Submitted:
08 February 2024
Posted:
12 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
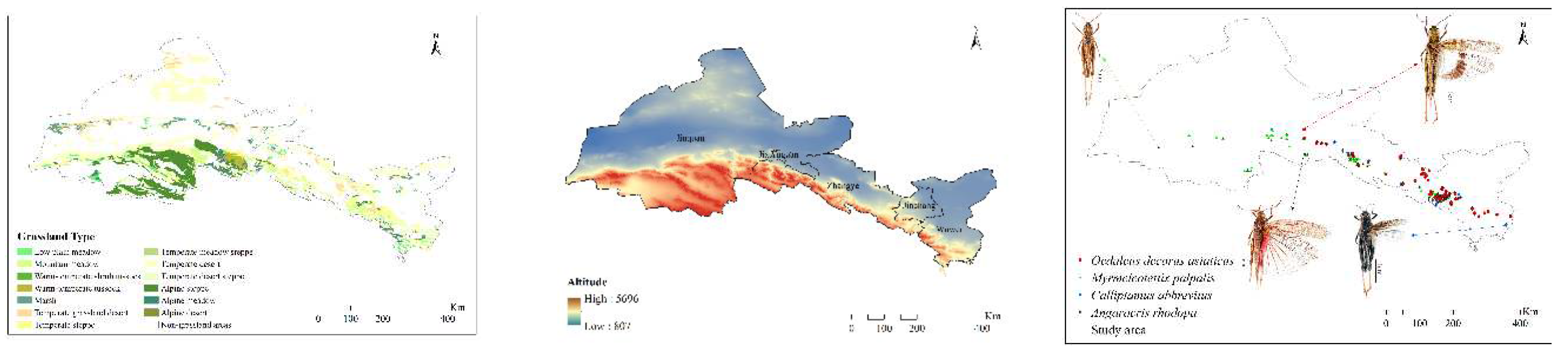
2.1. Study Area
2.2. Data Acquisition and Processing
2.2.1. Grasshopper Survey Data
2.2.2. Environmental Variables
| Type | Code | Variable name |
| Climatic | Bio1 | Annual mean temperature |
| Bio2 | Mean diurnal range (monthly mean (max temp minus min temp)) | |
| Bio3 | Isother mality (BIO2/BIO7) (×100) | |
| Bio4 | Temperature seasonality (standard deviation×100) | |
| Bio5 | Max temperature of warmest month | |
| Bio6 | Min temperature of coldest month | |
| Bio7 | Temperature annual range (BIO5 minus BIO6) | |
| Bio8 | Mean temperature of wettest quarter | |
| Bio9 | Mean temperature of driest quarter | |
| Bio10 | Mean temperature of warmest quarter | |
| Bio11 | Mean temperature of coldest quarter | |
| Bio12 | Annual precipitation | |
| Bio13 | Precipitation of wettest month | |
| Bio14 | Precipitation of driest month | |
| Bio15 | Precipitation seasonality (coefficient of variation) | |
| Bio16 | Precipitation of wettest quarter | |
| Bio17 | Precipitation of driest quarter | |
| Bio18 | Precipitation of warmest quarter | |
| Bio19 | Precipitation of coldest quarter | |
| LST | Land surface temperature | |
| Vegetation | NDVI | Normalized difference vegetation index |
| GT | Grassland type | |
| Topographical | Elevation | Elevation |
| Slop | Slop | |
| Aspect | Aspect | |
| Soil | AWC | Soil available water content |
| ST | Soil type | |
| PH | T_PH | |
| Human activity | HFP | Human Footprint Index |
2.2.3. Environment Variable De-correlation
2.3. MaxEnt Model Runs
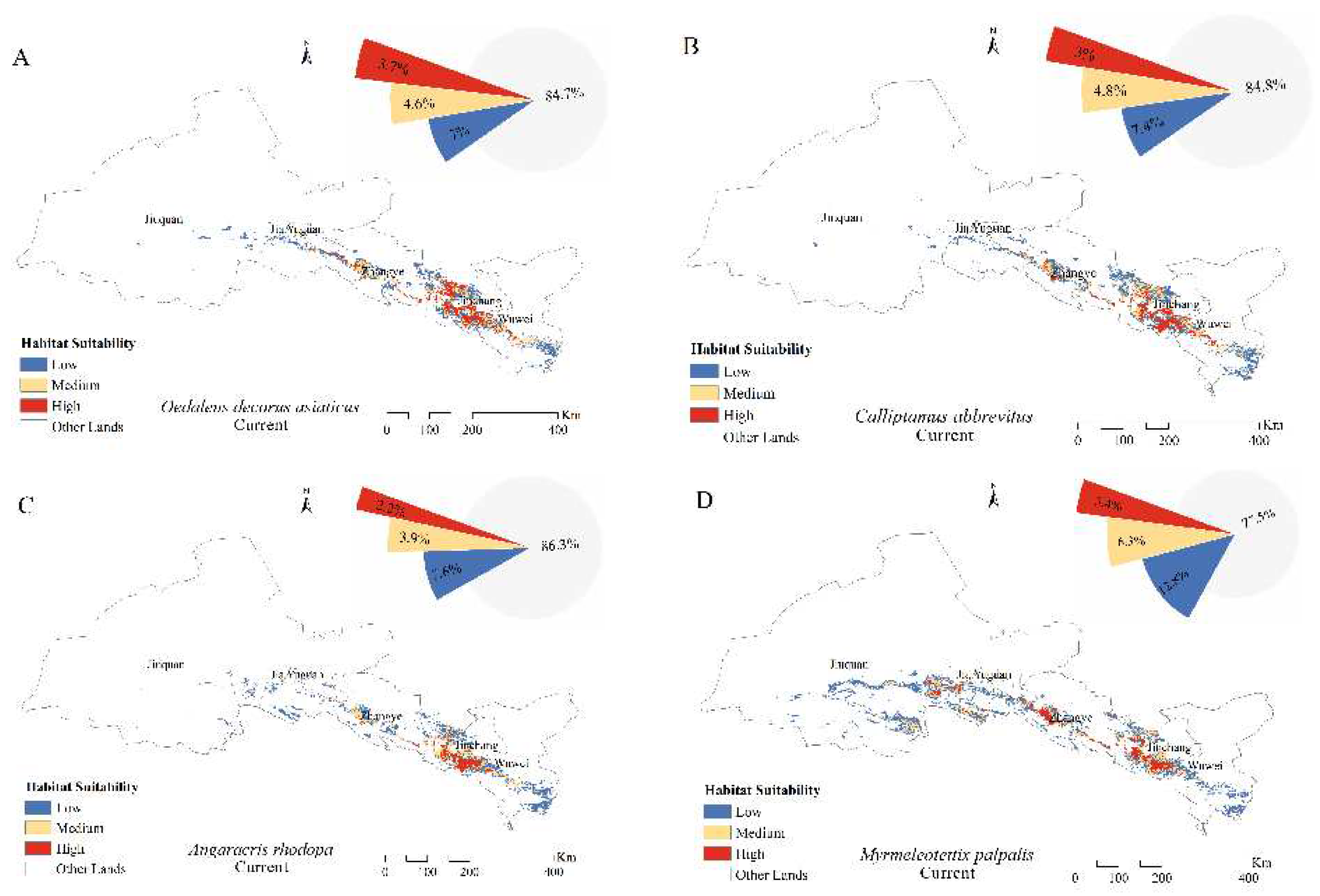
3. Results
3.1. Accuracy of the MaxEnt Model
3.2. Effects of Major Environmental Variables on the Distribution of Grasshoppers
| O. decorus asiaticus | C. abbreviatus | A. rhodopa | M. palpalis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| variables | Percent contribution (%) | Cumulative contribution rate (%) |
Variables | Percent contribution (%) | Cumulative contribution rate (%) |
Variables | Percent contribution (%) | Cumulative contribution rate (%) |
Variables | Percent contribution (%) | Cumulative contribution rate (%) |
| Bio12 | 33.6 | 33.6 | Bio12 | 33.5 | 33.5 | Bio12 | 35.5 | 35.5 | NDVI | 31.8 | 31.8 |
| AWC | 18.8 | 52.4 | HFP | 19.9 | 53.4 | NDVI | 30.3 | 65.8 | HFP | 12.4 | 44.2 |
| NDVI | 18.6 | 71 | AWC | 15.9 | 69.3 | Bio7 | 8.5 | 74.3 | Bio12 | 9.8 | 54 |
| Bio7 | 9.3 | 80.3 | NDVI | 9.7 | 79 | HFP | 5.8 | 80.1 | Bio1 | 9.5 | 63.5 |
| HFP | 8.9 | 89.2 | Bio7 | 7.3 | 86.3 | AWC | 5.4 | 85.5 | Bio2 | 8.2 | 71.7 |
| Slop | 3.8 | 93 | Slop | 4.8 | 91.1 | Bio15 | 4.1 | 89.6 | Bio7 | 8.1 | 79.8 |
| Bio15 | 2.8 | 95.8 | Bio3 | 2.7 | 93.8 | Slop | 2.8 | 92.4 | Slop | 5.9 | 85.7 |
| Elevation | 1.5 | 97.3 | Bio15 | 2 | 95.8 | LST | 2.5 | 94.9 | Bio19 | 5.6 | 91.3 |
