Submitted:
09 February 2024
Posted:
12 February 2024
You are already at the latest version
Abstract
Keywords:
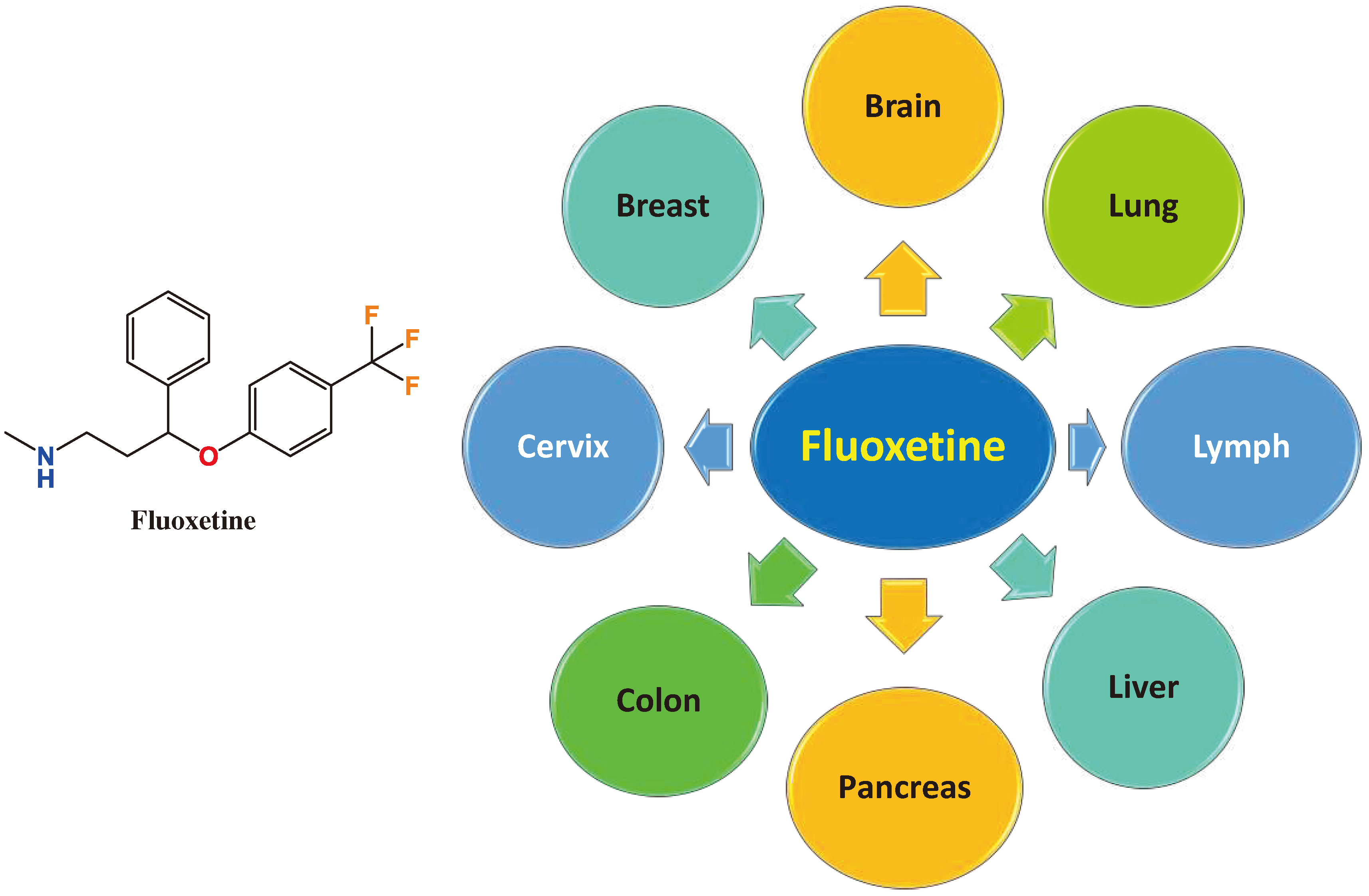
1. Introduction
2. Materials and Methods
3. Results
3.1. FLX in Brain Tumors
3.2. FLX in Breast Cancer
3.3. FLX in Hepatocellular Carcinoma (HCC)
3.4. FLX in Colon Cancer
3.5. FLX in Cervical Cancer
3.6. FLX in NSCLC
3.7. FLX in Pancreatic Cancer
3.8. FLX in Lymphoma
3.8. FLX in Multidrug Resistance (MDR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Oliveira, E.A.M.; Lang, K.L. Drug Repositioning: Concept, Classification, Methodology, and Importance in Rare/Orphans and Neglected Diseases. J. Appl. Pharm. Sci. 2018, 8, 157–165. [Google Scholar] [CrossRef]
- Song, Y.; Yang, X.; Yu, B. Repurposing Antidepressants for Anticancer Drug Discovery. Drug Discov. Today 2022, 27, 1924–1935. [Google Scholar] [CrossRef]
- Rascol, O.; Fabbri, M.; Poewe, W. Amantadine in the Treatment of Parkinson’s Disease and Other Movement Disorders. Lancet Neurol. 2021, 20, 1048–1056. [Google Scholar] [CrossRef]
- Menter, D.G.; Schilsky, R.L.; DuBois, R.N. Cyclooxygenase-2 and Cancer Treatment: Understanding the Risk Should Be Worth the Reward. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 1384–1390. [Google Scholar] [CrossRef]
- Sezer, A.; Halilović-Alihodžić, M.; Vanwieren, A.R.; Smajkan, A.; Karić, A.; Djedović, H.; Šutković, J. A Review on Drug Repurposing in COVID-19: From Antiviral Drugs to Herbal Alternatives. J. Genet. Eng. Biotechnol. 2022, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Bento Cunha, R.; Vassilevskaia, T.; Viveiros, M.; Cunha, C. Drug Repurposing for COVID-19: A Review and a Novel Strategy to Identify New Targets and Potential Drug Candidates. Mol. Basel Switz. 2022, 27, 2723. [Google Scholar] [CrossRef]
- Hijikata, A.; Shionyu, C.; Nakae, S.; Shionyu, M.; Ota, M.; Kanaya, S.; Shirai, T. Current Status of Structure-Based Drug Repurposing against COVID-19 by Targeting SARS-CoV-2 Proteins. Biophys. Physicobiology 2021, 18, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Lenze, E.J.; Reiersen, A.M.; Zorumski, C.F.; Santosh, P.J. Beyond “Psychotropic”: Repurposing Psychiatric Drugs for COVID-19, Alzheimer’s Disease, and Cancer. J. Clin. Psychiatry 2023, 84, 22r14494. [Google Scholar] [CrossRef]
- Radwan, M.O.; Abd-Alla, H.I.; Alsaggaf, A.T.; El-Mezayen, H.; Abourehab, M.A.S.; El-Beeh, M.E.; Tateishi, H.; Otsuka, M.; Fujita, M. Gypsogenin Battling for a Front Position in the Pentacyclic Triterpenes Game of Thrones on Anti-Cancer Therapy: A Critical Review—Dedicated to the Memory of Professor Hanaa M. Rady. Molecules 2023, 28, 5677. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–386. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, A.K.; Sakamoto, T.; Toma, T.; Sakamoto, M.; Abourehab, M.A.S.; Otsuka, M.; Fujita, M.; Tateishi, H.; Radwan, M.O. New Insights into the Structural Requirements of Isatin-Derived Pro-Apoptotic Agents against Acute Myeloid Leukemia. Pharm. 