Submitted:
09 February 2024
Posted:
12 February 2024
Read the latest preprint version here
Abstract
Keywords:
1. Clinical Case
1.1. Results of Laboratory Investigations
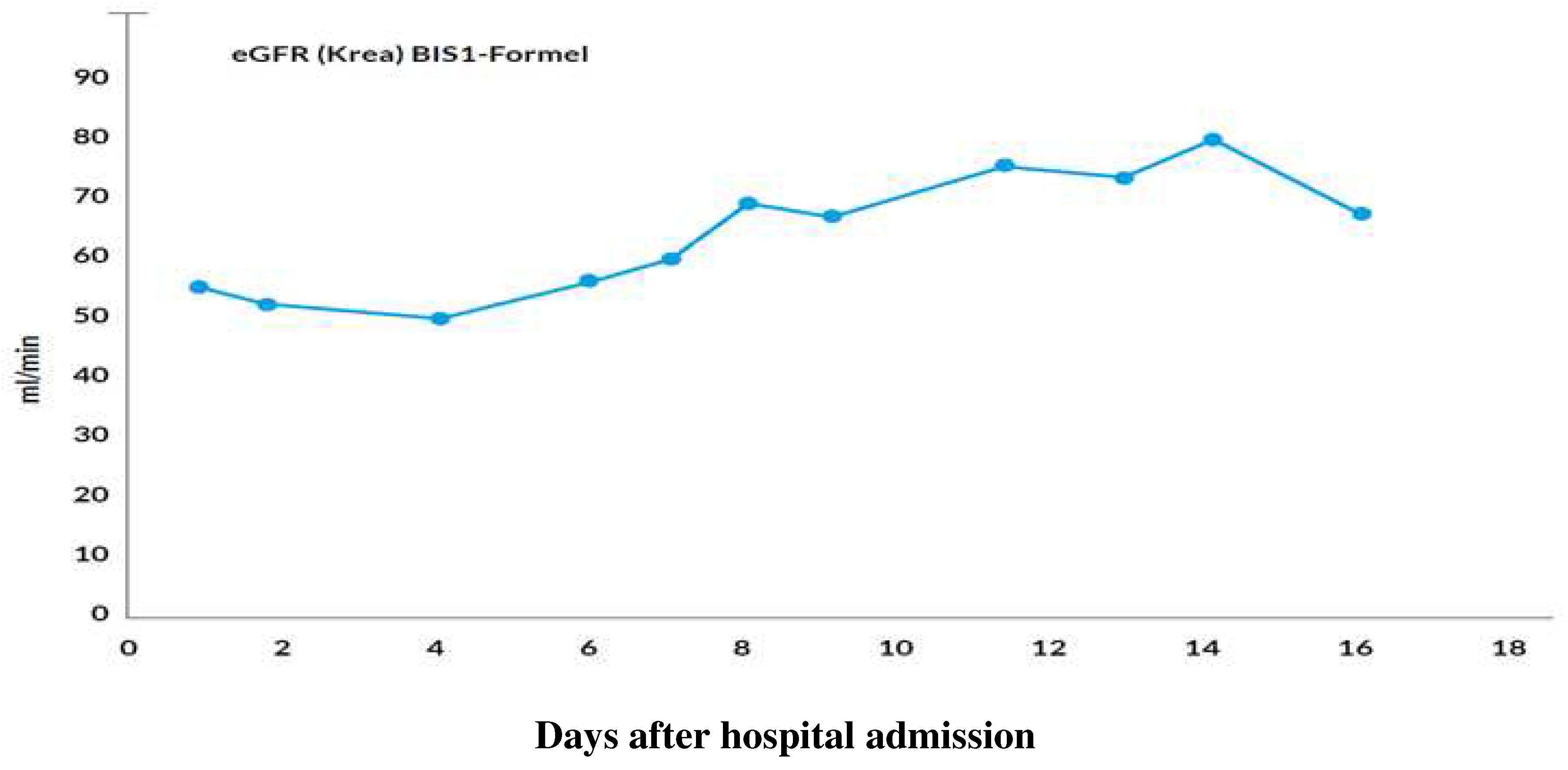
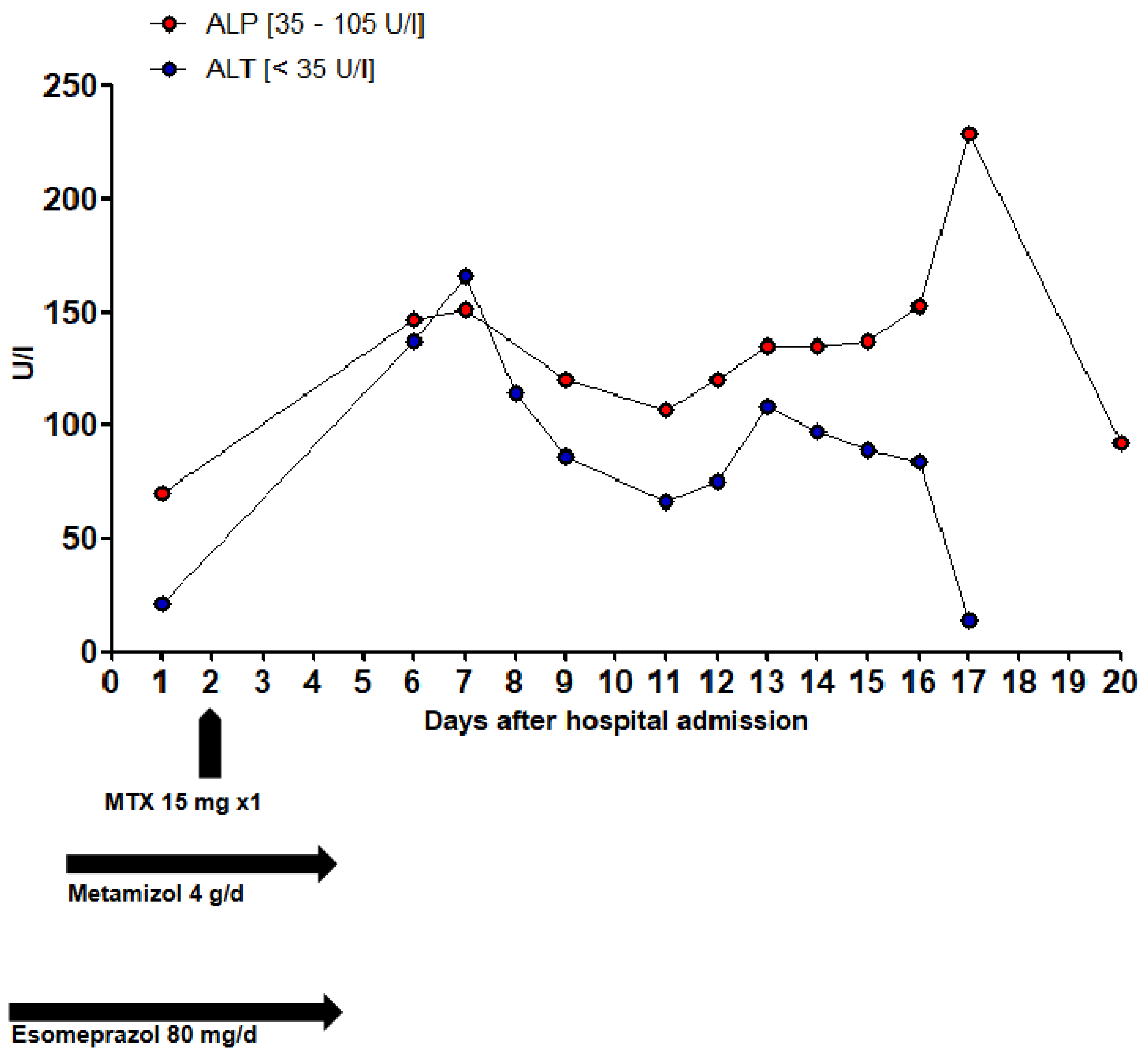
