1. Introduction
Enhancing forest cover is crucial for enhancing carbon sequestration and mitigating the impact of climate change (Aronson et al., 2006; Clewell and Aronson, 2007). Natural forest regeneration is a recommended, cost-effective, and sustainable method for achieving this goal (Stanturf et al., 2020). However, it is essential to acknowledge that natural regeneration may not be feasible in regions with adverse climates hindering its success (Stanturf et al., 2014, 2015). For instance, in the arid and semiarid lands of Central Asia, environmental conditions hinder natural forest regeneration necessitating additional management measures such as irrigation and fertilization to facilitate the growth of planted forests.
A significant portion of Mongolia’s territory faces a severe continental climate ranging from subarctic, to arid and semi-arid with 78% of the territory experiencing severe soil erosion and desertification (Country Programme of Mongolia, 2019; MNET 2010; Desertification Atlas of Mongolia, 2013). Climatic models predict a worsening of environmental conditions in these lands, leading to an expansion of desertification, loss of biodiversity, and a decline in residual forest cover (Yessekin et al., 2008; Hessl et al., 2016; Reyer et al., 2017; Stavi and Lal, 2015). In response to these challenges, the Government of Mongolia has initiated tree-planting projects, aiming to halt further land degradation and improve local livelihoods through economically viable agroforestry activities (Tsogtbaatar, 2013).
One tree-planting initiative is the Greenbelt project (Byambadorj et al. (2021 a,b), which commenced in 2009 and has contributed to afforestation in Mongolia’s arid and semiarid land. These plantations (approximately 3000 ha) required the construction of water wells due to limited water availability from rainfall. Consequently, thorough monitoring of the growth and developmental trend in these plantations was imperative to determine an optimal and sustainable amount of watering necessary to ensure the success of the planting initiative.
In a broad context, monitoring the growth and development of trees in a new plantation is a crucial step (McDonald et al., 2016) for at least three reasons. Firstly, it allows us to anticipate the success or failure of the plantation’s objective (Menz et al., 2011; Abiyu et al., 2016; Guariguata and Evans, 2020), and, if necessary, make adaptive management adjustments (Le et al., 2012). Secondly, monitoring provides the opportunity to accumulate knowledge and expertise essential for scaling up or planning similar initiatives in the future (Kanowski et al., 2010; Derhé, 2016), and contributes to transparency and accountability (Benayas et al., 2009). Thirdly, monitoring is essential for generating comprehensive reports for investors, securing additional funds, and facilitating the exchange of information with similar initiatives (Guariguata and Evans, 2020).
Extensive literature exists on the various methods for measuring the biophysical parameters of growing trees. These methods can be broadly categorized into three overlapping groups: 1) Digital Tracking (DT), 2) Remote Sensing (RS), and 3) Drone Fly-Overs (DFO). RS utilizes LiDAR, while DFO employs lightweight uncrewed aerial vehicles. Both RS and DFO measure aboveground biomass, density, vegetation structure and composition, natural seedlings recruitment, tree architecture, flowering flushes, etc. DT involves field visits to the stand where morphological traits are hand-measured and recorded directly in a digital platform such as FARM-TRACE. Therefore, it is reasonable to consider DT as a “boots on the ground” monitoring method, whereas RS and DFO could be defined as “remote” monitoring methods, as both approaches collect imagery for later analysis using specific software to provide a Digital Terrain Model (DTM) and a Canopy Height Model (CHM) (Farajelahl et al., 2022).
An important difference between these monitoring methods consists in the type of information they provide. RS and DFO monitor a stand at a community level (Ruiz-Jaén and Aide, 2006; Monie et al., 2013), whereas DT monitors the growth and development of individual trees within a stand (Mahamoudou and Arakwiye, 2020; Reytar et al., 2020). DT monitoring is particularly relevant at the initial stage of forest landscape restoration (FLR) implementation to establish the compatibility between plant species and the environmental factors specific to the selected site.
We have been monitoring the performance of the Greenbelt Project plantations since 2009 through a DT approach based on the yearly “in vivo” (non-destructive) measurement of various morphological parameters of aboveground organs (stem, branches, and leaves). In particular, these studies have focused on Populus sibirica and Ulmus pumila and the data are summarized in several published papers that show the growth and development trends followed by these trees from 2009 to 2019. This monitoring approach has established that the higher levels of irrigation (4 and 8 Lh-1) ensure the best growth and development trends (even in the absence of fertilization) (Byambadorj et al., 2021a,b; Nyam-Osor and al., 2021; Montagnoli et al., 2022). Regarding belowground organs, unfortunately, it has been impossible to use any “in vivo” monitoring approach due to the physical nature of the soil which limits the efficacy of the penetrating radar technology (Zhang et al., 2019). For this reason, in 2019, we utilized an “ex vivo” (destructive) approach (i.e., hand excavation) to measure the level of growth and development achieved by the root system of these trees after 11 years of growth. In particular, we measured various parameters such as root length, root diameter, and dry and fresh weight; for this purpose, the roots were categorized into taproots and lateral roots, divided into diameters classes and branching orders (Montagnoli et al., 2022).
The data collected in this study confirmed that Populus sibirica and Ulmus pumila trees subjected to the higher irrigation regime of 4 and 8L h-1, presented the highest values of all the morphological traits measured. Interestingly, the root-to-total-tree biomass ratio exceeded 40%. This ratio aligned with values derived from various allometric biomass equations found in the literature (He et al., 2018), indicating that at the time of excavation (2019), these trees exhibited a well-developed root system.
