1. Introduction
Periodontal diseases (PDs) are pathologies that affect a large part of the population. These diseases are caused by biofilms, and their main clinical manifestations are gingivitis and periodontitis [
1].
Several factors may be involved in the onset and progression of PDs. These include tobacco smoking, genetic factors, hormonal changes, stress, medication, diabetes mellitus, dietary deficiencies, and systemic diseases, among others. In general, all of these conditions can influence the immune response [
2].
On the other hand, PD, especially in advanced stages of periodontitis (stages III and IV), can trigger or aggravate several systemic conditions, including preeclampsia and preterm birth [
3], cardiovascular events [
4], respiratory conditions [
5], renal diseases [
6], rheumatoid arthritis [
7], and particularly diabetes mellitus (DM) [
8,
9], among others.
In brief, a bidirectional relationship between PD and DM has been suggested [
10].
The mechanism linked to the altered immune response in patients with DM and PD appears to be associated with advanced glycation end products (AGEs) and the presence of their receptors (RAGEs) [
11].
This interaction has direct effects on the endothelium, neutrophil function, cytokine activation (particularly TNFα), and collagen synthesis and degradation, hindering reparative processes [
12]. One way to assess the AGE level is through glycosylated hemoglobin (HbA1c), which measures the average level of glycosylated glucose in the blood over the last three months.
On the other hand, the persistence of poorly controlled periodontitis has been reported to contribute to systemic inflammatory response syndrome (SIRS), which leads to the development of glucose intolerance [
13]. In addition, in severe cases, this condition complicates glycemic control [
14,
15].
Periodontal treatment has been shown to be highly effective at controlling periodontal disease [
16]. The initial step in this treatment is motivation, involving plaque control by the patient and nonsurgical periodontal treatment (NSPT), followed by supportive periodontal treatment (SPT) [
16,
17,
18,
19]. Some authors consider NSPT to be effective at achieving glycemic control, comparable to the effect of adding a second oral antidiabetic drug [
20]. Therefore, the control of periodontal disease by NSPT could play a crucial role in the production of AGEs and the management of DM.
The aim of this case‒control study was to ascertain whether NSPT, when compared to no treatment, has an equivalent influence on the level of glycosylated hemoglobin (HbA1c) as the primary outcome variable. The plaque index (PlI), bleeding on probing (BOP), periodontal probing depth (PPD), and clinical attachment level (CAL) were included as secondary variables.
2. Materials and Methods
2.1. Ethical Approval
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Virgen de la Arrixaca Hospital (ID: 141/2013, March 3, 2013) in Murcia, Spain. This was a case‒control study.
This study followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement for case‒control studies.
2.2. Sample Size
A sample size of 15 patients in each group was estimated considering an α=0.05 [95% confidence interval] and β=0.2 [80% power]. These data were entered into the online calculator available at the following web address:
https://www.questionpro.com/es/calculadora-de-muestra.html. The following formula was used: Sample Size =
where Z = a confidence level of 1.96 (95%), p =.05 and c = the margin of error (.25%).
The formula substituted with actual values was as follows: (3.8416*(0.19208*0.95))/0.0625 = 11.213
The dropout rate during monitoring was assumed to be 30% = 3.363.
This sample size was in agreement with that in the study by Raman et al. [
21].
2.3. Sample Distribution
Thirty diabetic patients (15 periodontal and 15 nonperiodontal patients; 8 women and 22 men; 5 smokers and 25 nonsmokers) with a mean age of 55 years were included in this study. Regarding the type of diabetes, 24 patients had type II DM, 6 patients had type I DM, and the mean duration of diabetes was 19 years. The patients were referred by the endocrinology service of the Virgen de la Arrixaca University Hospital in Murcia (Spain) to the University Dental Clinic of the Morales Meseguer Hospital in Murcia (Spain) for periodontal clinical examination by the same qualified person (B.M-M.). This person was trained and calibrated until the data obtained during the periodontal examination reached significant reliability, with a kappa coefficient close to 0.97.
2.4. Inclusion Criteria
The inclusion criteria were as follows: diabetes mellitus patients of legal age with an HbA1c level >5.5 or <11 who signed the consent form to participate in this study.
2.5. Exclusion Criteria
The exclusion criteria were as follows: patients previously diagnosed and treated for periodontal disease; pregnant or lactating patients; individuals who had taken antibiotics, antiseptics, or medications that could affect the host response during the month prior to the periodontal assessment; and patients who did not sign the informed consent form.
