1. Introduction
Chronic hepatitis B (CHB) constitutes a pressing global health issue resulting in substantial morbidity and mortality [
1,
2,
3]. An estimated 257·5 million individuals worldwide were living with CHB, constituting 3.2% of the global population, with 555,000 deaths each year attributed to hepatitis B virus (HBV)-related sequelae [
2,
3]. The course of CHB infection varies among patients, resulting in a wide spectrum of disease severity [
4]. A large portion of CHB-infected patients are categorized as inactive carriers, also known as those with hepatitis B e antigen (HBeAg)-negative chronic HBV infection [
5].
As per the 2017 clinical practice guidelines of the European Association for the Study of the Liver (EASL), an inactive carrier state is identified by the absence of the hepatitis B e-antigen (HBeAg) and presence of the HBe antibody (anti-HBe), with consistent maintenance of normal alanine aminotransferase (ALT) levels, coupled with low or undetectable serum levels of HBV DNA (<2,000 IU/mL), although the latter may fluctuate up to 20,000 IU/mL in some cases [
6]. Inactive carriers generally have an excellent prognosis compared to other CHB-infected patients [
5,
7]. However, the evolving clinical profile of inactive carriers requires regular monitoring for HBV reactivation. Moreover, although rare, life-threatening complications like hepatocellular carcinoma (HCC) and cirrhosis can occur, underscoring the importance of frequent screening in this subset of patients [
8,
9].
Hepatic fibrosis is characterized by changes in the echotexture of the liver as a result of underlying inflammation [
10]. It is categorized into five stages from F0 to F4, based on the extent of scar tissue accumulation, with stage F2 or higher indicating significant fibrosis [
11]. While a liver biopsy remains the gold standard for assessing hepatic fibrosis, its invasive nature makes this procedure suboptimal for routine monitoring of CHB inactive carriers [
8]. International guidelines thus recommend non-invasive techniques like serum markers and elastography for initial fibrosis staging and monitoring [
12]. Notably, two-dimensional (2D) shear-wave elastography (SWE) is a relatively recent, non-invasive imaging modality which can be used to assess the degree of fibrosis by measuring the speed at which shear waves pass through liver tissue using a 2D ultrasound probe, providing a quantitative measure of hepatic tissue stiffness [
13].A recent systematic review and meta-analysis affirmed the efficacy of 2D SWE as an excellent technique for predicting significant fibrosis in CHB-infected patients [
14].
In Oman, a country with intermediate HBV endemicity (~2–7%), there is a notable absence of research on HBV inactive carriers and their associated complications and risk factors [
1,
15,