While the “ex vivo” approach provided valuable insights into root conformation, the data fell short of fully characterizing the growth trends in the root systems of Ulmus pumila over their 11-year lifespan. To address this gap, the present study employed a dendrometric analysis of xylogenetic activity (Rathgeber et al., 2016) to characterize the wood in the upper portion of the taproot and the lower portion of the stem in excavated Ulmus pumila trees.
This approach rested on the assumption that the growth and development of the overall root system including the production of new lateral roots in a given year, are intricately linked to the width of the annual ring produced in the same year in the upper portion of the taproot (Kozlowski and Pallardy, 1997). Similarly, we hypothesized that the overall production of new branches and leaves aboveground is intricately linked to the width of the annual ring produced in the same year in the lower portion of the stem.
Furthermore, the dendrometric analysis of the wood organization in the upper portion of the taproot and the lower portion of the stem could illuminate the correlation established during the tree’s lifespan between the transportation of raw sap and phloem sap produced by the leaves (Kozlowski and Pallardy, 1997).
The results obtained suggested the occurrence of similar growth and developmental trends in both above- and belowground organs in Ulmus pumila trees. In both cases, the trends feature an initial LAG phase, followed by an exponential LOG phase that decelerates without reaching the conventional STATIONARY phase observed in typical geometric growth trajectories.
Furthermore, this study revealed the occurrence of a difference between the trends obtained through the new dendrometric (i.e., “ex vivo”) or traditional DT (i.e., “in vivo”) approach. In this last case, the trends obtained by measuring stem height and diameter from 2009 to 2019 followed a typical geometric growth curve. The discussion in the paper delves into the difference between these trends emphasizing the importance of adopting both approaches for reliable monitoring of plantation performance.
2. Materials and Methods
2.1. The Experimental Site
The experimental site is in Lun soum, Tuv province, Mongolia (47°52′15.43″N, 105°10′46.4″E) at an elevation of 1,130 m a.s.l. The site extends for 0.2 ha, and it is described as the Middle Khalkha dry steppe (Ulziykhutag 1989) dominated by xerophytic and mesoxerophytic graminoids (e.g., Stipa sareptana subsp. krylovii (Roshev.), Cleistogenes squarrosa (Trin.), Agropyron cristatum (L.) Gaertn, Artemisia frigida (Willd.), Artemisia adamsii (Besser), Carex duriuscula C.A.Mey., Leymus chinensis (Trin.)) (Lavrenko et al. 1991). Soil type is classified as Loamic Kastanozems (IUSS Working Group WRB, 2015), with topsoil characterized by a hardness of 4.5 kg cm−2, while that of the subsoil was 1.7 kg cm−2.
2.2. Climatic Characteristics of the Experimental Site
According to the National Agency for Meteorology and Environmental Monitoring of Mongolia (NAMEM, 2019), the annual average temperature is 0.6 ± 0.45 °C with a summer (May–September) average temperature of 16.29 ± 0.41 °C. Annual mean precipitation during the whole experiment (2000–2019) was 196 mm, with a maximum value between June and August that accounted for 80–90% of the total annual rainfall. The mean annual potential evapotranspiration is 752.12 ± 30.68 mm (Cao et al. 2018). The warmest month is July (16 °C) whereas the coldest month is January (−22 °C).
2.3. Plant Material and Management: Watering and Fertilization
Two-year-old saplings of U. pumila (grown from seeds) were transplanted in May 2009, in 60–70 cm-deep holes with a diameter of 50–60 cm. Immediately after planting, a sufficient level of watering was supplied to individual trees by compensating non-leakage (CNL) button drippers placed 10 cm from the stem of each sapling. After sapling acclimatization, an irrigation regime of 8 L h−1=1.0 mm m−2 was applied: The 5-hour duration of watering was done twice a week for the entire vegetative season (from the beginning of May to the end of August) and was repeated every year from 2009 to 2019.
Each plot measured 20 × 10 m, and the seedlings were planted in rows with a north-south orientation to ensure maximum light availability during the whole day (Johnson and Brandle, 2009). The distance between rows was 2.5 m and distance between trees was 2.5 m.
2.4. Monitoring by “In Vivo” Approach
The methods used for trait measurement “in vivo,” are described extensively in Byambadorj et al. (2021 a,b). In particular, the height and RCD of the stem of elm was measured by ruler and calliper at 30-day intervals during the entire vegetative season during the period 2009–2019. The seedling recruitment (i.e., seedling survival) rate was calculated as the number of living plants divided by the number of plants originally planted.
2.5. Monitoring by “Ex Vivo” Approach
Tree excavation is described extensively in Montagnoli et al. (2022). In summary, six trees were randomly selected and hand-excavated to recover the intact root system. Before excavation, a single screw was driven into the bark of each tree at the root-stem interface to delineate the north. Two screws were partially drilled into the stump about 20 cm apart with their heads adjusted to indicate the horizontal level of the soil. Two more screws, perpendicular to the first two, were installed similarly. Excavation of the trees was done to a depth of ∼0.8–1m and the portions of roots extending more than 1m from the trunk were left in the soil. From six excavated trees an aliquot of disks was cut from the lower portion of the stem (10 cm above the soil surface) and from the upper portion of the taproot (10 cm below the soil surface) to be used for dendrometric studies. In particular, the disks were sanded with a series of increasingly fine grit sandpaper (ranging from 60 to 600-grit). The sanded sections were later scanned at 750 dpi sensitivity and images were analysed by a plug-in (ObjectJ) of ImageJ software to measure the annual ring thickness and the xylem diameter. The thickness of each annual ring was obtained as the mean of two measurements obtained by starting from the centre of the section and moving toward the cork in two opposite directions. This procedure was adopted to avoid the effect of the eccentricity of the section which could have a considerable effect on the thickness measurement.