2022 Vol 15 Page 1579 2022, 15, 1579. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming Cancer Therapeutic Bottleneck by Drug Repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Cruz-Burgos, M.; Losada-Garcia, A.; Cruz-Hernández, C.D.; Cortés-Ramírez, S.A.; Camacho-Arroyo, I.; Gonzalez-Covarrubias, V.; Morales-Pacheco, M.; Trujillo-Bornios, S.I.; Rodríguez-Dorantes, M. New Approaches in Oncology for Repositioning Drugs: The Case of PDE5 Inhibitor Sildenafil. Front. Oncol. 2021, 11, 627229. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Gennings, C.; Bear, H.D.; Graham, L.J.; Sheth, C.M.; White, K.L.; Gewirtz, D.A. Influence of the Phosphodiesterase-5 Inhibitor, Sildenafil, on Sensitivity to Chemotherapy in Breast Tumor Cells. Breast Cancer Res. Treat. 2010, 124, 349–360. [Google Scholar] [CrossRef] [PubMed]
- He, X.-K.; Su, T.-T.; Si, J.-M.; Sun, L.-M. Metformin Is Associated With Slightly Reduced Risk of Colorectal Cancer and Moderate Survival Benefits in Diabetes Mellitus: A Meta-Analysis. Medicine (Baltimore) 2016, 95, e2749. [Google Scholar] [CrossRef]
- Urpilainen, E.; Puistola, U.; Boussios, S.; Karihtala, P. Metformin and Ovarian Cancer: The Evidence. Ann. Transl. Med. 2020, 8, 1711. [Google Scholar] [CrossRef]
- Pan, X.; Mao, T.-Y.; Mai, Y.-W.; Liang, C.-C.; Huang, W.-H.; Rao, Y.; Huang, Z.-S.; Huang, S.-L. Discovery of Quinacrine as a Potent Topo II and Hsp90 Dual-Target Inhibitor, Repurposing for Cancer Therapy. Mol. Basel Switz. 2022, 27, 5561. [Google Scholar] [CrossRef]
- Oien, D.B.; Pathoulas, C.L.; Ray, U.; Thirusangu, P.; Kalogera, E.; Shridhar, V. Repurposing Quinacrine for Treatment-Refractory Cancer. Semin. Cancer Biol. 2021, 68, 21–30. [Google Scholar] [CrossRef]
- Gurova, K.V.; Hill, J.E.; Guo, C.; Prokvolit, A.; Burdelya, L.G.; Samoylova, E.; Khodyakova, A.V.; Ganapathi, R.; Ganapathi, M.; Tararova, N.D.; et al. Small Molecules That Reactivate P53 in Renal Cell Carcinoma Reveal a NF-kappaB-Dependent Mechanism of P53 Suppression in Tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 17448–17453. [Google Scholar] [CrossRef]
- Park, S.; Oh, A.-Y.; Cho, J.-H.; Yoon, M.-H.; Woo, T.-G.; Kang, S.-M.; Lee, H.-Y.; Jung, Y.-J.; Park, B.-J. Therapeutic Effect of Quinacrine, an Antiprotozoan Drug, by Selective Suppression of p-CHK1/2 in P53-Negative Malignant Cancers. Mol. Cancer Res. MCR 2018, 16, 935–946. [Google Scholar] [CrossRef]
- Radwan, M.O.; Toma, T.; Arakaki, Y.; Kamo, M.; Inoue, N.; Koga, R.; Otsuka, M.; Tateishi, H.; Fujita, M. New Insight into the Bioactivity of Substituted Benzimidazole Derivatives: Repurposing from Anti-HIV Activity to Cell Migration Inhibition Targeting hnRNP M. Bioorg. Med. Chem. 2023, 117294–117294. [Google Scholar] [CrossRef]
- Radwan, M.O.; Ciftci, H.I.; Ali, T.F.S.; Ellakwa, D.E.; Koga, R.; Tateishi, H.; Nakata, A.; Ito, A.; Yoshida, M.; Okamoto, Y.; et al. Antiproliferative S-Trityl-l-Cysteine -Derived Compounds as SIRT2 Inhibitors: Repurposing and Solubility Enhancement. Molecules 2019, 24, 3295. [Google Scholar] [CrossRef]