16]. Consequently, this study aimed to assess the prevalence of significant hepatic fibrosis and associated risk factors among a sample of Omani HBV inactive carriers visiting the Sultan Qaboos University Hospital (SQUH), a tertiary care unit in Muscat, the capital city of Oman. As one of the first studies focusing on HBV inactive carriers, findings from this study are expected to contribute to enhancing overall patient management and outcomes within this population.
2. Materials and Methods
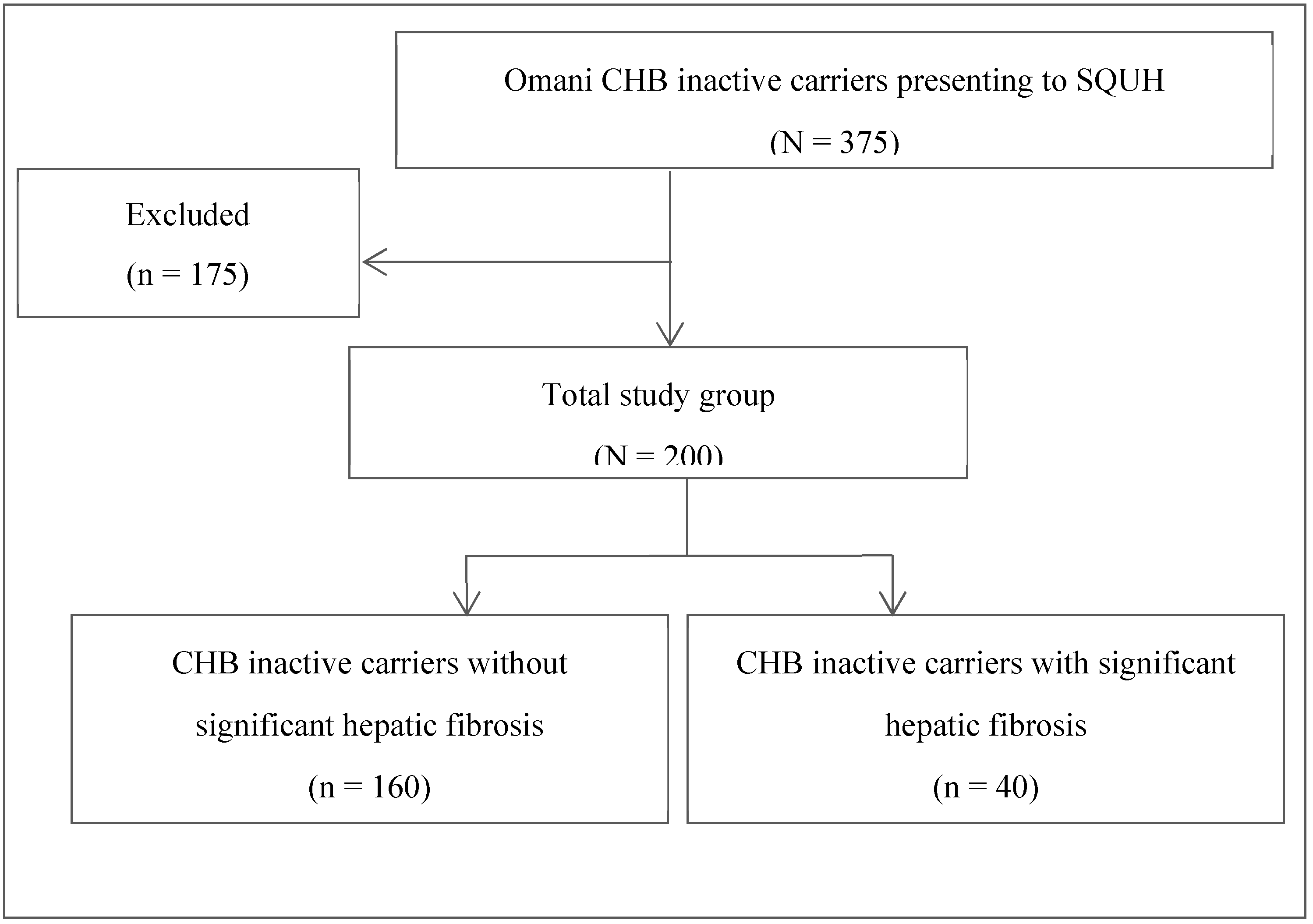
This single-center retrospective study targeted 200 Omani adult patients (aged ≥13 years) who were diagnosed as CHB inactive carriers and underwent 2D SWE at the adult hepatology clinic of SQUH between January 2017 and December 2018. As per the 2017 EASL guidelines, an inactive carrier state was defined based on the following criteria:
Patients for whom there was major data missing, pregnant women, and those with concurrent chronic liver diseases like hepatitis C, hepatitis D, and human immunodeficiency virus infections, autoimmune hepatitis, cholesteric liver disease, alpha-1 antitrypsin deficiency, Wilson’s disease, or hemochromatosis were excluded from the study.
The hospital’s healthcare information system was used to collect data from the patients’ electronic medical records (TrakCare, InterSystems Corp., Cambridge, Massachusetts, USA). Information was collected relating to potential risk factors for the development of fibrosis. Demographic and anthropometric factors included each patient’s age, gender, marital status, place of residence, and body mass index (BMI), while family-related factors assessed the presence of a family history of HBV, HCC, or cirrhosis. In addition, comorbidities such as type 2 diabetes mellitus (T2DM), sickle cell disease (SCD), thalassemia, dyslipidemia, renal disease, heart failure, fatty liver disease, and malignancy were considered, while nosocomial factors encompassed a history of hemodialysis, organ transplantation, surgery, and blood transfusions. Lastly, various biochemical parameters were assessed, including platelet and white blood cell counts, and levels of ALT, aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), albumin, total bilirubin, and alpha-fetoprotein.
All patients underwent simultaneous 2D SWE and liver ultrasound imaging under non-fasting conditions using the GE Logiq E9 2.0 with XDClear multi-purpose ultrasound system (GE Healthcare, Milwaukee, Wisconsin, USA). Changes in echotexture were assessed to diagnose cirrhosis and fatty liver disease and to classify the degree of hepatic fibrosis. Liver stiffness measurements (LSMs) of ≤7.1, >7.1–7.8, >7.8–8.0, >8.0–11.5, and >11.5 kPa corresponded to stages F0, F1, F2, F3, and F4, respectively, with stage F2 and higher indicating significant hepatic fibrosis [
17]. Subsequently, depending on the presence or absence of significant hepatic fibrosis, the study population was divided into patients with or without significant hepatic fibrosis (
Figure 1). Comparisons between groups were conducted in order to determine potential risk factors for the development of significant hepatic fibrosis in CHB inactive carriers.