| Bio2 | 0.9 | 98.2 | Elevation | 1.3 | 97.1 | Bio2 | 1.9 | 96.8 | GT | 2.9 | 94.2 |
| Bio1 | 0.8 | 99 | Bio1 | 0.9 | 98 | Aspect | 1.1 | 97.9 | Aspect | 1.8 | 96 |
| Aspect | 0.4 | 99.4 | Aspect | 0.7 | 98.7 | Bio1 | 0.7 | 98.6 | AWC | 1.4 | 97.4 |
| GT | 0.2 | 99.6 | GT | 0.5 | 99.2 | GT | 0.5 | 99.1 | ST | 0.9 | 98.3 |
| Bio19 | 0.2 | 99.8 | Bio2 | 0.3 | 99.5 | Bio19 | 0.4 | 99.5 | LST | 0.6 | 98.9 |
| ST | 0.1 | 99.9 | ST | 0.2 | 99.7 | Bio4 | 0.2 | 99.7 | Elevation | 0.6 | 99.5 |
| PH | 0.1 | 100 | PH | 0.1 | 99.8 | ST | 0.1 | 99.8 | Bio14 | 0.3 | 99.8 |
| LST | 0 | 100 | LST | 0.1 | 99.9 | Elevation | 0.1 | 99.9 | PH | 0.1 | 99.9 |
| Bio4 | 0.1 | 100 | PH | 0.1 | 100 | Bio4 | 0.1 | 100 | |||
3.3. Distribution and Size of Suitable Areas for Grasshoppers in the Current Climate
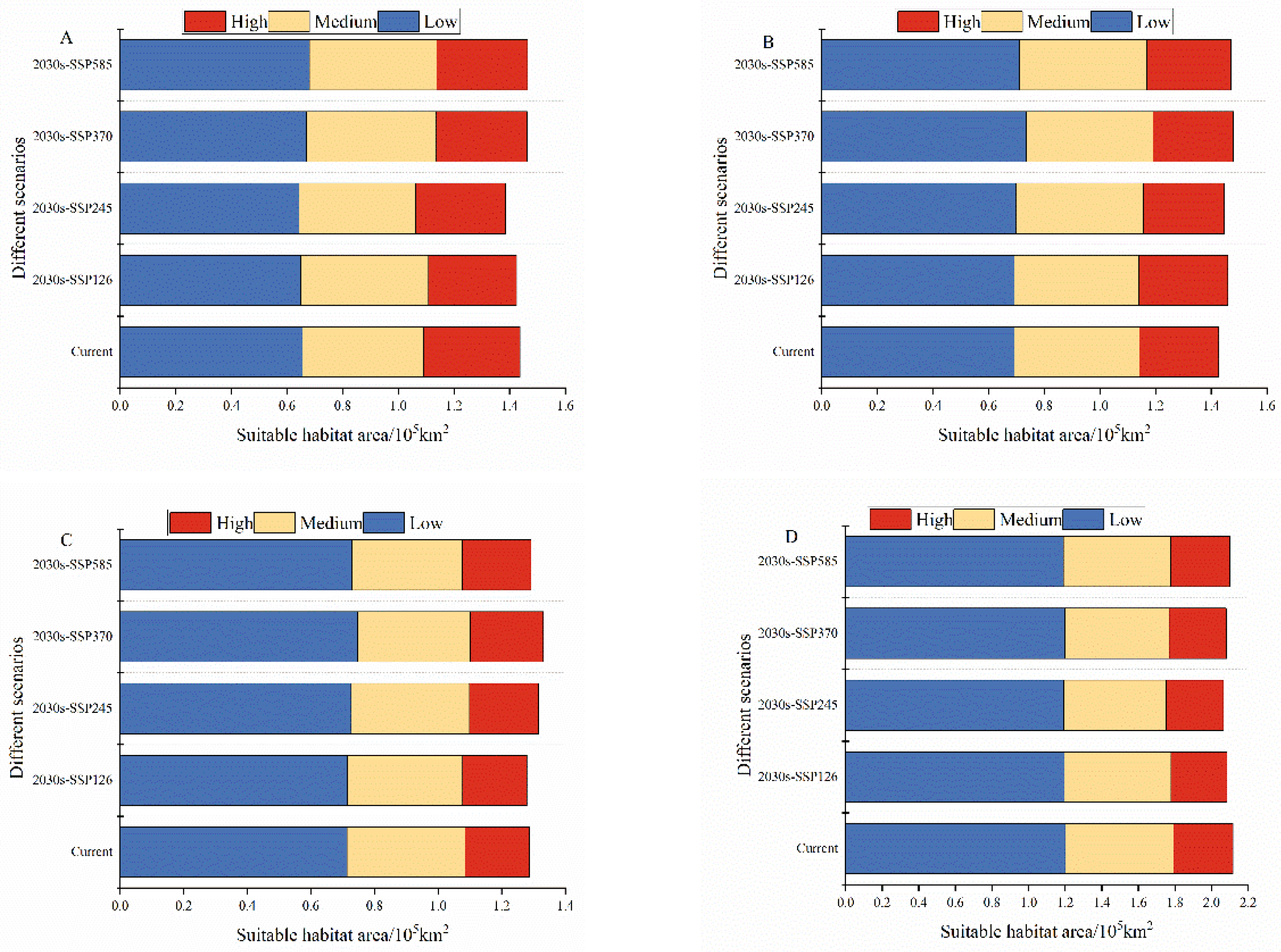
3.4. Potential Distribution of Grasshoppers under Future Climates
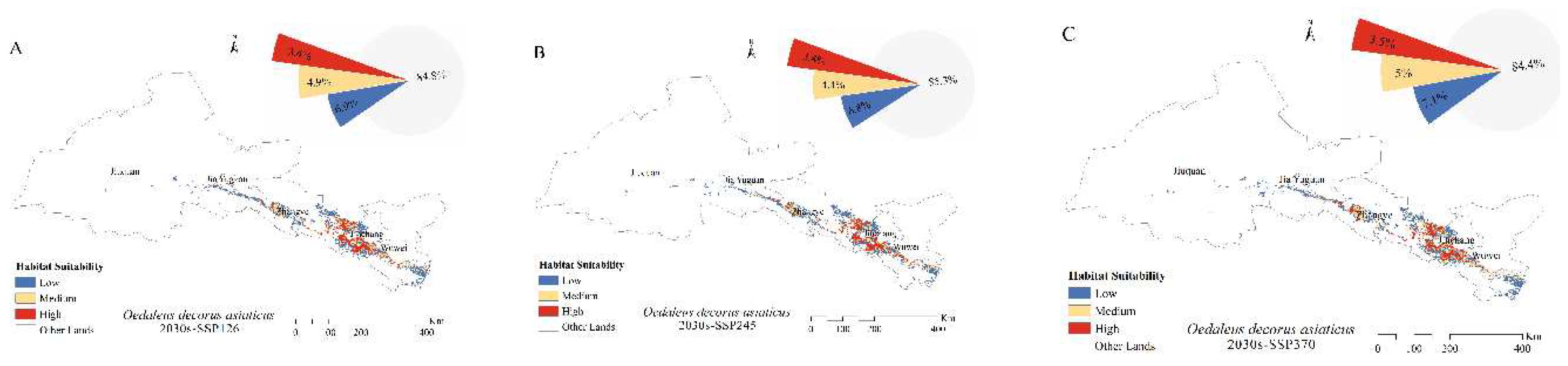
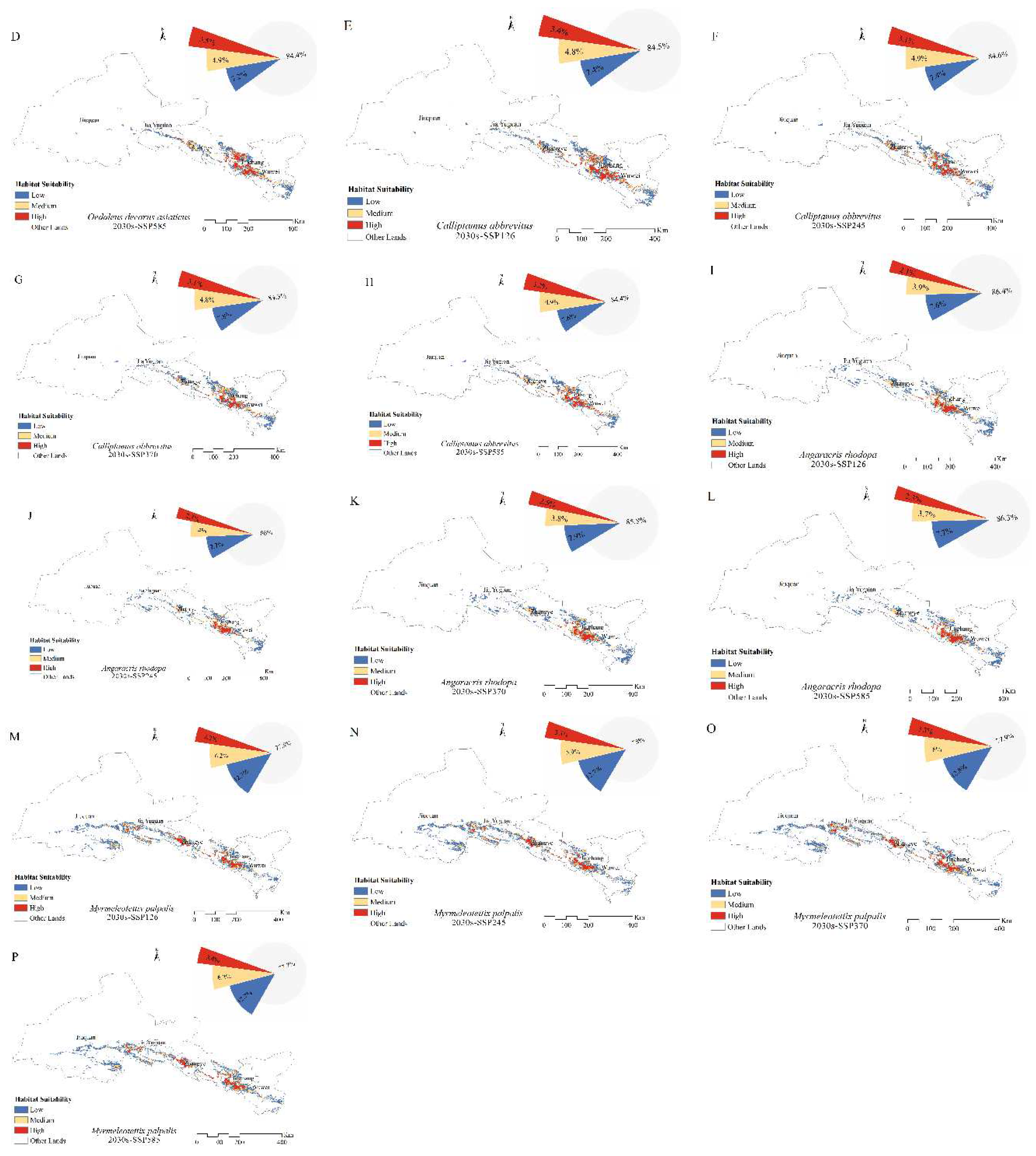
4. Discussion
4.1. Selection of Distribution Points and Variables for the MaxEnt Model
4.2. Influence of Environmental Variables on Grasshopper Distribution
4.3. Changes in the Distribution of Grasshopper Habitat Areas under Future Climate Scenarios
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teng, Y.M.; Wang, C.; Wei, X.Q.; Su, M.R.; Zhan, J.Y.; Wen, L.X. Characteristics of vegetation change and its climatic and anthropogenic driven pattern in the Qilian Mountains. Forests. 2023, 14, 1951. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, X.Y.; Zhao, C.Y.; Wang, H.; Zang, F. The trade-offs and synergies of the ecological-production-living functions of grassland in the Qilian Mountains by ecological priority. J. Environ. Manage. 2023, 327, 116883. [Google Scholar] [CrossRef]
- Yang, L.; Jia, W.X.; Shi, Y.; Zhang, Z.Y.; Xiong, H.; Zhu, G.F. Spatiotemporal differentiation of soil organic carbon of grassland and its relationship with soil physicochemical properties on the northern slope of Qilian Mountains, China. Sustainability. 2020, 12, 9396–9396. [Google Scholar] [CrossRef]
- Yang, X.T.; Fan, J.; Ge, J.M.; Du, M.G.; Jin, M. Soil physical and chemical properties and vegetation characteristics of different types of grassland in Qilian Mountains, China. J Appl Ecol. 2022, 33, 878–886. [Google Scholar] [CrossRef]
- Zhao, Z.; He, Y.; Li, Q.; Jia, S.F.; Yang, P.Y.; Jin, C.F.; Yang, X.L.; Sun, X.Y.; Li, J.; Gu, Z.L.; Qu, Y.; Zhang, W.J.; Qiao, Y.S.; Wang, J.; Yang, S.L. Investigation of grassland resources in Sunan Yugur Autonomous County. Acta Prataculturae Sin. 2010, 19, 231–247. [Google Scholar]
- Sun, T.; Long, R.J.; Liu, Z.Y. A comparative study of grasshopper species (Orthoptera:Acridoidea) diversity in different grasslands in the northern slopes of Qilian Mountains. Acta Entomologica Sin. 2010, 53, 702–707. [Google Scholar] [CrossRef]