2.6. Clinical Periodontal Examination
Once the patients were informed of the nature of this study and signed the informed consent form, the trained person proceeded to take a complete clinical history and a periodontogram. All periodontal recordings were performed with a manual periodontal probe (CP-15, Hu-Friedy Manufacturing Co., LLC, Chicago, USA). In addition, the examination was completed via orthopantomography to determine the periodontal status of the patient.
2.7. Blood Samples and Biometric Data
Endocrine-metabolic variables such as the type of DM, duration of disease, body mass index (BMI) and biochemical markers such as LDL, HDL, TG and HbA1c levels were determined by an endocrinologist at the Virgen de la Arrixaca Hospital in Murcia. No changes in diabetes treatment were made during the conduct of this study.
2.8. Group Assignment
The sample was divided into two groups: patients with periodontitis (test group) and patients without periodontitis (control group). Periodontitis was diagnosed in patients who presented with attachment losses due to inflammatory problems greater than or equal to 2 mm at the interproximal level or greater than or equal to 3 mm at the buccal or lingual/palatal level in two or more nonadjacent teeth.
All the samples were reviewed at 3 and 6 months after treatment, and all the periodontal and endocrine-metabolic variables mentioned above were noted.
2.9. Statistical Analysis
The data obtained were analyzed with SPSS statistical software (Statistical Package for the Social Sciences), version 25.0 (SPSS, Chicago, IL, USA). A descriptive and inferential analysis of both groups (test and control groups) was carried out to assess the existence of statistically significant differences between the groups. Student’s t test for paired samples was used to determine the existence or absence of statistically significant differences within the same group (test and control groups) regarding the HbA1c level at 3 and 6 months. A p value <0.05 was considered to indicate statistical significance.
3. Results
No individuals dropped out during this study, either at 3 months or 6 months.
3.1. Distribution and Characteristics of the Sample
The resulting groups (test and control) were homogeneous with respect to the following variables: sex, age, type of DM, duration of DM, BMI and biochemical markers (LDL, HDL, TG, and HbA1c levels) at baseline (
Table 1).
3.2. Evolution of Periodontal Variables
3.2.1. Clinical Attachment Level (CAL)
The mean periodontal attachment loss in the test group was 4.47 mm (SD: 1.09), with a minimum value of 2.85 and a maximum value of 7.38 mm. Three months after periodontal treatment, the CAL improved, reaching a mean value of 3.16 mm (SD: 1.22), with a minimum value of 1.95 mm and a maximum value of 6.52 mm. These values remained stable 6 months after treatment [mean CAL: 3.24 ±0.85 (1.97; 5.34)].
3.2.2. Gingival Bleeding Index (GBI)
The mean baseline GBI of the periodontal patients was 41%, and the GBI decreased to 7% and 13% at 3 and 6 months after periodontal treatment, respectively. In the control group, the mean baseline GBI was significantly lower (mean 12%, p <0.0001) than that in the test group, and although it decreased to 2% at 3 months after periodontal treatment, it was equal to that in the test group at 6 months after treatment (13%).
3.2.3. Hygiene Index (HI)
Although the HI was slightly greater in the periodontal patient group than in the control group at the beginning (87% versus 72%) and at 3 (62% versus 53%) and 6 months after treatment (63% versus 45%), the differences were not statistically significant.