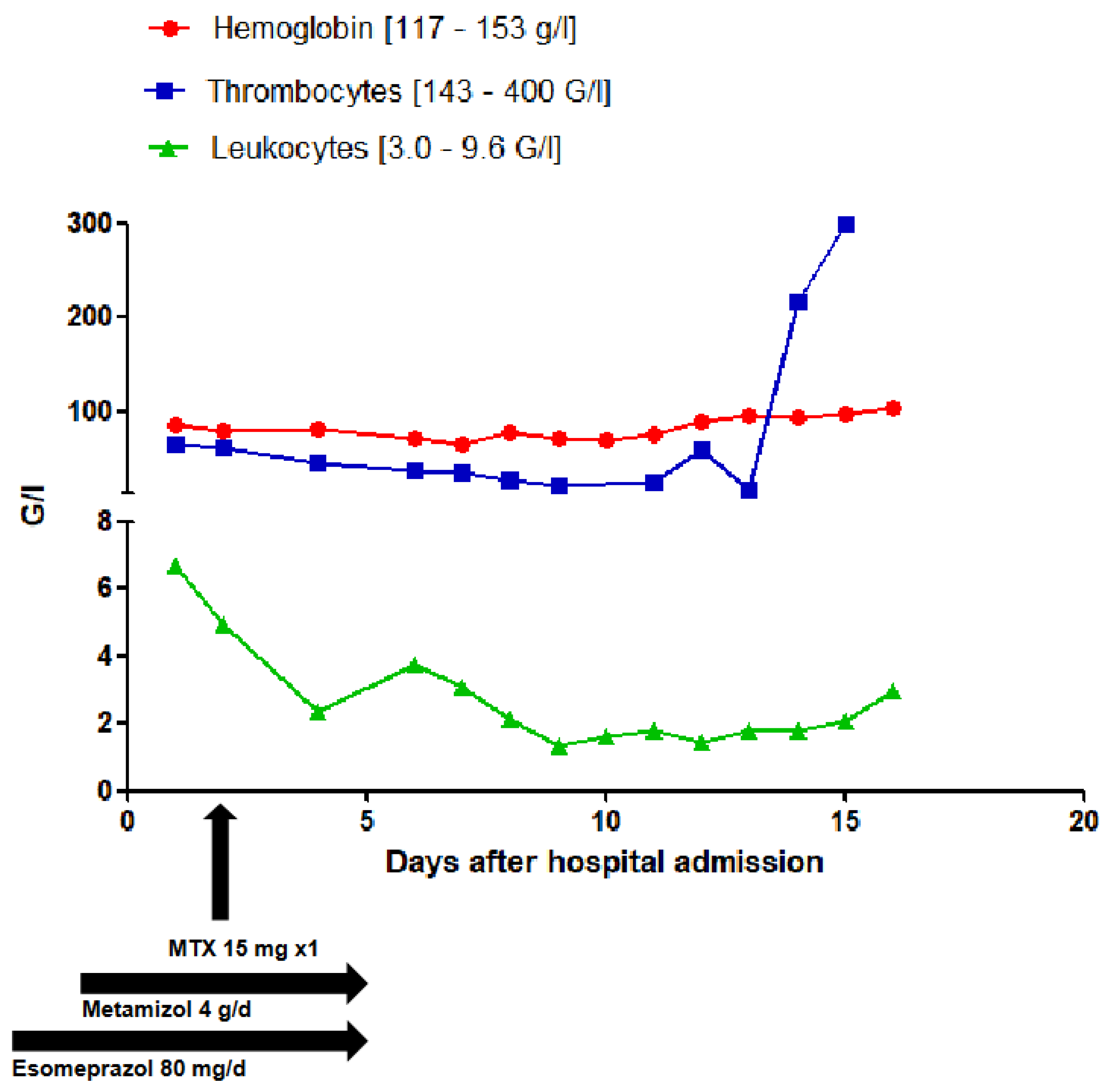
2. Pharmacology of Methotrexate
2.1. Pharmacodynamic Interactions of Methotrexate with Other Drugs
2.2. Pharmacokinetic Interactions of Methotrexate with Other Drugs
3. Case Evaluation
4. Conclusion
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998 Jul 15;102(2):322-8. [CrossRef]
- Chan ES, Cronstein BN. Methotrexate--how does it really work? Nat Rev Rheumatol. 2010 Mar;6(3):175-8. [CrossRef]
- Swiss Drug Information. Available online: www.swissmedicinfo.ch (accessed on 6 August 2023).