- Hao, H.W.; Bao, M.; Ke, J.; Li, L.L.; Ma, C.X.; Dan, Z.C.; Chen, Z.N. Fauna elements and eco-geographical distribution of locus in Qinghai province. J Biol. 2019, 36, 62–68. [Google Scholar] [CrossRef]
- Sun, T.; Liu, Z. Y.; Qin, L.P.; Long, R. J. Grasshopper (Orthoptera: Acrididae) community composition in the rangeland of the northern slopes of the Qilian Mountains in northwestern China. J. Insect Sci. 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Li. L.L.; Zhao, C.Z.; Zhao, X.W.; Wang, D.W.; Li, Y. Pattern of plant communities' influence to grasshopper abundance distribution in heterogeneous landscapes at the upper reaches of Heihe River, Qilian Mountains, China. Environ. Sci. Pollut. Res. Int. 2021, 29, 13177–13187. [Google Scholar] [CrossRef]
- Li, L.L.; Zhao, C.Z.; Yin, C.Q.; Wang, D.W.; Zhang, J.X. Species richness of grasshoppers ( Orthoptera: Acrididae ) on natural grasslands in relation with topography in the upper reaches of Heihe River,western China analyzed with generalized additive models (GAMs). Acta Entomologica Sin. 2011, 54, 1312–1318. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, C.Z.; Wang, K.M. Distribution characteristics of grasshoppers in the Black River Basin and their relationship with habitats. J Arid Land. 2010, 24, 147–152. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Yu, H.Y.; Chen, W.; Li, M.; Yi, S.H.; Meng, B.P. Predicting inhabitable areas for locust based on field observation and multi-environmental factors in alpine grassland—A case study in the Qilian Mountain National Park, China. Front. Ecol. Evol. 2023, 11, 1149952. [Google Scholar] [CrossRef]
- Xin, B.; Dang, Y.Q.; Wang, X.Y.; Yang, Z.Q. Mechanisms and influential factors of southern limits in insect. Acta Ecologica Sin. 2019, 39, 9379–9386. [Google Scholar]
- Wang, B.; Deveson, E. D.; Waters, C.; Spessa, A.; Lawton, D.; Feng, P.Y.; Li, D.L. Future climate change likely to reduce the Australian plague locust (Chortoicetes terminifera) seasonal outbreaks. Sci. Total Environ. 2019, 668, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Rahman, S.; Alam, S. Modeling current and future potential distributions of desert locust Schistocerca gregaria (Forskål) under climate change scenarios using MaxEnt. J Asia-Pacific Biodiversity. 2021, 14, 399–409. [Google Scholar] [CrossRef]
- Macfadyen, S.; McDonald, G.; Hill, M.P. From species distributions to climate change adaptation: knowledge gaps in managing invertebrate pests in broad-acre grain crops. Agric., Ecosyst. Environ. 2018, 253, 208–219. [Google Scholar] [CrossRef]
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Shabani, F. Suitable areas of Phakopsora pachyrhizi, Spodoptera exigua, and their host plant Phaseolus vulgaris are projected to reduce and shift due to climate change. Theor. Appl. Climatol. 2018, 135, 409–424. [Google Scholar] [CrossRef]
- Shen, Z.H.; Zhao, J. Prediction of spatial patterns of species richness based on plant-topography relationships: An application of GAMs approch. Acta Ecologica Sin. 2007, 953–963. [Google Scholar]