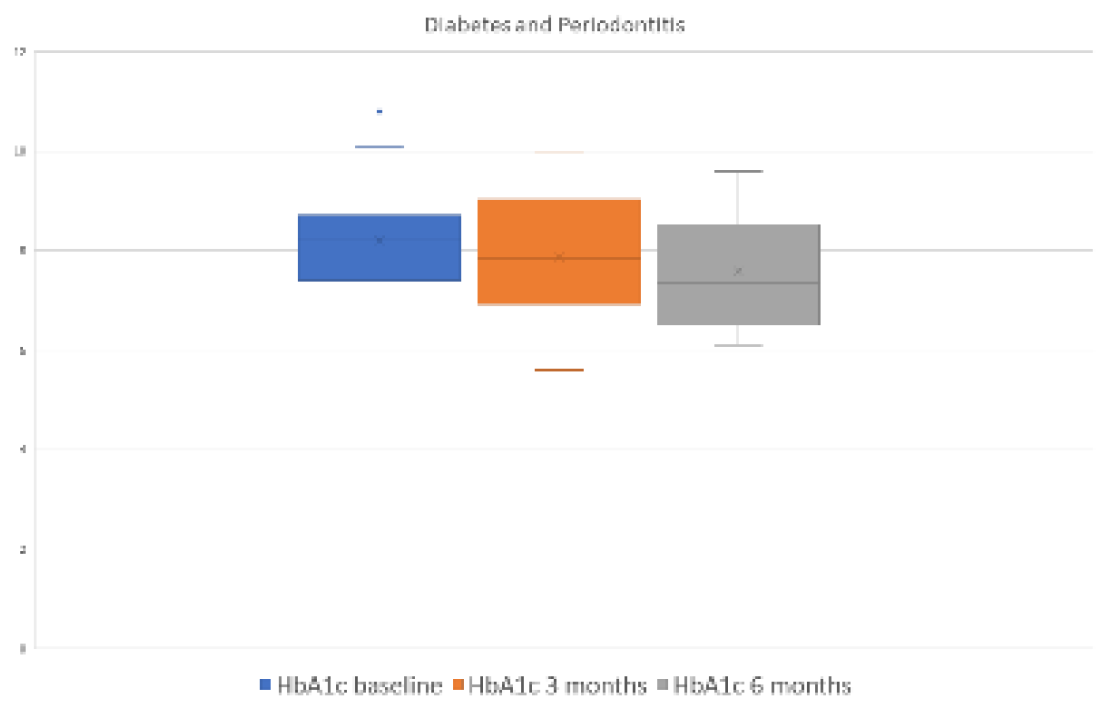
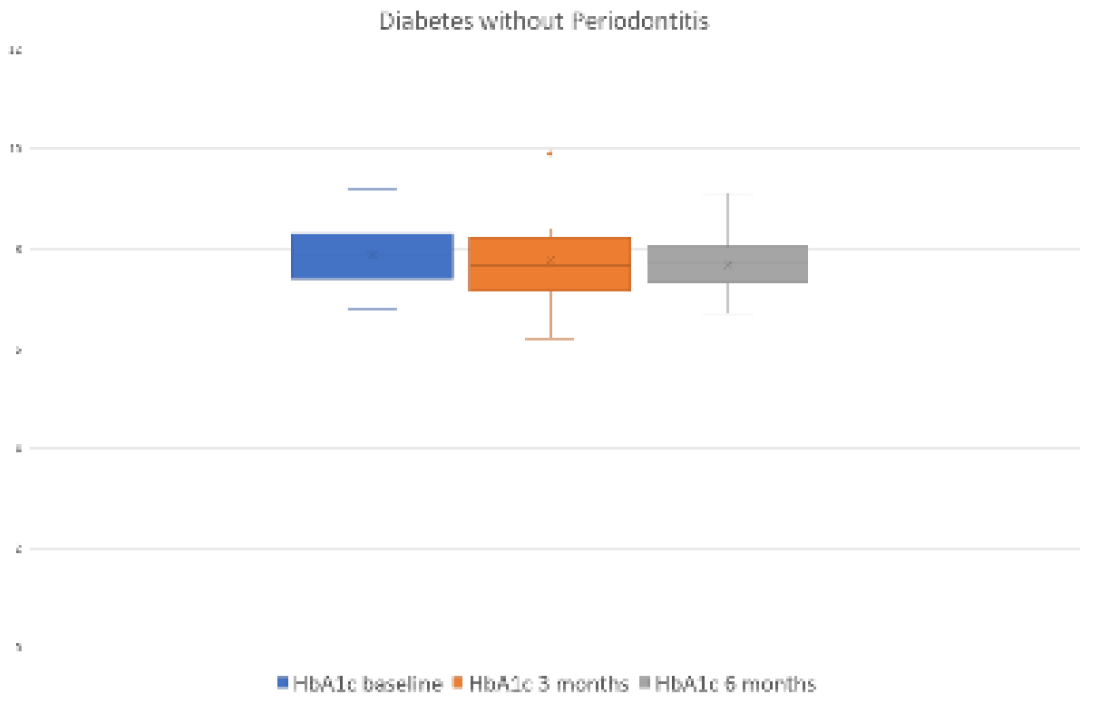
3.2.4. Evolution of the Endocrine-Metabolic Variable HbA1c
Significant differences were observed at 3 (p=0.039) and 6 (p=0.025) months after periodontal treatment with respect to the HbA1c level in the group of periodontal patients. However, these differences were not found in the control group at 3 (p=0.352) or 6 (p=0.379) months after periodontal treatment (
Figure 1 and
Figure 2 and
Table 2 and
Table 3).
