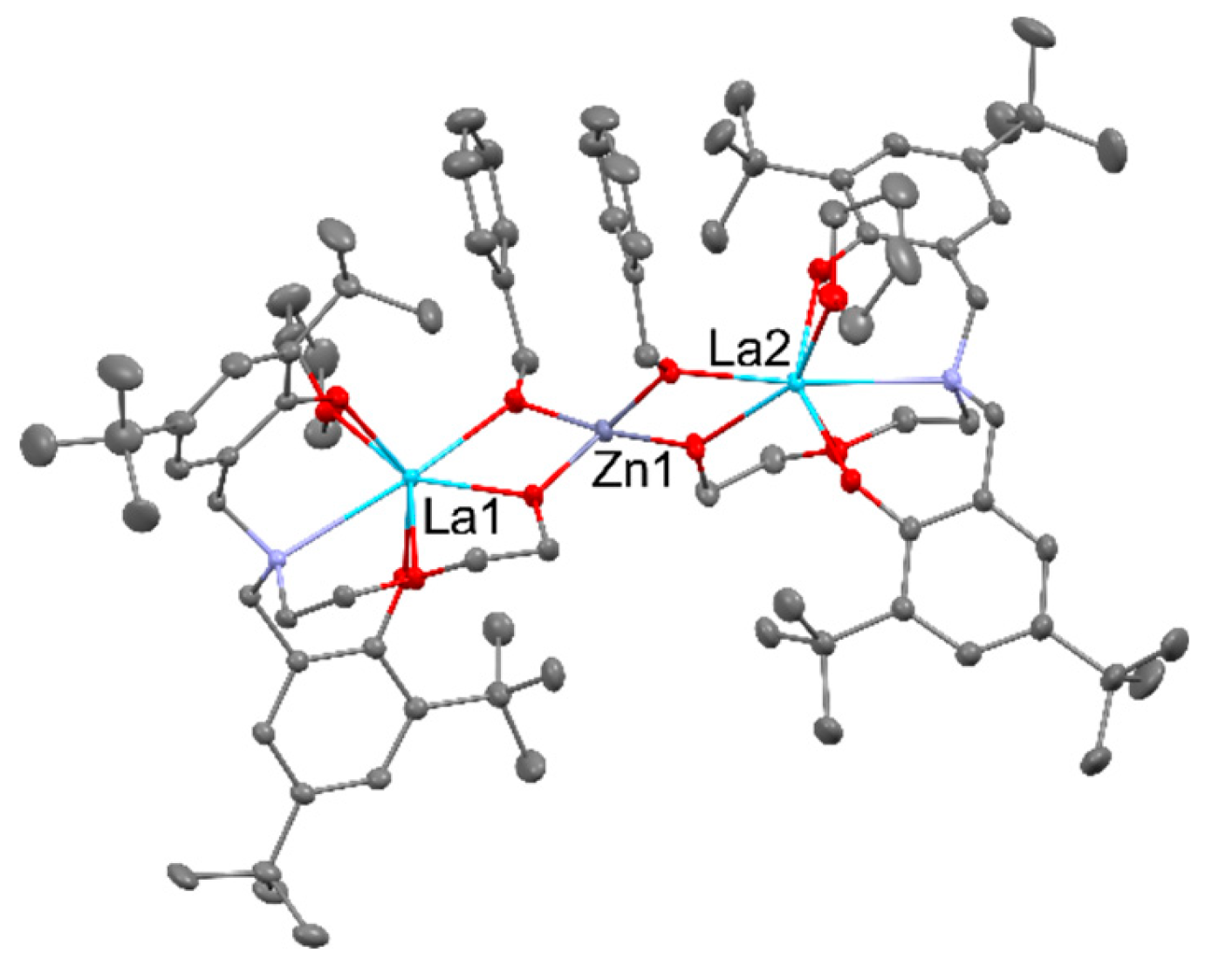
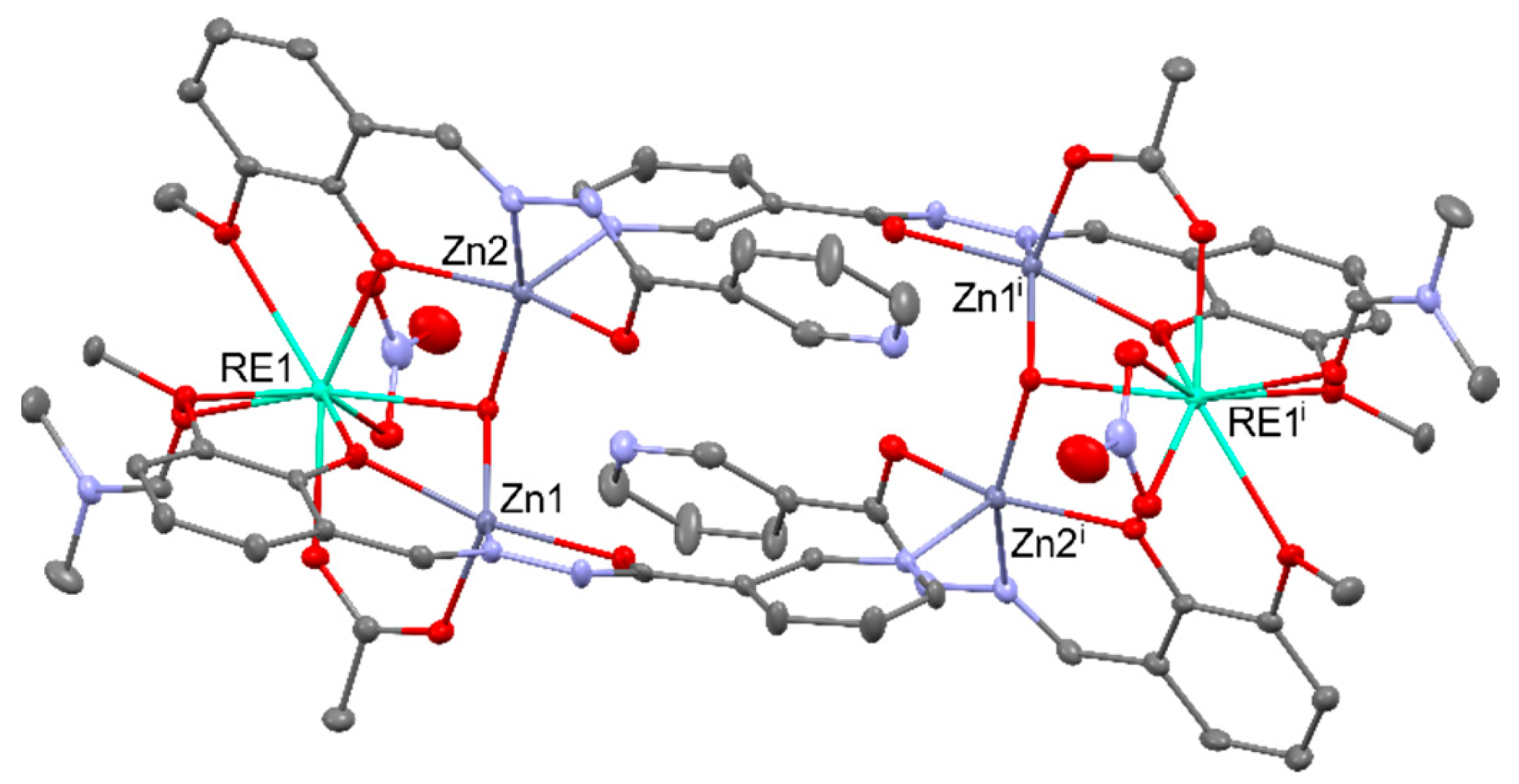
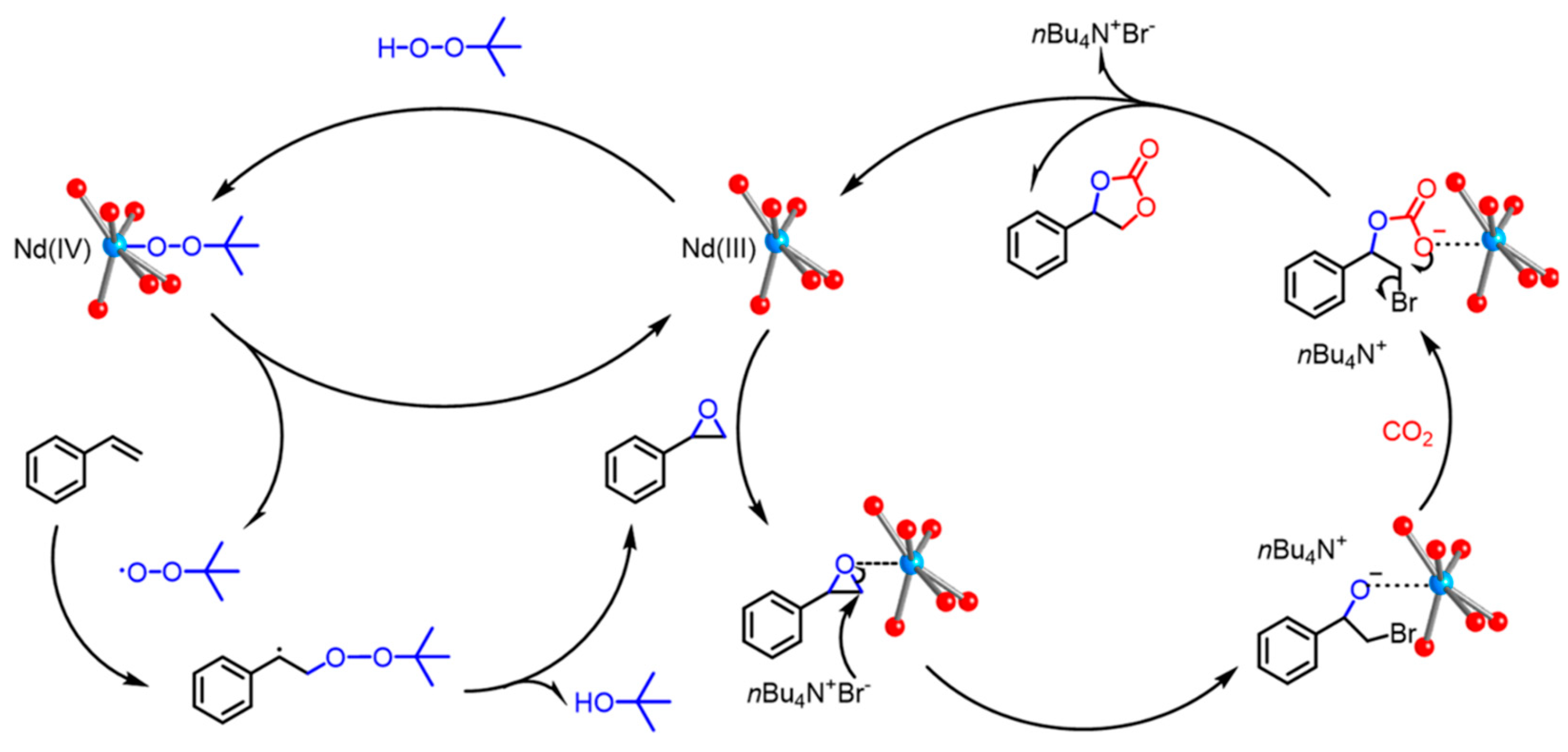
2.4. CO2 conversion
2.5. Catalysts for energy conversion processes
2.6. Molecular precursors of functional inorganic materials.
3. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Castor, S.B. and Hedrick, L.B. Rare Earth Elements in Kogel, J.E, Trivedi, N.C., Barker, J.M., and Krukowski, S.T., ed., Industrial Minerals volume, 7th edition: Society for Mining, Metallurgy, and Exploration, Littleton, Colorado, United States, 2006; pp. 769–792.