- Wood, S.N. mgcv: GAMs and generalized ridge regression for R. R news. 2001, 1, 20–25. [Google Scholar]
- Isley, F.B. The relations of Texas Acrididae to plants and soils. Ecol. Monogr. 1938, 8, 553–604. [Google Scholar] [CrossRef]
- Miao, H. T.; Liu, Y.; Shan, L. Y.; Wu, G. L. Linkages of plant-soil interface habitat and grasshopper occurrence of typical grassland ecosystem. Ecol. Indic. 2018, 90, 324–333. [Google Scholar] [CrossRef]
- VanDyke, K. A.; Latchininsky, A. V.; Schell, S. P. Importance of ecological scale in montane grasshopper (Orthoptera: Acrididae) species structure in similar habitat between differing soil textures and dominant vegetative canopy coverage. J Orthoptera Research. 2009, 18, 215–223. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, N.; He, B.; Liu, C.Y.; Li, Y.; Zhang, H.Y.; Chen, X.Y.; Lin, H. Construction of a GeogDetector-based model system to indicate the potential occurrence of grasshoppers in Inner Mongolia steppe habitats. Bull. Entomol. Res. 2015, 105, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Zhao, C.Z.; Yin, C.Q.; Wang, D.W.; Zhang, J.X. Grasshopper (Orthoptera: Acrididae) density on natural grasslands in upper reaches of Heihe River, Northwest China in relation with topography: An analysis with generalized additive models ( GAM). J Ecology. 2012, 31, 3121–3126. [Google Scholar] [CrossRef]
- Bernays, E.A.; Gonzalez, N.; Angel, J.; Bright, K.L. Food mixing by generalist grasshoppers: Plant secondary compounds structure the pattern of feeding. J. Insect Behav. 1994, 8, 161–180. [Google Scholar] [CrossRef]
- Branson, D.H. Influence of a large late summer precipitation event on food limitation and grasshopper population dynamics in a northern great plains grassland. Environ. Entomol. 2008, 37, 686–695. [Google Scholar] [CrossRef]
- Anderson, R.P.; Raza, A. The effect of the extent of the study region on GIS models of species geographic distributions and estimates of niche evolution: preliminary tests with montane rodents (genus Nephelomys) in Venezuela. J Biogeogr. 2010, 37, 1378–1393. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Wang, T.L.; Wang, G.Y.; Innes, J.; Nitschke, C.; Kang, H.J. Climatic niche models and their consensus projections for future climates for four major forest tree species in the Asia–Pacific region. For. Ecol. Manage. 2016, 360, 357–366. [Google Scholar] [CrossRef]
- Leng, W.F.; He, H. S.; Liu, H.J. Response of larch species to climate changes. J. Plant Ecol. 2008, 1, 203–205. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Modell. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Hirzel, A. H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: how to compute habitat-suitability maps without absence data? Ecol. 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Busby, J.R. BIOCLIM: a bioclimate analysis and prediction system. Plant Prot. Q. 1991, 6, 8–9. [Google Scholar]