In the test group, the HbA1c level decreased by 0.264% ± 0.11 [weighted mean difference (WMD) -0.264, 95% confidence interval (CI) = -0.156, -0.513, p= 0.039] at 3 months and by 0.571% ± 0.226 [WMD -0.571%, 95% CI = -0.083, -1.060, p= 0.025] at 6 months after periodontal treatment.
4. Discussion
4.1. Advanced Glycation end Products (AGEs) and Inflammation
A bidirectional relationship between DM and PD has long been suggested [
22,
23,
24]. On the one hand, the persistence of an infection involving mostly gram-negative bacteria seems to amplify the proinflammatory response via cytokines, leading to SIRS [
25,
26]. On the other hand, increased AGEs are associated with increased destruction of periodontal tissues [
11,
12,
21,
23], thus creating a vicious cycle.
The accumulation of AGEs can lead to changes in the extracellular matrix and inflammatory responses in diabetic patients with periodontitis [
11,
12]. These findings suggest that AGEs may play a role in the pathogenesis of periodontitis, contributing to periodontal destruction [
25]. The interaction of AGEs with their receptors, RAGEs, can lead to increased secretion of proinflammatory cytokines, further intensified by the presence of specific bacteria [
26].
On the other hand, the accumulation of AGEs may promote the development of more aggressive subgingival flora, resulting in increased periodontal destruction and elevated levels of proinflammatory cytokines [
10]. AGE inhibitors could mitigate these effects, offering a promising avenue for periodontal treatment in diabetes patients.
4.2. Primary Outcome
In our study, HbA1c levels decreased by 0.264% at 3 months and by 0.571% at 6 months after periodontal treatment; therefore, the values improved over time. These data are consistent with the results obtained in various systematic reviews [
27,
28], in which reductions of 0.27-0.56% were shown at 3-4 months after periodontal treatment. In another review of randomized controlled clinical trials, very similar reductions in HbA1c levels were observed at 3 and 6 months after periodontal treatment, with values of 0.514% and 0.548%, respectively [
29].
In other studies [
30], the statistically significant reduction in HbA1c levels at 3 or 4 months after periodontal treatment was not maintained over time and ceased to be significant at 6 months after treatment. In line with this study, in a recent review [
31], the authors found significant reductions in HbA1c levels at 3 and 6 months after NSPT, but the levels decreased over time, from 0.49% at 3 months to 0.38% at 6 months.
Other studies [
32,
33,
34,
35,
36] have not shown statistically significant differences in the HbA1c levels of periodontal patients with DM after periodontal treatment.
In the present study, both the test group and the control group underwent a hygiene education program and tartar removal. However, this treatment did not yield any improvements in terms of HbA1c levels in the control group. Although the HI improved, decreasing from 72% to 53% at 3 months after treatment and further to 45% at 6 months after treatment, these changes did not have a statistically significant impact on the HbA1c level in the control group. These findings align with those of Chen et al. [
37], who indicated that patients with a lower degree of periodontal damage did not experience improvements in HbA1c levels.
Therefore, we believe that, in our study, meticulous SPT played an important role in achieving and maintaining low HbA1c levels even at 6 months.
4.3. Secondary Outcomes
As in other studies, there was total uniformity in that the outcome of NSPT was effective in both diabetic and nondiabetic patients in our study [
16,
22,
38,
39,
40,
41]. All clinical variables associated with periodontitis showed marked improvement after NSPT and were maintained with adequate SPT.
Because both groups were initially similar, the only variable that could aggravate periodontal disease was the HbA1c level, which was greater in the group of periodontal patients (mean = 8.21%; SD =1.23%) than in the control group (mean = 7.87%; SD = 0.68%). The U.S. National Health and Nutrition Examination Survey showed that people with elevated glycosylated hemoglobin levels have a greater risk of developing severe periodontal disease [
42].
Regarding the GBI, before periodontal treatment, there was significantly more bleeding in the group of diabetic patients with periodontitis than in the control group. However, these differences disappeared after periodontal treatment and remained stable at 3 and 6 months after treatment. These findings show that NSPT is effective at reducing bleeding in diabetic patients with periodontal disease in the same way as it is in nondiabetic periodontal patients [
16].