- Wall, F. , in Gunn, G., ed., Critical Metals Handbook, John Wiley & Sons, Oxford, 2014; p. 312-339.
- Czerwiński, F. Critical Assessment 36: Assessing Differences between the Use of Cerium and Scandium in Aluminium Alloying. Mater. Sci. Technol. 2019, 36, 255–263. [Google Scholar] [CrossRef]
- Voncken, J. H. L. The Rare Earth Elements; 2016. [CrossRef]
- Vitova, T.; Roesky, P. W.; Dehnen, S. Open Questions on Bonding Involving Lanthanide Atoms. Commun. Chem. 2022, 5. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons, 2013.
- Wang, Z.; Guo, Y.; Gong, X.; Guo, Y.; Wang, Y.; Lu, G. Current Status and Perspectives of Rare Earth Catalytic Materials and Catalysis. Chin. J. Catal. 2014, 35, 1238–1250. [Google Scholar] [CrossRef]
- Van Gosen, B. S.; Verplanck, P. L.; Long, K. R.; Gambogi, J.; Seal, R. R. The Rare-Earth Elements: Vital to Modern Technologies and Lifestyles. Fact Sheet /. [CrossRef]
- Coey, J. M. D. Perspective and Prospects for Rare Earth Permanent Magnets. Engineering 2020, 6, 119–131. [Google Scholar] [CrossRef]
- Lucas, J.; Lucas, P.; Mercier, T. L.; Rollat, A.; Davenport, W. G. Rare Earths in Rechargeable Batteries. In Elsevier eBooks; 2015; pp 167–180. [CrossRef]
- Seddon, A. B.; Tang, Z.; Furniss, D.; Sujecki, S.; Benson, T. M. Progress in Rare-Earth-Doped Mid-Infrared Fiber Lasers. Opt. Express 2010, 18, 26704. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, M.; Murtaza, Q.; Kumar, P. Effect of Rare Earth Elements on Coatings Developed by Thermal Spraying Processes (TSP) – A Brief Review. Mater. Today: Proc. 2021, 44, 4053–4058. [Google Scholar] [CrossRef]
- Dubey, V.; Dubey, N.; Domanska, M. M.; Jayasimhadri, M.; Dhoble, S. J. Rare-Earth-Activated Phosphors: Chemistry and Applications; Elsevier, 2022.
- Hossain, Md. F.; Ahmed, M. H.; Khan, I.; Miah, Md. S.; Hossain, S. Recent Progress of Rare Earth Oxides for Sensor, Detector, and Electronic Device Applications: A Review. ACS Appl. Electron. Mater. 2021, 3, 4255–4283. [Google Scholar] [CrossRef]
- Hossain, M. K.; Raihan, G. A.; Akbar, M. A.; Rubel, M. H. K.; Ahmed, M. H.; Khan, I.; Hossain, S.; Sen, S. K.; Jalal, M. I. E.; El-Denglawey, A. Current Applications and Future Potential of Rare Earth Oxides in Sustainable Nuclear, Radiation, and Energy Devices: A Review. ACS Appl. Electron. Mater. 2022, 4, 3327–3353. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.; Sun, M.; Ding, W. Recent Developments and Applications on High-Performance Cast Magnesium Rare-Earth Alloys. J. Magnes. Alloys 2021, 9, 1–20. [Google Scholar] [CrossRef]
- Luo, Q.; Guo, Y.; Liu, B.; Feng, Y.; Zhang, J.; Li, Q.; Chou, K. Thermodynamics and Kinetics of Phase Transformation in Rare Earth–Magnesium Alloys: A Critical Review. J. Mater. Sci. Technol. 2020, 44, 171–190. [Google Scholar] [CrossRef]
- Смирнoв, Л. А.; Рoвнушкин, В. А.; Орыщенкo, А. С.; Kalinin, G. Yu.; Milyuts, V. G. Modification of Steel and Alloys with Rare-Earth Elements. Part 1. Metallurgist 2016, 59 (11–12), 1053–1061. [CrossRef]
- Zhao, K.; Gao, Y.; Wang, X.; Lis, B. M.; Liu, J.; Jin, B.; Smith, J.; Huang, C.; Gao, W.; Wang, X.; Wang, X.; Zheng, A.; Huang, Z.; Hu, J.; Schomaecker, R.; Wachs, I. E.; Li, F. Lithium Carbonate-Promoted Mixed Rare Earth Oxides as a Generalized Strategy for Oxidative Coupling of Methane with Exceptional Yields. Nat. Commun. 2023, 14. [Google Scholar] [CrossRef]
- Khodakov, Yu. S.; Nesterov, V. K.; Миначев, Х. М. Isomerization of 1-Butene on Oxides of Rare-Earth Elements. Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science 1975, 24, 1892–1894. [Google Scholar] [CrossRef]
- Wang, Z.; Fongarland, P.; Lu, G.; Wang, Z.; Essayem, N. Effect of Hydration on the Surface Basicity and Catalytic Activity of Mg-Rare Earth Mixed Oxides for Aldol Condensation. J. Rare Earths 2018, 36, 359–366. [Google Scholar] [CrossRef]
- Imamura, H.; Ohmura, A.; Haku, E.; Tsuchiya, S. Rare Earth Metals as Hydrogenation Catalysts of Unsaturated Hydrocarbons. J. Catal. 1985, 96, 139–145. [Google Scholar] [CrossRef]
- Sato, S.; Sato, F.; Gotoh, H.; Yamada, Y. Selective Dehydration of Alkanediols into Unsaturated Alcohols over Rare Earth Oxide Catalysts. ACS Catal. 2013, 3, 721–734. [Google Scholar] [CrossRef]
