Introduction
High Intensity Focused Ultrasound (HIFU) is a therapeutic technology based on ultrasound. It has been developing as a clinical treatment since it was first used clinically in the 1940′s. It uses similar physical principals to the more common diagnostic ultrasound, but at a much greater intensity. A diagnostic ultrasound beam may have a frequency of 2-16MHz with an intensity of 0.1 watts/cm
2, whereas HIFU treatments causing cell death will have frequencies in the range of 300 kHz to several MHz, with intensities reaching 1500 watts/cm
2. The focused waves can be targeted at precise areas of tissue, [
1] ablating the targeted area.
A newer technique of tissue emulsification rather than heat, known as histotripsy, is now being used in trials to treat some cancers including kidney and prostate [
2].
Since HIFU is a non-invasive technique, it is an attractive alternative to traditional surgical techniques. Prior to the point of focus, the emitted waves are insufficiently powerful to damage normal tissue and it is only when the waves meet at the focal point that the energy contained within them is sufficient to cause tissue necrosis [
3].
HIFU causes necrosis in two ways. Firstly, it generates high temperatures of over 80 °C, which is considerably higher than the 56 °C where it has been shown that an exposure for one second will cause coagulative necrosis and cellular death [
3]. These high temperatures are kept localised by keeping exposure times short – less than three seconds. There is a sharp temperature gradient between the targeted tissue and surrounding tissue, which results in a sharp delineation on histology between the treated tissue and untreated tissue. Furthermore, by ensuring that the time of exposure is kept under three seconds, the cooling effect of the surrounding blood flow is minimised [
4]. Secondly, HIFU causes tissue destruction through cavitation. Here tiny bubbles only a few microns in diameter vibrate under an ultrasound field, leading to an oscillating effect as the bubbles undergo alternating compression and rarefaction. Sometimes these bubbles may collapse and when this happens mechanical stresses are caused in the immediate microenvironment and in turn this may cause temperatures of up to 2000–5000
oC in those areas. This results in cell death.
Thermal damage from HIFU is easier both to replicate and calculate than cavitation [
5], and this in turn, at least at the current time, makes heating the favoured mode of treatment. However, a theoretical advantage of cavitation, which will disrupt the cells without totally destroying the contents of the cell, is that it may enhance the development of anti-tumour antibodies, which in turn might lead to a favourable effect on metastatic disease [
6].
Minimally invasive treatment techniques like HIFU have many potential benefits over traditional surgery. Some are obvious: the absence of an incision reduces infection risk, often means a more rapid recovery, and the lack of scar can be cosmetically valuable. HIFU may also have additional advantages not common to other minimally invasive techniques. For example, one downside of radiotherapy is its immunosuppressive effect [
7]. HIFU does not lead to immunosuppression, and there is some evidence to suggest that HIFU may be able to boost the anti-tumoral immune response [
8]. Unlike radiotherapy, HIFU has no maximum dose limit, meaning repeat administrations are possible to ensure satisfactory outcomes [
3].
One limitation of HIFU arises from the fact that ultrasound waves, unlike light, propagate poorly through gases, and so gas containing structures within the body such as the lungs or bowel are not generally suitable targets [
9]. Furthermore, these structures can be at risk when HIFU is delivered to other structures in close proximity. Another limitation is the length of time taken to deliver an effective HIFU treatment. Some sessions of HIFU on larger tumours can take many hours. Due to the precise nature of HIFU, the patient must remain still during the treatment, and so general anaesthetics are required in many instances [
9]. This is not always suitable for patients.
History of HIFU
The early properties of high-energy ultrasound waves were first described by Wood and Loomis in 1927 [
10], and in 1942, Lynn et al. [
11] outlined some of the possible clinical scenarios that might be treated in this way, It was not until the 1950s that William and Frank Fry in Illinois used it for the treatment of Parkinson’s disease [
12,
13,
14]. However, these treatments involved opening of the skull via craniotomy, and this highly invasive requirement inevitably meant that this use of HIFU was not further pursued, as new drugs for the treatment of Parkinson’s were coming onto the scene [
1].
The technology was explored again in the 1990s, with variable success. China has been one of the leaders in the clinical HIFU use in recent times. In the past 20 years, many patients have been treated in the Far East and China for conditions such as uterine fibroids and benign prostate hypertrophy [
15].
Professor Gail ter Haar and her colleagues in Surrey pioneered HIFU research in the UK, and in 2002, a dedicated HIFU unit was founded by Professor David Cranston in Oxford. This unit has carried out several trials on a variety of different clinical applications of HIFU [
16].
The Technology
HIFU machines are not all the same, and the monitoring of treatment can be down in two ways, with ultrasound or with magnetic resonance imaging (MRI). Ultrasound-guided HIFU combines the diagnostic and therapeutic forms of ultrasound. It is cheaper, smaller, and more widely available than the alternative: MRI-guided HIFU. This is more expensive, and the imaging is not done in ‘real time’, however the pictures produced are 3-dimensional and clearer than in ultrasound-guided HIFU. Recently, these methods have been used alongside each other to achieve the best of both worlds.