Our study presented a result similar to that reported by Raman et al. [
21] in their meta-analysis. The decrease in the bleeding rate was very significant at 3 months and was equal to the decrease in the bleeding rate in the control group at 6 months. The modification of maintenance visits based on a patient’s risk profile plays a fundamental role in inflammation control. In our study, the baseline bleeding rate ranged from 41% to 7% at 3 months and 13% at 6 months.
There was a slight relapse after 6 months, which we attributed to relaxation in the patients’ hygiene habits. These data highlight the importance of regular maintenance visits within the SPT protocol.
In the group of periodontal patients, a clinical attachment gain of more than 1 mm was observed three months after periodontal treatment, and this gain remained stable at six months post-treatment. These results are consistent with those obtained in periodontal patients without any systemic disease [
16]. A less favorable outcome has been reported, with a clinical attachment gain of only 0.22 mm observed three months after treatment [
43].
4.4. Alternative Periodontal Treatment Options
Other nonsurgical periodontal treatment options include photodynamic treatment and/or the use of antibiotics. A recent meta-analysis [
44] suggested that NSPT combined with photodynamic therapy and doxycycline achieves the best efficacy in reducing HbA1c levels. However, Taylor and Borgnakke [
24] showed that there was insufficient scientific evidence to support the recommendation of NSPT combined with local or systemic antibiotics. Currently, due to the problem of bacterial resistance emerging from the use of antibiotics, joint antibiotic administration with nonsurgical periodontal therapy is recommended only for young patients with advanced-stage disease [
45].
Finally, we must emphasize the importance of motivation in the maintenance of oral hygiene. It has been noted that, compared to individuals with better brushing efficiency, individuals with insufficient brushing efficiency have greater plaque levels, leading to increased levels of glycosylated hemoglobin [
21,
22].
4.5. Limitations
This study has several limitations. First, this study had a small sample size. Determination of our sample size was based on the agreement of patients to participate in this study, and a total of 30 patients agreed to participate. Our sample size is consistent with that of other similar studies; for example, Moeintaghavi et al. [
46] included 40 patients, Raman et al. [
21] included 32 patients, and Telgi et al. [
47] included 40 patients. Conversely, the study with the largest number of patients was conducted by Engebretson et al. [
40], who involved a total of 514 patients. This is noteworthy because, as mentioned by Li et al. [
48], small samples may detect statistically significant differences, whereas large samples may not. We believe this discrepancy could be attributed to variations in HbA1c levels at the beginning of the study, differences in the stages of periodontitis and, most importantly, challenges in maintaining adequate patient control with close professional follow-up.
Second, selection bias was present: nonrandom sampling is associated with the risk of selecting individuals who are not representative of the broader population. This can lead to inaccurate conclusions. Furthermore, this study had self-selection bias. In some cases, participants may have self-selected themselves for this study, introducing bias if those who chose to participate differed systematically from those who did not.
Third, the generalizability of the findings is limited: a small sample may not accurately represent the broader population from which findings are drawn.
Fourth, as a consequence of the above, an increase in variability occurred: smaller samples are more susceptible to random variations, making it difficult to establish the true underlying trends or patterns.
Finally, the follow-up time was limited; therefore, insight into the long-term effects of the treatment is limited. Studies with short follow-up times may miss important long-term effects or trends.
4.6. Future Research Directions
Additional studies are needed to determine both the influence of the different degrees of severity of periodontal disease in diabetic patients and the impact of different HbA1c levels on the severity of periodontal disease.
5. Conclusions
Nonsurgical periodontal treatment significantly decreased the degree of periodontal inflammation and the HbA1c level at 3 and 6 months after treatment. Therefore, due to the repercussions for public health and the costs associated with the treatment of DM, periodontal examination and nonsurgical treatment should be considered part of the medical treatment of patients with DM.
Author Contributions
Conceptualization; methodology; investigation, B.M.-M., M.A.-M. and P.P.-O.; data curation, B.M.-M.; writing—original draft preparation, M.J.M.-V. and A.S.-P.; writing—review and editing, M.J.M.-V., A.S.-P.; supervision, M.J.M.-V., B.M.-M., M.A.-M., P.P.-O. and A.S.-P.;. All the authors gave their final approval and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Virgen de la Arrixaca Hospital (ID: 141/2013, March 3, 2013) in Murcia, Spain.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data of this study are available to readers in Excel upon request to the corresponding author (arturosa@um.es).