Data analysis was conducted using the Statistical Package for the Social Sciences (SPSS) software, version 26.0 (IBM Corp., Armonk, New York, USA). A Chi-squared test was employed to evaluate associations between the development of significant hepatic fibrosis and potential risk factors. Normality of data distribution for continuous variables was assessed using the Kolmogorov-Smirnov test. Parametric variables were analyzed using a Student’s t-test, while non-parametric variables were assessed using a Mann-Whitney U test. A p value of <0.05 was considered statistically significant. Logistic regression was used for the multivariate adjusted analysis. Variables which showed a p value of <0.25 in the crude analysis were entered into the regression analysis.
This study received ethical approval from the Medical Research and Ethics Committee of the College of Medicine and Health Sciences, Sultan Qaboos University (MREC #1548). Additionally, permission to access patients’ medical records was obtained from the Directorate of Hospital Information Systems at SQUH.
3. Results
Of the 375 Omani CHB inactive carriers followed-up at the SQUH adult hepatology clinic during the study period, 200 fulfilled the inclusion criteria. Of these, 106 (53%) were male and 94 (47%) were female, and the mean age was 44.6 ± 9.3 years (range: 31–79 years). Mean ALT and median HBV DNA levels were 22.5 U/L and 139.5 IU/mL, respectively (
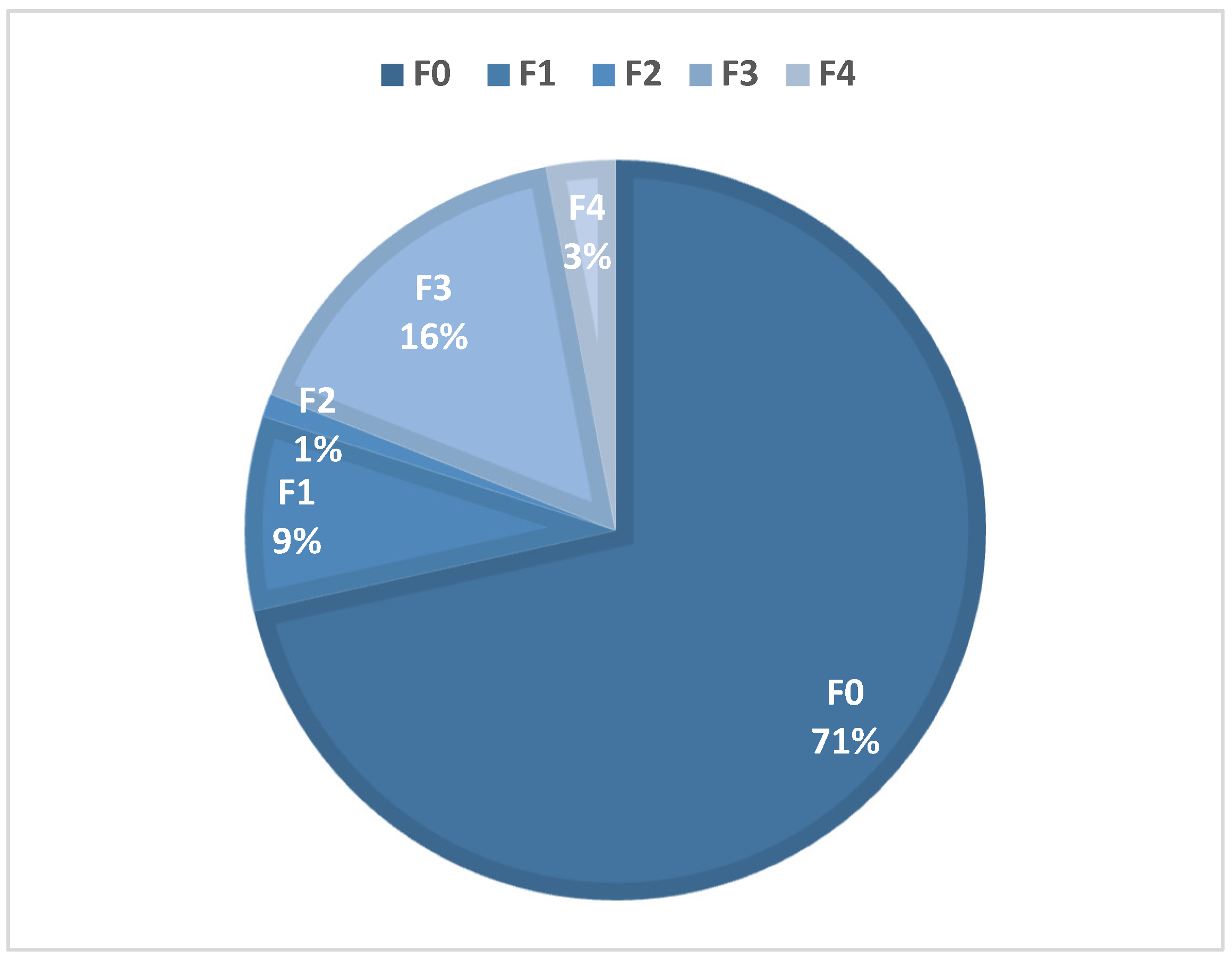
Table 1). Overall, a total of 40 patients (20%) developed significant hepatic fibrosis, defined as stage F2 fibrosis or higher (
Figure 2).