- Bochkarev, M. N.; Pushkarev, A. P. Synthesis and Luminescence of Some Rare Earth Metal Complexes. Org. Photonics Photovolt. 2016, 4. [Google Scholar] [CrossRef]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaro, G.; Cavazzini, M.; Tondello, E. Design of Luminescent Lanthanide Complexes: From Molecules to Highly Efficient Photo-Emitting Materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Li, S.; Zhou, L.; Zhang, H. Investigation Progresses of Rare Earth Complexes as Emitters or Sensitizers in Organic Light-Emitting Diodes. Light-Sci. Appl. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Evans, W. J.; Johnston, M. C.; Greci, M. A.; Ansari, M. A.; Brady, J. C.; Ziller, J. W. Synthesis of Arene-Soluble Mixed-Metal Zr/Ce, Zr/Y, and Related {[Zr2(OiPr)9]LnX2}n Complexes Using the Dizirconium Nonaisopropoxide Ligand. Inorg. Chem. 2000, 39, 2125–2129. [Google Scholar] [CrossRef]
- Artner, C.; Kronister, S.; Czakler, M.; Schubert, U. Ion-Size-Dependent Formation of Mixed Titanium/Lanthanide OXO Clusters. Eur. J. Inorg. Chem. 2014, 2014, 5596–5602. [Google Scholar] [CrossRef]
- Mashima, K.; Nakamura, A. Novel Synthesis of Lanthanoid Complexes Starting from Metallic Lanthanoid Sources. J. Chem. Soc.-Dalton Trans. 1999, 3899–3907. https://doi.org/10.1039/a905998i. (b) Deacon, G. B.; Hamidi, S.; Junk, P. C.; Kelly, R. L.; Wang, J. Direct Reactions of Iodine-Activated Rare-Earth Metals with Phenols of Varying Steric Bulk. Eur. J. Inorg. Chem. 2013, 2014, 460–468. Eur. J. Inorg. Chem. [CrossRef]
- Petrus, R.; Kowaliński, A.; Utko, J.; Matuszak, K.; Lis, T.; Sobota, P. Heterometallic 3D–4f Alkoxide Precursors for the Synthesis of Binary Oxide Nanomaterials. Inorg. Chem. 2023, 62, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Petrus, R.; Chomiak, K.; Utko, J.; Bieńko, A.; Lis, T.; Sobota, P. Heterometallic Group 4–Lanthanide Oxo-Alkoxide Precursors for Synthesis of Binary Oxide Nanomaterials. Inorg. Chem. 2020, 59, 16545–16556. [Google Scholar] [CrossRef] [PubMed]
- Botta, M.; Casellato, U.; Scalco, C.; Tamburini, S.; Tomasin, P.; Vigato, P. A.; Aime, S.; Barge, A. Heterodinuclear LnNa Complexes with an Asymmetric Macrocyclic Compartmental Schiff Base. Chem. A Eur. J. 2002, 8, 3917–3926. [Google Scholar] [CrossRef]
- Xu, X.; Ma, M.; Yao, Y.; Zhang, Y.; Shen, Q. Synthesis, Characterisation of Carbon-Bridged (Diphenolato)Lanthanide Complexes and Their Catalytic Activity for Diels–Alder Reactions. Eur. J. Inorg. Chem. 2005, 2005, 676–684. [Google Scholar] [CrossRef]
- Zheng, Z.-P.; Ou, Y.-J.; Hong, X.; Wei, L.-M.; Wan, L.-T.; Zhou, W.; Zhan, Q.-G.; Cai, Y. Anion-Dependent Assembly of Four Sensitized Near-Infrared Luminescent Heteronuclear ZnII–YbIII Schiff Base Complexes from a Trinuclear ZnII Complex. Inorg. Chem. 2014, 53, 9625–9632. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yin, K.; Chen, Y.; Zhao, B.; Zhang, Y.; Zhu, X.; Yuan, D.; Yao, Y. Synthesis of Heterometallic Rare Earth(III)–Cobalt(II) Complexes and Their Application in Alternating Copolymerization of Cyclohexene Oxide and Carbon Dioxide. Chin. J. Chem. 2023, 41, 805–813. [Google Scholar] [CrossRef]
- Langley, S. K.; Le, C.; Ungur, L.; Moubaraki, B.; Abrahams, B. F.; Chibotaru, L. F.; Murray, K. S. Heterometallic 3D–4f Single-Molecule Magnets: Ligand and Metal Ion Influences on the Magnetic Relaxation. Inorg. Chem. 2015, 54, 3631–3642. [Google Scholar] [CrossRef] [PubMed]
- Piquer, L. R.; Sañudo, E. C. Heterometallic 3d–4f Single-Molecule Magnets. Dalton Trans. 2015, 44, 8771–8780. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Acharya, J.; Chandrasekhar, V. Heterometallic 3D–4F Complexes as Single-Molecule Magnets. Chem.-Asian J. 2019, 14, 4433–4453. [Google Scholar] [CrossRef]
- Chakraborty, A.; Goura, J.; Kalita, P.; Swain, A.; Rajaraman, G.; Chandrasekhar, V. Heterometallic 3d–4f Single Molecule Magnets Containing Diamagnetic Metal Ions. Dalton Trans. 2018, 47, 8841–8864. [Google Scholar] [CrossRef]
- Kahn, M. L.; Mathonière, C.; Kahn, O. Nature of the Interaction between LnIII and CuII Ions in the Ladder-Type Compounds {Ln2[Cu(Opba)]3}·S (Ln = Lanthanide Element; Opba = Ortho-Phenylenebis(Oxamato), S = Solvent Molecules). Inorg. Chem. 1999, 38, 3692–3697. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M. L.; Lecante, P.; Verelst, M.; Mathonière, C.; Kahn, O. Structural Studies and Magnetic Properties of Polymeric Ladder-Type Compounds {Ln2[Ni(Opba)]3}·S (Ln = Lanthanide Element; Opba = o-Phenylenebis(Oxamato), S = Solvent Molecules). Chem. Mater. 2000, 12, 3073–3079. [Google Scholar] [CrossRef]