- Zhang, Z. D.; Zang, R. G.; Convertino, M. Predicting the distribution of potential natural vegetation based on species functional groups in fragmented and species-rich forests. Plant Ecol Evol. 2013, 146, 261–271. [Google Scholar] [CrossRef]
- Yang, X. Q.; Kushwaha, S. P. S.; Saran, S.; Xu, J.C.; Roy, P. S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Yi, Y. J.; Cheng, X.; Yang, Z. F.; Zhang, S. H. Maxent modeling for predicting the potential distribution of endangered medicinal plant (H. riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Bai, D.F.; Chen, P.J.; Atzeni, L.; Cering, L.; Li, Q.; Shi, K. Assessment of habitat suitability of the snow leopard (Panthera uncia) in Qomolangma National Nature Reserve based on MaxEnt modeling. Zool. Res. 2018, 39, 373–386. [Google Scholar] [CrossRef]
- Wu, T.T.; Pan, C.T.; Bian, T.; Wang, Q.X.; Kou, J.; Zhou, B.W. Response of a sylvan moss species (Didymodon validus Limpr.) with a narrow distribution range to climate change. Forests. 2023, 14, 2227. [Google Scholar] [CrossRef]
- Hu, J.H.; Liu, Y. Unveiling the conservation biogeography of a data-deficient endangered bird species under climate change. PLoS One. 2014, 9, e84529. [Google Scholar] [CrossRef]
- Puchałka, R.; Dyderski, M.K.; Vítková, M.; Sádlo, J.; Klisz, M.; Netsvetov, M.; Prokopuk, Y.; Matisons, R.; Mionskowski, M.; Wojda, T.; Koprowski, M.; Jagodziński, A.M. Black locust (Robinia pseudoacacia L.) range contraction and expansion in Europe under changing climate. Global Change Biol. 2021, 27, 1587–1600. [Google Scholar] [CrossRef]
- Cui, L.N.; Berger, U.; Cao, M.M.; Zhang, Y.Q.; He, J.M.; Pan, L.H.; Jiang, J. Conservation and restoration of mangroves in response to invasion of spartina alterniflora based on the MaxEnt Model: a case study in China. Forests. 2023, 14, 1220. [Google Scholar] [CrossRef]
- Wang, X. ;Jiang, Y.Y.; Wu, W.P.; He, X.Z.; Wang, Z.H.; Guan, Y.Y.; Xu, N.; Chen, Q.L.; Shen, Y.J.; Cao, J.P. Cryptosporidiosis threat under climate change in China: prediction and validation of habitat suitability and outbreak risk for human-derived Cryptosporidium based on ecological niche models. Infect dis poverty. 2023, 12, 35. [Google Scholar] [CrossRef]
- Cao, B.; Bai, C.K.; Wu, K.Y.; La, T.; Su, Y.Y.; Che, L.Y.; Zhang, M.; Lu, Y.M.; Gao, P.F.; Yang, J.J.; Xue, Y.; Li, G.S. Tracing the future of epidemics: coincident niche distribution of host animals and disease incidence revealed climate-correlated risk shifts of main zoonotic diseases in China. Global Change Biol. 2023, 29, 3723–3746. [Google Scholar] [CrossRef]
- Wang, L.Y.; Hu, W.B.; Soares Magalhaes, R.J.; Bi, P.; Ding, F.; Sun, H.L.; Li, S.L.; Yin, W.W.; Wei, L.; Liu, Q.Y.; Haque, U.; Sun, Y.S.; Huang, L.Y.; Tong, S.L.; Clements, A.C.; Zhang, W.Y.; Li, C.Y. The role of environmental factors in the spatial distribution of Japanese encephalitis in mainland China. Environ int. 2014, 73, 1–9. [Google Scholar] [CrossRef]