Several risk factors were found to be significantly associated with the development of significant hepatic fibrosis in the studied population (
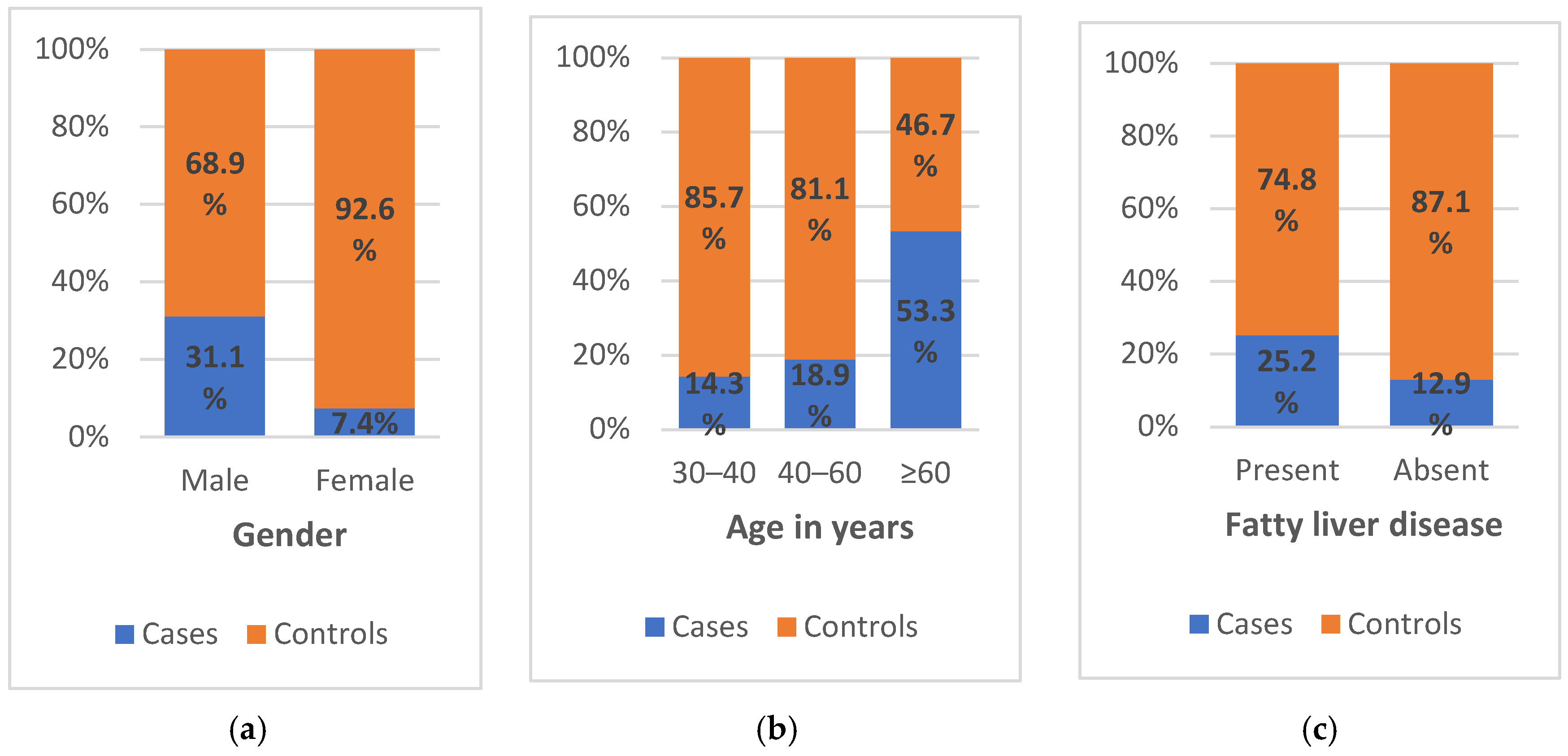
Table 2). Overall, 31.1% of male patients exhibited significant fibrotic changes compared to 7.4% of female patients (
Figure 3a). The likelihood of developing significant fibrosis was significantly greater for male compared to female patients (odds ratio [OR] = 5.618, 95% confidence interval [CI] = 2.347–13.450;
p <0.001). Similarly, 53.3% of patients aged ≥60 years exhibited stage F2 or higher fibrosis compared to 14.3% and 18.9% of those aged 30–40 and 40–60 years, respectively (
Figure 3b). According to the univariate analysis, patients aged ≥60 years had a five-times increased risk of developing this complication compared to those in other age groups (OR = 5.618, 95% CI = 2.347–13.450;
p = 0.003).
