1. Introduction
Neisseria gonorrhoeae, also known as Neisser’s gonococcus, is the causative agent responsible for gonorrhea (or blenorrhagia) [
1,
2]. This Gram-negative bacterium belongs to the Neisseriaceae family and is morphologically similar to Neisseria meningitidis, the causative agent of meningococcal meningitis. Both are gram-negative cocci that often occur in pairs [
1,
2,
3,
4,
5,
6]. Neisseria gonorrhoeae is transmitted mainly through sexual contact and can cause a variety of symptoms involving the genital system and, in some cases, other areas such as the throat or anus [
1,
2,
3,
4,
5,
6]. Increasing resistance to antibiotics has made the management of gonorrhea a challenge, underscoring the importance of safe sexual practices and timely identification and appropriate treatment of infections [
1,
2,
3,
4].
This Gram-negative diplococcus primarily infects the genital and reproductive tract mucosa but can also affect the throat and rectum [
1,
2].
Neisseria gonorrhoeae is transmitted through unprotected sexual contact and can lead to a range of symptoms, including genital discharge, pain during urination, and in some cases, complications such as pelvic inflammatory disease [
1,
2,
3,
4,
5,
6]. Antibiotic resistance in N. gonorrhoeae is a growing concern, highlighting the need for alternative treatment options [
1,
2,
3,
4,
5,
6].
The emergence of antibiotic-resistant strains of Neisseria gonorrhoeae has posed challenges for treatment, emphasizing the importance of early detection and appropriate antibiotic therapy. Regular testing, safe sexual practices, and awareness are crucial for preventing and managing infections caused by Neisseria gonorrhoeae [
5,
6]
2. Material and Methods
-Fe(3+) ions import ATP-binding protein fbpC (PDB Code: 3fvq) Grid box Coordinates of binding Center X ( -18,1105), Y( -13,5136), Z(39,4644)
3. Results and Discussion
This short theoretical computational study based on Molecular Docking [
7,9,10] aims to investigate natural molecules against Neisseria gonorrhoea [
1,
2,
3,
4,
5,
6]. Neisseria gonorrhoeae is a bacterium responsible for the sexually transmitted infection (STI) known as gonorrhea. This bacterium infects the mucous membranes of the reproductive tract, including the cervix, uterus, and fallopian tubes in women, and the urethra in both men and women. It can also infect the throat, rectum, and eyes [
1,
2,
3,
4,
5,
6].
Gonorrhea is primarily transmitted through sexual contact, including vaginal, anal, and oral sex.Transmission from an infected mother to her child can also occur during childbirth [
1,
2,
3,
4,
5,
6]. Particular attention docking calculation was performed against Fe(3+) ions import ATP-binding protein fbpC by Neisseria gonorrhoeae [
8].
Performing a docking calculation allows to understand the potential binding modes and strength of interaction between the Fe(3+) ions and the fbpC protein [
8].
This information can be crucial in understanding the role of this protein in iron import processes in Neisseria gonorrhoeae. Docking analysis was conducted by Mcule Server [
9] by Autodock Vina [
10].
The observation that Hypericin demonstrated an excellent binding energy of about -13.2 kcal/mol against the Fe(3+) ions import ATP-binding protein fbpC in Neisseria gonorrhoeae FA 1090 is noteworthy. A lower binding energy typically indicates a stronger and more stable interaction between the molecule and the target protein.
This calculation suggests its potential utility as a candidate for further exploration in combating Neisseria gonorrhoeae infections. However, it’s important to note that in silico docking studies provide predictions, and experimental validation is crucial to confirm the actual biological activity and effectiveness of Hypericin against Neisseria gonorrhoeae.
