Submitted:
12 February 2024
Posted:
13 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Greenhouse Trial
2.2. Field Trial
3. Results
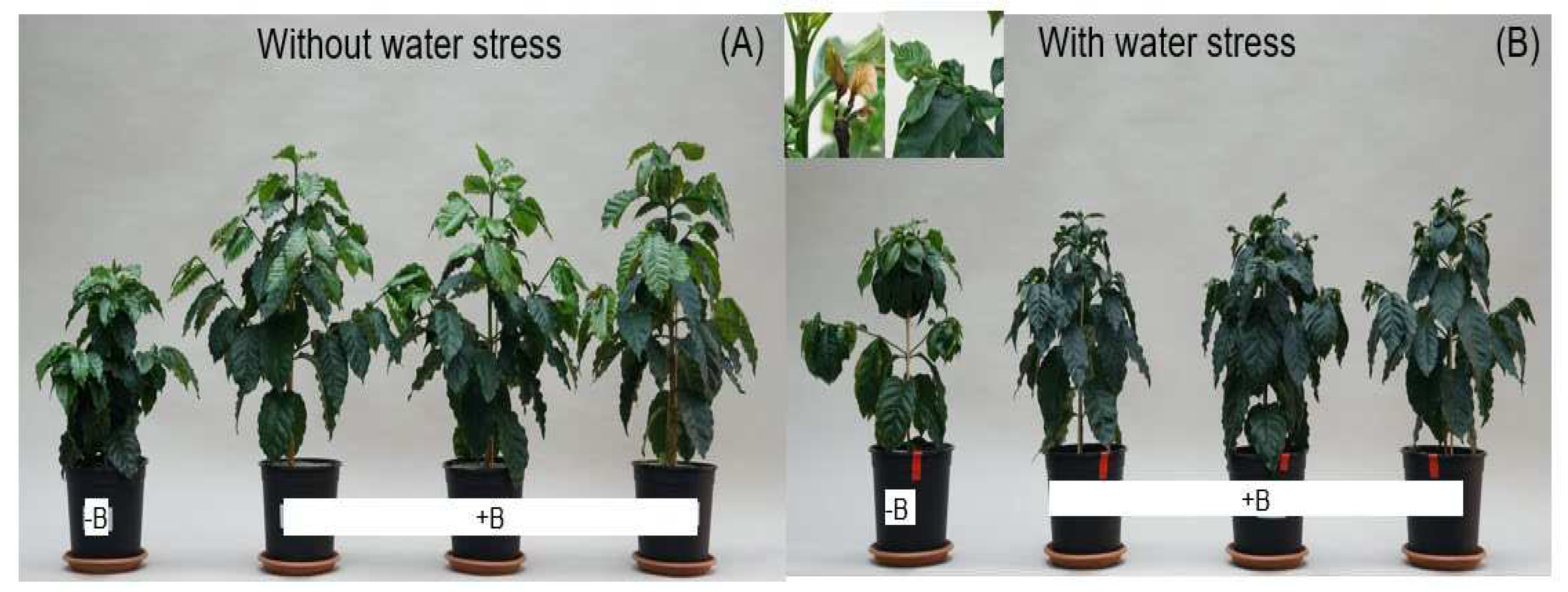
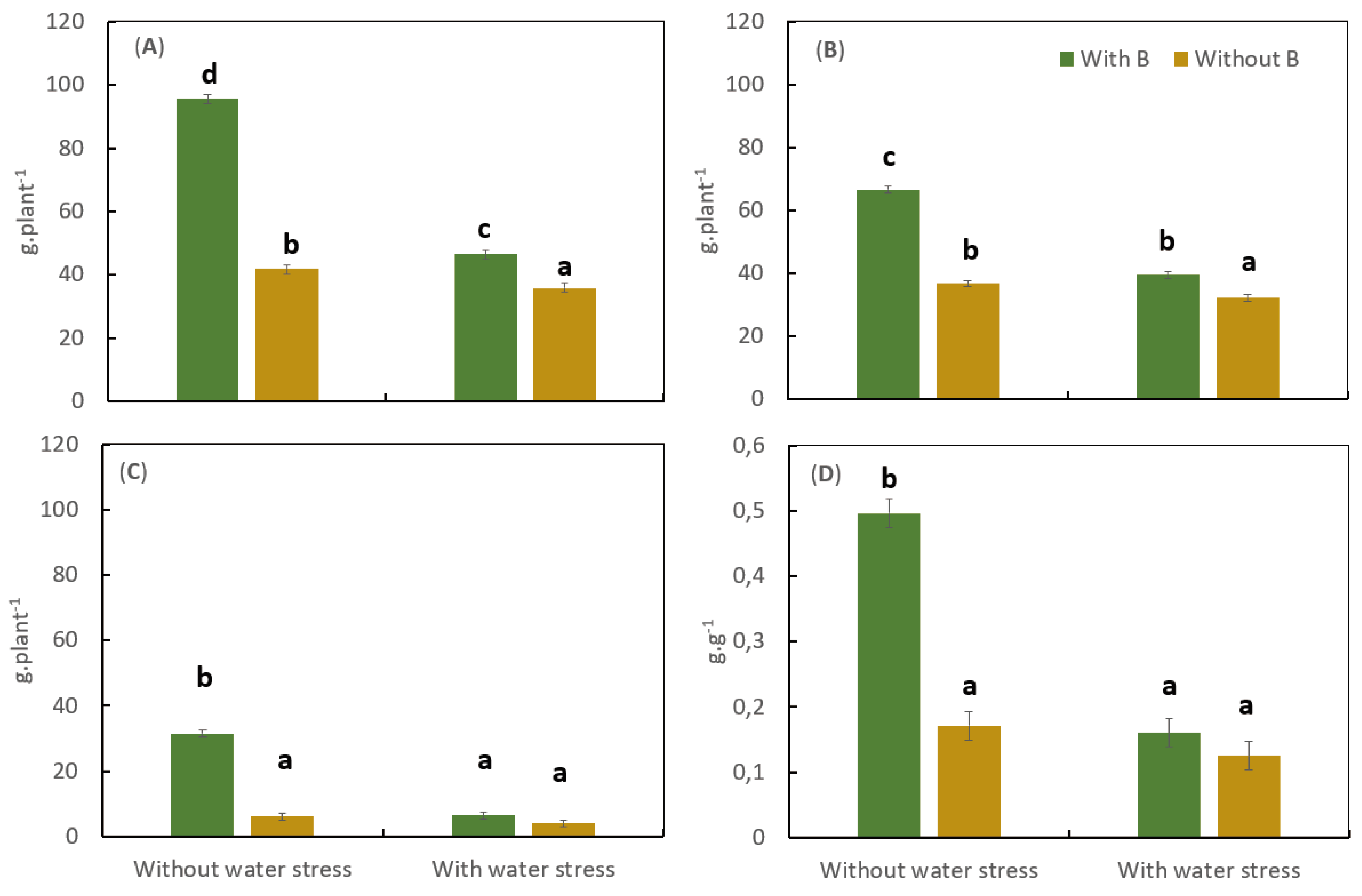
3.1. Effect of Water Stress and Boron on Biomass Accumulation, Boron, and Nitrogen Uptake Under Controlled Conditions