- Guo, J.; Lu, L.H.; Dong, Y.Y.; Huang, W.J.; Zhang, B.; Du, B.B.; Ding, C.; Ye, H.C.; Wang, K.; Huang, Y.R.; Hao, Z.Q.; Zhao, M.X.; Wang, N. Spatiotemporal distribution and main influencing factors of grasshopper potential habitats in two steppe types of inner mongolia, China. Remote Sens. 2023, 15, 866. [Google Scholar] [CrossRef]
- Wen, F.; Lu, L.H.; Nie, C.J.; Sun, Z.X.; Liu, R.H.; Huang, W.J.; Ye, H.C. Analysis of spatiotemporal variation in habitat suitability for Oedaleus decorus asiaticus Bei-Bienko on the Mongolian Plateau using Maxent and multi-source remote sensing data. Insects. 2023, 14, 492. [Google Scholar] [CrossRef] [PubMed]
- Du, B.B.; Wei, J.; Lin, K.J.; Lu, L.H.; Ding, X.L.; Ye, H.C.; Huang, W.J.; Wang, N. Spatial and temporal variability of grassland grasshopper habitat suitability and its main influencing factors. Remote Sens. 2022, 14, 3910. [Google Scholar] [CrossRef]
- Zhang, X.W.; Huang, W.J.; Ye, H.C.; Lu, L.H. Study on the identification of habitat suitability areas for the dominant locust species Dasyhippus barbipes in inner Mongolia. Remote Sens. 2023, 15, 1718. [Google Scholar] [CrossRef]
- Zheng, S.L. Natural grassland resources and their evaluation in Gansu Hexi Corridor. Chin. J. Grassland. 1989, 5–10. [Google Scholar]
- Yang, L.; Jia, W.X.; Shi, Y.; Zhang, Z.Y.; Xiong, H.; Zhu, G.F. Spatiotemporal differentiation of soil organic carbon of grassland and its relationship with soil physicochemical properties on the northern slope of Qilian Mountains, China. Sustainability. 2020, 12, 9396. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Jia, W.X.; Zhao, Y.F.; Liu, Y.R.; Zhao, Z.; Chen, J.H. Spatio-temporal variations of net primary productivity of Qilian Mountains vegetation based on CASA model. Acta Bot. Boreali-Occident. Sin. 2014, 34, 2085–2091. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, T.; Chen, N.L. Vertical distribution patterns of soil organic carbon and total nitrogen and related affecting factors along northern slope of Qilian Mountains. J. Appl. Ecol. 2009, 20, 518–524. [Google Scholar]
- Dong, R.; Hua, L. M.; Hua, R.; Ye, G. H.; Bao, D.; Cai, X. C.; Cai, B.; Zhao, X.C.; Chu, B.; Tang, Z. S. Prediction of the potentially suitable areas of Ligularia virgaurea and Ligularia sagitta on the Qinghai–Tibet Plateau based on future climate change using the MaxEnt model. Front Plant Sci. 2023, 14, 1193690. [Google Scholar] [CrossRef]
- Hu, J.H.; Liu, Y. Unveiling the conservation biogeography of a data-deficient endangered bird species under climate change. PLoS One. 2014, 9, e84529. [Google Scholar] [CrossRef] [PubMed]
- Naudiyal, N.; Wang, J.N.; Ning, W.; Gaire, N. P.; Shi, P.L.; Wei, Y.Q.; He, J.L.; Shi, N. Potential distribution of Abies, Picea, and Juniperus species in the sub-alpine forest of Minjiang headwater region under current and future climate scenarios and its implications on ecosystem services supply. Ecol. Indic. 2021, 121, 107131. [Google Scholar] [CrossRef]