- Radwan, M.O.; Ciftci, H.I.; Ali, T.F.S.; Koga, R.; Tateishi, H.; Nakata, A.; Ito, A.; Yoshida, M.; Fujita, M.; Otsuka, M. Structure Activity Study of S-Trityl-Cysteamine Dimethylaminopyridine Derivatives as SIRT2 Inhibitors: Improvement of SIRT2 Binding and Inhibition. Bioorg. Med. Chem. Lett. 2020, 30, 127458–127458. [Google Scholar] [CrossRef]
- Singhal, S.; Maheshwari, P.; Krishnamurthy, P.T.; Patil, V.M. Drug Repurposing Strategies for Non-Cancer to Cancer Therapeutics. Anticancer Agents Med. Chem. 2022, 22, 2726–2756. [Google Scholar] [CrossRef] [PubMed]
- Sternbach, H. Are Antidepressants Carcinogenic? A Review of Preclinical and Clinical Studies. J. Clin. Psychiatry 2003, 64, 1153–1162. [Google Scholar] [CrossRef]
- Bielecka, A.M.; Obuchowicz, E. Antidepressant Drugs as a Complementary Therapeutic Strategy in Cancer. Exp. Biol. Med. Maywood NJ 2013, 238, 849–858. [Google Scholar] [CrossRef]
- Tutton, P.J.; Barkla, D.H. Influence of Inhibitors of Serotonin Uptake on Intestinal Epithelium and Colorectal Carcinomas. Br. J. Cancer 1982, 46, 260–265. [Google Scholar] [CrossRef]
- Zheng, Y.; Chang, X.; Huang, Y.; He, D. The Application of Antidepressant Drugs in Cancer Treatment. Biomed. Pharmacother. Biomedecine Pharmacother. 2023, 157, 113985. [Google Scholar] [CrossRef] [PubMed]
- Radin, D.P.; Patel, P. A Current Perspective on the Oncopreventive and Oncolytic Properties of Selective Serotonin Reuptake Inhibitors. Biomed. Pharmacother. Biomedecine Pharmacother. 2017, 87, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-W.; Kim, E.-J.; Nyiramana, M.; Shin, E.-J.; Jin, H.; Ryu, J.; Kang, K.; Lee, G.-W.; Kim, H.; Han, J.; et al. Paroxetine Induces Apoptosis of Human Breast Cancer MCF-7 Cells through Ca2+-and P38 MAP Kinase-Dependent ROS Generation. Cancers 2019, 11, 64. [Google Scholar] [CrossRef]
- Gillman, P.K. Tricyclic Antidepressant Pharmacology and Therapeutic Drug Interactions Updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Ashbury, J.E.; Lévesque, L.E.; Beck, P.A.; Aronson, K.J. A Population-Based Case-Control Study of Selective Serotonin Reuptake Inhibitors (SSRIs) and Breast Cancer: The Impact of Duration of Use, Cumulative Dose and Latency. BMC Med. 2010, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.; García Rodríguez, L.A.; Hernández-Díaz, S. Use of Antidepressants and Risk of Lung Cancer. Cancer Causes Control 2007, 18, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tamim, H.; Shapiro, S.; Stang, M.R.; Collet, J.-P. Use of Antidepressants and Risk of Colorectal Cancer: A Nested Case-Control Study. Lancet Oncol. 2006, 7, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Coogan, P.F.; Strom, B.L.; Rosenberg, L. Antidepressant Use and Colorectal Cancer Risk. Pharmacoepidemiol. Drug Saf. 2009, 18, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Wernli, K.J.; Hampton, J.M.; Trentham-Dietz, A.; Newcomb, P.A. Antidepressant Medication Use and Breast Cancer Risk. Pharmacoepidemiol. Drug Saf. 2009, 18, 284–290. [Google Scholar] [CrossRef]