2.6. Statistical Analysis
Statistical analysis used the SAS software package, version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). One-way analysis of variance (ANOVA) with Duncan’s multiple range test (DMRT) was used for multiple comparisons among the 2019 data. Permanent plots were considered independent replicates. During the 10-year monitoring period, the morphological traits (height and RCD) in stems of trees within each plot were measured and the data treated as a mean.
3. Results and Discussion
The widely accepted definition of growth and development in plant biology suggests that while plant growth involves organ dimension increase over time, plant development refers to structural changes. These processes are closely related and should be studied together as part of a single “integrative development” event (Dambreville et al., 2015), influenced by both genetic properties and environmental factors (Kholmanskiy, 2015). Therefore, optimal and coordinated growth and development of both above and belowground organs are crucial for plant health. Consequently, monitoring plantation performance should involve evaluating the simultaneous growth and developmental trends of all tree organs, considering the anatomical and physiological differences between aboveground and belowground organs and their distinct living environments (i.e., air and soil).
Regrettably, current plantation monitoring, whether at the level of single trees or communities, relies solely on “in vivo” measurements of aboveground tree organs (Padua et al., 2017). It is surprising that this limitation exists, considering the critical role played by belowground organs in the life of higher plants (Ryan et al., 2016). However, it is not difficult to understand this limitation, given that the opaque nature of soil still hinders the “in vivo” study of belowground organs, such as the root system.
Since 2009, our laboratories have been involved in monitoring the performance of plantations established in arid and semiarid sites of Mongolia under the Greenbelt project (Byambadorj et al., 2021a,b; Nyam-Osor et al., 2021; Montagnoli et al., 2022). Until 2019, we monitored these plantations through a DT approach by measuring “in vivo” year after year the morphological traits of aboveground (stem, branches, and leaves) organs. These studies succeeded in establishing that the irrigation regime of 8 L h−1 was the management measure that better ensured an optimal growth and development trend of Ulmus pumila trees even in the absence of fertilization (Byambadorj et al., 2021a,b; Nyam-Osor et al., 2021; Montagnoli et al., 2022). Unfortunately, until 2019 no monitoring had been possible to investigate the growth and development trends of belowground organs of these trees.
For this purpose, in 2019 we adopted an “ex vivo” (i.e., after excavation) monitoring approach and excavated six Ulmus pumila trees to analyze the architectural conformation of their root system present at the time of tree excavation (Montagnoli et al., 2022). These “ex vivo” studies succeeded in providing a clear picture of the health condition of the root systems at the time of tree excavation but were useless in reconstructing the growth and developmental trends followed by the root systems in the period 2009-2019.
The study presented here shows for the first time how it is possible to obtain an indication of the growth and development trend followed by the root system of a tree using a dendrometric approach. This approach is obviously to be considered as an “ex vivo” monitoring approach and is based on the analysis of annual ring width over the years. In this regard,
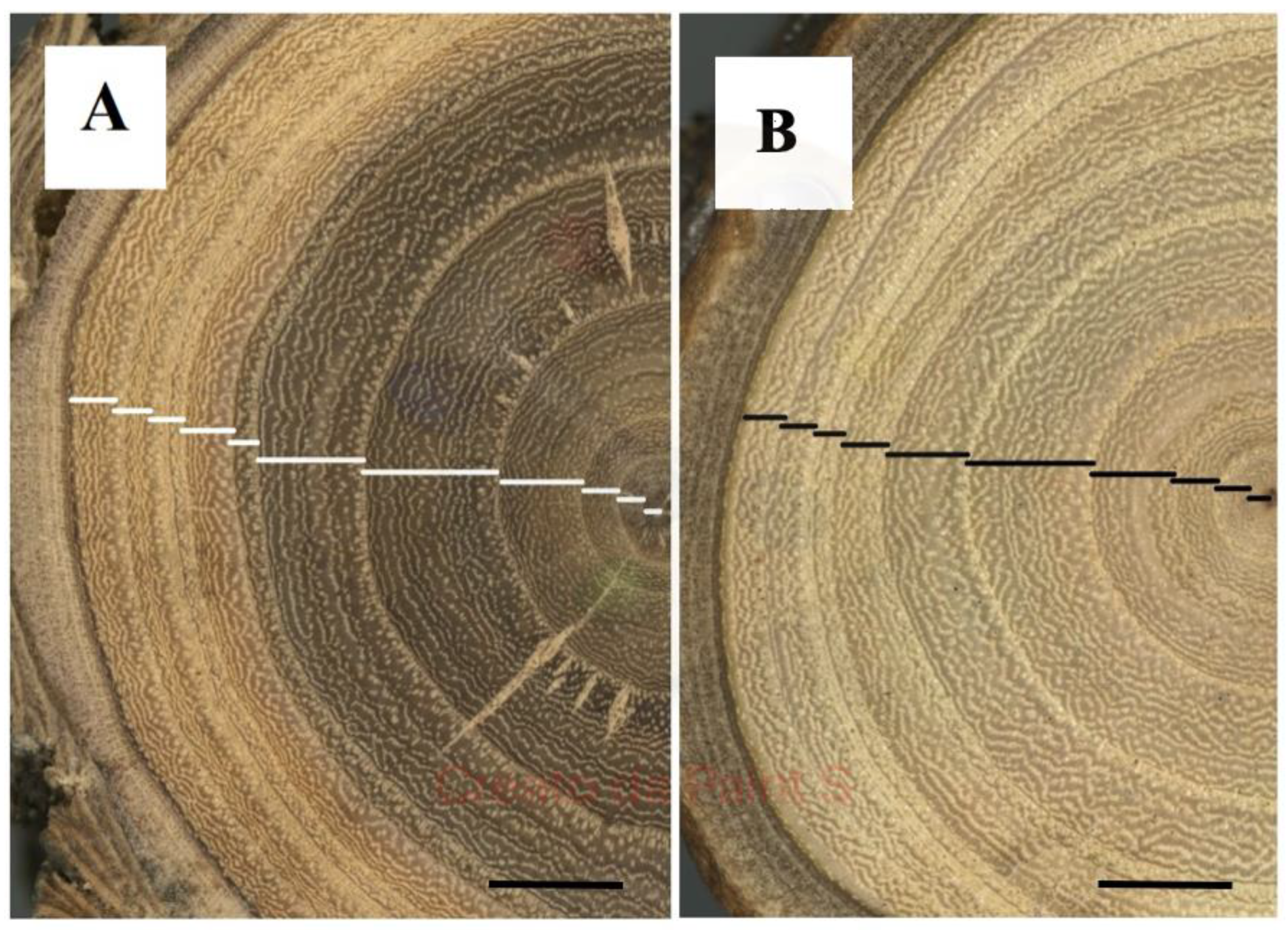
Figure 1 A shows the aspect of a wood section of the upper portion of a taproot of
Ulmus pumila where a considerable variation can be observed in the width of the annual rings took place in the years 2009-2019.