Acknowledgments
The authors would like to thank María de los Ángeles Pérez-Albacete for her collaboration in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 Suppl 1, S1–S8. [Google Scholar] [CrossRef]
- Arowojolu, M.O.; Dosumu, E.B.; Onyeaso, C.O.; Lawoyin, J.O. Effects of some risk factors and immunodeficiencies on the periodontium--a review. Afr. J. Med. Med. Sci. 2002, 31, 195–199. [Google Scholar]
- Xiong, X.; Buekens, P.; Vastardis, S.; Yu, S.M. Periodontal disease and pregnancy outcomes: state-of-the-science. Obstet. Gynecol. Surv. 2007, 62, 605–615. [Google Scholar] [CrossRef]
- Tonetti, M.S. Periodontitis and risk for atherosclerosis: an update on intervention trials. J. Clin. Periodontol. 2009, 36 Suppl 10, 15–19. [Google Scholar] [CrossRef]
- Scannapieco, F.A. Role of oral bacteria in respiratory infection. J. Periodontol. 1999, 70, 793–802. [Google Scholar] [CrossRef]
- Craig, R.G. Interactions between chronic renal disease and periodontal disease. Oral Dis. 2008, 14, 1–7. [Google Scholar] [CrossRef]
- Kaur, S.; White, S.; Bartold, P.M. Periodontal disease and rheumatoid arthritis: a systematic review. J. Dent. Res. 2013, 92, 399–408. [Google Scholar] [CrossRef]
- Mealey, B.L. Diabetes and periodontal disease: two sides of a coin. Compend. Contin. Educ. Dent. 2000, 21, 943–946, 948, 950, passim; quiz 956. [Google Scholar]
- Mealey, B.L.; Ocampo, G.L. Diabetes mellitus and periodontal disease. Periodontol 2000 2007, 44, 127–153. [Google Scholar] [CrossRef]
- Zhao, M.; Xie, Y.; Gao, W.; Li, C.; Ye, Q.; Li, Y. Diabetes mellitus promotes susceptibility to periodontitis-novel insight into the molecular mechanisms. Front. Endocrinol. (Lausanne) 2023, 14, 1192625. [Google Scholar] [CrossRef]
- Lalla, E.; Lamster, I.B.; Stern, D.M.; Schmidt, A.M. Receptor for advanced glycation end products, inflammation, and accelerated periodontal disease in diabetes: mechanisms and insights into therapeutic modalities. Ann. Periodontol. 2001, 6, 113–118. [Google Scholar] [CrossRef]
- Lalla, E.; Lamster, I.B.; Feit, M.; Huang, L.; Spessot, A.; Qu, W.; Kislinger, T.; Lu, Y.; Stern, D.M.; Schmidt, A.M. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J. Clin. Invest. 2000, 105, 1117–1124. [Google Scholar] [CrossRef]
- Saito, T.; Shimazaki, Y.; Kiyohara, Y.; Kato, I.; Kubo, M.; Iida, M.; Koga, T. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study. J. Dent. Res. 2004, 83, 485–490. [Google Scholar] [CrossRef]
- Hugoson, A.; Thorstensson, H.; Falk, H.; Kuylenstierna, J. Periodontal conditions in insulin-dependent diabetics. J. Clin. Periodontol. 1989, 16, 215–223. [Google Scholar] [CrossRef]
- Thorstensson, H.; Hugoson, A. Periodontal disease experience in adult long-duration insulin-dependent diabetics. J. Clin. Periodontol. 1993, 20, 352–358. [Google Scholar] [CrossRef]
- Sanz, M.; Bäumer, A.; Buduneli, N.; Dommisch, H.; Farina, R.; Kononen, E.; Linden, G.; Meyle, J.; Preshaw, P.M.; Quirynen, M.; et al. Effect of professional mechanical plaque removal on secondary prevention of periodontitis and the complications of gingival and periodontal preventive measures: consensus report of group 4 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases. J. Clin. Periodontol. 2015, 42 Suppl 16, S214–S220. [Google Scholar] [CrossRef]
- Herrera, D.; Sanz, M.; Kebschull, M.; Jepsen, S.; Sculean, A.; Berglundh, T.; Papapanou, P.N.; Chapple, I.; Tonetti, M.S. Treatment of stage IV periodontitis: the EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2022, 49 Suppl 24, 4–71. [Google Scholar] [CrossRef]