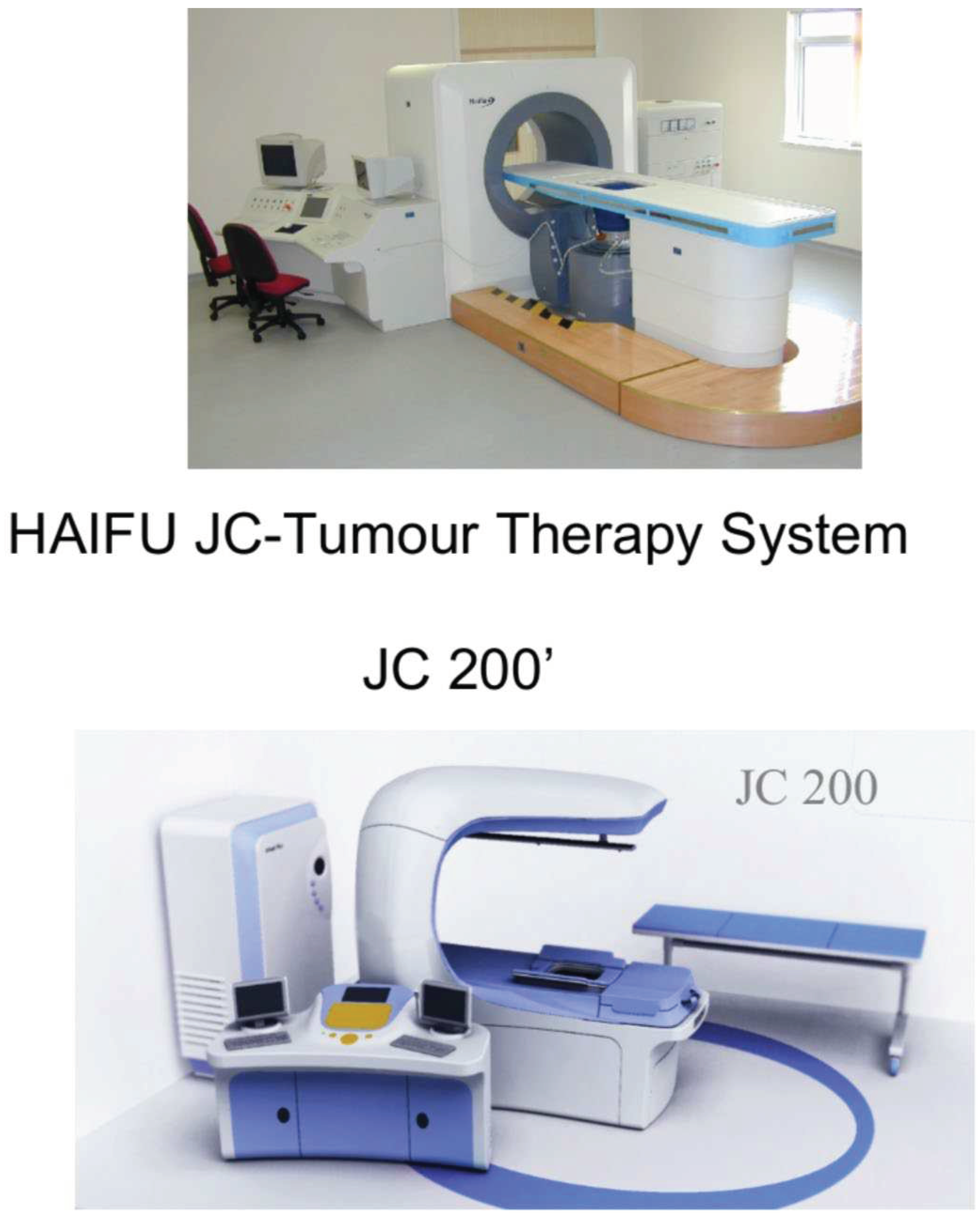
Figure 1.
The JC200 ultrasound machine.
Figure 1.
The JC200 ultrasound machine.
Kidney
The treatment of renal tumours with HIFU has been reported in the literature. A 2010 study by the Oxford HIFU team tested HIFU treatment on 17 patients with renal tumours [
17]. All the patients had a tumour size of under 5cm and were treated under a general anaesthetic and then spent one night in hospital after their treatments. The machine used was an ultrasound-guided HIFU machine. The patients were then followed up with an MRI scan, initially after 12 days, and then every 6 months for a mean duration of 36 months. Of the 17 original patients, 15 had the full HIFU treatment – two of the procedures had to be stopped due to bowel obstruction. Of those 15, 7 showed tumour ablation at the 12-day follow-up. After the 36 months, the overall results showed that two thirds of the patients experienced some tumour ablation, with a mean reduction of tumour volume of 30%.