- Shiga, T.; Ohba, M.; Ōkawa, H. A Series of Trinuclear CuIILnIIICuII Complexes Derived from 2,6-Di(Acetoacetyl)Pyridine: Synthesis, Structure, and Magnetism. Inorg. Chem. 2004, 43, 4435–4446. [Google Scholar] [CrossRef] [PubMed]
- Păsătoiu, T. D.; Sutter, J.; Mădălan, A. M.; Fellah, F. Z. C.; Duhayon, C.; Andruh, M. Preparation, Crystal Structures, and Magnetic Features for a Series of Dinuclear [NIIILNIII] Schiff-Base Complexes: Evidence for Slow Relaxation of the Magnetization for the DYIII Derivative. Inorg. Chem. 2011, 50, 5890–5898. [Google Scholar] [CrossRef] [PubMed]
- Koner, R.; Lin, H.; Wei, H.; Mohanta, S. Syntheses, Structures, and Magnetic Properties of Diphenoxo-Bridged MIILnIII Complexes Derived from N,N‘-Ethylenebis(3-Ethoxysalicylaldiimine) (M = Cu or Ni; Ln = Ce−Yb): Observation of Surprisingly Strong Exchange Interactions. Inorg. Chem. 2005, 44, 3524–3536. [Google Scholar] [CrossRef]
- Costes, J.; Dahan, F.; Dupuis, A.; Laurent, J. Nature of the Magnetic Interaction in the (CU2+, LN3+) Pairs: An Empirical Approach Based on the Comparison between Homologous (CU2+, LN3+) and (NILS2+, LN3+) Complexes. Chem.-A Eur. J. 1998, 4, 1616–1620. [Google Scholar] [CrossRef]
- Osa, S.; Kido, T.; Matsumoto, N.; Re, N.; Pochaba, A. A.; Mroziński, J. A Tetranuclear 3d−4f Single Molecule Magnet: [CuIILTbIII(Hfac)2]2. Journal of the American Chemical Society 2003, 126, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tan, R.; Yi, T.; Gao, S.; Yan, C.; Cao, L. Preparation of Nanosized La2CuO4 Perovskite Oxide Using an Amorphous Heteronuclear Complex as a Precursor at Low-Temperature. Journal of Alloys and Compounds 2000, 311, 16–21. [Google Scholar] [CrossRef]
- Bridonneau, N.; Gontard, G.; Marvaud, V. A New Family of Hetero-Tri-Metallic Complexes [M(CuTb)]n (n = 1, 2, ∞; M = Co, Cr, Fe): Synthesis, Structure and Tailored Single-Molecule Magnet Behavior. Dalton Trans. 2015, 44, 5170–5178. [Google Scholar] [CrossRef]
- Bridonneau, N.; Gontard, G.; Marvaud, V. A New Family of Hetero-Tri-Metallic Complexes [M(CuTb)]n (n = 1, 2, ∞; M = Co, Cr, Fe): Synthesis, Structure and Tailored Single-Molecule Magnet Behavior. Dalton Trans. 2015, 44, 5170–5178. [Google Scholar] [CrossRef]
- Ghazali, N. F.; Vignesh, K. R.; Phonsri, W.; Murray, K. S.; Junk, P. C.; Deacon, G. B.; Turner, D. R. Efficient Synthetic Route to Heterobimetallic Trinuclear Complexes [Ln–Mn–Ln] and Their Single Molecule Magnetic Properties. Dalton Trans. 2022, 51, 18502–18513. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-Y.; Liu, Y.; Deng, X.; Zhu, Z.; Yao, M.; Jing, S. Synthesis, Structures and Magnetism of Heterodinuclear Ni–Ln Complexes: Field-Induced Single-Molecule Magnet Behavior in the Dysprosium Analogue. New J. Chem. 2015, 39, 3467–3473. [Google Scholar] [CrossRef]
- Colacio, E.; Ruíz, J.; Mota, A. J.; Palacios, M. A.; Cremades, E.; Ruiz, E.; White, F.; Brechin, E. K. Family of Carboxylate- and Nitrate-Diphenoxo Triply Bridged Dinuclear NIIILNIII Complexes (LN = EU, GD, TB, HO, ER, Y): Synthesis, Experimental and Theoretical Magneto-Structural Studies, and Single-Molecule Magnet Behavior. Inorg. Chem. 2012, 51, 5857–5868. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Das, C.; Vaidya, S.; Langley, S. K.; Murray, K. S.; Shanmugam, M. Nickel(II)-Lanthanide(III) Magnetic Exchange Coupling Influencing Single-Molecule Magnetic Features in {NI2LN2} Complexes. Chem.-A Eur. J. 2014, 20, 14235–14239. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Fujinami, T.; Nishi, K.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Re, N. Carbonato-Bridged NiII2LnIII2 (LnIII = GdIII, TbIII, DyIII) Complexes Generated by Atmospheric CO2 Fixation and Their Single-Molecule-Magnet Behavior: [(Μ4-CO3)2{NiII(3-MeOsaltn)(MeOH or H2O)LnIII(NO3)}2]·solvent [3-MeOsaltn = N,N′-Bis(3-Methoxy-2-Oxybenzylidene)-1,3-Propanediaminato]. Inorganic Chemistry 2013, 52, 7218–7229. [Google Scholar] [CrossRef] [PubMed]
- Mondal, K. C.; Kostakis, G. E.; Lan, Y.; Wernsdorfer, W.; Anson, C. E.; Powell, A. K. Defect-Dicubane NI2LN2 (LN = DY, TB) Single Molecule Magnets. Inorg. Chem. 2011, 50, 11604–11611. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pineda, E.; Chilton, N. F.; Tuna, F.; Winpenny, R. E. P.; McInnes, E. J. L. Systematic Study of a Family of Butterfly-Like {M2LN2} Molecular Magnets (M = MGII, MNIII, COII, NIII, and CUII; LN = YIII, GDIII, TBIII, DYIII, HOIII, and ERIII). Inorg. Chem. 2015, 54, 5930–5941. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, L.; Xu, X.; Xu, G.; Guo, Y.; Tang, J.; Liu, Z. Heterometallic Cubanes: Syntheses, Structures, and Magnetic Properties of Lanthanide(III)−Nickel(II) Architectures. Inorg. Chem. 2011, 50, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Canaj, A. B.; Tzimopoulos, D. I.; Siczek, M.; Lis, T.; Inglis, R.; Milios, C. J. Enneanuclear [NI6LN3] Cages: [LNIII3] Triangles Capping [NIII6] Trigonal Prisms Including a [NI6DY3] Single-Molecule Magnet. Inorg. Chem. 2015, 54, 7089–7095. [Google Scholar] [CrossRef]