In terms of family-related factors, a total of 58 (29%), nine (4.5%), and 22 (11%) patients had a family history of HBV, HCC, and cirrhosis, respectively. Although none of these factors were found to be significantly associated with the development of significant fibrosis, it should be noted that 25% of patients in the case group had a family history of at least one of these three conditions. Moreover, only two comorbidities were significantly associated with the development of significant fibrosis, namely hypertension (OR = 5.516, 95% CI = 1.974–15.416;
p = 0.002) and fatty liver disease (OR = 2.268, 95% CI = 1.060–4.853;
p = 0.049). Specifically, 52.9% of hypertensive patients developed this complication, compared to only 16.9% of non-hypertensive patients, while 25.2% vs. 12.9% of those with and without fatty liver disease, respectively, developed stage F2 or higher fibrosis (
Figure 3c). No significant associations were identified with regards to nosocomial factors.
Various biochemical parameters were significantly associated with the development of significant fibrosis in the case group (
Table 3). In particular, mean hemoglobin levels were significantly higher in the case group compared to the controls (13.76 ± 1.66 vs. 12.92 ± 1.81 g/dL;
p = 0.009). Similarly, ALT levels showed a significant positive correlation with the development of significant fibrotic changes in Omani CHB inactive carriers (
p = 0.027). In contrast, there was a significant negative correlation with mean platelet count, which was significantly decreased among patients with stage F2 or higher fibrosis (218.45 ± 70.19 vs. 249.73 ± 65.93 × 10
9/L;
p = 0.009).
A total of 182 participants were included in the regression analysis. Gender showed an independent association with significant fibrosis (
p = 0.007). In this regard, males were six- times more likely to have significant fibrosis (OR = 6.568, 95% CI = 1.673–25.785). In addition, age showed a significant association with fibrosis (
p = 0.024), with those aged >60 years 10-times more likely to develop fibrosis compared to participants aged 30–40 years (OR = 10.900; 95% CI = 1.371–86.631). Fatty liver disease was also an independent risk factor (OR = 2.650, 95% CI = 1.025–6.855;
p = 0.044). All other variables showed non-significant results in the multivariate analysis (
Table 4).
4. Discussion
Previous studies from countries with low HBV endemicity have indicated that CHB inactive carriers generally demonstrate good prognoses, with a minimum prevalence of HBV-related hepatic fibrosis [
18,
19,
20]. However, in high and intermediate endemic regions, the incidence of such complications tends to increase [
21,
22,
23]. For example, a longitudinal, cross-sectional study from Taiwan, a country with an intermediate-to-high HBV prevalence, found that 16.1% of CHB inactive carriers experienced viral reactivation, while 15% developed hepatic fibrosis over the 25-year study period [
9]. Another study from Egypt, an intermediate HBV prevalence country, reported that 20% of inactive carriers exhibited significant fibrotic changes as determined by liver biopsy [
22]. To the best of the authors’ knowledge, the present study is the first to shed light on CHB inactive carriers and their prevalence of significant fibrosis, an important HBV-related complication. The results, therefore, provide essential baseline data regarding the prevalence of and possible contributory risk factors for developing significant fibrosis in this subpopulation, which could help ensure early detection and optimal patient management.
The overall prevalence of significant fibrosis (20%) among Omani CHB inactive carriers identified in the current study aligns with findings reported in similar research conducted in intermediate HBV endemic areas (15–20%) [
9,
22]. In contrast, studies from Saudi Arabia and Hong Kong have reported considerably higher prevalence rates for this complication (30.9% and 33.3%, respectively) [
24,
25]. These variations in findings may be attributed to the use of liver biopsies instead of SWE for hepatic fibrosis staging in the latter two studies. However, a study from Spain, another intermediate HBV endemic country, indicated a prevalence rate of 25% using a non-invasive elastography technique similar to that utilized in the present study [
26]. In this case, the discrepancy in findings might be explained by differences in the LSM criteria used (>7.5 vs. >7.8 kPa), ultrasound equipment used, or the study duration.