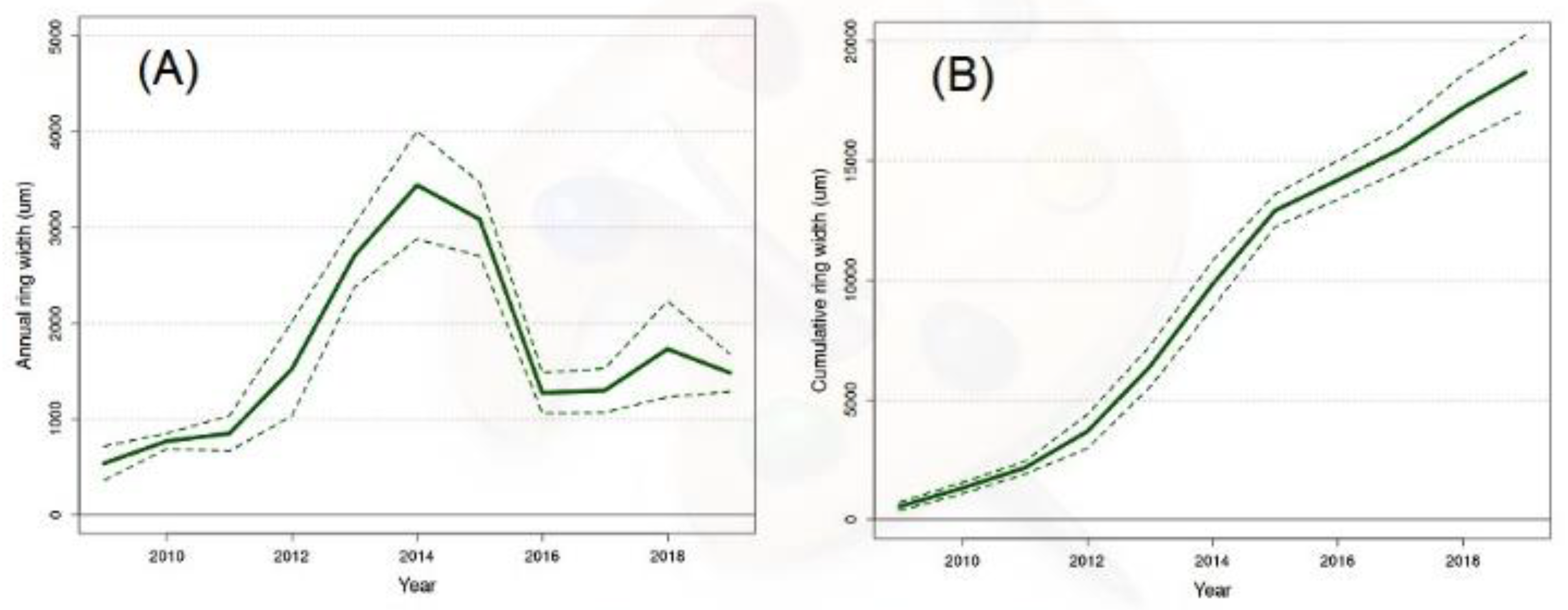
When we plotted each annual ring width over the years (
Figure 2A), we observed a curve with the highest value in 2014. When we plotted the cumulative sum of values of the annual rings year after year, then we obtained a curve of “cumulative root biomass” (
Figure 2B). We assumed that each annual ring present in the upper portion of a taproot could represent an indicator of the overall biomass that the plant yearly invested in the growth and development of the root system. In particular, this curve showed an initial increase followed by a continuous exponential phase of width increase. Moreover, the standard deviation reported in both panels suggested clearly that the same pattern characterized the variation of annual ring width in the woody sections of all the excavated trees.
In plant biology, the literature predicts the occurrence of a theoretical and empirical geometrical pattern of growth where an initial slow increase (LAG phase), is followed by an exponential increase (LOG phase), that ends with an equilibrium (STATIONARY phase) (Deng et al., 2012). According to this theoretical definition of a growth trend, the data collected by our dendrometric approach on one hand confirm the possibility to reconstruct the growth and development trend of the root system, but on the other hand, suggest that our data do not follow a geometrical trend. The initial LAG phase (2009-2012) seems to be followed by a continuous LOG phase (lasting at least until the time of root excavation).