- Guo, Y.L.; Wei, H.Y.; Lu, C.Y.; Gao, B.; Gu, W. Predictions of potential geographical distribution and quality of schisandra sphenanthera under climate change. PeerJ. 2016, 4, e2554. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S. J.; Anderson, R. P.; Schapire, R. E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Fang, Y.Q.; Zhang, X.H.; Wei, H.Y.; Wang, D.J.; Chen, R.D.; Wang, L.K.; Gu, W. Predicting the invasive trend ofexotic plants in China based on the ensemble model under climate change: a case for three invasive plants of asteraceae. Sci. Total Environ. 2021, 756, 143841. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Huang, Y.; Jiang, X.; Chen, H.; Liu, M.; Wang, R.L. Potential geographical distribution of the edangred plant Isoetes under human activities using MaxEnt and GARP. Glob Ecol Conserv. 2022, 38, e02186. [Google Scholar] [CrossRef]
- He, F.L.; Legendre, P.; LaFrankie, J. Spatial pattern of diversity in a tropical rain forest in Malaysia. J Biogeogr. 1996, 23, 57–74. [Google Scholar] [CrossRef]
- Ge, F. Challenges facing entomologists in a changing global climate. J App Entomol. 2011, 48, 1117–1122. [Google Scholar]
- Zhao, Y.D.; Wu, F.S.; Liu, Y.; Wu, M.H.; Wang, S.J.; Sun, H. J.; Liu, G.X.; Zhang, Y.Y.; Cui, X.W.; Zhang, W.; Chen, T.; Zhang, G.S. The distribution and influencing factors of hypolithic microbial communities in the Hexi Corridor. Microorganisms. 2023, 11, 1212. [Google Scholar] [CrossRef]
- Tian, H.D.; Stige, L. C.; Cazelles, B.; Kausrud, K. L.; Svarverud, R. , Stenseth, N. C.; Zhang, Z.B. Reconstruction of a 1,910-y-long locust series reveals consistent associations with climate fluctuations in China. Proc. Natl. Acad. Sci. 2011, 108, 14521–14526. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. C. Study on long-term prediction of locust population fluctuations. Acta entomologica sin. 1965, 14, 319–338. [Google Scholar]
- Yamashita, O.; Yaginuma, T.; Hasegawa, K. Hormonal and metabolic control of egg diapause of the silkworm, Bombyx mori (Lepidoptera: Bombycidae). Entomol. Gen. 1981, 7, 195–211. [Google Scholar] [CrossRef]
- Leonard, A.; Egonyu, J. P.; Tanga, C. M.; Kyamanywa, S.; Tonnang, H. Z.; Azrag, A. G.; Khamis, F. M.; Ekesi, S.; Subramanian, S. Predicting the current and future distribution of the edible long-horned grasshopper Ruspolia differens (Serville) using temperature-dependent phenology models. J. Therm. Biol. 2021, 95, 102786. [Google Scholar] [CrossRef]
- Knop, E.; Schmid, B.; Herzog, F. Impact of regional species pool on grasshopper restoration in hay meadows. Restoration Ecology. 2008, 16, 34–38. [Google Scholar] [CrossRef]
- Yu, F. Vegetation growth based on MODIS and the relationship between vegetation and grasshoppers of grasshoppers plague areas in Altay. Xinjiang Normal University, 2008.