- Gil-Ad, I.; Zolokov, A.; Lomnitski, L.; Taler, M.; Bar, M.; Luria, D.; Ram, E.; Weizman, A. Evaluation of the Potential Anti-Cancer Activity of the Antidepressant Sertraline in Human Colon Cancer Cell Lines and in Colorectal Cancer-Xenografted Mice. Int. J. Oncol. 2008, 33, 277–286. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, W.; Shen, X.; Wang, Q.; Lv, J.; Liu, M.; Cheng, F.; Zhao, Z.; Pang, X. Repurposing Sertraline Sensitizes Non–Small Cell Lung Cancer Cells to Erlotinib by Inducing Autophagy. JCI Insight 3 e98921. [CrossRef]
- Ozunal, Z.G.; Cakil, Y.D.; Isan, H.; Saglam, E.; Aktas, R.G. Sertraline in Combination with Sorafenib: A Promising Pharmacotherapy to Target Both Depressive Disorders and Hepatocellular Cancer. Biol. Futura 2019, 70, 341–348. [Google Scholar] [CrossRef]
- Geeraerts, S.L.; Kampen, K.R.; Rinaldi, G.; Gupta, P.; Planque, M.; Louros, N.; Heylen, E.; De Cremer, K.; De Brucker, K.; Vereecke, S.; et al. Repurposing the Antidepressant Sertraline as SHMT Inhibitor to Suppress Serine/Glycine Synthesis–Addicted Breast Tumor Growth. Mol. Cancer Ther. 2021, 20, 50–63. [Google Scholar] [CrossRef]
- Baldissera, A.B.; Boia-Ferreira, M.; Basílio, A.B.C.; Resende, J.S. de S.; Castro, M.A.A.; Chaim, O.M.; Gremski, L.H.; Veiga, S.S.; Senff-Ribeiro, A. Sertraline as a Potential Cancer Therapeutic Approach: Biological Relevance of TCTP in Breast Cancer Cell Lines and Tumors. Adv. Med. Sci. 2023, 68, 227–237. [Google Scholar] [CrossRef]
- Wang, K.; Gong, Q.; Zhan, Y.; Chen, B.; Yin, T.; Lu, Y.; Zhang, Y.; Wang, H.; Ke, J.; Du, B.; et al. Blockage of Autophagic Flux and Induction of Mitochondria Fragmentation by Paroxetine Hydrochloride in Lung Cancer Cells Promotes Apoptosis via the ROS-MAPK Pathway. Front. Cell Dev. Biol. 2019, 7, 397. [Google Scholar] [CrossRef]
- Jang, W.-J.; Jung, S.K.; Vo, T.T.L.; Jeong, C.-H. Anticancer Activity of Paroxetine in Human Colon Cancer Cells: Involvement of MET and ERBB3. J. Cell. Mol. Med. 2019, 23, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.C.-H.; Hsieh, Y.-H.; Chen, L.-J.; Hsu, T.-C.; Tzang, B.-S. Escitalopram Oxalate Induces Apoptosis in U-87MG Cells and Autophagy in GBM8401 Cells. J. Cell. Mol. Med. 2018, 22, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Yuan, I.; Horng, C.-T.; Chen, V.C.-H.; Chen, C.-H.; Chen, L.-J.; Hsu, T.-C.; Tzang, B.-S. Escitalopram Oxalate Inhibits Proliferation and Migration and Induces Apoptosis in Non-Small Cell Lung Cancer Cells. Oncol. Lett. 2018, 15, 3376–3382. [Google Scholar] [CrossRef] [PubMed]
- Bavadekar, S.; Panchal, P.; Hanbashi, A.; Vansal, S. Cytotoxic Effects of Selective Serotonin- and Serotonin-Norepinephrine Reuptake Inhibitors on Human Metastatic Breast Cancer Cell Line, MCF-7 (842.3). FASEB J. 2014, 28, 842–3. [Google Scholar] [CrossRef]