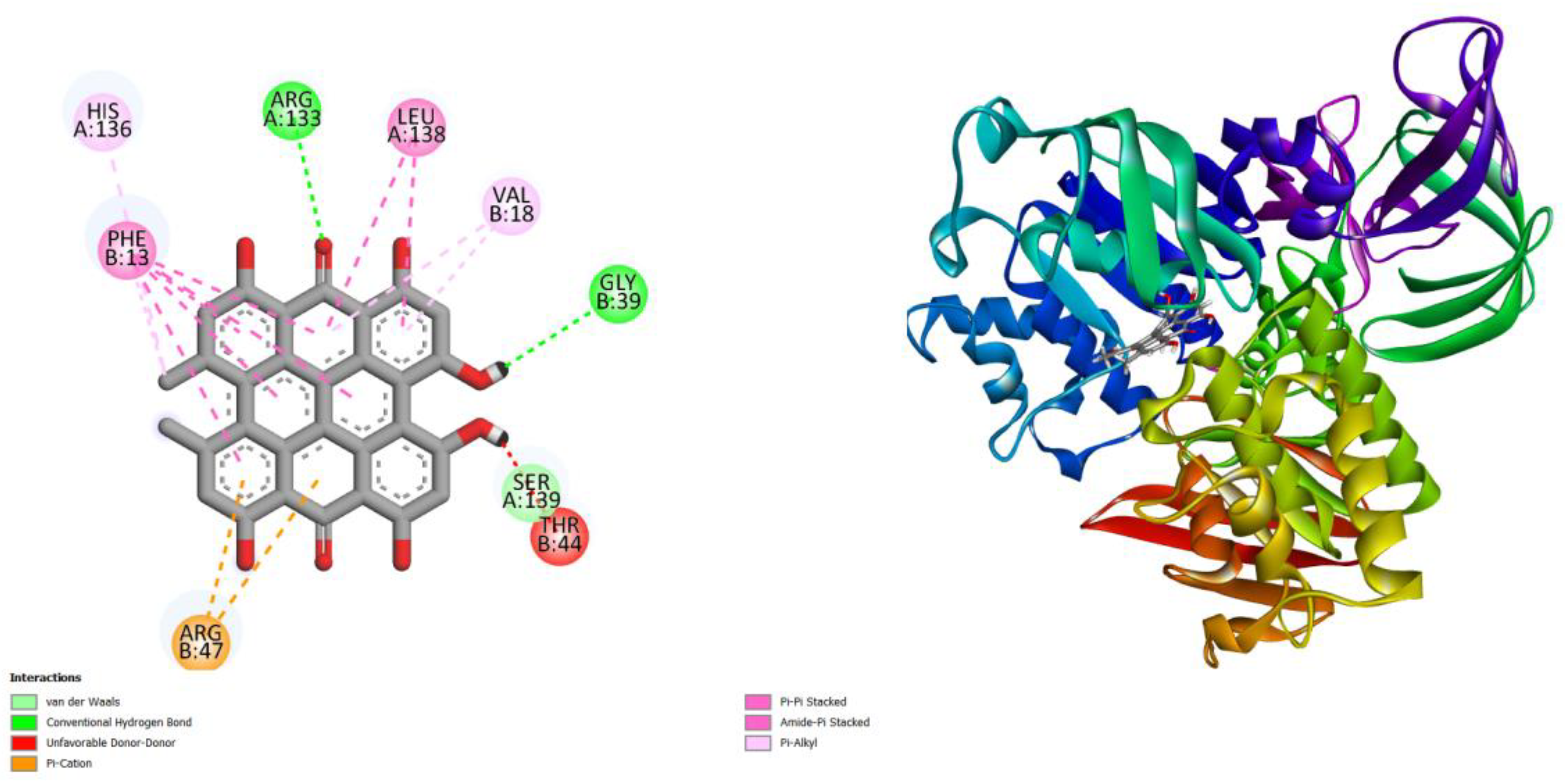
Figure 1.
displays the docking outcomes of Fe(3+) ions import ATP-binding protein fbpC in conjunction with Hypericin -13.2 kcal/mol within the Ligand Binding Site, as analyzed by Autodock Vina through the Mcule Database. On the left side, 2D diagrams illustrate the residue interactions between the protein and Hypericin. Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of Hypericin.