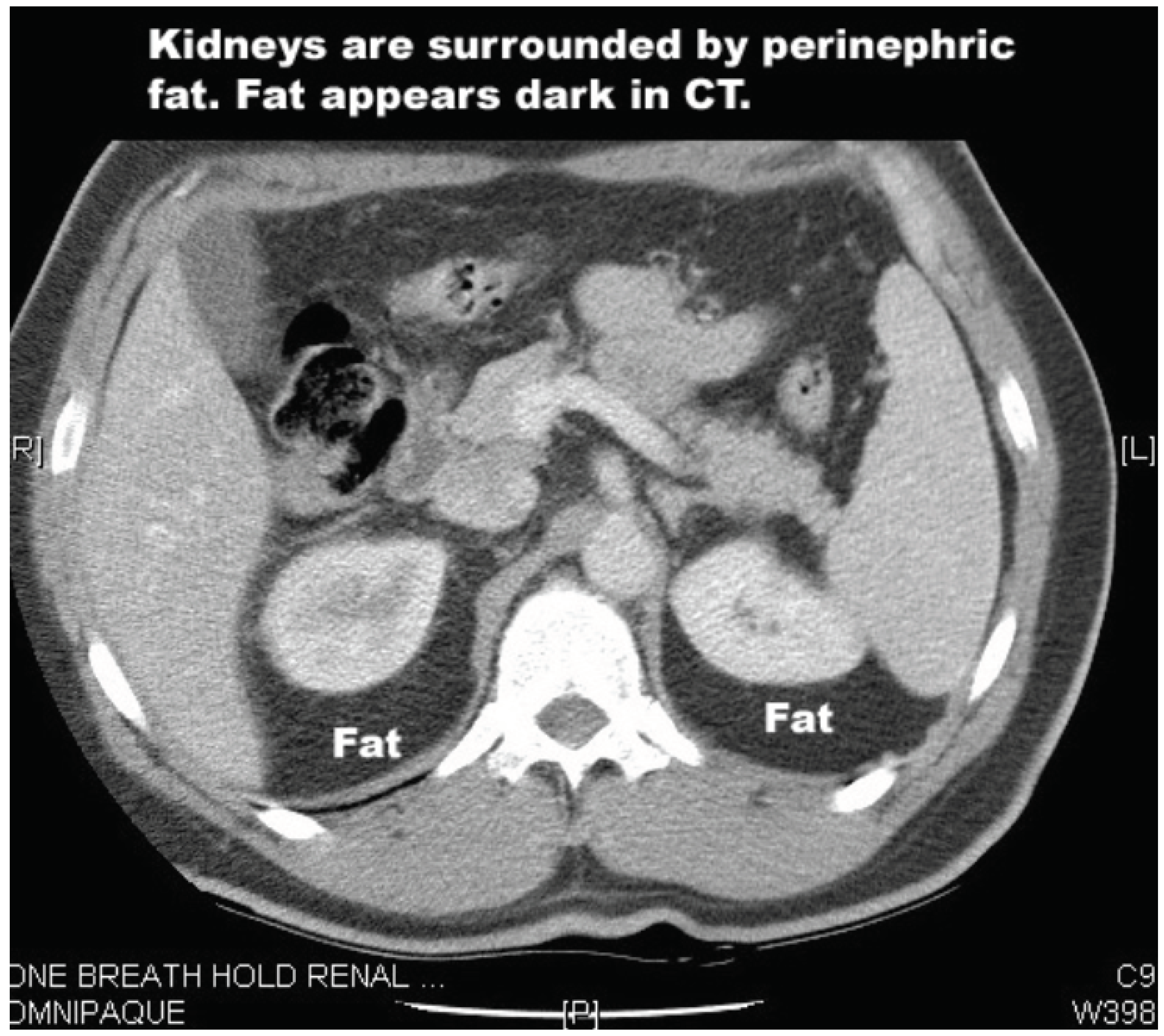
Renal tumours are a potentially challenging target for HIFU given the dense layers of perinephric fat that surround the kidneys. These layers absorb some of the energy of the HIFU beam. A 2020 study investigated this issue to determine the percentage drop of HIFU energy compared to the depth of the perinephric fat [
18]. The reduction was significant: from 58% of the output energy level at 2cm deep, to 26% at 5cm. To compensate for this, higher output energies are required, but this increases risk of surrounding tissue damage. However, transplanted kidneys have had their perinephric fat removed, and so transplant patients are perhaps better candidates for HIFU treatment.
Two patients who had undergone renal transplants and had developed tumours in their transplanted kidneys have been treated with HIFU in Oxford. The first treatment was unsuccessful due to technical issues. The second patient [
19] received two treatments of HIFU over 4 months. On both occasions, the patient experienced no pain and was discharged within 24 hours. Post-treatment analysis of the tumour showed a 90% reduction in tumour size.
A laparoscopic HIFU system (Sonatherm, manufactured be Misonix Inc.) has also been used for the treatment of small kidney cancers, and a Phase 1 study was undertaken in Oxford (12 patients) and Vienna (10 patients) with encouraging results [
20]. This demonstrated that it was safe and effective, however it is invasive treatment, unlike extracorporeal HIFU. Due to financial constraints, and the fact that this remains an invasive procedure, no further studies were performed.