The present study also investigated possible risk factors contributing to the development of significant fibrosis, including demographic, family-related, nosocomial, and comorbidity factors, as well as biochemical parameters. In terms of demographic factors, significant associations were observed between stage F2 or higher fibrosis and both gender and age. Such findings are consistent with those reported in previous research from Taiwan and Bangladesh, in which both male gender and advanced age were significantly linked to the development of hepatic fibrosis in CHB inactive carriers [
9,
27]. On the other hand, studies from Spain and Hong Kong did not find significant associations for these two factors [
25,
26]. Moreover, while age was associated with the development of significant fibrosis in another Taiwanese study, the highest risk presented for patients aged 40–60 years rather than those aged ≥60 years [
28]. Based on these findings, both male and elderly patients with inactive CHB infections should be closely monitored to ensure early detection of hepatic fibrosis.
In a recent study conducted in Oman, intra-familial transmission emerged as the predominant mode of HBV transmission, while nosocomial transmission was found to be relatively uncommon [
16]. Nonetheless, given the highly infective nature of HBV, both nosocomial and family-related factors were explored as possible risk factors for fibrotic changes in the present study. Although none of the family-related factors exhibited statistically significant associations, it is noteworthy that a quarter of HBV inactive carriers with stage F2 fibrosis or higher had a family history of either CHB, HCC, or cirrhosis. The precise relationship between family history of HBV-related conditions and the development of significant fibrosis is uncertain; however, the frequency of the latter complication might be related to the fact that individuals with a family history of HBV are prone to infection earlier in life, resulting in a longer duration of infection and, consequently, an increased risk of advanced liver scarring [
22]. Nosocomial factors also failed to show significant associations with the development of significant fibrosis in the present study. This outcome aligns with contemporary standards of disinfection, sterilization, and screening practices observed during surgical and medical procedures. As elucidated in previous research, stringent adherence to these protocols over the last several decades has substantially mitigated the role of nosocomial factors in HBV transmission [
16].
In the current study, among all evaluated comorbidities, only hypertension and fatty liver disease exhibited statistically significant associations with significant fibrosis. Notably, there is a paucity of research exploring the association between hypertension and its impact on HBV inactive carriers. Following a review of the literature, only one study from Spain was identified assessing these factors, reporting non-significant associations with both of these comorbidities [
26]. The coexistence of fatty liver disease and CHB has been studied extensively; however, with the exception of the aforementioned Spanish study, there is a dearth of studies illuminating the prevalence of these conditions, specifically among inactive carriers. A recent review highlighted the synergistic escalation of liver fibrosis risk when fatty liver disease coexists with CHB, consistent with the observations of the present study [
28]. The mechanism behind this is multifactorial, encompassing genetic, metabolic, and immune factors [
28].
Interest in non-invasive diagnostic tests for hepatic fibrosis in chronic liver diseases has grown considerably in recent decades. Such tests would mitigate the need for invasive procedures like liver biopsy and their associated complications, making regular follow-ups more convenient. As a result, researchers have explored the potential of different biochemical parameters as risk factors for fibrosis development and their efficacy in predicting such complications [
29,
30]. In particular, ALT stands out as a notable example, with previous research revealing a significant correlation between elevated ALT levels and fibrosis development, establishing it as a reliable predictor for such complications in CHB inactive carriers [
30,
31]. In contrast, conflicting findings have been reported in other research suggesting that ALT does not accurately predict significant liver injury [
22,
25].
Nonetheless, ALT also demonstrated a positive significant correlation with significant fibrosis in the present study, as did hemoglobin level, while a negative correlation was observed for platelet count. A negative correlation with platelet count has also been reported in Chinese and Iranian populations, suggesting the potential utility of this factor for predicting hepatic fibrosis [
25,
32]. The association of hemoglobin with significant fibrosis lacks prior investigation, and reasons for this association remain unclear. A recent study also identified a negative correlation between platelet count and the development of significant fibrosis in CHB patients, supporting the current study’s findings of this variable as an independent predictor of fibrosis [
33]. Such findings support the effectiveness of implementing non-invasive biochemical parameters—especially platelet count—in the Omani population.
This study possesses several strengths. First, it is the first in Oman to document the prevalence of lung fibrosis among CHB inactive carriers. Moreover, a diverse array of risk factors was assessed, thereby providing a more holistic understanding of this particular subset of patients. However, certain limitations were encountered. The primary constraint lies in the restricted timeframe, which necessitated the recruitment of a limited sample size. A larger sample would enhance patient representation, bolstering the reliability of comparisons between the case and control groups. Additionally, the retrospective nature of the study resulted in missing data related to crucial biochemical parameters, such as high-density and low-density lipoproteins, thyroxine, and thyroid-stimulating hormone; consequently, analysis of these variables as potential risk factors was not possible. Finally, the current research targeted a single center in Muscat, potentially leading to an underestimation of the prevalence of significant fibrosis in the broader Omani population of inactive carriers. Additional prospective studies in Oman, including a larger sample size, are recommended to address these limitations, further investigate the HBV inactive carrier stage and its related complications, and confirm causality for the associations identified in the present study.