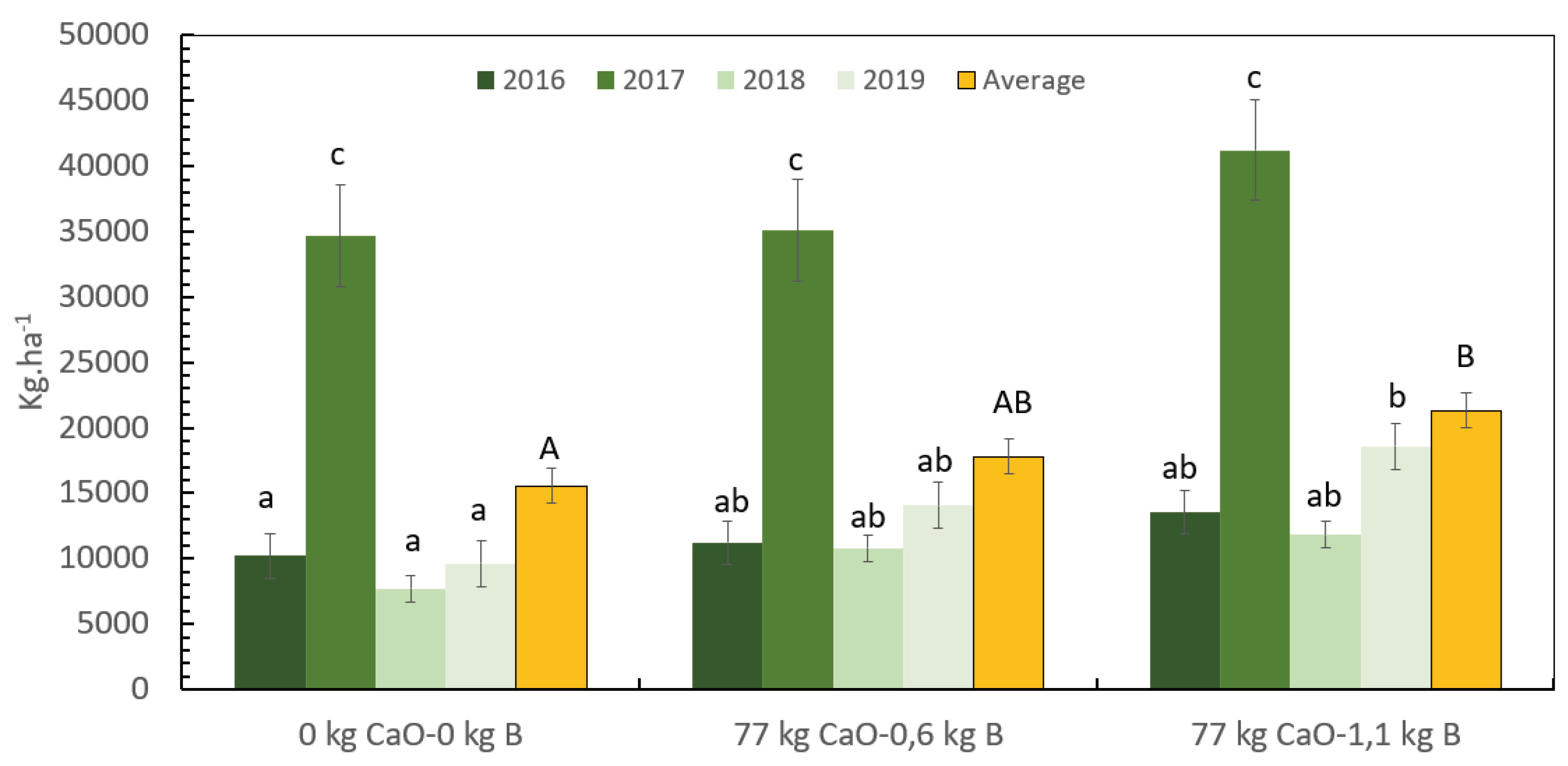
3.2. B Influence on Coffee Yield in Field Conditions
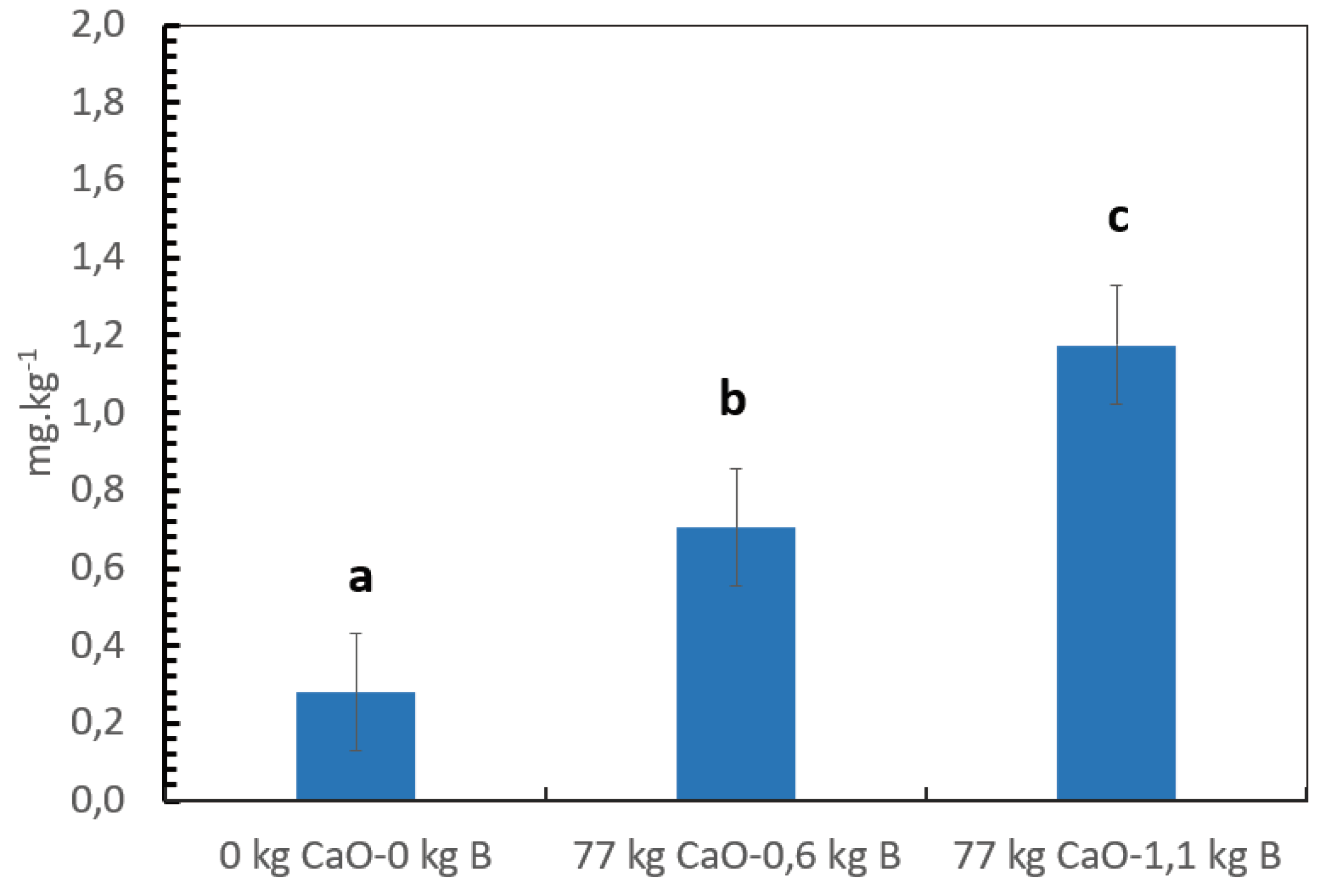
3.3. B in the Soil After Five Years of Application in the Field Trial
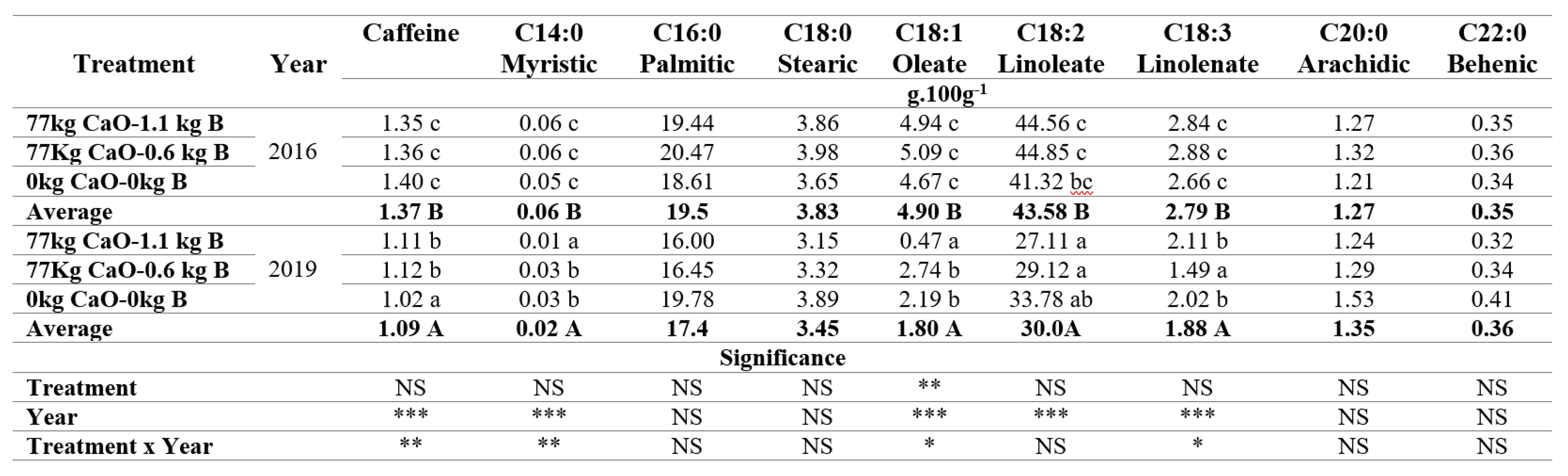
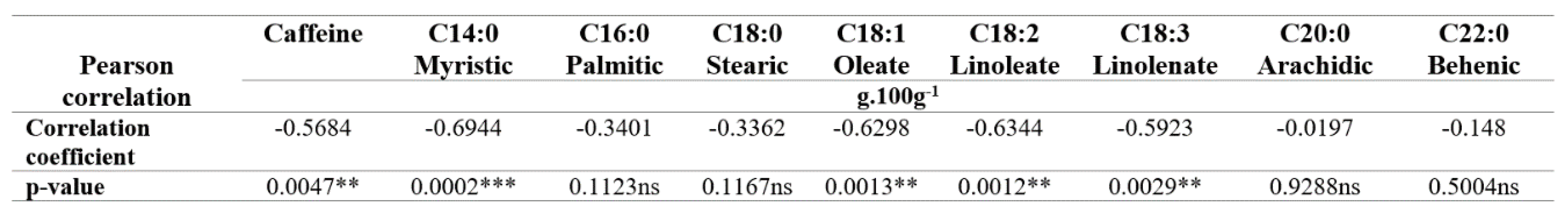
3.4. B and the Biochemical Composition of the Green Coffee Beans Produced under Field Conditions
4. Discussion
4.1. Interaction Water and B Deficits on Biomass Accumulation, B and N Uptake under Controlled Conditions
4.2. Sinergy Ca-B on Coffee Productivity
4.3. B in the Soil After Five Years Application
4.4. B and the Biochemical Composition of the Green Coffee Beans in Field Conditions
5. Conclusions
Author Contributions
Funding
Data Availability
Acknowledgments
Conflicts of interest
References
- Samper, F.L.; Giovannucci, D.; Marques, V.L. The powerful role of intangibles in the coffee value chain. Economic Research Working Paper/World Intellectual Property Organization-WIPO. 2017, No.39. 79p.