Furthermore, our dendrometric approach reveals consistent trends in taproot and stem (
Figure 1 A, B) suggesting that
Ulmus pumila in the Greenbelt Project plantations adheres to the “pipe model theory”. This theory as proposed by Shinozaki in 2017 posits a robust correlation between plant development and the connectivity between belowground (roots) and aboveground (leaves) organs. Moreover, the absence of discernible differences in the trends in our trees indicates both ontogenetic and environmental factors, such as variation in soil nutrient concentration, have not affected the allocation of biomass between above- or belowground organs as shown by other authors (Helmisari et al., 2002; Poorter et al., 2011; Mathew et al., 2016; Jevsenak and Levanic, 2018; Holland et al., 2019; Soong et al., 2020). Regarding environmental factors, a meticulous analysis of meteorological data (air relative humidity, air temperature, precipitation, soil water content, soil temperature, wind strength) recorded from 2009 to 2019 did not reveal any variability that could have influenced the biomass production and allocation of our trees (data not shown).
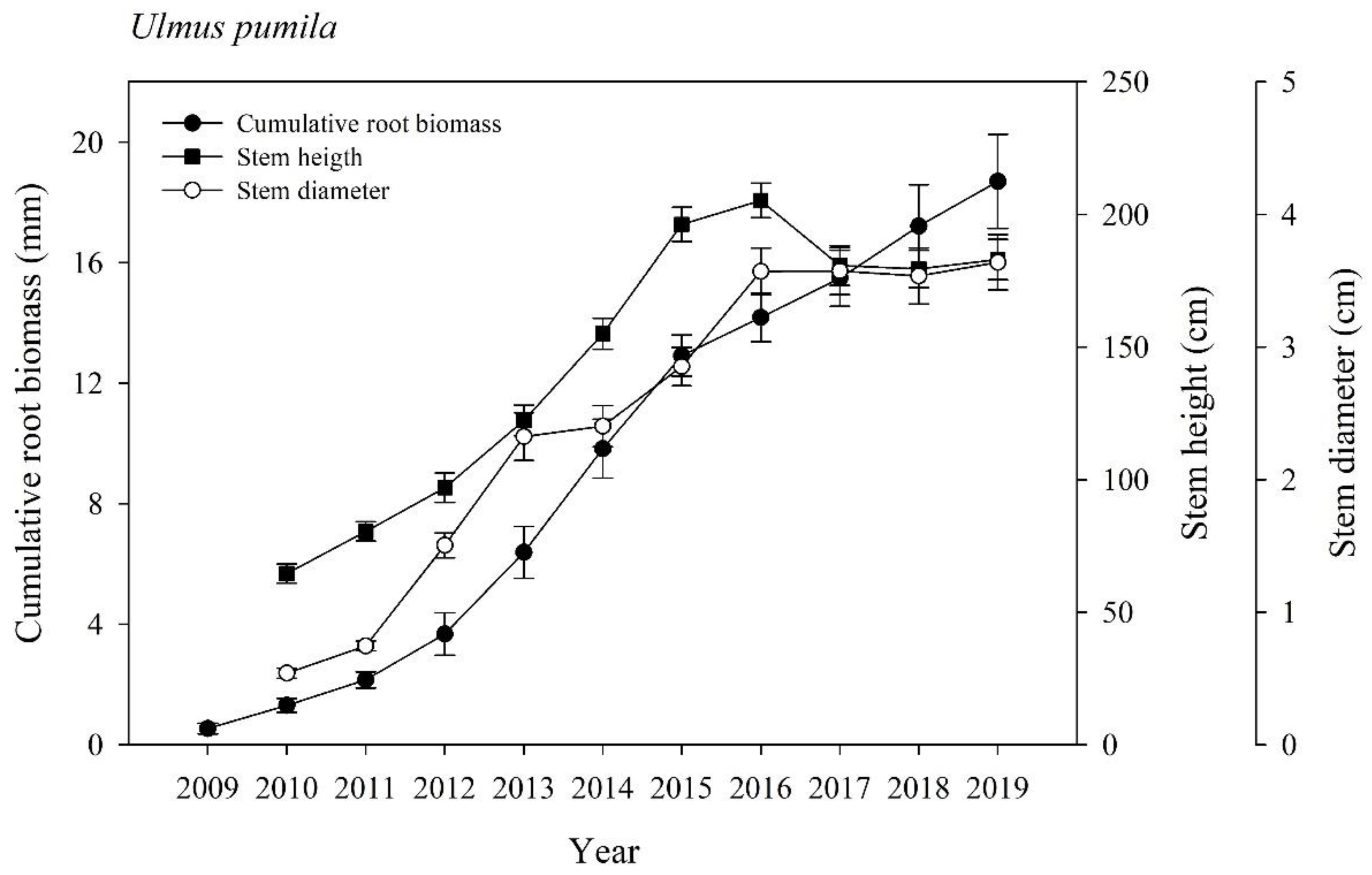
The difference emerging from the present study between the trend described by this novel “ex vivo” dendrometric approach, with the trend obtainable by the traditional plotting of the variation of morphological traits (stem height and RCD) collected through DT monitoring (Byambadorj et al., 2021a,b; Nyam-Osor et al., 2021) is surprising. This difference is reported in
Figure 3 wherein the same graph we have reported the trends obtained through “in vivo” (stem height and RCD) and “ex vivo” approaches (annual ring width measured in the taproot wood). In this figure, it is possible to observe that both height and RCD trends can be characterized as geometric trends (LAG, LOG, and STATIONARY) contrary to the annual ring trends that lack the STATIONARY phase.