5. Conclusions
The prevalence of significant fibrosis among a sample of Omani CHB inactive carriers visiting a tertiary hepatology clinic was 20%. Risk factors associated with the development of this complication included male gender, advanced age (≥60 years), and the presence of hypertension and fatty liver disease; hence, periodic liver function assessments should be considered in patients with these risk factors. Several routine biochemical parameters— platelet count and hemoglobin and ALT levels—also showed significant correlations; as such, these metrics may be useful as cost-effective predictors of disease prognosis in this population. Since the present study represents the first of its kind in Oman, additional multi-center studies involving larger sample sizes are recommended to validate these findings.
Author Contributions
Conceptualization, S.A-B.; methodology, B.A-M. and H.A-S.; validation, S.A-B. and H.A-S.; formal analysis, B.A-M. and H.A-S.; investigation, A.A-S. and B.A-M; resources, S.A-B.; data curation, A.A-S. and B.A-M; writing—original draft preparation, B.A-M.; writing—review and editing, S.A-B. and H.A-S.; visualization, B.A-M. and H.A-S.; supervision, S.A-B. and H.A-S.; project administration, S.A-B.; funding acquisition, S.A-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Medical Research and Ethics Committee of the College of Medicine and Health Sciences, Sultan Qaboos University (MREC #1548).
Informed Consent Statement
Patient consent was waived due to the absence of identifying information.
Data Availability Statement
The raw data supporting the results reported in this study can be obtained upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fact sheet: Hepatitis B. Available online: www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 13 November 2023).
- GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol 2022, 7, 796–829. [Google Scholar] [CrossRef] [PubMed]
- Polaris Observatory Collaborators (2023). Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: a modelling study. The lancet. Gastroenterology & hepatology, 8(10), 879–907. [CrossRef]
- McMahon, B.J. The natural history of chronic hepatitis B virus infection. Hepatology 2009, 49, S45–S55. [Google Scholar] [CrossRef]
- Sharma, S.K.; Saini, N.; Chwla, Y. Hepatitis B virus: inactive carriers. Virol J 2005, 2, 82. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Invernizzi, F.; Viganò, M.; Grossi, G.; Lampertico, P. The prognosis and management of inactive HBV carriers. Liver Int 2016, 36, 100–104. [Google Scholar] [CrossRef]
- Pita, I.; Horta-Vale, A.M.; Cardoso, H.; Macedo, G. Hepatitis B inactive carriers: An overlooked population? GE Port J Gastroenterol 2014, 21, 241–249. [Google Scholar] [CrossRef]
- Chu, C.M.; Liaw, Y.F. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol 2009, 104, 1693–1699. [Google Scholar] [CrossRef]
- Horowitz, J.M.; Venkatesh, S.K.; Ehman, R.L.; Jhaveri, K.; Kamath, P.; Ohliger, M.A.; et al. Evaluation of hepatic fibrosis: A review from the Society of Abdominal Radiology Disease focus panel. Abdom Radiol (NY) 2017, 42, 2037–2053. [Google Scholar] [CrossRef]
- Pavlov, C.S.; Casazza, G.; Nikolova, D.; Tsochatzis, E.; Burroughs, A.K.; Ivashkin, V.T., et al. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database Syst Rev 2015, 1, CD010542. [CrossRef]
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017, 66, 153–1594. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; El Shimy, A.; Abd El Aziz, M.M. 2D shear wave elastography (SWE) performance versus vibration-controlled transient elastography (VCTE/fibroscan) in the assessment of liver stiffness in chronic hepatitis. Insights Imaging 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Jiang, H.Y.; Li, M.; Zhang, T.; Song, B. Two-dimensional shear wave elastography for significant liver fibrosis in patients with chronic hepatitis B: A systematic review and meta-analysis. Eur J Radiol 2020, 124, 108839. [Google Scholar] [CrossRef] [PubMed]
- Custer, B.; Sullivan, S.D.; Hazlet, T.K.; Iloeje, U.; Veenstra, D.L.; Kowdley, K.V. Global epidemiology of hepatitis B virus. J Clin Gastroenterol 2004, 38, S158–S168. [Google Scholar] [CrossRef] [PubMed]
- Al-Busafi, S.A.; Al-Harthi, R.; Al-Naamani, K.; Al-Zuhaibi, H.; Priest, P. Risk factors for hepatitis B virus transmission in Oman. Oman Med J 2021, 36, e287. [Google Scholar] [CrossRef] [PubMed]