- Kebschull, M.; Chapple, I. Evidence-based, personalised and minimally invasive treatment for periodontitis patients - the new EFP S3-level clinical treatment guidelines. Br. Dent. J. 2020, 229, 443–449. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I-III periodontitis-the EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 Suppl 22, 4–60. [Google Scholar] [CrossRef]
- Sabharwal, A.; Gomes-Filho, I.S.; Stellrecht, E.; Scannapieco, F.A. Role of periodontal therapy in management of common complex systemic diseases and conditions: an update. Periodontol 2000 2018, 78, 212–226. [Google Scholar] [CrossRef]
- Raman, R.P.; Taiyeb-Ali, T.B.; Chan, S.P.; Chinna, K.; Vaithilingam, R.D. Effect of nonsurgical periodontal therapy verses oral hygiene instructions on type 2 diabetes subjects with chronic periodontitis: a randomised clinical trial. BMC Oral Health 2014, 14, 79. [Google Scholar] [CrossRef]
- Chapple, I.L.; Genco, R. Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J. Periodontol. 2013, 84, S106–S112. [Google Scholar] [CrossRef]
- Grossi, S.G.; Genco, R.J. Periodontal disease and diabetes mellitus: a two-way relationship. Ann. Periodontol. 1998, 3, 51–61. [Google Scholar] [CrossRef]
- Taylor, G.W.; Borgnakke, W.S. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. 2008, 14, 191–203. [Google Scholar] [CrossRef]
- Chopra, A.; Jayasinghe, T.N.; Eberhard, J. Are inflamed periodontal tissues endogenous source of advanced glycation end-products (AGEs) in individuals with and without diabetes mellitus? A systematic review. Biomolecules 2022, 12, 642. [Google Scholar] [CrossRef]
- Makiura, N.; Ojima, M.; Kou, Y.; Furuta, N.; Okahashi, N.; Shizukuishi, S.; Amano, A. Relationship of Porphyromonas gingivalis with glycemic level in patients with type 2 diabetes following periodontal treatment. Oral Microbiol Immunol 2008, 23, 348–351. [Google Scholar] [CrossRef]
- Corbella, S.; Francetti, L.; Taschieri, S.; De Siena, F. & Fabbro, M. D. Effect of periodontal treatment on glycemic control of patients with diabetes: a systematic review and meta-analysis. J. Diabetes Investig. 2013, 4, 502–509. [CrossRef]
- .Li, Q.; Hao, S.; Fang, J.; Xie, J.; Kong, X.H.; Yang, J.X. Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials 2015, 16, 291. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zhan, Q.; Wu, C.Z.; Yuan, Y.H.; Chen, W.; Yu, F.Y.; Li, Y.; Li, L.J. Baseline HbA1c level influences the effect of periodontal therapy on glycemic control in people with type 2 diabetes and periodontitis: a systematic review on randomized controlled trails. Diabetes Ther. 2021, 12, 1249–1278. [Google Scholar] [CrossRef]
- Madianos, P. N. & Koromantzos, P. A. An update of the evidence on the potential impact of per-iodontal therapy on diabetes outcomes. J. Clin. Periodontol. 2018, 45, 188–195. [CrossRef]
- Di Domenico, G. L.; Minoli, M.; Discepoli, N.; Ambrosi, A. & De Sanctis, M. Effectiveness of periodontal treatment to improve glycemic control: an umbrella review. Acta Diabetol. 2023, 60, 101–113. [CrossRef]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Ef-fect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J. Appl. Oral Sci. 2020, 28, e20190248. [Google Scholar] [CrossRef]
- Engebretson, S. P.; Hyman, L.G.; Michalowicz, B.S.; Schoenfeld, E.R.; Gelato, M.C.; Hou, W.; Seaquist, E.R.; Reddy, M.S.; Lewis, C.E.; Oates, T.W.; Tripathy, D.; Katancik, J.A.; Orlander, P.R.; Paquette, D.W.; Hanson, N.Q.; Tsai, M. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA 2013, 310, 2523–2532. [Google Scholar] [CrossRef]
- Auyeung, L.; Wang, P.W.; Lin, R.T.; Hsieh, C.J.; Lee, P.Y.; Zhuang, R.Y.; Chang, H.W. Evalua-tion of periodontal status and effectiveness of non-surgical treatment in patients with type 2 dia-betes mellitus in Taiwan for a 1-year period. J. Periodontol. 2012, 83, 621–628. [Google Scholar] [CrossRef]