An explanation for this difference could be that in the case of the arrest of stem height increase, the trees achieve a maturity stage (i.e., after the LOG phase) when biomass is used for the production and development of branches (branching plasticity) as reported by Yoshihira et al. (2020). Alternatively, it could be suggested that at a certain stage (tree maturity?), there is the onset of a “diminishing return” effect that arrests stem height increase. The diminishing return consists of a change in biomass allocation between organs that responds to the need of a plant to enable physiological adjustments (Shi et al., 2019). For example, this effect has been reported in natural forests where variations in the carbon allocation among organs are part of the plant’s strategy for responding to competition for light (Mensah et al., 2016).
The onset of a STATIONARY phase in the RCD trend could be the consequence of the onset of a bark peeling event. In fact, despite there being no data regarding bark shedding for Ulmus pumila, this event would hide the overall diameter increase of the stem that should take place as a consequence of the yearly production of new secondary xylem and phloem.
Our finding that a difference occurs when monitoring the growth and development trends of trees through an “in vivo” or “ex vivo” approach is similar to the lack of linearity that has been observed in other tree species when morphological traits (such as leaf area index) are compared to the biomass-related relative growth rate (Weraduwage et al., 2015). Moreover, a loss of correlation has been observed during the development of a leaf when comparing the relationship between specific traits such as dry or fresh leaf biomass, leaf thickness, and leaf area (Shi et al., 2020).
4. Conclusions
This study presents a novel monitoring approach allowing simultaneous comparison of growth and developmental trends in both aboveground and belowground organs of the same tree. Currently, radar scanning technology for “in vivo” monitoring of root system growth and development lacks reliability (Zhang et al., 2019), necessitating the use of the “ex vivo” monitoring approach, especially in harsh soil environments like arid and semiarid lands.
The combined use of “in vivo” and “ex vivo” monitoring approaches demonstrates the effectiveness of management measures under the Greenbelt Project in promoting optimal growth and development performance. In fact, it confirms that an irrigation regime of 8 Lh-1 enables coordinated growth and development of belowground organs (Montagnoli et al., 2022), and avoids the need for measuring numerous morphological traits, preventing the collection of disconnected and unreliable trait measurements (Viani et al., 2018).
The observed differences in trends between “in vivo” and “ex vivo” monitoring approaches affirm the possibility of predicting aboveground organ trait variations from root trait variations, while reverse prediction is not feasible (Caldwell and O’Hara, 2017). The significance of monitoring root system growth and development trends supports previous conclusions on the critical nature of investigating the root system, given its high sensitivity to environmental and mechanical factors (Montagnoli et al., 2022; James et al., 2022).
Author Contributions
BN-O, SO-B, and DC conceived the research project. BN-O provided primary funding. SO-B, BN-O, AM, and DC developed the methodological approaches. FD performed the dendrometry data analysis. AM, GSS, JS, and DC provided important insights into the research process. DC and JS wrote the manuscript.
Funding
This work was funded by the Fellowship Grant (P2019-3635) by the National University of Mongolia and the Korea-Mongolia Joint Green Belt Plantation Project.
Acknowledgements
The authors gratefully thank the staff of the Korea-Mongolia Joint Green Belt Plantation Project and the members of the Laboratory of Forest Genetics and Ecophysiology, at the National University of Mongolia for their assistance in the laboratory and field works. AM and DC acknowledge the Department of Biotechnology and Life Science, the University of Insubria for providing the necessary support to the joint research project. This work is included in the activities of Task Force IUFRO “Transforming Forest Landscapes for Future Climates and Human Well-being”
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
References
- Abiyu, A., Teketay, D., Glatzel, G., Gratzer, G. (2016). Seed production, seed dispersal and seedling establishment of two afromontane tree species in and around a church forest: Implications for forest restoration. For. Ecosyst. 3. [CrossRef]
- Aronson, J., Clewell, A.F., Blignaut, J.N., Milton, S.J. (2006). Ecological restoration: A new frontier for nature conservation and economics. J. Nat. Conserv. 14, 135–139. [CrossRef]
- Benayas, J.M.R., Newton, A.C., Diaz, A., Bullock, J. (2009). Enhancement of biodiversity and ecosystem services by ecological restoration: a meta-analysis. Science, 325: 1121-1124. [CrossRef]
- Byambadorj, S-O., Chiatante, D., Akhmadi, K., Lunten, J., Ochirbat, B., Park, B.B., Scippa, G.S., Montagnoli, A., Nyam-Osor, B. (2021b). The effect of different watering regimes and fertilizer addition on the growth of tree species used to afforest the semi-arid steppe of Mongolia. Plant Bios. 155, 747-758. [CrossRef]
- Byambadorj, S-O., Park, B.B., Hernandez, J. O., Tsedensodnom E., Byambasuren, Montagnoli, A., Chiatante, D., Nyam-Osor, B. (2021a). Effects of irrigation and fertilization on the morphophysiological traits of Populus sibirica Hort. Ex Tausch and Ulmus pumila L. in the semiarid steppe region of Mongolia. Plants, 10, 2407. [CrossRef]
- Byambadorj, S-O., Nyam-Osor, B., Park, B.B., Avirmed, T., Scippa, G.S., Chiatante, D., Montagnoli, A., Dimitrova, A. (2022). Afforestation of Mongolian steppe: patterns of biomass partitioning in Populus sibirica and Ulmus pumila trees in response to management supporting measures, Plant Bios. [CrossRef]
- Cao, X.-J., Gao, Q.-Z, Hasbagan, G., Liang, Y, Li, W.-H, Hu, G.-Z. 2018. Influence of climatic factors on variation in the Normalised Difference Vegetation Index in Mongolian Plateau grasslands. Rangel. J. 40(2):91–100.
- Clewell, A. F., and Aronson, J. (2007). Ecological Restoration: Principles, Values, and Structure of an Emerging Profession. Island Press: Washington, DC, USA.