Figure 1.
displays the docking outcomes of Fe(3+) ions import ATP-binding protein fbpC in conjunction with Hypericin -13.2 kcal/mol within the Ligand Binding Site, as analyzed by Autodock Vina through the Mcule Database. On the left side, 2D diagrams illustrate the residue interactions between the protein and Hypericin. Meanwhile, the right side exhibits the Ligand Binding Site of the protein, highlighting the specific location of Hypericin.
4. Conclusion
In conclusion, the molecular docking study revealed that Hypericin exhibits a notable and favorable binding affinity with the Fe(3+) ions import ATP-binding protein fbpC in Neisseria gonorrhoeae FA 1090, as evidenced by a robust binding energy of approximately -13.2 kcal/mol. This finding suggests the potential of Hypericin as a candidate for further investigation as a therapeutic agent against Neisseria gonorrhoeae infections. However, it is crucial to acknowledge that these computational results serve as a preliminary step, and experimental validation through in vitro and in vivo studies is essential to confirm the actual antimicrobial efficacy and safety of Hypericin.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Belcher, T.; Rollier, C.S.; Dold, C.; Ross, J.D.; MacLennan, C.A. Immune responses to Neisseria gonorrhoeae and implications for vaccine development. Front. Immunol. 2023, 14, 1248613. [Google Scholar] [CrossRef]
- Williams, E.; Seib, K.L.; Fairley, C.K.; Pollock, G.L.; Hocking, J.S.; McCarthy, J.S.; Williamson, D.A. Neisseria gonorrhoeae vaccines: a contemporary overview. Clin. Microbiol. Rev. 2024, e0009423. [Google Scholar] [CrossRef]
- Vaezzadeh, K.; Sepidarkish, M.; Mollalo, A.; As’ adi, N.; Rouholamin, S.; Rezaeinejad, M.; Mojtahedi, M.F.; Hosseini, S.M.M.; Taheri, M.; Mahjour, S.; et al. Global prevalence of Neisseria gonorrhoeae infection in pregnant women: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; McSheffrey, G.G.; Gray-Owen, S.D.; Zhang, J.R.; Tang, Y.W. Neisseria gonorrhoeae. In Molecular Medical Microbiology; Academic Press: Cambridge, MA, USA, 2024. [Google Scholar]
- RRubin, D.H.; Ma, K.C.; Westervelt, K.A.; Hullahalli, K.; Waldor, M.K.; Grad, Y.H. CanB is a metabolic mediator of antibiotic resistance in Neisseria gonorrhoeae. Nat. Microbiol. 2023, 8, 28–39. [Google Scholar] [CrossRef]
- kakooza, F.; Kiggundu, R.; Mboowa, G.; Kateete, P.D.; Nsangi, O.T.; Kabahita, J.M.; Unemo, M. Antimicrobial susceptibility surveillance and antimicrobial resistance in Neisseria gonorrhoeae in Africa from 2001 to 2020: A mini-review. Front. Microbiol. 2023, 14, 1148817. [Google Scholar] [CrossRef]
- Azad, I. Molecular Docking in the Study of Ligand-Protein Recognition: An Overview. Mol. Docking-Recent Adv. 2023. [Google Scholar] [CrossRef]
- Di Cesare, M.; Kaplan, E.; Rendon, J.; Gerbaud, G.; Valimehr, S.; Gobet, A.; Ngo, T.-A.T.; Chaptal, V.; Falson, P.; Martinho, M.; et al. The transport activity of the multidrug ABC transporter BmrA does not require a wide separation of the nucleotide-binding domains. J. Biol. Chem. 2024, 300, 105546. [Google Scholar] [CrossRef] [PubMed]
- Odhar, H.A.; Rayshan, A.M.; Ahjel, S.W.; Hashim, A.A.; Albeer AA, M.A. Molecular docking enabled updated screening of the matrix protein VP40 from Ebola virus with millions of compounds in the MCULE database for potential inhibitors. Bioinformation 2019, 15, 627. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Tang, S.; Mei, Z.; Wang, L.; Huang, Q.; Hu, H.; Ling, M.; Wu, J. Vina-GPU 2.0: Further Accelerating AutoDock Vina and Its Derivatives with Graphics Processing Units. J. Chem. Inf. Model. 2023, 63, 7–1982. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).