- Cordes, Y.K.; Sagan, M.; Kennedy, S. Responsible coffee sourcing: Towards a living income for producers. Columbia Center on Sustainable investment. Columbia law school and Earth Institute, Columbia University. 2021, 72p.
- International Coffee Organization-ICO. Coffee development report. 2019, 98p. Available online: https://www.internationalcoffeecouncil.com/_files/ugd/0dd08e_b2c2768ae87045e383962ce14ef44925.pdf.
- DaMatta, M.F.; Ramalho, J.D.C. Impacts of drought and temperature stress on coffee physiology and production: A Review. Braz. J. Plant. Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Ramirez, B.V.H.; Peña, Q.A.; Jaramillo, R.A.; Giraldo, E.; Suarez, A.; Duque, R. Agroclimatic risk for the Colombian coffee zone: Method to regionalize the climate variability. Cenicafé 2012, 63, 98–115. (in Spanish). [Google Scholar]
- Ramirez, B.V.H.; Jaramillo, R.A.; Arcila, P.J. The climate factors that influence the coffee crop productivity in Colombia.in. Manual del Cafetero Colombiano-Cenicafé (Colombia). 2013, 1, 205–238. (in Spanish). [Google Scholar]
- Ramalho, J.C.; Rodrigues, A.P.; Lidon, F.C.; Marques, L.M.C. , Leitao, A.E.; Fortunato, A.S.; Pais, J.P.; Silva, M.J.; Scotti-Campos, P.; Lopes, A.; Reboredo, F.H.; Ribeiro-Barros, A.I. Stress cross-response of the antioxidative system promoted by superimposed drought and cold conditions in Coffee spp. PLoS ONE 2018, 16, e0198694. [Google Scholar] [CrossRef] [PubMed]
- Leon-Burgos, A.F.; Unigarro, C.; Balaguera-López, H.E. Can prolonged conditions of water deficit alter photosynthetic performance and water relations of coffee plants in central-west Colombia. South African Journal of Botany. 2022, 149, 1–10. [Google Scholar] [CrossRef]
- Bunn, C.; Läderach, P.; Ovalle, R.O.; Kirschke, D. A bitter cup: Climate change profile of global production of Arabica and Robusta coffee. Climate Change. 2015, 129, 89–101. [Google Scholar] [CrossRef]
- Grüter, R.; Traschel, T.; Laube, P.; Jaisli, I. Expected global suitability of coffee, cashew and avocado due to climate change. PLoS ONE 2022, 17, e0261976. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.C. Introduction. In boron and its role in crops production. Ed. U C Gupta.p 1; CRC Press: Boca Raton, FL, USA, 1993; 256p. [Google Scholar]
- Welch, R.M.; Allaway, W.H.; House, W.A.; Kubota, J. Geographic distribution of trace elements problems, In Micronutrients in Agriculture, 2nd ed.; Montvelt, J.J., Cox, F.R., Shuman, L.M., Welch, R.M., Eds.; SSSA Book Ser. 4; SSSA: Madision, Wisconsin, 1991; pp. 31–57. [Google Scholar]
- Sarkar, D.; Mandal, B.; Mazumdar, D. Plant availability of boron in acid soils as assessed by different extractants. J. Plant Nutr. Soil Sci. 2008, 171, 249–254. [Google Scholar] [CrossRef]
- Antoniadis, V.; Chatzissavvidis, C.; Paparnakis, A. Boron behavior in apple plants in acidic and limed soil. J. Plant Nutr. Soil Sci. 2013, 176, 267–272. [Google Scholar] [CrossRef]
- Brown, H.P.; Shelp, J.B. Boron mobility in plants. Plant and Soil. 1997, 193, 85–101. [Google Scholar] [CrossRef]
- Malavolta, E.; Lima-Filho, O.F. Mineral nutrition and fertilization of traditional, high plant density irrigated and organic coffee plantation. In I Simposio de Pesquisa dos Cafés do Brasil; (in Portuguese). 2000; pp. 331–353. [Google Scholar]
- Ramirez, F.; Bertsch, F.; Mora, L. Nutrients consumption by fruits and branches of coffee during development and maturation in Aquiares, Turrialba, Costa Rica. Agronomia costaricence. 2002, 26, 33–42. (in Spanish). [Google Scholar]
- Laviola, B.G.; Martines, H.E.P.; Salomão, L.C.C.; Cruz, C.D.; Mendoza, S.M. Nutrients accumulation by the coffee beans in two elevations: Micronutrients. Rev. Bras. Ciên. Solo 2007, 31, 1439–1449. (in Portuguese). [Google Scholar] [CrossRef]