- Magagnoli, J.; Narendran, S.; Pereira, F.; Cummings, T.H.; Hardin, J.W.; Sutton, S.S.; Ambati, J. Association between Fluoxetine Use and Overall Survival among Patients with Cancer Treated with PD-1/L1 Immunotherapy. Pharmaceuticals 2023, 16, 640. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, H.I.; Radwan, M.O.; Sever, B.; Hamdy, A.K.; Emirdağ, S.; Ulusoy, N.G.; Sozer, E.; Can, M.; Yayli, N.; Araki, N.; et al. EGFR-Targeted Pentacyclic Triterpene Analogues for Glioma Therapy. Int. J. Mol. Sci. 2021, 22, 10945. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-H.; Yang, S.-T.; Lin, Y.-K.; Lin, J.-W.; Lee, Y.-H.; Wang, J.-Y.; Hu, C.-J.; Lin, E.-Y.; Chen, S.-M.; Then, C.-K.; et al. Fluoxetine, an Antidepressant, Suppresses Glioblastoma by Evoking AMPAR-Mediated Calcium-Dependent Apoptosis. Oncotarget 2014, 6, 5088–5101. [Google Scholar] [CrossRef]
- You, F.; Zhang, C.; Liu, X.; Ji, D.; Zhang, T.; Yu, R.; Gao, S. Drug Repositioning: Using Psychotropic Drugs for the Treatment of Glioma. Cancer Lett. 2022, 527, 140–149. [Google Scholar] [CrossRef]
- Bi, J.; Khan, A.; Tang, J.; Armando, A.M.; Wu, S.; Zhang, W.; Gimple, R.C.; Reed, A.; Jing, H.; Koga, T.; et al. Targeting Glioblastoma Signaling and Metabolism with a Re-Purposed Brain-Penetrant Drug. Cell Rep. 2021, 37, 109957. [Google Scholar] [CrossRef]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The Glutamate Receptor Ion Channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar] [PubMed]
- Sobolevsky, A.I.; Rosconi, M.P.; Gouaux, E. X-Ray Structure, Symmetry and Mechanism of an AMPA-Subtype Glutamate Receptor. Nature 2009, 462, 745–756. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.-R.; Chen, W.; Chen, M.-H.; Wang, H.; Wang, X.-D.; Sun, L.-L.; Wang, F.-Z.; Wang, D.-C. Fluoxetine Synergizes with Temozolomide to Induce the CHOP-Dependent Endoplasmic Reticulum Stress-Related Apoptosis Pathway in Glioma Cells. Oncol. Rep. 2016, 36, 676–684. [Google Scholar] [CrossRef]
- Song, T.; Li, H.; Tian, Z.; Xu, C.; Liu, J.; Guo, Y. Disruption of NF-κB Signaling by Fluoxetine Attenuates MGMT Expression in Glioma Cells. OncoTargets Ther. 2015, 8, 2199–2208. [Google Scholar] [CrossRef]
- Bibbo’, S.; Lamolinara, A.; Capone, E.; Purgato, S.; Tsakaneli, A.; Panella, V.; Sallese, M.; Rossi, C.; Ciufici, P.; Nieddu, V.; et al. Repurposing a Psychoactive Drug for Children with Cancer: p27Kip1-Dependent Inhibition of Metastatic Neuroblastomas by Prozac. Oncogenesis 2020, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J.; Najafi, S.H.; Shafiee, F.; Hassanzadeh, S.; Farzipour, S.; Ghasemi, A.; Asgarian-Omran, H. Fluoxetine as an Antidepressant Medicine Improves the Effects of Ionizing Radiation for the Treatment of Glioma. J. Bioenerg. Biomembr. 2020, 52, 165–174. [Google Scholar] [CrossRef]