Figure 2.
CT scan showing perinephric fat.
Figure 2.
CT scan showing perinephric fat.
Prostate
The technique for using HIFU to target the prostate involves inserting a small probe into the rectum, which then emits the ultrasound waves. For benign prostatic disease, HIFU has been trialled and compared with transurethral resection of the prostate (TURP), however HIFU has not yet demonstrated to be superior [
21].
The first reports that HIFU could be used to treat prostate carcinoma were published in 1995 be Madersbacher et al. [
22] and subsequently a many more studies have been carried out. Two commercial systems have been used: the Ablatherm
® (EDAP-Technomed, Lyon, France) and the Sonablate
®500 (Focus Surgery, Indianapolis, IN, USA). Both are examples of ultrasound guided HIFU and use rectal probes which combine imaging and a treatment transducer.
In the context of prostate cancer, HIFU may have many advantages over the traditional methods of surgical radical prostatectomy and radiotherapy. In 2019, the UK National Prostate Cancer Audit published the results of a study involving patient-reported outcomes from over 25,000 men who had received the traditional prostate cancer treatment methods [
23]. This audit highlighted the significant risk of side effects and complications from traditional treatments for prostate cancer. 1 in 10 men experienced ‘severe urinary complications’ following surgery or ‘severe bowel complications’ following radiotherapy. These results demonstrate the significant negative impact of established treatments for prostate cancer, and the importance of carefully controlled trials for proper evaluation of new technology [
24].
A paper by the French Urological Association of a six-year [
25] study (2009–2015), which looked at 111 patients with medium- or low-grade localised prostate cancer. This showed a 95% absence of clinically significant cancer at 12 months, and a treatment-free survival rate of 89% at 24 months. These results show the potential efficacy of HIFU as a treatment for prostate cancer. It is important to note though that two years is a short follow up in the context of evaluating the effectiveness of radical treatments for prostate cancer. Follow up duration of 10-15 years may be required to compare treatments more effectively [
26].
Dickinson et al. [
27] described a national cohort of 569 men who received HIFU to the whole prostate for localised prostate cancer. 754 interventions were carried out in total as the study protocol allowed for further treatment sessions where necessary. They carried out the majority of these as day cases with low rates of side effects in terms of incontinence (12%). Furthermore, the erectile dysfunction rate was similar to those having other whole-gland treatments (61%). One patient did develop a recto-urethral fistula, a much more serious complication. These results demonstrate the possible advantages that HIFU has over other prostate cancer treatment options in terms of its low side effect and complication rates.
In 2019, Stabile et al. [
28] described the medium-term results following focal treatment in 1032 men, 80% of which had a Gleason score of 7 (3 + 4) or above. They found that at 96 months post HIFU, 81% of the patients had avoided any further radical treatment. Furthermore, survival rate at 96 months was 97%. As a result of this they felt that focal HIFU for prostatic carcinoma is treatment with with good results survival rates and a low incidence of re-treatment at 96 months.
The first study of HIFU treatment of prostate cancer in the United States was published in 2020 [
29]. The report was retrospective but showed similarly positive results to the Stabile et al. study, with a sample size of 100 men. HIFU was shown to be effective (91% of the men avoided further radical treatment within the first 2 years after treatment), and all 100 of the patients maintained full urinary continence after the treatment.
Houstiou et al. evaluated HIFU for recurrent prostate cancer after previous brachytherapy and radiotherapy treatment [
30]. They treated 50 patients over 12 years between 2003 and 2015. They found encouraging results, both in terms of oncological and complication outcomes. Progression free survival was 45%, with an overall survival was 93%. The HIFU was delivered with specific post-brachytherapy and post-radiotherapy parameters.
Crouzet et al. carried out a retrospective study on 418 patients and examined the outcomes of their salvage treatments from 1995-2009 [
31]. The patients had all received salvage HIFU for locally recurrent prostate cancer following external beam radiotherapy. The 7-year cancer-specific survival rate was over 80%, however they initially reported worse morbidity results than the Houstiou et al. study. Complication rates of incontinence, outflow obstruction, and recto-urethral fistula were all higher in the period 1995-2002. In 2002, however, treatment-specific parameters were introduced for the salvage HIFU. These, in a similar way to the Houstiou et al. study, accounted for the tissue changes that previous radiotherapy induces, in particular damage to the blood supply to the prostate from radiotherapy. These alterations to the HIFU treatment improved complication rates post-2002, resulting in the rate of recto-urethral fistula decreasing from 9% to 0.6%.