- Micromedex™. Available online: www.micromedexsolutions.com (accessed on 6 August 2023).
- UpToDate™. Available online: www.uptodate.com (accessed on 6 August 2023).
- Chabner BA, Allegra CJ, Curt GA, Clendeninn NJ, Baram J, Koizumi S, Drake JC, Jolivet J. Polyglutamation of methotrexate. Is methotrexate a prodrug? J Clin Invest. 1985 Sep;76(3):907-12. [CrossRef]
- García-Carrasco M, Mendoza-Pinto C, Macías-Díaz S, Etchegaray-Morales I, Méndez-Martínez S, Soto-Santillán P, Pérez-Romano B, Jiménez-Herrera EA, Guzmán-Ruiz O, Ruiz-Argüelles A. Clinical relevance of P-glycoprotein activity on peripheral blood mononuclear cells and polymorphonuclear neutrophils to methotrexate in systemic lupus erythematosus patients. Clin Rheumatol. 2017 Oct;36(10):2267-2272. [CrossRef]
- De Graaf D, Sharma RC, Mechetner EB, Schimke RT, Roninson IB. P-glycoprotein confers methotrexate resistance in 3T6 cells with deficient carrier-mediated methotrexate uptake. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1238-42. [CrossRef]
- Riquelme-Mc Loughlin C, Giavedoni P, Mascaró JM Jr. More than skin deep. Am J Emerg Med. 2018 Sep;36(9):1719.e3-1719.e4. [CrossRef]
- Van der Klauw MM, Wilson JHP, & Stricker BHC: Drug-associated agranulocytosis: 20 years of reporting in the netherlands (1974-1994). Am J Hematol 1998; 57:206-211. [CrossRef]
- Hoffmann F, Bantel C, Jobski K. Agranulocytosis attributed to metamizole: An analysis of spontaneous reports in EudraVigilance 1985-2017. Basic Clin Pharmacol Toxicol. 2020 Feb;126(2):116-125. [CrossRef]
- Blaser LS, Tramonti A, Egger P, Haschke M, Krähenbühl S, Rätz Bravo AE. Hematological safety of metamizole: retrospective analysis of WHO and Swiss spontaneous safety reports. Eur J Clin Pharmacol. 2015 Feb;71(2):209-17. [CrossRef]
- Blanchet E, Beau P, Frat JP. Bone marrow aplasia following dipyrone treatment in a patient with Crohn's disease receiving long-term methotrexate. Gastroenterol Clin Biol. 2004 May;28(5):502-3. French. [CrossRef]
- Trautmann A, Brockow K, Stoevesandt J. Metamizole-induced reactions as a paradigm of drug hypersensitivity: Non-allergic reactions, anaphylaxis, and delayed-type allergy. Clin Exp Allergy. 2020 Sep;50(9):1103-1106. [CrossRef]
- Stone SR, Morrison JF. Mechanism of inhibition of dihydrofolate reductases from bacterial and vertebrate sources by various classes of folate analogues. Biochim Biophys Acta. 1986 Feb 14;869(3):275-85. [CrossRef]
- Hamid M, Lashari B, Ahsan I, Micaily I, Sarwar U, Crocetti J. A deadly prescription: combination of methotrexate and trimethoprim-sulfamethoxazole. J Community Hosp Intern Med Perspect. 2018 Jun 12;8(3):149-151. [CrossRef]
- Thomas DR, Dover JS, Camp RD. Pancytopenia induced by the interaction between methotrexate and trimethoprim-sulfamethoxazole. J Am Acad Dermatol. 1987 Dec;17(6):1055-6. [CrossRef]