- Xiong, K.; Wang, X.; Jiang, F.; Gai, Y.; Xu, W.; Su, K.; Li, X.; Yuan, D.; Hong, M. Heterometallic Thiacalix[4]Arene-Supported Na2NiII12LnIII2 Clusters with Vertex-Fused Tricubane Cores (Ln = Dy and Tb). Chem. Commun. 2012, 48, 7456. [Google Scholar] [CrossRef]
- Takehara, C.; Then, P. L.; Kataoka, Y.; Nakano, M.; Yamamura, T.; Kajiwara, T. Slow Magnetic Relaxation of Light Lanthanide-Based Linear LnZn2 Trinuclear Complexes. Dalton Trans. 2015, 44, 18276–18283. [Google Scholar] [CrossRef] [PubMed]
- Fondo, M.; Corredoira-Vázquez, J.; Herrera-Lanzós, A.; García-Deibe, A. M.; Sanmartín-Matalobos, J.; Herrera, J. M.; Colacio, E.; Núñez, C. Improving the SMM and Luminescence Properties of Lanthanide Complexes with LnO9cores in the Presence of ZnII: An Emissive Zn2Dy Single Ion Magnet. Dalton Trans. 2017, 46, 17000–17009. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, P.; Wang, C.-Y.; Liu, Y.; Liu, W.; Zhang, M. Three Sandwich-Type Zinc(Ii)–Lanthanide(Iii) Clusters: Structures, Luminescence and Magnetic Properties. RSC Adv. 2017, 7, 22692–22698. [Google Scholar] [CrossRef]
- Long, J. Luminescent Schiff-Base Lanthanide Single-Molecule Magnets: The Association between Optical and Magnetic Properties. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Long, J.; Vallat, R.; Ferreira, R. a. S.; Carlos, L. D.; Paz, F. a. A.; Guari, Y.; Larionova, J. A Bifunctional Luminescent Single-Ion Magnet: Towards Correlation between Luminescence Studies and Magnetic Slow Relaxation Processes. Chem. Commun. 2012, 48, 9974. [Google Scholar] [CrossRef] [PubMed]
- Yamagiwa, N.; H, Q.; Matsunaga, S.; Shibasaki, M. Lewis Acid−Lewis Acid Heterobimetallic Cooperative Catalysis: Mechanistic Studies and Application in Enantioselective Aza-Michael Reaction. J. Am. Chem. Soc. 2005, 127, 13419–13427. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Kumagai, N.; Matsunaga, S.; Moll, G.; Ohshima, T.; Suzuki, T.; Shibasaki, M. Direct Catalytic Asymmetric Aldol Reaction: Synthesis of Either Syn- or Anti-α,β-Dihydroxy Ketones. J. Am. Chem. Soc. 2001, 123, 2466–2467. [Google Scholar] [CrossRef]
- Tosaki, S. Y.; Hara, K.; Gnanadesikan, V.; Morimoto, H.; Harada, S.; Sugita, M.; Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. Mixed LA−LI Heterobimetallic Complexes for Tertiary Nitroaldol Resolution. J. Am. Chem. Soc. 2006, 128, 11776–11777. [Google Scholar] [CrossRef]
- Kakei, H.; Sone, T.; Sohtome, Y.; Matsunaga, S.; Shibasaki, M. Catalytic Asymmetric Cyclopropanation of Enones with Dimethyloxosulfonium Methylide Promoted by a La−Li3−(Biphenyldiolate)3 + NaI Complex. J. Am. Chem. Soc. 2007, 129, 13410–13411. [Google Scholar] [CrossRef]
- Gnanadesikan, V.; Horiuchi, Y.; Ohshima, T.; Shibasaki, M. Direct Catalytic Asymmetric Aldol-Tishchenko Reaction. J. Am. Chem. Soc. 2004, 126, 7782–7783. [Google Scholar] [CrossRef]
- Tian, J.; Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. An Asymmetric Cyanation Reaction and Sequential Asymmetric Cyanation–Nitroaldol Reaction Using a [YLi3{tris(Binaphthoxide)}] Single Catalyst Component: Catalyst Tuning with Achiral Additives. Angew. Chem. Int. Ed. 2002, 41, 3636–3638. [Google Scholar] [CrossRef]
- Robinson, J. R.; Booth, C. H.; Carroll, P. J.; Walsh, P. J.; Schelter, E. J. Dimeric Rare-Earth BINOLate Complexes: Activation of 1,4-Benzoquinone through Lewis Acid Promoted Potential Shifts. Chem. - A Eur. J. 2013, 19, 5996–6004. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Harwood, S. J.; Gröger, H.; Shibasaki, M. The First Catalytic Asymmetric Nitro-Mannich-Type Reaction Promoted by a New Heterobimetallic Complex. Angew. Chem. Int. Ed. 1999, 38, 3504–3506. [Google Scholar] [CrossRef]
- Emori, E.; Arai, T.; Sasai, H.; Shibasaki, M. A Catalytic Michael Addition of Thiols to α,β-Unsaturated Carbonyl Compounds: Asymmetric Michael Additions and Asymmetric Protonations. J. Am. Chem. Soc. 1998, 120, 4043–4044. [Google Scholar] [CrossRef]