Liver
A 2005 study conducted in Oxford examined the feasibility and safety of HIFU for the treatment of both liver and kidney tumours in a Western population [
32]. Thirty patients were recruited, with 22 having liver tumours. They were treated under anaesthetic in a single session. Of the 30 patients treated, 27 were able to have their response to treatment evaluated, primarily through an MRI scan at 12 days post HIFU. Tumour ablation was seen in 25 of the 30 patients (93%). The accuracy of the treatment was also evaluated. This involved observing the zones of ablation to see if they fell within the tumour area or impacted the surrounding tissue. Accuracy was said to be ‘good’ if the ablation only effected the tumour (21 patients), and ‘poor’ if it was outside the tumour (4 patients). The patients were also monitored for side effects post-treatment. The only clinically relevant symptom reported was ‘discomfort’ around the site of treatment, which was reported as ‘mild’ in severity in 80% of cases. Tiny skin blisters were seen in seven patients and a slightly larger area in one other. All resolved spontaneously.
A study in 2020 by Yang, T et al. also investigated the treatment of liver tumours [
33]. This phase 1 clinical trial aimed to evaluate HIFU as a treatment for colorectal liver metastases (CRLM), specifically metastases that were deemed ‘difficult’ and unsuitable for resection or radiological ablation. 13 patients between the ages of 20 to 80 were recruited. Ultrasound-guided HIFU was used to ablate the liver tumours. The surgical method of hepatectomy can achieve a 5-year 50% survival rate for colorectal liver metastasis (CRLM) [
34,
35]. However, up to 80% of these patients will not be suitable for surgery and have a median survival time of less than a year [
36]. The results of this study were positive. Firstly, adverse events were minimal, with the most common reported being pain and fatigue. The median follow-up to the treatment was 25 months. The 2-year overall survival was 77.8%, and the median overall survival time was 25 months. The authors suggested that HIFU was a safe treatment and could be considered for patients who were unsuitable for other treatment options.
Uterine Fibroids
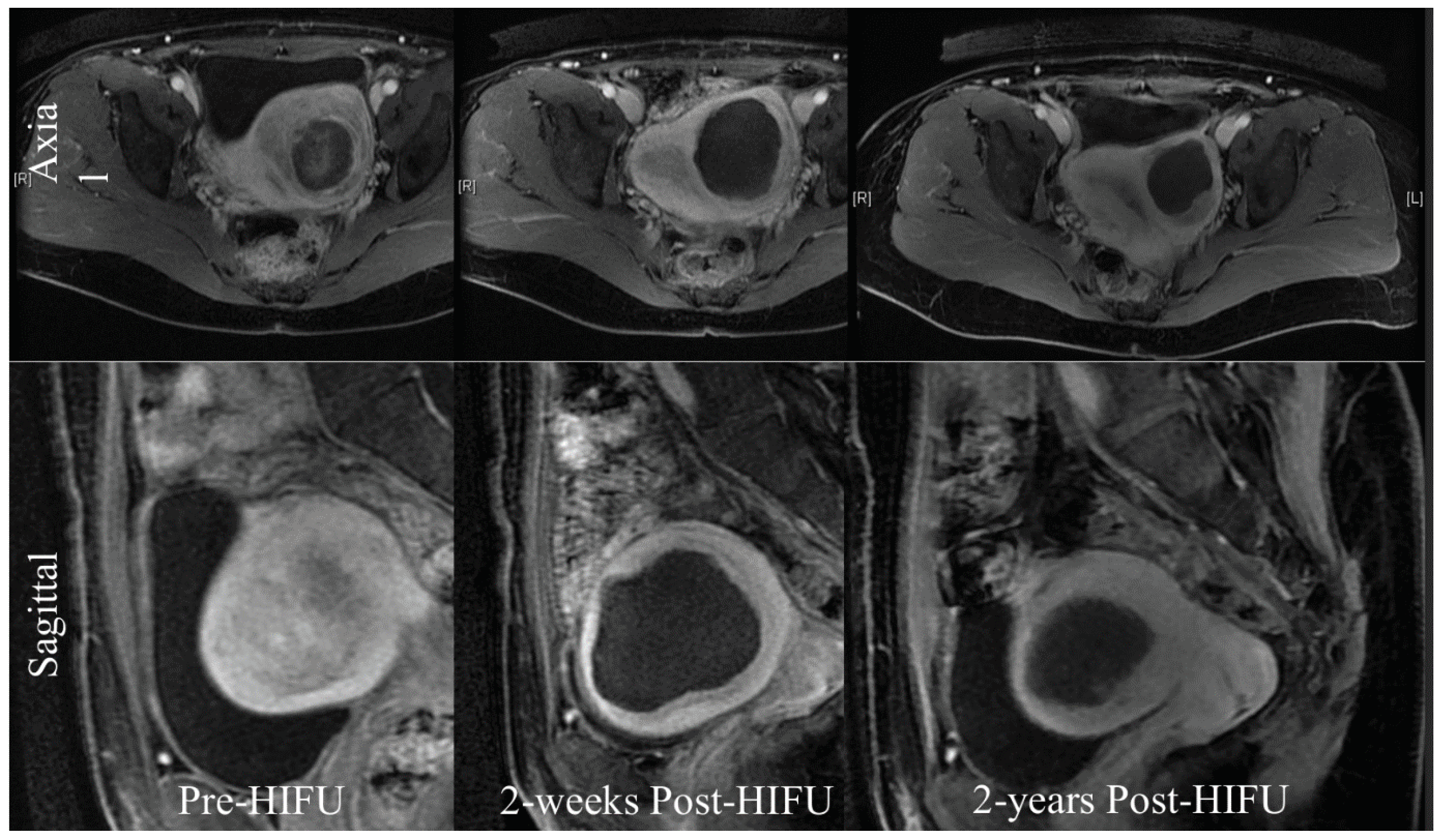
Figure 3.