- Groenendal H, Rampen FH. Methotrexate and trimethoprim-sulphamethoxazole--a potentially hazardous combination. Clin Exp Dermatol. 1990 Sep;15(5):358-60. [CrossRef]
- Cody V, Pace J, Makin J, Piraino J, Queener SF, Rosowsky A. Correlations of inhibitor kinetics for Pneumocystis jirovecii and human dihydrofolate reductase with structural data for human active site mutant enzyme complexes. Biochemistry. 2009 Mar 3;48(8):1702-11. [CrossRef]
- Ferrazzini G, Klein J, Sulh H, Chung D, Griesbrecht E, Koren G. Interaction between trimethoprim-sulfamethoxazole and methotrexate in children with leukemia. J Pediatr. 1990 Nov;117(5):823-6. [CrossRef]
- Kümpornsin K, Modchang C, Heinberg A, Ekland EH, Jirawatcharadech P, Chobson P, Suwanakitti N, Chaotheing S, Wilairat P, Deitsch KW, Kamchonwongpaisan S, Fidock DA, Kirkman LA, Yuthavong Y, Chookajorn T. Origin of robustness in generating drug-resistant malaria parasites. Mol Biol Evol. 2014 Jul;31(7):1649-60. [CrossRef]
- Liu H, Qin Y, Zhai D, Zhang Q, Gu J, Tang Y, Yang J, Li K, Yang L, Chen S, Zhong W, Meng J, Liu Y, Sun T, Yang C. Antimalarial Drug Pyrimethamine Plays a Dual Role in Antitumor Proliferation and Metastasis through Targeting DHFR and TP. Mol Cancer Ther. 2019 Mar;18(3):541-555. [CrossRef]
- Ueda H, Narumi K, Sato Y, Furugen A, Kobayashi M, Iseki K. Evaluation of possible pharmacokinetic interaction between methotrexate and proton pump inhibitors in rats. Pharmacol Rep. 2020 Oct;72(5):1426-1432. [CrossRef]
- Ueda H, Narumi K, Sato Y, Furugen A, Kobayashi M, Iseki K. Evaluation of possible pharmacokinetic interaction between methotrexate and proton pump inhibitors in rats. Pharmacol Rep. 2020 Oct;72(5):1426-1432. [CrossRef]
- Chioukh R, Noel-Hudson MS, Ribes S, Fournier N, Becquemont L, Verstuyft C. Proton pump inhibitors inhibit methotrexate transport by renal basolateral organic anion transporter hOAT3. Drug Metab Dispos. 2014 Dec;42(12):2041-8. [CrossRef]
- Narumi K, Sato Y, Kobayashi M, Furugen A, Kasashi K, Yamada T, Teshima T, Iseki K. Effects of proton pump inhibitors and famotidine on elimination of plasma methotrexate: Evaluation of drug-drug interactions mediated by organic anion transporter 3. Biopharm Drug Dispos. 2017 Dec;38(9):501-508. [CrossRef]
- Santucci R, Levêque D, Kemmel V, Lutz P, Gérout AC, N'guyen A, Lescoute A, Schneider F, Bergerat JP, Herbrecht R. Severe intoxication with methotrexate possibly associated with concomitant use of proton pump inhibitors. Anticancer Res. 2010 Mar;30(3):963-5.
- Santucci R, Levêque D, Lescoute A, Kemmel V, Herbrecht R. Delayed elimination of methotrexate associated with co-administration of proton pump inhibitors. Anticancer Res. 2010 Sep;30(9):3807-10.