- Sadeghian, K.S.; Salamanca, J.A. Micronutrients in coffee fruits and leaves. Cenicafé 2015, 66, 73–87. (in Spanish). [Google Scholar]
- Lima-Filho, O.F.; Malavolta, E. Evaluation of extraction procedure on determination of critical soil and foliar level of boron and zinc in coffee plants. Comm. Soil Sci. Plant Anal 1998, 29, 825–833. [Google Scholar] [CrossRef]
- Malavolta, E. Manual of plant nutrition; Editorial Agronomica Ceres Ltd.a.: Sao Paulo, Brasil, 2006; 631p. (in Portuguese) [Google Scholar]
- Favarin, J.L.; Teles de Souza, L.; Mazzafera, P.; Dimenstein, L. Soil correction and fertilization of irrigated coffee trees in production (in Portuguese). In: Cafeicultura do Cerrado/editores técnicos Gladyston Rodrigues/Carvalho et al-Belo Horizonte:EPAMIG. 2021; 564p. [Google Scholar]
- Gonzales, F.A. Why boron is an essential element for vascular plants. New Phytologist. 2020, 226, 1228–1230. [Google Scholar] [CrossRef]
- Brown, H.P.; Bellaloui, N.; Wimmer, A.M.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F.; Römheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- Goldbach, E.H. , Wimmer, A.M. Boron in plants and animals: Is there a role beyond cell-wall structure? J. Plant. Nutr. Soil Sci. 2007, 170, 39–48. [Google Scholar] [CrossRef]
- Huang, L.; Ye, S.; Bell, W.R.; Dell, B. Boron nutrition and chilling tolerance of warm climate crop species. Ann. Bot. 2005, 96, 755–767. [Google Scholar] [CrossRef]
- Lima de Souza, T.; Pádua, O.D.; Ferreira, S.C.; Pereira, R.T.H.; Campos, C.J.P.; da Silva Resende, R.E.; Fernandes, T.J.; de Souza, T.R.; Ramirez, B.V.; Guelfi, D. Nitrogen fertilizer technologies: Opportunities to improve nutrient use efficiency towards sustainable coffee production systems. Agric. Ecosyst. Environ. 2023, 345, 108317. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Leite, M.V. Coffee leaf and stem anatomy under boron deficiency. R. Bras. Ci. Solo. 2007, 31, 477–483. [Google Scholar] [CrossRef]
- Malavolta, E.; Fernandes, D.R.; Casale, H.; Peres, R.J. Be the doctor of your coffee plantation. Informaciones Agronomicas. Brazilian Association for Potash and Phosphorus 1993, 64(36p). (in Portuguese). [Google Scholar]
- Nyomora, A.M.S.; Brown, P.H.; Pinney, K.; Polito. Foliar Application of Boron to Almond Trees Affects Pollen Quality. J. Amer. Soc. Hort. Sci. 2000, 125, 265–270. [Google Scholar] [CrossRef]
- Ramirez, B.V.H.; Küsters, J.; de Souza, T.R.; Simmes, C. Calcium nutrition in coffee and its influences on growth, stress tolerance, cations uptake, and productivity. Front. Agron. 2020, 2, 590892. [Google Scholar] [CrossRef]
- Arcila, P.J.; Buhr, L.; Bleiholder, H.; Hack, H.; Meier, U.; Wicke., H. Application of the extended BBCH scale for the description of the growing stages of the coffee (Coffea spp.). Ann. Appl. Biol 2002, 141, 19–27. [Google Scholar] [CrossRef]
- González, O.H.; Salamanca, J.A. Representative soil units of the colombian coffee Zone, Chinchiná Cenicafé: Caldas, Colombia. (in Spanish). 2008; 25p. [Google Scholar]
- Ramirez, B.V.H. Coffee phenology is a useful tool to support decision-making. Avances Técnicos Cenicafé (in Spanish). 2014, 441(8p). [Google Scholar]
- Puerta, Q.G.I.; Echeverry, M.J.G. Controlled coffee fermentation: Technology to add value to the quality. Avances Técnicos Cenicafé 2015, 454(12p). (in Spanish). [Google Scholar]
- Ashoor, H.S.; Seperich, J.G.; Woodrow, C.; Monte, C.; Welty, J. High-Performance liquid chromatography determination of caffeine in decaffeinated coffee, tea, and beverage Products. J. Assoc. Off. Anal. Chem. 1983, 66, 606–609. [Google Scholar]
- Villarreal, D.; Laffargue, A.; Posada, H.; Bertands, B.; Lashermes, P.; Dussert, S. Genotypic and environmental effects on Coffee (Coffea arabica L.) bean fatty acid profile: Impact on variety and origen chemometric determination. J. Agric. Food Chem 2009, 57, 11321–11327. [Google Scholar] [CrossRef]