The treatment of uterine fibroids with HIFU (MRI).
Figure 3.
The treatment of uterine fibroids with HIFU (MRI).
In females, HIFU can be used to treat uterine fibroids, which occur between 20% and 25% of women of childbearing age [
37]. A study from 20 centres in China in collaboration with the University of Oxford under the IDEAL framework [
24] of a prospective collaborative cohort study looked at 2411 Chinese women with symptomatic fibroids and compared the outcomes from (HIFU) and surgery. 1353 women received HIFU, 472 hysterectomy and 586 myomectomies. Quality of life improved more rapidly after HIFU than after surgery, with fewer side effects and a shorted hospital stay longer-term quality of life outcomes were similar.
Oxford University Hospitals conducted the first study of HIFU treatment for this condition on an NHS population [
38], [M1] treating a total of 14 fibroids. These patients were then monitored for a two-year period. No serious adverse events were recorded, but one patient with a surgical scar from a previous caesarean section had a secondary degree skin burn. Significant improvements were noted in mean symptom severity scores. In summary this study suggested that HIFU ablation led to a substantial improvement in symptoms over a 2-year time period. The occurrence of adverse events was low, with the exception of the skin burn which led to a change in protocol with no further patients being treated though a previous scar.
Chordomas
Chordomas are malignant tumours that arise from remnants of the notochord, and form in areas such as the sacrum [
40]. Surgical resection is usually the best treatment, but this is not always possible due to their location and relationship to essential structures nearby. Radiotherapy is an alternative to surgery, however but recurrence is common, and the prognosis is poor [
41]. HIFU has great potential in this area. In a 2017 clinical trial at Churchill Hospital, Oxford, successfully treated four patients with sacral chordomas [
42]. three of the four patients were treated under general anaesthetic, and the other was given only sedation (allowing verbal feedback to be given throughout the treatment). Only three of the patients were able to be followed up as one lived abroad. All three saw a decrease in tumour volume over time, and tumour necrosis was demonstrated in two patients. The side effects were minimal, with the most prominent symptom being mild discomfort.
Pancreas and Breast
Pancreatic cancers generally have a poor prognosis, and surgery, which gives the best chance of cure, is only possible in 15-20% of patients at the time of diagnosis. [
43]. In Oxford, a multi-centre clinical trial is being carried in patients with pancreatic cancer. Patients are initially treated with chemotherapy for three months, and then receive HIFU. They are then followed up for two years. Multiple patients so far have been successfully treated with no significant side effects. This is therefore a promising direction for HIFU treatment.
Breast cancer is another area where trials are ongoing for HIFU therapy [
44]. Breast tissue is an ideal location for HIFU beams as it sits superficially and so does not have vulnerable or gas filled structures nearby. HIFU is an established treatment for breast cancer in China [
1], and a trial in Oxford has been approved.
Possible Immunological Benefits of HIFU
HIFU may have positive immunological impacts with regards to cancer specific immunity. Murine studies suggest that this might arise from destroyed tumour cells that remain in-situ and which can act as sources of antigens that provoke a tumour specific immune response. [
47] Another study involving rats showed that this phenomenon may be mediated in part via heat shock proteins (HSPs) [
48]. Cancer cells express these proteins during time of stress, such as when targeted with HIFU therapy. These proteins can then stimulate cytotoxic T-cell activity. Whilst more research needs to be done in this area, the fact that HIFU may potentially benefit a patients’ immune response puts it into stark contrast with other non-surgical cancer treatments. Both chemotherapy and radiotherapy, for example, have the opposite effect.
Conclusion
There is little doubt that HIFU is becoming an increasingly important non-invasive addition to the medical armamentarium in many conditions. It is being used more commonly for prostate cancer and in many parts of the world a definitive treatment for uterine fibroids. Other areas continue to progress and with increased understanding of the physics and improvements in the technological development of different HIFU machines treatment of other abdominal tumours will become more of a reality. Other areas of targeted drug delivery including within the brain will improve and with an increased understanding of HIFU in combination with other treatments, the clinical possibilities for treatment will continue to expand.
References
- Kennedy, J.E., Ter Haar, G.R. and Cranston, D. (2003) ‘High intensity focused ultrasound: Surgery of the future?’, The British Journal of Radiology, 76(909), pp. 590–599. [CrossRef]
- Kennedy, J.E. (2005) ‘High-intensity focused ultrasound in the treatment of solid tumours’, Nature Reviews Cancer, 5(4), pp. 321–327. [CrossRef]
- Chen, L.; ter Haar, G.R.; Hill, C.R.; Dworkin, M,; Carnochan,P,; Young, H,; Bensted, JPM. Effect of blood perfusion on the ablation of liver parenchyma with high-intensity focused ultrasound. Phys. Med. Biol. 1993, 38, 1661–1673.