- Boerrigter E, Crul M. A non-interventional retrospective cohort study of the interaction between methotrexate and proton pump inhibitors or aspirin. Ann Pharm Fr. 2017 Sep;75(5):344-348. [CrossRef]
- Tröger U, Stötzel B, Martens-Lobenhoffer J, Gollnick H, Meyer FP. Drug points: Severe myalgia from an interaction between treatments with pantoprazole and methotrexate. BMJ. 2002 Jun 22;324(7352):1497. [CrossRef]
- Uwai Y, Saito H, Inui K. Interaction between methotrexate and nonsteroidal anti-inflammatory drugs in organic anion transporter. Eur J Pharmacol. 2000 Dec 1;409(1):31-6. [CrossRef]
- Maeda A, Tsuruoka S, Kanai Y, Endou H, Saito K, Miyamoto E, Fujimura A. Evaluation of the interaction between nonsteroidal anti-inflammatory drugs and methotrexate using human organic anion transporter 3-transfected cells. Eur J Pharmacol. 2008 Oct 31;596(1-3):166-72. [CrossRef]
- Iwaki M, Shimada H, Irino Y, Take M, Egashira S. Inhibition of Methotrexate Uptake via Organic Anion Transporters OAT1 and OAT3 by Glucuronides of Nonsteroidal Anti-inflammatory Drugs. Biol Pharm Bull. 2017;40(6):926-931. [CrossRef]
- Uwai Y, Suzuki R, Iwamoto K. [Effect of nonsteroidal anti-inflammatory drugs on pharmacokinetics of methotrexate: a meta-analysis]. Yakugaku Zasshi. 2011;131(5):853-61. Japanese. [CrossRef]
- Dupuis LL, Koren G, Shore A, Silverman ED, Laxer RM. Methotrexate-nonsteroidal antiinflammatory drug interaction in children with arthritis. J Rheumatol. 1990 Nov;17(11):1469-73.
- Liang CA, Su YC, Lin SJ, Tsai TH. Risk factors for acute kidney injury after high-dose methotrexate therapy: a single-center study and narrative review. Eur J Clin Pharmacol. 2023 Jun;79(6):789-800. [CrossRef]
- Stewart CF, Fleming RA, Germain BF, Seleznick MJ, Evans WE. Aspirin alters methotrexate disposition in rheumatoid arthritis patients. Arthritis Rheum. 1991 Dec;34(12):1514-20. [CrossRef]
- Liegler DG, Henderson ES, Hahn MA, Oliverio VT. The effect of organic acids on renal clearance of methotrexate in man. Clin Pharmacol Ther. 1969 Nov-Dec;10(6):849-57. [CrossRef]
- Aherne GW, Piall E, Marks V, Mould G, White WF. Prolongation and enhancement of serum methotrexate concentrations by probenecid. Br Med J. 1978 Apr 29;1(6120):1097-9. [CrossRef]
- Lilly MB, Omura GA. Clinical pharmacology of oral intermediate-dose methotrexate with or without probenecid. Cancer Chemother Pharmacol. 1985;15(3):220-2. [CrossRef]
- El-Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Ther. 2007 Jan;320(1):229-35. [CrossRef]
- Maeda A, Tsuruoka S, Ushijima K, Kanai Y, Endou H, Saito K, Miyamoto E, Fujimura A. Drug interaction between celecoxib and methotrexate in organic anion transporter 3-transfected renal cells and in rats in vivo. Eur J Pharmacol. 2010 Aug 25;640(1-3):168-71. [CrossRef]
- Statkevich P, Fournier DJ, Sweeney KR. Characterization of methotrexate elimination and interaction with indomethacin and flurbiprofen in the isolated perfused rat kidney. J Pharmacol Exp Ther. 1993 Jun;265(3):1118-24.
- Thyss A, Milano G, Kubar J, Namer M, Schneider M. Clinical and pharmacokinetic evidence of a life-threatening interaction between methotrexate and ketoprofen. Lancet. 1986 Feb 1;1(8475):256-8. [CrossRef]
- Tracy TS, Krohn K, Jones DR, Bradley JD, Hall SD, Brater DC. The effects of a salicylate, ibuprofen, and naproxen on the disposition of methotrexate in patients with rheumatoid arthritis. Eur J Clin Pharmacol. 1992;42(2):121-5. [CrossRef]
- Dupuis LL, Koren G, Shore A, Silverman ED, Laxer RM. Methotrexate-nonsteroidal antiinflammatory drug interaction in children with arthritis. J Rheumatol. 1990 Nov;17(11):1469-73.