- Robinson, J. R.; Carroll, P. J.; Walsh, P. J.; Schelter, E. J. Uranium(IV) BINOLate Heterobimetallics: Synthesis and Reactivity in an Asymmetric Diels–Alder Reaction. Organometallics 2013, 32, 1493–1499. [Google Scholar] [CrossRef]
- Shibasaki, M.; Yoshikawa, N. Lanthanide Complexes in Multifunctional Asymmetric Catalysis. Chem. Rev. 2002, 102, 2187–2210. [Google Scholar] [CrossRef]
- Nieto, I.; Wooten, A. J.; Robinson, J. R.; Carroll, P. J.; Schelter, E. J.; Walsh, P. J. Synthesis and Catalytic Activity of Heterobimetallic Rare Earth–Zinc Ethyl BINOLate Analogues of Shibasaki’s Catalysts. Organometallics 2013, 32, 7431–7439. [Google Scholar] [CrossRef]
- Ramirez, B.; Lu, C. C. Rare-Earth Supported Nickel Catalysts for Alkyne Semihydrogenation: Chemo- and Regioselectivity Impacted by the Lewis Acidity and Size of the Support. J. Am. Chem. Soc. 2020, 142, 5396–5407. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Silva, A. R.; Fu, L.; Shi, F. Ionothermal Synthesis, Crystal Structure, Topology and Catalytic Properties of Heterometallic Coordination Polymers Constructed from N-(Phosphonomethyl) Iminodiacetic Acid. Dalton Trans. 2015, 44, 13745–13751. [Google Scholar] [CrossRef]
- Chisholm, M. H. Concerning the Ring-Opening Polymerization of Lactide and Cyclic Esters by Coordination Metal Catalysts. Pure Appl. Chem. 2010, 82, 1647–1662. [Google Scholar] [CrossRef]
- Sheng, H.; Shi, J.; Feng, Y.; Wang, H.; Jiao, Y.; Sheng, H.; Zhang, Y.; Shen, Q. Remarkable Effect of Alkali Metal on Polymerization of Cyclic Esters Catalyzed by Samarium–Alkali Metal Multinuclear Alkoxide Clusters. Dalton Trans. 2012, 41, 9232. [Google Scholar] [CrossRef] [PubMed]
- Hultzsch, K. C.; Spaniol, T. P.; Okuda, J. Chiral Lanthanocene Derivatives Containing Two Linked Amido−Cyclopentadienyl Ligands: Heterobimetallic Structure and Lactone Polymerization Activity. Organometallics 1997, 16, 4845–4856. [Google Scholar] [CrossRef]
- Sánchez-Barba, L. F.; Hughes, D. L.; Humphrey, S. M.; Bochmann, M. Ligand Transfer Reactions of Mixed-Metal Lanthanide/Magnesium Allyl Complexes with β-Diketimines: Synthesis, Structures, and Ring-Opening Polymerization Catalysis. Organometallics 2006, 25, 1012–1020. [Google Scholar] [CrossRef]
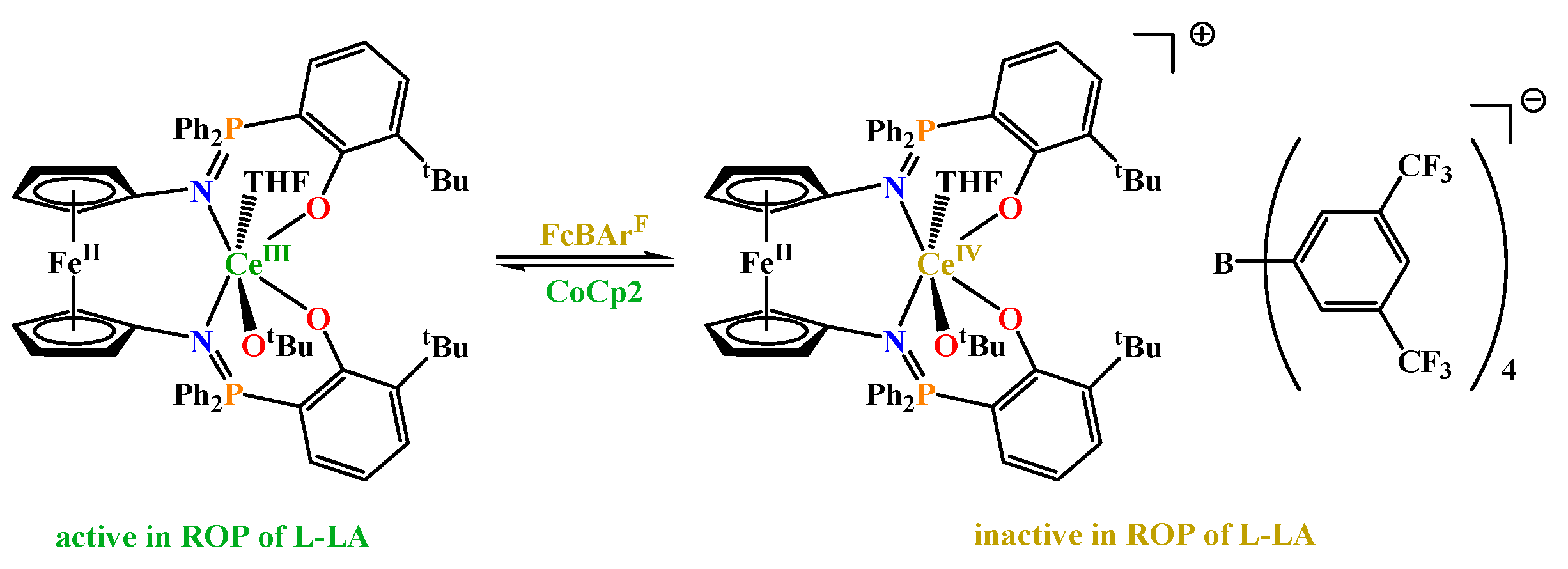
- Broderick, E. M.; Thuy-Boun, P. S.; Guo, N.; Vogel, C.; Sutter, J.; Miller, J. T.; Meyer, K.; Diaconescu, P. L. Synthesis and Characterization of Cerium and Yttrium Alkoxide Complexes Supported by Ferrocene-Based Chelating Ligands. Inorg. Chem. 2011, 50, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Broderick, E. M.; Guo, N.; Wu, T.; Vogel, C.; Xu, C.; Sutter, J.; Miller, J. T.; Meyer, K.; Cantat, T.; Diaconescu, P. L. Redox Control of a Polymerization Catalyst by Changing the Oxidation State of the Metal Center. Chem. Commun. 2011, 47, 9897. [Google Scholar] [CrossRef] [PubMed]
- Broderick, E. M.; Guo, N.; Vogel, C.; Xu, C.; Sutter, J.; Miller, J. T.; Meyer, K.; Mehrkhodavandi, P.; Diaconescu, P. L. Redox Control of a Ring-Opening Polymerization Catalyst. J. Am. Chem. Soc. 2011, 133, 9278–9281. [Google Scholar] [CrossRef] [PubMed]
- Jin, W. J.; Ding, L.; Chu, Z.; Chen, L.; Lü, X.; Zheng, X.; Song, J.; Fan, D. Controllable Bulk Solvent-Free Melt Ring-Opening Polymerization (ROP) of l-Lactide Catalyzed by Ni(II) and Ni(II)–Ln(III) Complexes Based on the Salen-Type Schiff-Base Ligand. J. Mol. Catal. A-Chem. [CrossRef]
- Yang, T.; Silva, A. R.; Shi, F. Six New 3d–4f Heterometallic Coordination Polymers Constructed from Pyrazole-Bridged CuIILnIII Dinuclear Units. Dalton Trans. 2013, 42, 13997. [Google Scholar] [CrossRef] [PubMed]
- Cancino, P.; Paredes-Garcı́A, V.; Torres, J.; Martínez, S.; Kremer, C.; Spodine, E. {[Cu3Lu2(ODA)6(H2O)6]·10H2O}n: The First Heterometallic Framework Based on Copper(Ii)/Lutetium(Iii) for the Catalytic Oxidation of Olefins and Aromatic Benzylic Substrates. Catal. Sci. Technol. 2017, 7, 4929–4933. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The Role of Carbon Capture and Utilization, Carbon Capture and Storage, and Biomass to Enable a Net-Zero-CO2 Emissions Chemical Industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef]
- Xin, X.; Shan, H.; Tian, T.; Wang, Y.; Yuan, D.; You, H.; Yao, Y. Conversion of CO2 into Cyclic Carbonates under Ambient Conditions Catalyzed by Rare-Earth Metal Complexes Bearing Poly(Phenolato) Ligand. ACS Sustain. Chem. Eng. 2020, 8, 13185–13194. [Google Scholar] [CrossRef]