- Country Programme of Mongolia (Green Climate Fund) 2019.
- Dambreville, A., Lauri, P.-E, Normand, F., Guédon, Y. (2015). Analyzing growth and development of plants jointly using developmental growth stages. Ann. Bot. 115, 93105. [CrossRef]
- Derhé, M.A., Murphy, H., Monteith, G., Menéndez, R. (2016). Measuring the success of reforestation for restoring biodiversity and ecosystem functioning. J. Appl. Ecol., 53, 1714–1724. [CrossRef]
- Desertification Atlas of Mongolia, 2013. https://www.researchgate.net/publication/296313726.
- Duggal, V., Sukhwani, M., Bipin, K., Reddy, G.S., Krishna, K. M. (2016). Plantation monitoring and yield estimation using autonomous quadcopter for precision agriculture. International Conference on Robotics and Automation. [CrossRef]
- Farajelahl, B., Eya, F.F., Arefi, H. (2022). Forest modeling and inventory estimation using lidar data. ISPRS Annals of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Volume X-4/W1-2022 GeoSpatial Conference 2022 – Joint 6th SMPR and 4th GIResearch Conferences, 19–22 February 2023, Tehran, Iran (virtual). [CrossRef]
- Guariguata, M.R. and Evans, K. (2020) A diagnostic for collaborative monitoring in forest landscape restoration. Restoration Ecology Vol. 28, No. 4, pp. 742–749. [CrossRef]
- Helmisaari, H.-S., Derome, J., Kukkola, P.N.M. (2002). Fine root biomass in relation to site and stand characteristics in Norway spruce and Scots pine stands. Tree Physiol. 27, 1493-504. [CrossRef]
- He, H, Zhang, C, Zhao, X, Fousseni, F, Wang, J, Dai, H, et al. (2018) Allometric biomass equations for 12 tree species in coniferous and broadleaved mixed forests, Northeastern China. PLoS ONE 13(1): e0186226. [CrossRef]
- Hessl, A.E., Brown, P., Byambasuren, O., Cockrell, S., Leland, C., Cook, E., Nachin, B., Pederson, N., Saladyga, T., Suran, B. (2016). Fire and climate in Mongolia (1532–2010 Common Era). Geophys. Res. Lett. 43, 6519–6527. [CrossRef]
- Holland, B.L., Monk, N.A.M., Clayton, R.H., Osborn, C.P. (2019). A theoretical analysis of how plant growth is limited by carbon allocation strategies and respiration. Plants. 1, 2517-5025. [CrossRef]
- IUSS Working Group WRB. (2015). World Reference Base for Soil Resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. FAO, Rome.
- James, K., Haritos, N., Ades, P.K. (2022). Mechanical stability of trees under dynamic loads. Am. J. Bot. 93, 1522-30. [CrossRef]
- Jevsenak, J. and Levanic, T. (2018). dendroTools:R package for studying linear and nonlinear responses between tree-rings and daily environmental data. Dendrochronologia 48, 32-39. [CrossRef]
- Johnson H, Brandle J. (2009). Shelterbelt design. [accessed 2024 Feb 6]. https://agriculture.vic.gov.au/__data/assets/pdf_file/0008/1004579/Shelterbelt-design-.pdf.
- Kanowski, J., Catterall, C.P., Freebody, K., Freeman, A.N.D., Harrison, D.A. (2010). Monitoring Revegetation Projects in Rainforest Landscapes: Toolkit Version 3; Reef and Rainforest Research Centre Ltd.: Cairns, Australia.
- Kholmanskiy, A.S. (2015). Modeling of growth kkinetics of conifer trees. Open J. For. 5, 21-27. [CrossRef]
- Kozlowski, T.T., and Pallardy, S.G. (1997). Physiology of Woody Plants. Cambridge: Academic Press, 411.
- Le, H.D., Smith, C., Herbohn, J., Harrison, S. (2012) More than just trees: assessing reforestation success in tropical developing countries. Journal of Rural Studies 28:5–19. [CrossRef]
- Lavrenko, E.M., Karamysheva, Z.V., Nikulina, R.I. (1991). [Stepi Evrazii]. Leningrad: Nauka. Russian.
- Mahamoudou, S. and Arakwiye, B. (2020). Improving the monitoring of forest and landscape restoration in Africa. ETFRN NEWS, 60.
- Mathew, M.M., Majule, A. E., Sinclair, F., and Marchant, R. (2016) Effect of soil properties on tree distribution across an agricultural landscape on a tropical mountain, Tanzania. Open J. Ecol. 6, 264-276. [CrossRef]
- McDonald, T., Gann, G.D., Jonson, J., Dixon, K.W. (2016) International standards for the practice of ecological restoration – Including principles and key concepts, Washington, DC: SER.
- Mensah, S, Veldtman, R., Du Toit, B., Kakai, R.G., Seifert, T. (2016) Aboveground biomass and carbon in a South African mist belt forest and the relationships with tree species diversity and forest structures. Forests. 7, 79. [CrossRef]
- Menz, M.H.M., Phillips, R.D., Winfree, R., Kremen, C., Aizen, M.A., Johnson, S.D., Dixon, K.W. (2011). Reconnecting plants and pollinators: Challenges in the restoration of pollination mutualisms. Trends Plant Sci. 16, 4–12. [CrossRef]
- MNET (Ministry for Nature, Environment and Tourism). (2010). National action program for combating desertification in Mongolia 2010–2020. National Report on the United Nations Convention to Combat Desertification. Fourth Conference of Parties.