-
https://academic.oup.com/stmcls/article/39/9/1155/6513599.
- Hill, C.R.; Rivens, I.; Vaughan, M.G.; ter Haar, G. Lesion development in focused ultrasound surgery: A general model. Ultrasound Med. Biol. 1994, 20, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Kepp, O.; Marabelle, A.; Zitvogel, L. Oncolysis without viruses–inducing systemic anticancer immune responses with local therapies. Nat. Rev. Clin. Oncol. 2020, 17, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Ruckert, M et al (2021) Radiotherapy and the immune system: More than just immune suppression, Stem Cells, 39 (9) pp 1155-1165. [CrossRef]
- Kennedy, J.E. et al. (2004) ‘High-intensity focused ultrasound for the treatment of liver tumours’, Ultrasonics, 42(1–9), pp. 931–935. [CrossRef]
- Izadifar, Zahra et al. (2020) ‘An introduction to high intensity focused ultrasound: Systematic review on principles, devices, and clinical applications’, Journal of Clinical Medicine, 9(2), p. 460. [CrossRef]
- Wood, R.W.; Loomis, A.L. The physical and biological effects of high-frequency sound waves of great intensity. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1927, 4, 417–436. [Google Scholar] [CrossRef]
- Lynn, J.G.; Zwemer, R.L.; Chick, A.J.; Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J. Gen. Physiol. 1942, 26, 179–193. 1942; 26, 179–193.
- Fry, W.J.; Barnard, J.W.; Fry, F.J.; Krumins RF, Brennan JF.. Ultrasonic lesions in the mammalian central nervous system. Science 1955, 122, 517–518.
- Fry, F.J. Precision high-intensity focusing ultrasonic machines for surgery. Am. J. Phys. Med. 1958, 37, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Burov, A.K. High-intensity ultrasonic vibrations for action on animal and human malignant tumours. Dokl Akad. Nauk SSSR 1956, 106, 239–241. [Google Scholar]
- Full article: The Asian perspective on HIFU (tandfonline.com).
- Haar, G. ter, Sinnett, D. and Rivens, I. (1989) ‘High intensity focused ultrasound-a surgical technique for the treatment of discrete liver tumours’, Physics in Medicine and Biology, 34(11), pp. 1743–1750. [CrossRef]
- Ritchie, R.W. et al. (2010) ‘Extracorporeal high intensity focused ultrasound for renal tumours: A 3-year follow-up’, BJU International, 106(7), pp. 1004–1009. [CrossRef]
- Mancini, M.; Righetto, M.; Baggio, G. Gender-related approach to kidney cancer management: Moving forward. Int. J. Mol. Sci. 2020, 21(9) 3378–3391.
- Chakera, A.; Leslie, T.; Roberts, I.; O’Callaghan, C.A.; Cranston, D. A lucky fall? Case report. Transplant. Proc. 2010, 42, 3883–3886. [Google Scholar] [CrossRef] [PubMed]
- Klingler, H.C.; Susani, M.; Seip, R.; Mauermann J,; Sanghvi, N,; Marberger, MJ. A novel approach to energy ablative therapy of small renal tumours: Laparoscopic high-intensity focused ultrasound. Eur. Urol. 2008, 53, 810–816.
-
https://www.sciencedirect.com/science/article/pii/S0929826699000129?casa_token=H-kan1Y35KAAAAAA:FJG8-1Jvxs96ujKSm8b3-UrvPQ7SJaY6pHESEVH6LRXltI42uZLUXNA2czQ9ALiuk5OUVl8a.
- Madersbacher, S.; Pedevilla, M.; Vingers, l.; Susani, M,; Marberger, M. Effect of High Intensity Focused Ultrasound on human prostate cancer in vivo. Cancer Res. 1995, 55, 3346–3351.
- NPCA Annual Report 2019 - National Prostate Cancer Audit.
- McCulloch, P.; Altman, D.G.; Campbell, W.B.; Flum, DR,; Glasziou,P,; Marshall JC,; Nicholl,J. No surgical intervention without evaluation. The IDEAL recommendations. Lancet 2009, 374, 1105–1112.
- Rischmann, P.; Gelet, A.; Riche, B.; Villers, A,; Pasticier, G,;Bondil,P,; Jung, JL,; Bugel, H,; Petit, J, Toledano, H.. et al Focal High Intensity Focused Ultrasound of Unilateral Localized Prostate Cancer: A Prospective Multicentric Hemiablation Study of 111 Patients. Eur. Urol. 2017, 71, 267–273.
-
https://www.nejm.org/doi/full/10.1056/NEJMoa2214122.