- Kremer JM, Petrillo GF, Hamilton RA. Pharmacokinetics and renal function in patients with rheumatoid arthritis receiving a standard dose of oral weekly methotrexate: association with significant decreases in creatinine clearance and renal clearance of the drug after 6 months of therapy. J Rheumatol. 1995 Jan;22(1):38-40.
- Takeda M, Khamdang S, Narikawa S, Kimura H, Hosoyamada M, Cha SH, Sekine T, Endou H. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Ther. 2002 Aug;302(2):666-71. [CrossRef]
- Najjar TA, Abou-Auda HS, Ghilzai NM. Influence of piperacillin on the pharmacokinetics of methotrexate and 7-hydroxymethotrexate. Cancer Chemother Pharmacol. 1998;42(5):423-8. [CrossRef]
- Iven H, Brasch H. The effects of antibiotics and uricosuric drugs on the renal elimination of methotrexate and 7-hydroxymethotrexate in rabbits. Cancer Chemother Pharmacol. 1988;21(4):337-42. [CrossRef]
- Iven H, Brasch H. Influence of the antibiotics piperacillin, doxycycline, and tobramycin on the pharmacokinetics of methotrexate in rabbits. Cancer Chemother Pharmacol. 1986;17(3):218-22. [CrossRef]
- Williams WM, Chen TS, Huang KC. Effect of penicillin on the renal tubular secretion of methotrexate in the monkey. Cancer Res. 1984 May;44(5):1913-7.
- Ronchera CL, Hernández T, Peris JE, Torres F, Granero L, Jiménez NV, Plá JM. Pharmacokinetic interaction between high-dose methotrexate and amoxycillin. Ther Drug Monit. 1993 Oct;15(5):375-9. [CrossRef]
- Titier K, Lagrange F, Péhourcq F, Moore N, Molimard M. Pharmacokinetic interaction between high-dose methotrexate and oxacillin. Ther Drug Monit. 2002 Aug;24(4):570-2. [CrossRef]
- Ueo H, Motohashi H, Katsura T, Inui K. Human organic anion transporter hOAT3 is a potent transporter of cephalosporin antibiotics, in comparison with hOAT1. Biochem Pharmacol. 2005 Oct 1;70(7):1104-13. [CrossRef]
- Takeda M, Babu E, Narikawa S, Endou H. Interaction of human organic anion transporters with various cephalosporin antibiotics. Eur J Pharmacol. 2002 Mar 8;438(3):137-42. Erratum in: Eur J Pharmacol. 2002 Aug 16;450(1):111. [CrossRef]
- Bubik RJ, Osmon DR, Oravec CP, Rivera CG. Two cases of severe neutropenia in patients on low-dose methotrexate and ceftriaxone. Am J Health Syst Pharm. 2019 May 17;76(11):804-809. [CrossRef]
- Jarfaut A, Santucci R, Levêque D, Herbrecht R. Severe methotrexate toxicity due to a concomitant administration of ciprofloxacin. Med Mal Infect. 2013 Jan;43(1):39-41. [CrossRef]
- Dalle JH, Auvrignon A, Vassal G, Leverger G. Interaction between methotrexate and ciprofloxacin. J Pediatr Hematol Oncol. 2002 May;24(4):321-2. [CrossRef]
- Aouinti I, Gaïes E, Trabelsi S, Salouage I, Jebabli N, Charfi R, Lakhal M, Klouz A. Delayed elimination of methotrexate in a patient receiving ciprofloxacin. Therapie. 2013 May-Jun;68(3):175-7. [CrossRef]
- Kamangar F, Berger TG, Fazel N, Koo JY. Methotrexate toxicity induced by ciprofloxacin leading to psoriatic plaque ulceration: a case report. Cutis. 2013 Sep;92(3):148-50.