- Yang, Y.H.; Rao, S.; Hu, H.F.; Chen, A.P.; Ji, C.J.; Zhu, B.; Zuo, W.Y.; Li, X.R.; Shen, H.H.; Wang, Z.H.; Tang, Y.H.; Fang, J.Y. Plant species richness of alpine grasslands in relation to environmental factors and biomass on the Tibetan Plateau. Biodiversity. 2004, 12, 200–205. [Google Scholar] [CrossRef]
- Zhao, H.T.; Liu, T.; Lei, J.Q.; Gui, D.E.; Zhao, X.Q. β diversity characteristic of vegetation community on south part of Gurbantunggut Desert and its interpretation. Acta Prataculturae Sin. 2010, 19, 29–37. [Google Scholar]
- Austin, M. P. Searching for a model for use in vegetation analysis. Vegetatio. 1980, 42, 11–21. [Google Scholar] [CrossRef]
- Shi, R.X.; Liu, G.; Li, D.M.; Xie, B.Y. Distribution of Locusts migratoria and soil in the locust plague area at Baiyangdian. J. Appl. Entomol. 2004, 29–33. [Google Scholar]
- Wu, T.J.; Hao, S.G.; Kang, L. Effects of soil temperature and moisture on the development and survival of grasshopper eggs in inner mongolian grasslands. Front. Ecol. Evol. 2021, 9, 727911. [Google Scholar] [CrossRef]
- Zhong, Z.W.; Wang, D.L.; Zhu, H.; Wang, L.; Feng, C.; Wang, Z.N. Positive interactions between large herbivores and grasshoppers, and their consequences for grassland plant diversity. Ecology. 2014, 95, 1055–1064. [Google Scholar] [CrossRef]
- Pener, M. P.; Simpson, S. J. Locust phase polyphenism: an update. Adv. Insect Physiol. 2009, 36, 1–272. [Google Scholar] [CrossRef]
- Hao, S.; Wang, S.; Cease, A.; Knag, L. Landscape level patterns of grasshopper communities in Inner Mongolia: Interactive effects of livestock grazing and a precipitation gradient. Landscape Ecol. 2015, 30, 1657–1668. [Google Scholar] [CrossRef]
- Cease, A.J; Elser, J.J.; Ford, C.F.; Hao, S.G.; Kang, L.; Harrison, J.F. Heavy livestock grazing promotes locust outbreaks by lowering plant nitrogen content. Science. 2012, 335, 467–469. [Google Scholar] [CrossRef]
- Zhang, L.; Hunter, D. M. Management of locusts and grasshoppers in China. Journal of Orthoptera Research. 2017, 26, 155–159. [Google Scholar] [CrossRef]
- TANAKA, H. Embryonic diapause and life cycle in the migratory locust, Locusta migratoria L. (Orthoptera: Acrididae), in Kyoto. Appl. Entomol. Zool. 1994, 29, 179–191. [Google Scholar] [CrossRef]
- Meynard, C. N.; Gay, P. E.; Lecoq, M.; Foucart, A.; Piou, C.; Chapuis, M. P. Climate-driven geographic distribution of the desert locust during recession periods: Subspecies’ niche differentiation and relative risks under scenarios of climate change. Global Change Biol. 2017, 23, 4739–4749. [Google Scholar] [CrossRef] [PubMed]
| Time | Emission scenarios | O. decorus asiaticus | C. abbreviatus | A. rhodopa | M. palpalis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Training AUC | Test AUC | Training AUC | Test AUC | Training AUC | Test AUC | Training AUC | Test AUC | ||
| Current | 0.971 | 0.946 | 0.970 | 0.949 | 0.972 | 0.958 | 0.954 | 0.929 | |
| 2021-2040 | SSP126 | 0.973 | 0.947 | 0.971 | 0.944 | 0.972 | 0.953 | 0.955 | 0.932 |
| SSP245 | 0.974 | 0.950 | 0.967 | 0.942 | 0.973 | 0.955 | 0.956 | 0.933 | |
| SSP370 | 0.974 | 0.951 | 0.972 | 0.943 | 0.972 | 0.952 | 0.956 | 0.931 | |
| SSP585 | 0.972 | 0.946 | 0.970 | 0.946 | 0.972 | 0.954 | 0.956 | 0.929 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