- Patanaphan, V.; Salazar, O.M.; Risco, R. Breast Cancer: Metastatic Patterns and Their Prognosis. South. Med. J. 1988, 81, 1109–1112. [Google Scholar] [CrossRef]
- Nelson, H.D.; Smith, M.E.B.; Griffin, J.C.; Fu, R. Use of Medications to Reduce Risk for Primary Breast Cancer: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 158, 604–614. [Google Scholar] [CrossRef]
- Ong, J.C.-L.; Sun, F.; Chan, E. Development of Stealth Liposome Coencapsulating Doxorubicin and Fluoxetine. J. Liposome Res. 2011, 21, 261–271. [Google Scholar] [CrossRef]
- Zhou, T.; Duan, J.; Wang, Y.; Chen, X.; Zhou, G.; Wang, R.; Fu, L.; Xu, F. Fluoxetine Synergys with Anticancer Drugs to Overcome Multidrug Resistance in Breast Cancer Cells. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2012, 33, 1299–1306. [Google Scholar] [CrossRef]
- Kabel, A.M.; Elkhoely, A.A. Ameliorative Potential of Fluoxetine/Raloxifene Combination on Experimentally Induced Breast Cancer. Tissue Cell 2016, 48, 89–95. [Google Scholar] [CrossRef]
- Tatar, O.; Ilhan, N.; Ilhan, N.; Susam, S.; Ozercan, I.H. Is There Any Potential Anticancer Effect of Raloxifene and Fluoxetine on DMBA-induced Rat Breast Cancer? J. Biochem. Mol. Toxicol. 2019, 33, e22371. [Google Scholar] [CrossRef]
- Duarte, D.; Cardoso, A.; Vale, N. Synergistic Growth Inhibition of HT-29 Colon and MCF-7 Breast Cancer Cells with Simultaneous and Sequential Combinations of Antineoplastics and CNS Drugs. Int. J. Mol. Sci. 2021, 22, 7408. [Google Scholar] [CrossRef]
- Duarte, D.; Rêma, A.; Amorim, I.; Vale, N. Drug Combinations: A New Strategy to Extend Drug Repurposing and Epithelial-Mesenchymal Transition in Breast and Colon Cancer Cells. Biomolecules 2022, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Falcão, S.I.; El Mehdi, I.; Vilas-Boas, M.; Vale, N. Honeybee Venom Synergistically Enhances the Cytotoxic Effect of CNS Drugs in HT-29 Colon and MCF-7 Breast Cancer Cell Lines. Pharmaceutics 2022, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-A.; Chu, P.-Y.; Tan, Z.-L.; Hsu, F.-T.; Lee, Y.-C.; Wu, H.-J. STAT3 Inactivation and Induction of Apoptosis Associate With Fluoxetine-Inhibited Epithelial-Mesenchymal Transition and Growth of Triple-Negative Breast Cancer In Vivo. Anticancer Res. 2022, 42, 3807–3814. [Google Scholar] [CrossRef]
- Bowie, M.; Pilie, P.; Wulfkuhle, J.; Lem, S.; Hoffman, A.; Desai, S.; Petricoin, E.; Carter, A.; Ambrose, A.; Seewaldt, V.; et al. Fluoxetine Induces Cytotoxic Endoplasmic Reticulum Stress and Autophagy in Triple Negative Breast Cancer. World J. Clin. Oncol. 2015, 6, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhu, L.; Zhao, Y.; Jiang, Y.; Chen, L.; Yu, Y.; Ouyang, L. Fluoxetine Induces Autophagic Cell Death via eEF2K-AMPK-mTOR-ULK Complex Axis in Triple Negative Breast Cancer. Cell Prolif. 2018, 51, e12402. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA. Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, J.; Yamada, T.; Egashira, N.; Ueda, M.; Zukeyama, N.; Ushio, S.; Masuda, S. Comparison of the Anti-Tumor Effects of Selective Serotonin Reuptake Inhibitors as Well as Serotonin and Norepinephrine Reuptake Inhibitors in Human Hepatocellular Carcinoma Cells. Biol. Pharm. Bull. 2015, 38, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Mun, A.-R.; Lee, S.-J.; Kim, G.-B.; Kang, H.-S.; Kim, J.-S.; Kim, S.-J. Fluoxetine-Induced Apoptosis in Hepatocellular Carcinoma Cells. Anticancer Res. 2013, 33, 3691–3697. [Google Scholar] [PubMed]
- Hsu, L.-C.; Tu, H.-F.; Hsu, F.-T.; Yueh, P.-F.; Chiang, I.-T. Beneficial Effect of Fluoxetine on Anti-Tumor Progression on Hepatocellular Carcinoma and Non-Small Cell Lung Cancer Bearing Animal Model. Biomed. Pharmacother. 2020, 126, 110054. [Google Scholar] [CrossRef]
- Chen, W.-T.; Hsu, F.-T.; Liu, Y.-C.; Chen, C.-H.; Hsu, L.-C.; Lin, S.-S. Fluoxetine Induces Apoptosis through Extrinsic/Intrinsic Pathways and Inhibits ERK/NF-κB-Modulated Anti-Apoptotic and Invasive Potential in Hepatocellular Carcinoma Cells In Vitro. Int. J. Mol. Sci. 2019, 20, 757. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Bar, H.M.; Khater, S.E.; Ghorab, D.M.; Al-mahallawi, A.M. Hexosomes as Efficient Platforms for Possible Fluoxetine Hydrochloride Repurposing with Improved Cytotoxicity against HepG2 Cells. ACS Omega 2020, 5, 26697–26709. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2023. CA. Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and Number of Cancer Cases and Deaths Attributable to Potentially Modifiable Risk Factors in the United States. CA. Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Engelmann, B.J.; Ryan, J.J.; Farrell, N.P. Antidepressants and Platinum Drugs. Anticancer Res. 2014, 34, 509–516. [Google Scholar]
- Kang, B.-G.; Shende, M.; Inci, G.; Park, S.-H.; Jung, J.-S.; Kim, S.B.; Kim, J.H.; Mo, Y.W.; Seo, J.-H.; Feng, J.-H.; et al. Combination of Metformin/Efavirenz/Fluoxetine Exhibits Profound Anticancer Activity via a Cancer Cell-Specific ROS Amplification. Cancer Biol. Ther. 2023, 24, 2161803. [Google Scholar] [CrossRef]
- Marcinkute, M.; Afshinjavid, S.; Fatokun, A.A.; Javid, F.A. Fluoxetine Selectively Induces P53-Independent Apoptosis in Human Colorectal Cancer Cells. Eur. J. Pharmacol. 2019, 857, 172441. [Google Scholar] [CrossRef]
- Minagawa, Y.; Kigawa, J.; Itamochi, H.; Kanamori, Y.; Shimada, M.; Takahashi, M.; Terakawa, N. Cisplatin-resistant HeLa Cells Are Resistant to Apoptosis via P53-dependent and -independent Pathways. Jpn. J. Cancer Res. Gann 1999, 90, 1373–1379. [Google Scholar] [CrossRef]
- Charles, E.; Hammadi, M.; Kischel, P.; Delcroix, V.; Demaurex, N.; Castelbout, C.; Vacher, A.-M.; Devin, A.; Ducret, T.; Nunes, P.; et al. The Antidepressant Fluoxetine Induces Necrosis by Energy Depletion and Mitochondrial Calcium Overload. Oncotarget 2016, 8, 3181–3196. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Xu, M.; Che, X.; Jiang, X. Fluoxetine Enhances Cellular Chemosensitivity to Cisplatin in Cervical Cancer.