- Villarreal, D.; Baena, L.; Posada, H. Lipids and Fatty acids analysis in green coffee beans in advanced lines of Coffea arabica planted in Colombia. Cenicafé 2012, 63, 19–40. (In Spanish) [Google Scholar]
- Ramirez, B.V.H.; Küsters, J. Calcium and potassium nutrition increase the water use efficiency in coffee: A promising strategy to adapt to climate change. Hydrology. 2021, 8, 75. [Google Scholar] [CrossRef]
- Brown, H.P.; Hu, H. Phloem mobility of boron in species dependent. Evidences for phloem mobility in sorbitol-rich species. Ann. Bot 1996, 77, 497–505. [Google Scholar] [CrossRef]
- Leite, M.V.; Brown, H.P.; Rosolem, A.C. Boron translocation in coffee tress. Plant Soil. 2007, 290, 221–229. [Google Scholar] [CrossRef]
- Du, W.; Pan, Z.Y.; Hussain, S.B.; Han, Z.X.; Peng, S.A.; Liu, Y.Z. Foliar supplied boron can be transported to roots as a boron-sucrose complex via phloem in citrus trees. Front. Plant Sci. 2020, 11, 250. [Google Scholar] [CrossRef]
- Brown, H.P.; Hu, H. Phloem boron mobility in diverse plant species. Bot Acta. 1998, 111, 331–335. [Google Scholar] [CrossRef]
- Noiraud, N.; Maurousset, L.; Lemonie, R. Transport of polyols in higher plants. Plant Phys. Bio. 2001, 39, 717–728. [Google Scholar] [CrossRef]
- Olivera-Silva, S.L.; Prado, R.d.M.; Abreu, C.H., Jr.; Silva, G.P.d.; Silva, G.B.d., Jr.; Silva, J.L. Fd. 10Boron is mobile in cowpea plants. Front. Plant Sci. 2021, 12, 717219. [Google Scholar] [CrossRef]
- Balleto, S.A.C.; Menegario, A.A.; Giné, M.F. Boron isotope dilution in cellular fractions of coffee leaves evaluated by inductively coupled plasma mass spectrometry with direct injection nebulization (DIN-ICP-MS). J. Braz Chem Soc. 2003, 14, 269–273. [Google Scholar] [CrossRef]
- Boaretto, R.M.; Quaggio, J.A.; Mourao Filho, F.A.A.; Giné, M.F.; Boaretto, A.E. Absorption and mobility of boton in young citrus plants. Communications in Soil Science and Plant Analysis 2008, 39, 2501–2514. [Google Scholar] [CrossRef]
- Boaretto, R.M.; Quaggio, J.A.; Mattos, D., Jr.; Muraoka, T.; Boaretto, A.E. Boron Uptake and Distribution in Field Grown Citrus Trees. J. Plant. Nutr. 2011, 34, 839–849. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Wang, S.; Shi, L.; Xu, F. Genotype differences in the synergy effect of nitrogen and boron on the seed yield and nitrogen uses efficiency of Brasicca napus. Journal of the Science of Food and Agriculture. 2021, 102, 3563–3571. [Google Scholar] [CrossRef]
- Karim, M.; Zhang, Y.Q.; Zhao, R.R.; Chen, X.P.; Zhang, F.S.; Zou, C.Q. Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. J. Plant Nutr. Soil Sci. 2012, 175, 142–151. [Google Scholar] [CrossRef]
- Cakmak, I.; Römheld, V. Boron deficiency-induced impairments of cellular functions in plants. Plant Soil. 1997, 193, 71–83. [Google Scholar] [CrossRef]
- Yan, L.; Du, C.; Riaz, M.; Jiang, C. Boron mitigates citrus root injuries by regulating intracellular pH and reactive oxygen species to resist H+-toxicity. Environmental Pollution. 2019, 255, 113254. [Google Scholar] [CrossRef]
- DaMatta, M.F.; Ronchi, P.C.; Maestri, M.; Barros, S.R. Ecophysiology of coffee growth and production: A Review. Braz. J. Plant. Physiol. 2007, 19, 485–510. [Google Scholar] [CrossRef]
- Santinato, F.; de Oliveira, T.T.; de Mello, P.R.; Caione, G.; de Albuquerque, S.V.; Santinato, R. Boron doses applied to soil during coffee development. Comunicata Scientiae. 2016, 7, 49–55. [Google Scholar] [CrossRef]
- Trenberth, E.K. The Definition of El Niño. Bull. Am. Meteorol. Soc. 1997, 78, 2771–2777. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration-NOAA/National Weather Service/Climate Prediction Center. ONI Data Base. Available online: https://origin.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ONI_v5.php (accessed on 1 January 2023).