- Urata S, Yoshikawa N, Saito K, Tazaki T, Ohno R, Takeshima H, Ikeda R. Delayed methotrexate elimination in a patient with primary central nervous system lymphoma: A case report. J Clin Pharm Ther. 2021 Dec;46(6):1796-1799. [CrossRef]
- Livertox® (www.livertox.nih.gov). Available online: www.swissmedicinfo.ch (accessed on 6 August 2023).
- Weiler S, Merz M, Kullak-Ublick GA. Drug-induced liver injury: the dawn of biomarkers? F1000Prime Rep. 2015 Mar 3;7:34. [CrossRef]
- Ortland I, Mirjalili M, Kullak-Ublick GA, Peymani P. Drug-induced liver injury in Switzerland: an analysis of drug-related hepatic disorders in the WHO pharmacovigilance database VigiBase from 2010 to 2020. Swiss Med Wkly. 2021 May 12;151:w20503. [CrossRef]
| Medication and Dosage | Dose Interval * | Remarks |
|---|---|---|
| Acetylsalicyclic acid 100 mg | 1-0-0-0 | |
| Atorvastatin 40 mg | 0-0-1-0 | |
| Bisoprolol 2.5 mg | 0.5-0-1-0 | |
| Escitalopram 10 mg | 1-0-0-0 | |
| Esomeprazole 40 mg | 2-0-0-0 | |
| Folic acid 5 mg | 1-0-0-0 | Saturdays at 8 am |
| Hydroxychloroquin 200 mg | 1-0-0-0 | |
| Iron, Folic acid 80/0.38 mg | 1-0-0-0 | before meals |
| Levothyroxin 0.05 mg | 1-0-0-0 | every other day |
| Methotrexat 15 mg | 1-0-0-0 | |
| Mirtazapin 30 mg | 0.5-0-0-0 | |
| Roflumilast 500 mcg | 0.5-0-0-0 | |
| Prednison 5 mg | 1.5-0-0-0 | |
| Temazepam 10 mg | 0-0-0-1 | |
| Torasemid 10 mg | 1-0-0-0 |
| Medication and Dosage | Dose Interval * | Remarks |
|---|---|---|
| Atorvastatin 40 mg | 0-0-1-0 | |
| Betamethasone 0.5 mg | 1-1-1-1 | started on admission day |
| Bisoprolol 2.5 mg | 0.5-0-1-0 | |
| Cholecalciferol 4000 EI/ml | 1-0-0-0 | started on day 4 |
| Chlorhexidine, Lidocaine 2 mg/ml | 1-1-1-1 | started on admission day |
| Dalteparin 5000 EI | 0-0-1-0 | started on admission day |
| Escitalopram 10 mg | 1-0-0-0 | |
| Esomeprazole 40 mg | 1-0-0-0 | |
| Folic acid 5 mg | 1-0-0-0 | 1 day before and after MTX |
| Hydroxychloroquin 200 mg | 1-0-0-0 | |
| Isopropanol, Isopropylalcohol 0.2 % | 1-1-1-1 | started on admission day |
| Levothyroxin 0.05 mg | 1-0-0-0 | every other day |
| Magnesium 10 mmol | 0-1-0-0 | started on admission day |
| Metamizol 500 mg | 2-2-2-2 | started on admission day |
| Methotrexat 15 mg | 1-0-0-0 | Fridays at 8 am |
| Mirtazapin 30 mg | 0.5-0-0-0 | |
| Oxycodon, Naloxone 15/7.5 mg | 1-0-1-0 | started on admission day |
| Paracetamol 500 mg | 1-1-1-1 | started on admission day |
| Potassium chloride 10 mmol | 2-2-2-0 | started on admission day |
| Roflumilast 500 mcg | 0.5-0-0-0 | |
| Prednison 5 mg | 1.5-0-0-0 | |
| Temazepam 10 mg | 0-0-0-1 | |
| Torasemid 10 mg | 1-0-0-0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