- Sporea, I.; Bota, S.; Gradinaru-Taşcău, O.; Sirli, R.; Popescu, A.; Jurchiş, A. Which are the cut-off values of 2D-shear wave elastography (2D-SWE) liver stiffness measurements predicting different stages of liver fibrosis, considering transient elastography (TE) as the reference method? Eur J Radiol 2014, 83, e118–e122. [Google Scholar] [CrossRef]
- Martinot-Peignoux, M.; Boyer, N.; Colombat, M.; Akremi, R.; Pham, B.N.; Ollivier, S.; et al. Serum hepatitis B virus DNA levels and liver histology in inactive HBsAg carriers. J Hepatol 2002, 36, 543–546. [Google Scholar] [CrossRef]
- Delle Monache, M.; Petrelli, A.; Rossi, A; Cecere, R.; Mirisola, C.; Costanzo, G.; et al. Noninvasive evaluation of liver fibrosis in a sample of putative inactive hbv carriers in Rome, Italy. Can J Infect Dis Med Microbiol 2021, 2021, 3068690. [CrossRef]
- Malik, R.; Kennedy, P.; Suri, D.; Brown, A.; Goldin, R.; Main, J.; et al. The role of liver fibrosis assessment in the management of patients with chronic hepatitis B infection: lessons learned from a single centre experience. Hepat Res Treat 2011, 2011, 524027. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Manesis, E.K.; Manolakopoulos, S.; Elefsiniotis, I.S.; Goulis, J.; Giannousis, J.; et al. Is there a meaningful serum hepatitis B virus DNA cutoff level for therapeutic decisions in hepatitis B e antigen-negative chronic hepatitis B virus infection? Hepatology 2008, 48, 1451–1459. [Google Scholar] [CrossRef]
- Fateen, A.A.; Shahin, R.Y.; Farres, M.N.; Eldeeb, M.A.; Amer, H.A. Assessment of hepatic fibrosis and necroinflammation among inactive HBsAg carriers in Egypt. Ann Hepatol 2012, 11, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sarin, S.K.; Hissar, S.; Pande, C.; Sakhuja, P.; Sharma, B.C.; et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology 2008, 134, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Sanai, F.M.; Babatin, M.A.; Bzeizi, K.I.; Alsohaibani, F.; Al-Hamoudi, W.; Alsaad, K.O.; et al. Accuracy of international guidelines for identifying significant fibrosis in hepatitis B e antigen--negative patients with chronic hepatitis. Clin Gastroenterol Hepatol 2013, 11, 1493.e2–1499.e2. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.K.; Lai, C.L.; Ip, P.P.; Fung, J.; Wong, D.K.; Yuen, J.C.; et al. A large population histology study showing the lack of association between ALT elevation and significant fibrosis in chronic hepatitis B. PLoS One 2012, 7, e32622. [Google Scholar] [CrossRef] [PubMed]
- Mena, Á.; Pedreira, J.D.; Castro, Á.; López, S.; Vázquez, P.; Poveda, E. Metabolic syndrome association with fibrosis development in chronic hepatitis B virus inactive carriers. J Gastroenterol Hepatol 2014, 29, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Al Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Guillen, G.; Penton, E.; Tuero, A.; et al. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial). PLoS One 2018, 13, e0201236. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Jiang, D.; Li, M.; Chen, Y.; Li, J.; et al. Chronic hepatitis B and non-alcoholic fatty liver disease: Conspirators or competitors? Liver Int 2020, 40, 496–508. [Google Scholar] [CrossRef]
- Zhou, K.; Gao, C.F.; Zhao, Y.P.; Liu, H.L.; Zheng, R.D.; Xian, J.C.; et al. Simpler score of routine laboratory tests predicts liver fibrosis in patients with chronic hepatitis B. J Gastroenterol Hepatol 2010, 25, 1569–1577. [Google Scholar] [CrossRef]
- Tan, Y.; Ye, Y.; Zhou, X.; Chen, L.; Wen, D. Age as a predictor of significant fibrosis features in HBeAg-negative chronic hepatitis B virus infection with persistently normal alanine aminotransferase. PLoS One 2015, 10, e0123452. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Manolakopoulos, S.; Liaw, Y.F.; Lok, A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: A systematic review. J Hepatol 2012, 57, 196–202. [Google Scholar] [CrossRef]
- Mohamadnejad, M.; Montazeri, G.; Fazlollahi, A.; Zamani, F.; Nasiri, J.; Nobakht, H.; et al. Noninvasive markers of liver fibrosis and inflammation in chronic hepatitis B-virus related liver disease. Am J Gastroenterol 2006, 101, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Zheng, J.; Huang, D.; Wang, Y.; Li, X.; Zhou, X.; et al. INR-to-platelet ratio (INPR) as a novel noninvasive index for predicting liver fibrosis in chronic hepatitis B. Int J Med Sci 2021, 18, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).