- Monie, K., Florentine, S., Palmer, G. (2013). Recruitment and functionality traits as bioindicators of ecological restoration success in the Lurg Hills district, Victoria, Australia. Ecol. Process., 2. [CrossRef]
- Montagnoli, A., Lasserre, B., Terzaghi, M., Byambadorj, S.-O., Nyam-Osor, B., Scippa, G. S., Chiatante, D. (2022). Fertilization reduces root architecture plasticity in Ulmus pumila used for afforesting Mongolian semi-arid steppe. Front Plant Sci. 113, 878299. [CrossRef]
- NAMEM: The National Agency for Meteorology and Environmental Monitoring of Mongolia. 2019. Weather data 2000–2019. [accessed 2019 Sep 25]. http://namem.gov.mn/eng/?p¼56.
- Nyam-Osor, B., Byambadorj, S.-O., Park, B.B., Terzaghi, M., Scippa, G.S., Stanturf, J.A., Chiatante, D. and Montagnoli, A. (2021) Root biomass distribution of Populus sibirica and Ulmus pumila afforestation stands is affected by watering regimes and fertilization in the Mongolian semi-arid steppe. Front. Plant Sci. 12:638828. [CrossRef]
- Pádua, L., Vanko, J., Hruška, J., Adão, T., Sousa, J.J., Peres, E., et al. (2017). UAS, sensors, and data processing in agroforestry: a review towards practical applications. Int J Remote Sens. 38:1–43. [CrossRef]
- Poorter, H., Niklas, K.J., Reich, P.B., Oleksyn, J., Poot, P., Mommer, L. (2011). Biomass allocation to leaves stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist 193, 30–50. [CrossRef]
- Rathgeber, C.B.K., Cuny, H.E., Fonti, P. (2016). Biological basis of tree-ring formation: a crash course. Front. Plant Sci. [CrossRef]
- Reytar, K., Landsberg, F., Stolle, F,, Anderson, W., Brandt, J., Matsumoto, M. (2020). The challenge of tracking how a trillion trees grow. www.wri.org/blog/2020/07/trillion-trees-tracking-challenges.
- Ruiz-Jaén, M.C., Aide, T.M. (2006). An integrated approach for measuring urban forest restoration success. Urban For. Urban Green. 4, 55–68. [CrossRef]
- Ryan, P.R., Delhaize, E., Watt, M., Richardson, A.E. (2016). Plant roots: understanding structure and function in an ocean of complexity. Ann. Bot. 118, 555–9. [CrossRef]
- Shi, P., Li, Y., Hui, C., Ratkowsky, D.A., Yu, X., Niinemets, U. (2020). Does the law of diminishing returns in leaf scaling apply to vines? Evidence from 12 species of climbing plants. Global Ecology and Conservation 21. [CrossRef]
- Shinozaki, K., Yoda, K., Hozumi, K., Kira, T. (1964). A quantitative analysis of plant form-the pipe model theory-further evidence of the theory and its application in forest ecology. Japanese J. Ecol. 14, 133-139. [CrossRef]
- Soong, J. L., Janssens, I. A., Grau, O., Margalef, O., Stahl, C., Van Langenhove, L., et al. (2020) Soil properties explain tree growth and mortality, but not biomass, across phosphorus-depleted tropical forests. Sci. Rep. 10, 2302. [CrossRef]
- Stanturf, J.A., Botman, E., Kalachev, A., Borissova, Y., Kleine, M., Rajapbaev, M., Chyngozhoev, N., Nyam-Osor, B. (2020). Dryland forest restoration under a changing climate in Central Asia and Mongolia. Mong. J. Biol Sci. 18, 3-18. [CrossRef]
- Stanturf, J.A., Kant, P., Lilleso, J-PB., Mansourian, S., Kleine, M., Graudal, L., Madsen, P. (2015). Forest landscape restoration as a key component of climate change mitigation and adaptation. Vienna, Austria: International Union of Forest Research Organizations (IUFRO); p. 72.
- Stanturf, J.A., Palik, B.J., Williams, M.I., Dumroese, R.K., Madsen, P. (2014). Forest restoration paradigms. J. Sustainable For. 33, S161–S194. [CrossRef]
- Stavi, I., and Lal, I. (2015). Achieving zero net land degradation: challenges and opportunities. J. Arid Envir. 111, 44-51. [CrossRef]
- Tsogtbaatar, J. (2013). Deforestation and reforestation of degraded forestland in Mongolia. In: The Mongolian ecosystem network. Tokyo: Springer; p. 83–98.
- Ulziykhutag, N. (1989). [Overview of the Flora of Mongolia]. Ulaanbaatar: State Publishing. Mongolian.
- Viani, R.A.G., Barreto, T.E., Farah, F.T., Rodrigues, R.R., Brancalion, P.H.S. (2018) Monitoring young tropical forest restoration sites: how much to measure? Tropical Conserv. Sci. 7. [CrossRef]
- Weraduwage, S.M., Chen, J., Anozie, F.C., Morales, A., Weise, S.E., Sharkey, T.D. (2015). The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. [CrossRef]
- Yessekin, B., Burlibayev, M., Medvedeva, N., Stafin, S. (2008). Water ecosystems of Central Asia: important factors affecting the environmental and social prosperity of the region. In: Moerlins, J.E, Khankhasayev, M.K., Leitman, S.F., Makhmudov, E.J. (eds.) Transboundary Water Resources: A Foundation for Regional Stability in Central Asia. Springer Netherlands, Dordrecht, pp. 43-64.
- Zhang, X., Derival, M., Albrecht, U., Ampatzitis, Y. (2019). Evaluation of a ground penetrating radar to map the root architecture of HLB-infected citrus trees. Agronomy, 9, 354. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).