- Naz, A.; Hashim, F.; Ali, S.A.; Badshah, M. Fabrication, Characterization and Therapeutic Evaluation of Fluoxetine-Dextran Nanoparticles. ChemistrySelect 2023, 8, e202204110. [Google Scholar] [CrossRef]
- Sagnella, S.M.; Duong, H.; MacMillan, A.; Boyer, C.; Whan, R.; McCarroll, J.A.; Davis, T.P.; Kavallaris, M. Dextran-Based Doxorubicin Nanocarriers with Improved Tumor Penetration. Biomacromolecules 2014, 15, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Kusumoto, S.; Ando, K.; Ohba, M.; Ohmori, T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3491. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Lin, S.-S.; Hsu, F.-T.; Chung, J.-G. Fluoxetine Inhibits DNA Repair and NF-ĸB-Modulated Metastatic Potential in Non-Small Cell Lung Cancer. Anticancer Res. 2018, 38, 5201–5210. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Aakjær, M.; Kristiansen, S.B.; Pape, K.; Sessa, M.; Dalhoff, K.P.; De Bruin, M.L.; Andersen, M. Investigation of the Potential Association between the Use of Fluoxetine and Occurrence of Acute Pancreatitis: A Danish Register-Based Cohort Study. Int. J. Epidemiol. 2022, 51, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Shang, Y.-Y.; Li, Y.-Y. Effect of Antidepressants on Body Weight, Ethology and Tumor Growth of Human Pancreatic Carcinoma Xenografts in Nude Mice. World J. Gastroenterol. 2008, 14, 4377–4382. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.A.; Heeb, L.; Beffinger, M.M.; Pantelyushin, S.; Linecker, M.; Roth, L.; Lehmann, K.; Ungethüm, U.; Kobold, S.; Graf, R.; et al. Attenuation of Peripheral Serotonin Inhibits Tumor Growth and Enhances Immune Checkpoint Blockade Therapy in Murine Tumor Models. Sci. Transl. Med. 2021, 13, eabc8188. [Google Scholar] [CrossRef] [PubMed]
- Matasar, M.J.; Zelenetz, A.D. Overview of Lymphoma Diagnosis and Management. Radiol. Clin. North Am. 2008, 46, 175–198. [Google Scholar] [CrossRef]
- Mugnaini, E.N.; Ghosh, N. Lymphoma. Prim. Care 2016, 43, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; Faderl, S.; O’Brien, S.; Bueso-Ramos, C.; Cortes, J.; Garcia-Manero, G.; Giles, F.J.; Verstovsek, S.; Wierda, W.G.; Pierce, S.A.; et al. Chemoimmunotherapy with Hyper-CVAD plus Rituximab for the Treatment of Adult Burkitt and Burkitt-Type Lymphoma or Acute Lymphoblastic Leukemia. Cancer 2006, 106, 1569–1580. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Drozgowska, A.; Fayne, D.; Williams, D.C. The Antidepressants Maprotiline and Fluoxetine Have Potent Selective Antiproliferative Effects against Burkitt Lymphoma Independently of the Norepinephrine and Serotonin Transporters. Leuk. Lymphoma 2010, 51, 523–539. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Williams, D.C. The Antidepressants Maprotiline and Fluoxetine Induce Type II Autophagic Cell Death in Drug-Resistant Burkitt’s Lymphoma. Int. J. Cancer 2011, 128, 1712–1723. [Google Scholar] [CrossRef]
- Di Rosso, M.E.; Sterle, H.A.; Cremaschi, G.A.; Genaro, A.M. Beneficial Effect of Fluoxetine and Sertraline on Chronic Stress-Induced Tumor Growth and Cell Dissemination in a Mouse Model of Lymphoma: Crucial Role of Antitumor Immunity. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Tan, B.; Piwnica-Worms, D.; Ratner, L. Multidrug Resistance Transporters and Modulation. Curr. Opin. Oncol. 2000, 12, 450–458. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, Mohd.F.-R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al.Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12. [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug Resistance in Cancer: Role of ATP–Dependent Transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Dekel, Y.; Melikhov, D.; Margalit, R. Fluoxetine Inhibits Multidrug Resistance Extrusion Pumps and Enhances Responses to Chemotherapy in Syngeneic and in Human Xenograft Mouse Tumor Models. Cancer Res. 2004, 64, 7562–7569. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, S. Structure and Mechanism of ABC Transporters. F1000Prime Rep. 2015, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Peña-Solórzano, D.; Stark, S.A.; König, B.; Sierra, C.A.; Ochoa-Puentes, C. ABCG2/BCRP: Specific and Nonspecific Modulators. Med. Res. Rev. 2017, 37, 987–1050. [Google Scholar] [CrossRef] [PubMed]
- Stefan, S.M.; Wiese, M. Small-Molecule Inhibitors of Multidrug Resistance-Associated Protein 1 and Related Processes: A Historic Approach and Recent Advances. Med. Res. Rev. 2019, 39, 176–264. [Google Scholar] [CrossRef]
- Bin Kanner, Y.; Teng, Q.-X.; Ganoth, A.; Peer, D.; Wang, J.-Q.; Chen, Z.-S.; Tsfadia, Y. Cytotoxicity and Reversal Effect of Sertraline, Fluoxetine, and Citalopram on MRP1- and MRP7-Mediated MDR. Front. Pharmacol. 2023, 14. [Google Scholar] [CrossRef]
- Wt, C.; Yh, T.; P, T.; Ft, H.; Hd, W.; Wc, L.; Fh, L.; Ct, W. Fluoxetine Inhibits STAT3-Mediated Survival and Invasion of Osteosarcoma Cells. Anticancer Res. 2023, 43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