- Dickinson, L.; Arya, M.; Afzal, N.; Cathcart, P,; Charman, SC,; Cornaby,A,; Hindley, RG,; Lewi, H,; McCartan,N; Moore, CM,;et al Medium-term Outcomes After Whole-gland High-intensity Focused Ultrasound for the Treatment of Nonmetastatic Prostate Cancer From a Multicentre Registry Cohort. Eur. Urol. 2016, 70, 668–674.
- Stabile, A.; Orczyk, C.; Hosking-Jervis, F.; Giganti,F,; Arya,M,; Hindley,RG,; Dickinson,L,; Allen, L,; Punwani,S,; Jameson, C.et al. Medium-term Oncological Outcomes in a Large Cohort of Men Treated With Either Focal or Hemi-Ablation Using High-Intensity Focused Ultrasonography for Primary Localized Prostate Cancer BJU Int. 2019, 124, 431–440.
- Abreu, A.L.; Peretsman, S.; Iwata, A.; Shakir, A,; Iwata, T,; Brooks, J,; Tafuri, A,; Ashrafi, A,; Park, D,; Cacciamani, GE.et al. High Intensity Focused Ultrasound Hemi-gland Ablation for Prostate Cancer: Initial Outcomes of a United States Series. J. Urol. 2020, 204, 741–747.
- Hostiou, T.; Gelet, A.; Chapelon, J.Y.; Rouviere, O,; Mege-Lechevalier, F,; Lafon, C,; Tonoli-Catez, H,; Badet, L. Crouzet, S. Salvage High-Intensity Focused Ultrasound for Locally Recurrent Prostate Cancer After Low-Dose-Rate Brachytherapy: Oncological and Functional Outcomes BJU Int. 2019, 124, 746–757.
- Crouzet, S.; Blana, A.; Murat, F.J.; Pasticier, G,; Brown, SC,; Conti, GN,; Ganzer, R,; Chapet, O,; Gelet,A,; Chaussy, CG..et al. Salvage High-Intensity Focused Ultrasound (HIFU) for Locally Recurrent Prostate Cancer After Failed Radiation Therapy: Multi-institutional Analysis of 418 Patients. BJU Int. 2017, 119, 896–904.
- Illing, R.O. et al. (2005) ‘The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population’, British Journal of Cancer, 93(8), pp. 890–895. [CrossRef]
-
https://link.springer.com/content/pdf/10.1007/s00464-020-07644-y.pdf.
- Rees M, Tekkis PP, Welsh FK et al (2008) Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 247:125–135.
- Pawlik TM, Scoggins CR, Zorzi D et al (2005) Efect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 241:715–722.
- Nordlinger B, Sorbye H, Glimelius B et al (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14:1208–1215.
- Chen, J. et al. (2017) ‘Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: An ideal prospective exploration study’, BJOG: An International Journal of Obstetrics & Gynaecology, 125(3), pp. 354–364. [CrossRef]
- Lyon, P. et al Ultrasound-guided High Intensity Focused Ultrasound Ablation for Symptomatic Uterine Fibroids: A Preliminary Clinical Experience Ultraschall in Med 2019; 40: 1–7.
- Chetan MR, Lyon PC, Wu F, et al., Role of diffusion-weighted imaging in monitoring treatment response following high-intensity focused ultrasound ablation of recurrent sacral chordoma. Radiol Case Rep. 2019 Aug 1;14(10):1197-1201.
- Y. Yang, X. Niu, Y. Li, W. Liu, H. Xu Recurrence and survival factors analysis of 171 cases of sacral chordoma in a single institute Eur Spine J, 26 (2017), pp. 1910-1916.
- S. Radaelli, S. Stacchiotti, P. Ruggieri, et al. Sacral chordoma Spine (Phila. Pa. 1976), 41 (2016), pp. 1049-1057.
- Gillies, M.J. et al. (2016) ‘High-intensity focused ultrasonic ablation of sacral chordoma is feasible: A series of four cases and details of a national clinical trial’, British Journal of Neurosurgery, 31(4), pp. 446–451. [CrossRef]
- Prachee I, Wu F, Cranston D. (2021) The Oxford Experience of High Intensity Focused Ultrasound International Journal of Hyperthermia, 38:2, 81-88. [CrossRef]
- Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia. 2015; 31:302–309.
-
https://spj.science.org/doi/full/10.34133/2022/9807347.
- Lyon, P.C. et al. (2018) ‘Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): A single-centre, open-label, phase 1 trial’, The Lancet Oncology, 19(8), pp. 1027–1039. [CrossRef]
- den Brok, M.H. et al. (2004) ‘In situ tumor ablation creates an antigen source for the generation of antitumor immunity’, Cancer Research, 64(11), pp. 4024–4029. [CrossRef]
- Schueller, G. et al. (2004) ‘Expression of heat shock proteins in human hepatocellular carcinoma after radiofrequency ablation in an animal model’, Oncology Reports [Preprint]. [CrossRef]
| 1 |
Ben Turner Medical Student Bristol University |
| 2 |
David Cranston Associate Professor of Surgery (Emeritus) NDS and Clinical Director Oxford HIFU unit |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).