- Correa, F. O.; Gonçalves, D.; Figueredo, C.M.S.; Bastos, A.S.; Gustafsson, A.; Orrico, S.R.P. Ef-fect of periodontal treatment on metabolic control, systemic inflammation and cytokines in pa-tients with type 2 diabetes. J. Clin. Periodontol. 2010, 37, 53–58. [Google Scholar] [CrossRef]
- .Mirnic, J.; Djuric, M.; Gusic, I.; Veljovic, T.; Cakic, S.; Katanic, J.; Vukoje, K.; Ramic, B.; Brkic, S. Effects of nonsurgical periodontal therapy on salivary 8-hydroxy-deoxyguanosine levels and glycemic control in diabetes mellitus type 2 patients. Biomedicines 2022, 10, 2269. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zhan, Q.; Wu, C.Z.; Yuan, Y.H.; Chen, W.; Yu, F.Y.; Li, Y.; Li, L.J. Baseline HbA1c level influences the effect of periodontal therapy on glycemic control in people with type 2 diabetes and periodontitis: a systematic review on randomized controlled trails. Diabetes Ther. 2021, 12, 1249–1278. [Google Scholar] [CrossRef]
- Auyeung, L.; Wang, P.W.; Lin, R.T.; Hsieh, C.J.; Lee, P.Y.; Zhuang, R.Y.; Chang, H.W. Evaluation of periodontal status and effectiveness of non-surgical treatment in patients with type 2 diabetes mellitus in Taiwan for a 1-year period. J. Periodontol. 2012, 83, 621–628. [Google Scholar] [CrossRef]
- Correa, F.O.; Gonçalves, D.; Figueredo, C.M.; Bastos, A.S.; Gustafsson, A.; Orrico, S.R. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J. Clin. Periodontol. 2010, 37, 53–58. [Google Scholar] [CrossRef]
- Engebretson, S.P.; Hyman, L.G.; Michalowicz, B.S.; Schoenfeld, E.R.; Gelato, M.C.; Hou, W.; Seaquist, E.R.; Reddy, M.S.; Lewis, C.E.; Oates, T.W.; et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA 2013, 310, 2523–2532. [Google Scholar] [CrossRef]
- Mirnic, J.; Djuric, M.; Gusic, I.; Veljovic, T.; Cakic, S.; Katanic, J.; Vukoje, K.; Ramic, B.; Brkic, S. Effects of nonsurgical periodontal therapy on salivary 8-hydroxy-deoxyguanosine levels and glycemic control in diabetes mellitus type 2 patients. Biomedicines 2022, 10, 2269. [Google Scholar] [CrossRef]
- Tsai, C.; Hayes, C.; Taylor, G.W. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent. Oral Epidemiol. 2002, 30, 182–192. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, J.; Bansal, D.; Sood, S.; Gupta, S.; Jain, A. Effect of scaling and root planing as monotherapy on glycemic control in patients of Type 2 diabetes with chronic periodontitis: a systematic review and meta-analysis. J. Indian Soc. Periodontol. 2019, 23, 303–310. [Google Scholar] [CrossRef]
- Cao, R.; Li, Q.; Wu, Q.; Yao, M.; Chen, Y.; Zhou, H. Effect of non-surgical periodontal therapy on glycemic control of type 2 diabetes mellitus: a systematic review and Bayesian network meta-analysis. BMC Oral Health 2019, 19, 176. [Google Scholar] [CrossRef]
- Sanz, M.; Del Castillo, A.M.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Moeintaghavi, A.; Arab, H.R.; Bozorgnia, Y.; Kianoush, K.; Alizadeh, M. Non-surgical periodontal therapy affects metabolic control in diabetics: a randomized controlled clinical trial. Aust. Dent. J. 2012, 57, 31–37. [Google Scholar] [CrossRef]
- Telgi, R.L.; Tandon, V.; Tangade, P.S.; Tirth, A.; Kumar, S.; Yadav, V. Efficacy of nonsurgical periodontal therapy on glycaemic control in type II diabetic patients: a randomized controlled clinical trial. J. Periodontal Implant Sci. 2013, 43, 177–182. [Google Scholar] [CrossRef]
- Li, Q.; Hao, S.; Fang, J.; Xie, J.; Kong, X.H.; Yang, J.X. Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials 2015, 16, 291. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).