- Wang, L.; Xu, C.; Han, Q.; Tang, X.; Zhou, P.; Zhang, R.; Gao, G.; Xu, B.; Qin, W.; Liu, W. Ambient Chemical Fixation of CO2 Using a Highly Efficient Heterometallic Helicate Catalyst System. Chem. Commun. 2018, 54, 2212–2215. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Hua, L.; Qu, L.; Yao, Q.; Wang, Y.; Yuan, D.; You, H.; Yao, Y. Heterobimetallic Rare Earth Metal–Zinc Catalysts for Reactions of Epoxides and CO2 under Ambient Conditions. Dalton Trans. 2021, 50, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Del Rosal, I.; Li, Q.; Wang, Y.; Yuan, D.; Yao, Y.; Maron, L. Efficient CO2 Transformation under Ambient Condition by Heterobimetallic Rare Earth Complexes: Experimental and Computational Evidences of a Synergistic Effect. Journal of CO2 Utilization 2019, 33, 413–418. [Google Scholar] [CrossRef]
- Gao, G.; Wang, L.; Zhang, R.; Xu, C.; Yang, H.; Liu, W. Hexanuclear 3d–4f Complexes as Efficient Catalysts for Converting CO2 into Cyclic Carbonates. Dalton Trans. 2019, 48, 3941–3945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, L.; Xu, C.; Yang, H.; Chen, W.; Gao, G.; Liu, W. Anion-Induced 3d–4f Luminescent Coordination Clusters: Structural Characteristics and Chemical Fixation of CO2 under Mild Conditions. Dalton Trans. 2018, 47, 7159–7165. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Li, B.; Han, C.-T.; Gao, P.; Wang, Y.; Yuan, D.; Yao, Y. Synthesis of Homo- and Heteronuclear Rare-Earth Metal Complexes Stabilized by Ethanolamine-Bridged Bis(Phenolato) Ligands and Their Application in Catalyzing Reactions of CO2 and Epoxides. Inorg. Chem. 2019, 58, 8775–8786. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Tran, Y. B. N.; Nguyen, T. C.; Gándara, F.; Nguyen, P. L. T. A Series of Metal–Organic Frameworks for Selective CO2 Capture and Catalytic Oxidative Carboxylation of Olefins. Inorg. Chem. 2018, 57, 13772–13782. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, C.; Zhao, B.; Yao, Y. Synthesis and Characterization of Amidato Divalent Lanthanide Complexes and Their Use in Forming 2,4-Quinazolidinones from CO2 and 2-Aminobenzonitriles. European Journal of Organic Chemistry 2016, 2016, 2555–2559. [Google Scholar] [CrossRef]
- Cheng, H.; Zhao, B.; Yao, Y.; Lu, C. Carboxylation of Terminal Alkynes with CO2 Catalyzed by Bis(Amidate) Rare-Earth Metal Amides. Green Chem. 2015, 17, 1675–1682. [Google Scholar] [CrossRef]
- Bhat, G. A.; Darensbourg, D. J. Progress in the Catalytic Reactions of CO2 and Epoxides to Selectively Provide Cyclic or Polymeric Carbonates. Green Chem. 2022, 24, 5007–5034. [Google Scholar] [CrossRef]
- Grignard, B.; Gennen, S.; Jérôme, C.; Kleij, A. W.; Detrembleur, C. Advances in the Use of CO2 as a Renewable Feedstock for the Synthesis of Polymers. Chem. Soc. Rev. 2019, 48, 4466–4514. [Google Scholar] [CrossRef]
- Darensbourg, D. J. Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2. Chemical Reviews 2007, 107, 2388–2410. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Xu, B.; Zhang, Y.; Yuan, D.; Yao, Y. Cooperative Rare Earth Metal–Zinc Based Heterometallic Catalysts for Copolymerization of CO2 and Cyclohexene Oxide. Green Chem. 2016, 18, 4270–4275. [Google Scholar] [CrossRef]
- Nagae, H.; Aoki, R.; Akutagawa, S.; Kleemann, J.; Tagawa, R.; Schindler, T.; Choi, G.; Spaniol, T. P.; Tsurugi, H.; Okuda, J.; Mashima, K. Lanthanide Complexes Supported by a Trizinc Crown Ether as Catalysts for Alternating Copolymerization of Epoxide and CO2: Telomerization Controlled by Carboxylate Anions. Angew. Chem. Int. Ed. 2018, 57, 2492–2496. [Google Scholar] [CrossRef]
- Pan, Z.-H.; Weng, Z.-Z.; Kong, X.; Liu, L.; Zheng, L. Lanthanide-Containing Clusters for Catalytic Water Splitting and CO2 Conversion. Coord. Chem. Rev. 2022, 457, 214419. [Google Scholar] [CrossRef]
- Asaba, H.; Iwasaki, T.; Hatazawa, M.; Deng, J.; Nagae, H.; Mashima, K.; Nozaki, K. Alternating Copolymerization of CO2 and Cyclohexene Oxide Catalyzed by Cobalt–Lanthanide Mixed Multinuclear Complexes. Inorg. Chem. 2020, 59, 7928–7933. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liao, P.; Jin, P.; Zhang, L.; Ling, B.; Wang, S.; Chan, Y.; Chen, X.; Zheng, Y. The Gigantic {NI36GD102} Hexagon: A Sulfate-Templated “Star-of-David” for Photocatalytic CO2 Reduction and Magnetic Cooling. J. Am. Chem. Soc. 2020, 142, 4663–4670. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, C.; Du, M.; Wang, X.; Wang, C.; Liu, L.; Kong, X.; Zheng, L. Soluble Lanthanide-Transition-Metal Clusters Ln36Co12 as Effective Molecular Electrocatalysts for Water Oxidation. Chem. Commun. 2021, 57, 3611–3614. [Google Scholar] [CrossRef]
- Lan, T.; Gao, W.-S.; Chen, C.; Wang, H.; Wang, M.; Yu, F. Two Tetranuclear 3d–4f Heterometal Complexes Mn2Ln2 (Ln = Dy, Gd): Synthesis, Structure, Magnetism, and Electrocatalytic Reactivity for Water Oxidation. New J. Chem. 2018, 42, 5798–5805. [Google Scholar] [CrossRef]
- Evangelisti, F.; Moré, R.; Hodel, F. H.; Luber, S.; Patzke, G. R. 3D–4F {COII3LN(OR)4} Cubanes as Bio-Inspired Water Oxidation Catalysts. J. Am. Chem. Soc. 2015, 137, 11076–11084. [Google Scholar] [CrossRef]
- Chen, R.; Zhuang, G.; Wang, Z.; Gao, Y.; Li, Z.; Wang, C.; Yang, Z.; Du, M.; Zeng, S.; Liu, L.; Kong, X.; Zheng, L. Integration of Bio-Inspired Lanthanide-Transition Metal Cluster and P-Doped Carbon Nitride for Efficient Photocatalytic Overall Water Splitting. Natl. Sci. Rev. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yan, Z.; Kong, X.; Liu, L.; Zheng, L. Integration of Lanthanide–Transition-Metal Clusters onto CdS Surfaces for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 16796–16800. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Sun, C.; Qin, C.; Wang, X.; Wang, H. N.; Zhou, E. L.; Li, W. E.; Su, Z. Iodine-Templated Assembly of Unprecedented 3d–4f Metal–Organic Frameworks as Photocatalysts for Hydrogen Generation. Chem. Commun. 2013, 49, 3564. [Google Scholar] [CrossRef]
- Alsowayigh, M. M.; Timco, G. A.; Borilović, I.; Alanazi, A. M.; Vitórica-Yrezábal, I. J.; Whitehead, G. F. S.; McNaughter, P. D.; Tuna, F.; O’Brien, P.; Winpenny, R. E. P.; Lewis, D. J.; Collison, D. Heterometallic 3D–4F Complexes as Air-Stable Molecular Precursors in Low Temperature Syntheses of Stoichiometric Rare-Earth Orthoferrite Powders. Inorg. Chem. 2020, 59, 15796–15806. [Google Scholar] [CrossRef] [PubMed]
- Deligne, N.; Gonze, V.; Bayot, D.; Devillers, M. Yttrium, Lanthanide and Mixed Y-Ln Vanadates Prepared from Molecular Precursors Based on EDTA. Eur. J. Inorg. Chem. 2008, 2008, 896–902. [Google Scholar] [CrossRef]
- Koroteev, P. S.; Dobrokhotova, Z. V.; Ilyukhin, A. B.; Ефимoв, Н. Н.; Kirdyankin, D. I.; Tyurin, A. V.; Гаврикoв, А. В.; Нoвoтoрцев, В. М. Polymeric Lanthanide Acetates with Peripheral Cymantrenecarboxylate Groups – Synthesis, Magnetism and Thermolysis. Polyhedron 2015, 85, 941–952. [Google Scholar] [CrossRef]
- Дoбрoхoтoва, Ж. В.; Koroteev, P. S.; Kirdyankin, D. I.; Кискин, М. А.; Kovba, M. L.; Ефимoв, Н. Н.; Гаврикoв, А. В.; Tyurin, A. V.; Нoвoтoрцев, В. М. Synthesis of Lanthanide Manganites LnMnO3 and LnMn2O5 from Individual Molecular Precursors. Russ. J. Inorg. Chem. 2015, 60, 1433–1443. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, R.; Yi, T.; Gao, S.; Yan, C.; Cao, L. Preparation of Nanosized La2CuO4 Perovskite Oxide Using an Amorphous Heteronuclear Complex as a Precursor at Low-Temperature. Journal of Alloys and Compounds 2000, 311, 16–21. [Google Scholar] [CrossRef]
- Dell’Amico, D. B.; Di Giacomo, A.; Falchi, L.; Labella, L.; Marelli, M.; Evangelisti, C.; Lezzerini, M.; Marchetti, F.; Samaritani, S. A Convenient Preparation of La2CuO4 from Molecular Precursors. Polyhedron 2017, 123, 33–38. [Google Scholar] [CrossRef]
- Xu, Q.; Pan, W.; Jing-Dong, W.; Wan, C.; Liu, Q.; Miao, H.; Mori, K.; Torigoe, T. Rare-Earth Zirconate Ceramics with Fluorite Structure for Thermal Barrier Coatings. J. Am. Ceram. Soc. 2005, 89, 340–342. [Google Scholar] [CrossRef]
- Liu, Z. -g.; Ouyang, J.; Sun, K. Improvement of Electrical Conductivity of Trivalent Rare-earth Cation-doped Neodymium Zirconate by Co-doping Gadolinium and Ytterbium. Fuel Cells 2010, 10, 1050–1056. [Google Scholar] [CrossRef]
- Zuniga, J. P.; Gupta, S. K.; Abdou, M.; Mao, Y. Effect of Molten Salt Synthesis Processing Duration on the Photo- and Radioluminescence of UV-, Visible-, and X-Ray-Excitable LA2HF2O7:EU3+ Nanoparticles. ACS Omega 2018, 3, 7757–7770. [Google Scholar] [CrossRef] [PubMed]
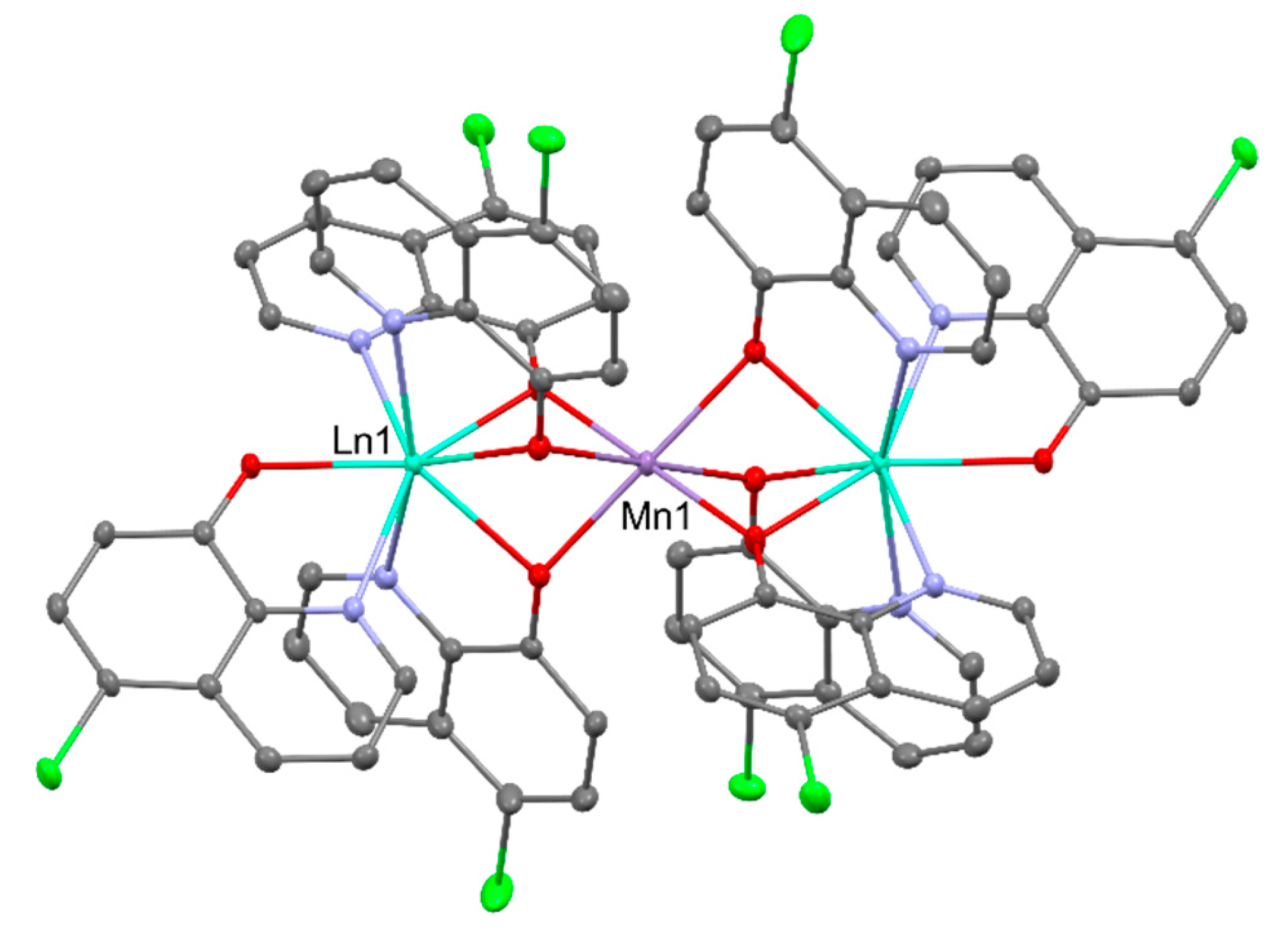
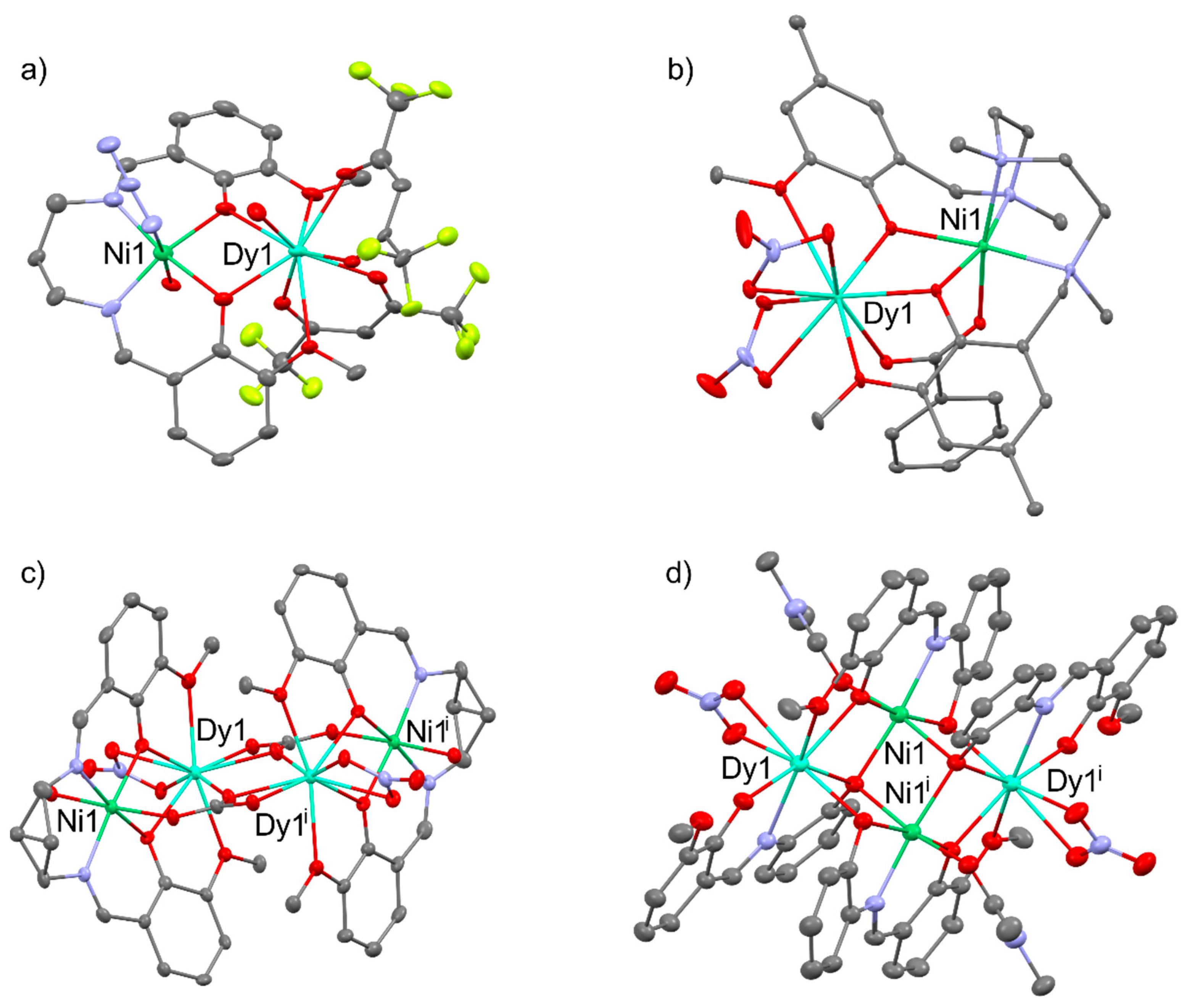
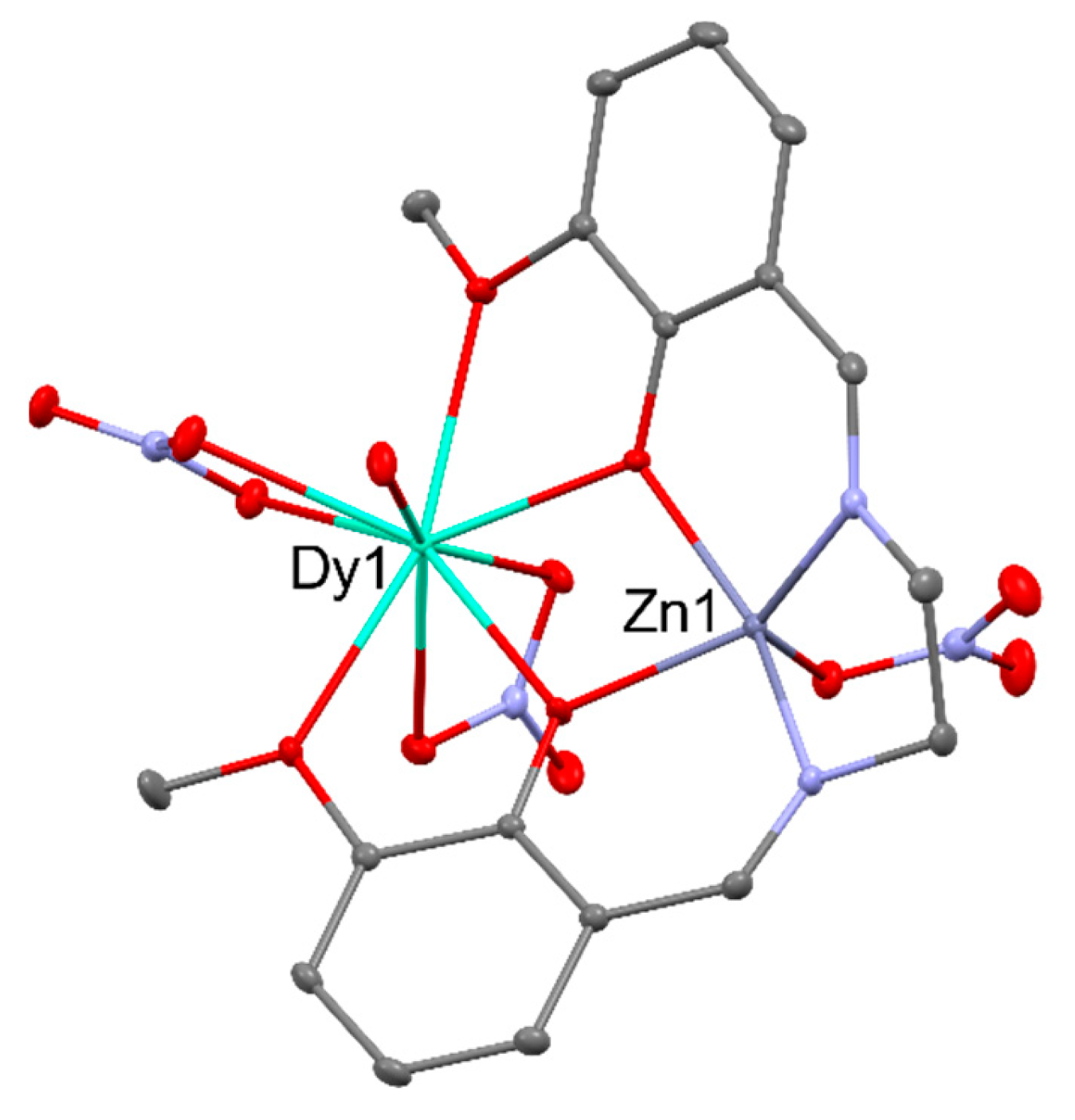
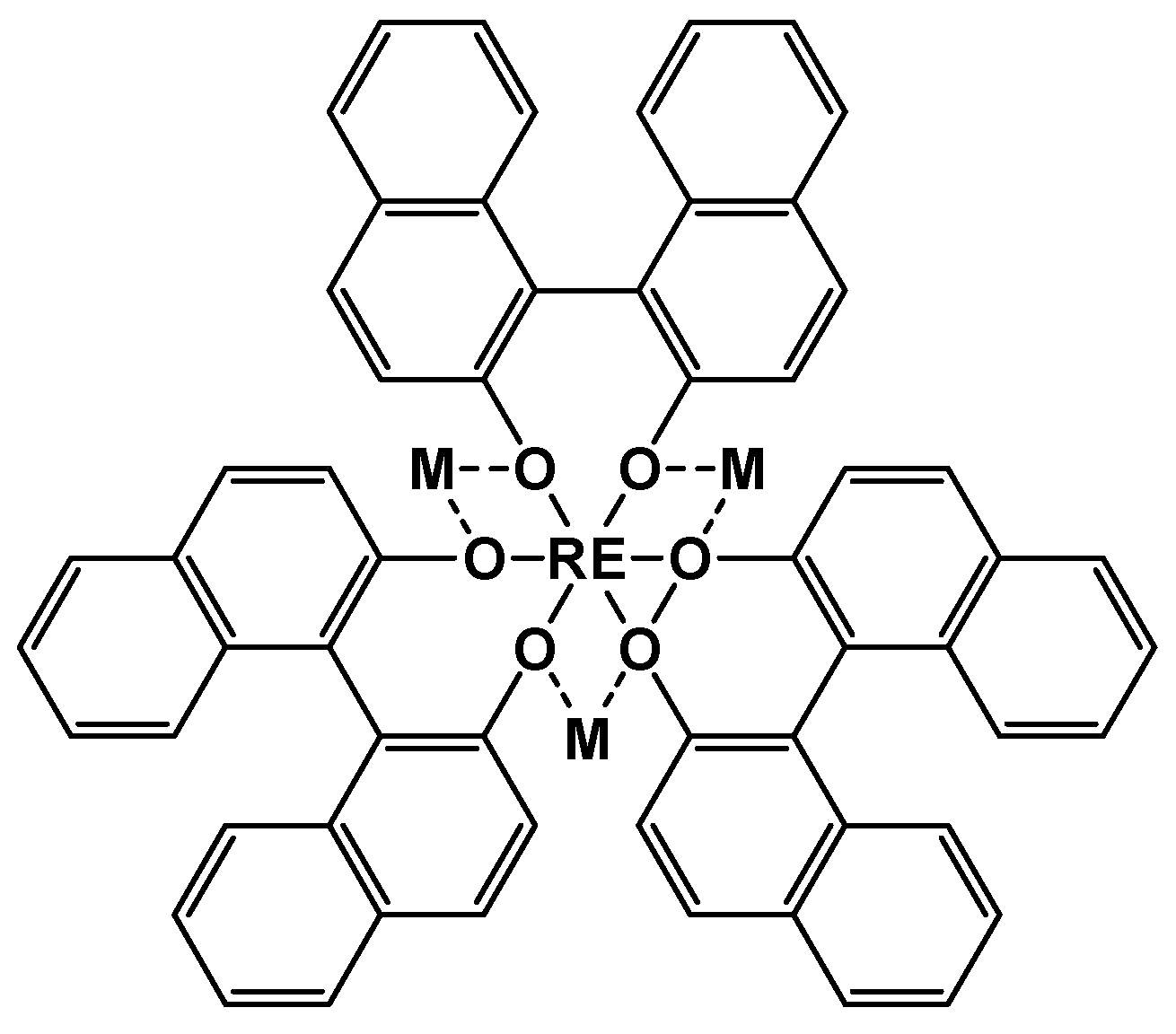
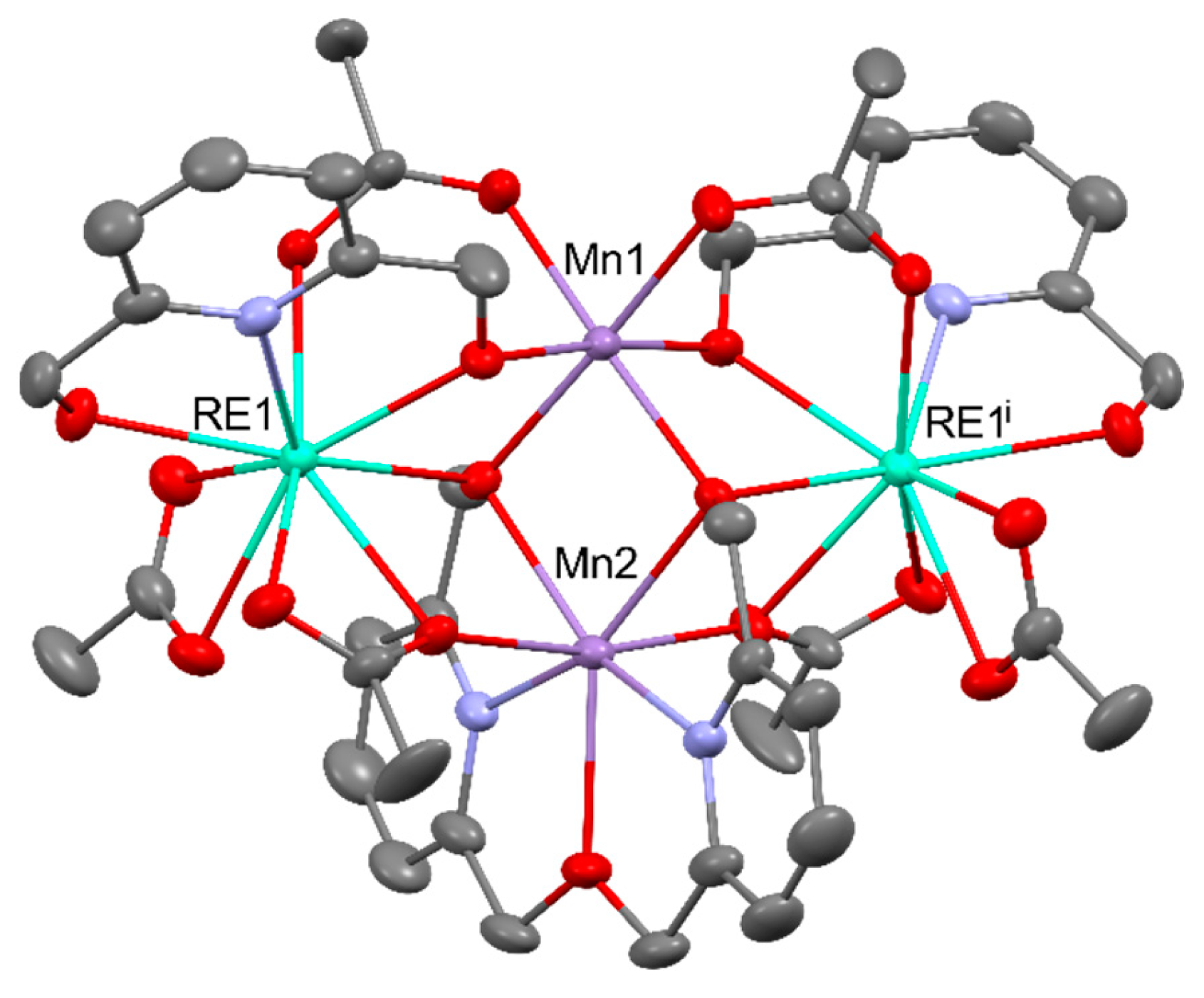
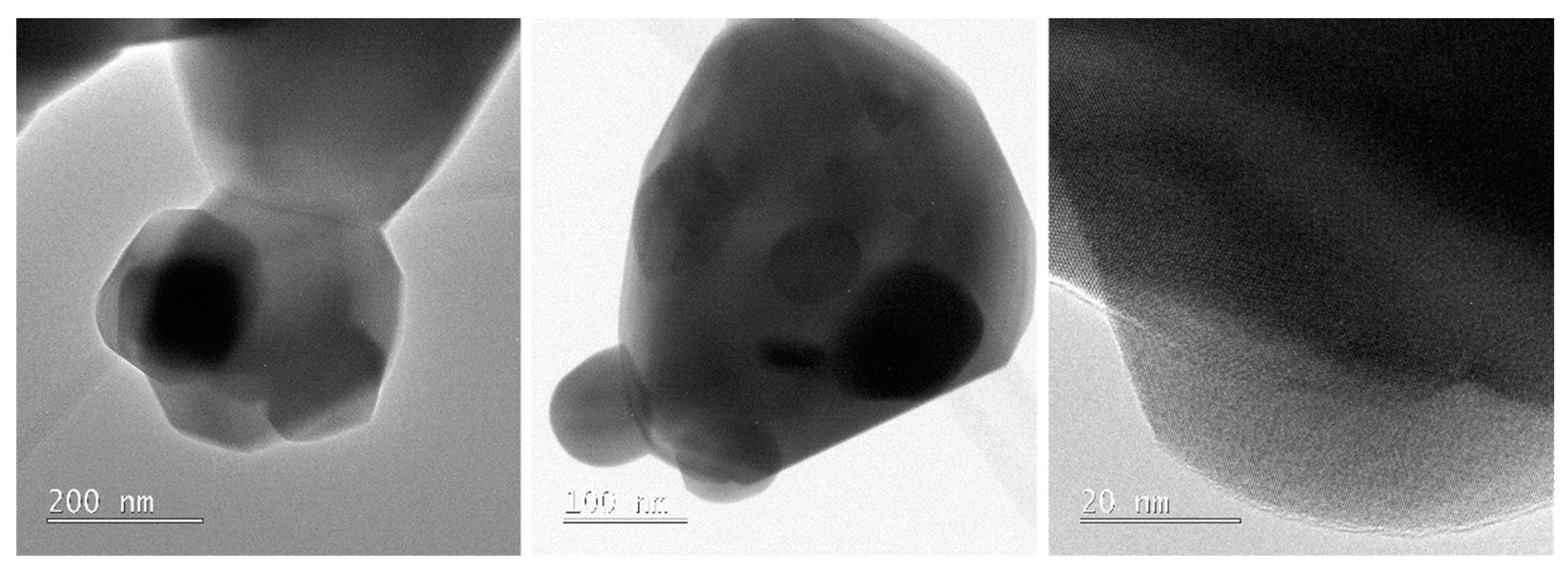
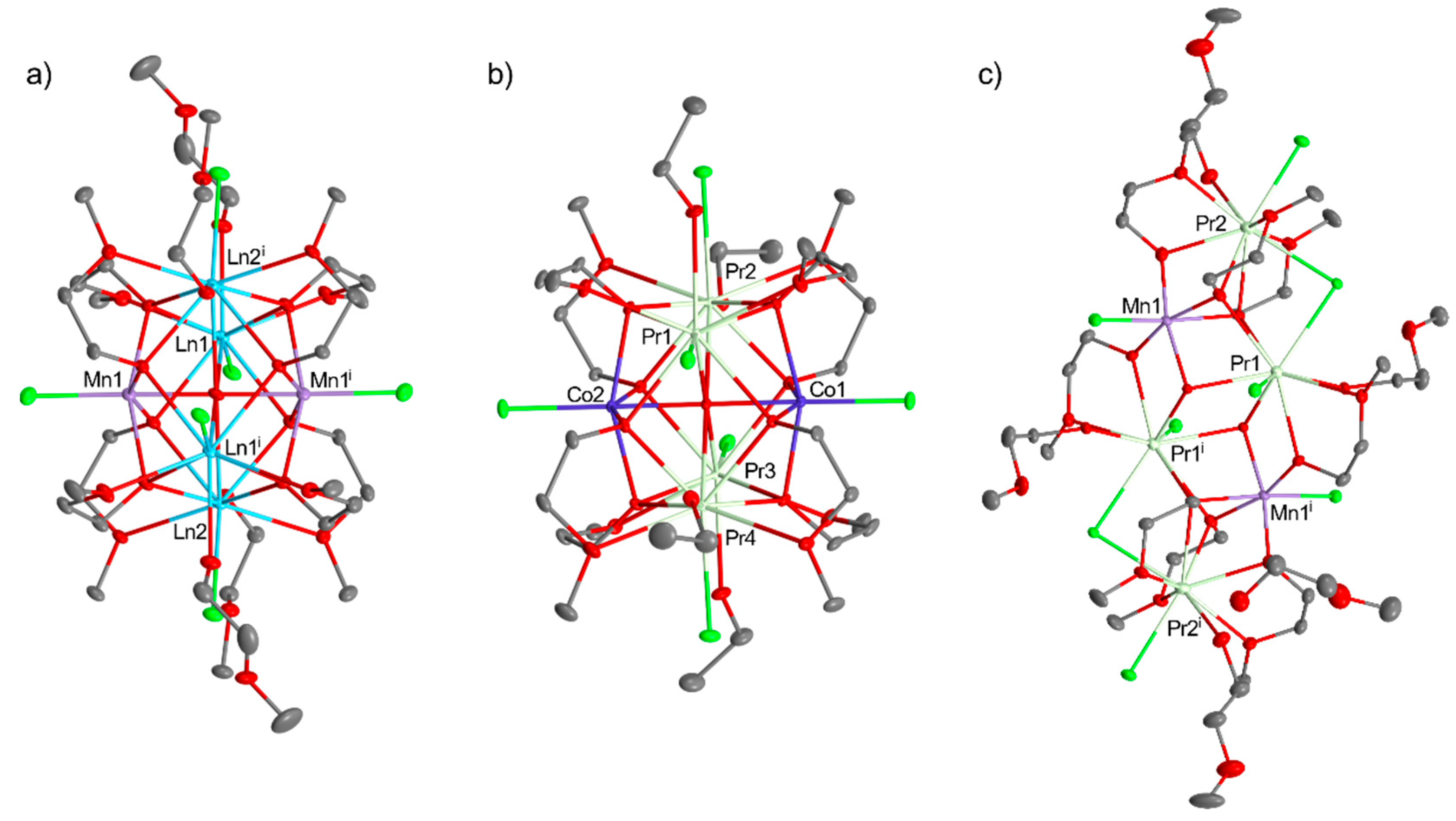
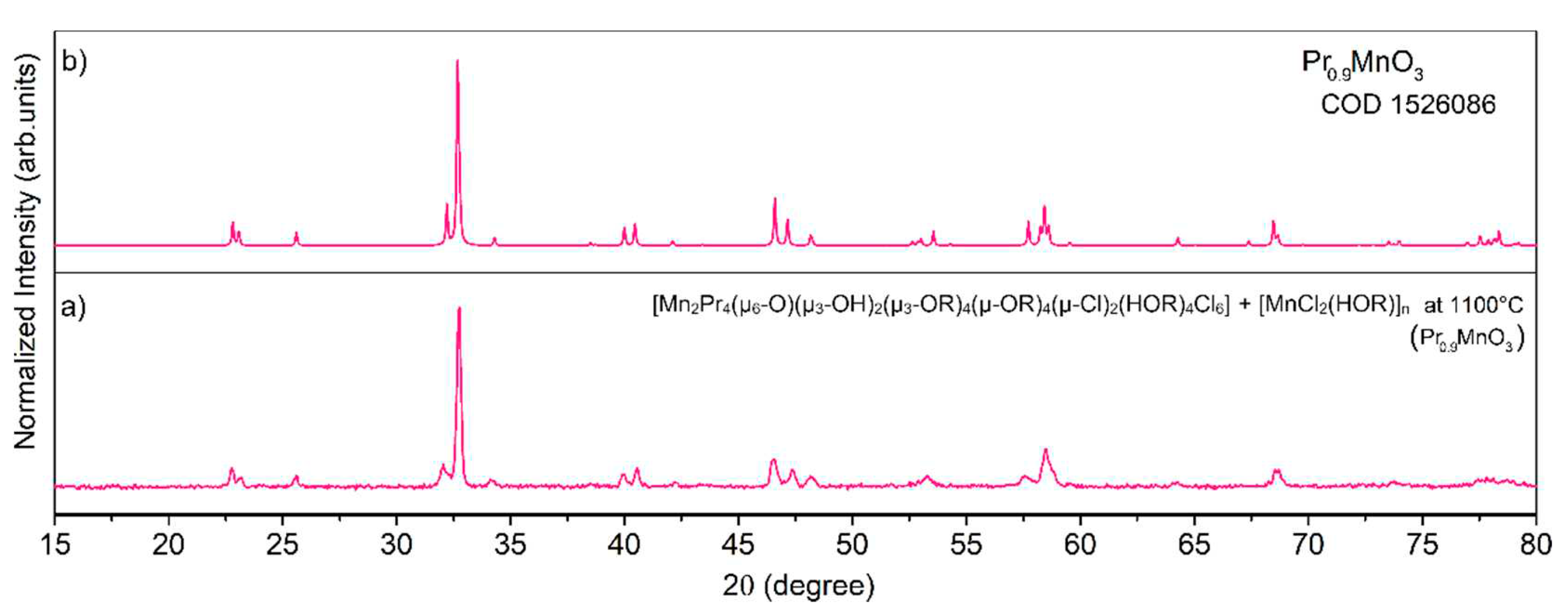
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