- Ramírez, B.V.H.; Arcila, P.J.; Jaramillo, R.A.; Rendón, S.J.S.; Cuesta, G.G.; Menza, F.H.D.; Mejia, M.C.G.; Montoya, D.F.; Mejia, M.J.W.; Torres, N.J.C.; Sánchez, A.P.M.; Baute, B.J.E.; Peña, Q.A. The Coffee crop flowering and its relationship with water, thermal, and energy availability. Cenicafé 2010, 61, 132–158. (in Spanish). [Google Scholar]
- Peña, Q.A.; Ramirez, B.V.H.; Jaramillo, R.A.; Rendon, S.J.R.; Arcila, P.J. Effects of the daylength and soil humidity on the Flowering of Coffea arabica L. in Colombia. Rev. Fac. Nal. Agr. Medellin. 2011, 64, 5745–5754. [Google Scholar]
- Sheng, O.; Zhou, G.; Wie, Q.; Peng, S.; Deng, X. Effects of excess boron on growth, gas exchange, and boron status of four orange scion-rootstock combinations. J. Plant Nutr. Soil Sci. 2010, 173, 469–476. [Google Scholar] [CrossRef]
- Da Silva, C.R.; Baird, R.; Degryse, F.; McLaughlin, J.M. Slow and fast-release boron sources in potash fertilizers: Spatial variability, nutrient dissolution, and plant uptake. Soil. Sci. Soc. Am. J. 2018, 82, 1437–1448. [Google Scholar] [CrossRef]
- Terraza, P.M.F.; Summer, E.M.; Cabrera, L.M.; Thomson, A. Boron adsorption and desorption on volcanic ash-derived soils. Soil. Sci. Soc. Am. J. 2018, 82, 66–75. [Google Scholar] [CrossRef]
- Cong, T.T. Study on supplying boron to coffee on basaltic soil in Central Highlands of Vietnam. J. Fertil. Pestic. 2017, 8, 000179. [Google Scholar] [CrossRef]
- Silva, S.L.O.; Prado, R.D.M.; Abreu, C.H.; Silva, G. P.; Silva, G. Bd.; Silva, J.L. Fd. Fd.. 10Boron is mobile in cowpea plants. Front. Plant Sci 2021, 12, 717219. [Google Scholar] [CrossRef]
- Mühling, K.H.; Wimmer, M.; Goldbach, H.E. Apoplastic and membrane-associated Ca+2 in leaves and roots as affected by boron deficiency. Physiol. Plant. 1998, 102, 179–184. [Google Scholar] [CrossRef]
- DeMoranville, C.; Deubert, K. Effect of commercial calcium-boron and manganese-zinc formulations on fruit set of cranberries. J. Hortic. Sci. 1987, 62, 163–169. [Google Scholar] [CrossRef]
- Galeriani, T.M.; Nieves, G.O.; Santos Fereira, J.H.; Oliveira, R.N.; Calonego, J.C.; Crusciol, C.A.C. Calcium and boron fertilization improves soybean photosynthetic efficiency and grain yield. Plants 2022, 11, 2937. [Google Scholar] [CrossRef]
- Simard, R.R.; Charron, G.; Pageau, D. Field calibration of boron soil test for barley. Comm Soil Sci and Plant Analysis. 1996, 27, 1631–1646. [Google Scholar] [CrossRef]
- Basak, P.; Sarkar, D.; Mandal, B.; Mal, S.; Adjikary, S.; Kundu, R.; Dutta, J.; Deb, S.; Rahman, F. Determination of critical concentration of boron in soils and plants of cauliflower (Brassica oleracea var. botrytis L.) using polynomial equations. Comm Soil Sci and Plant Analysis. 2022, 53, 2388–2399. [Google Scholar] [CrossRef]
- Kidder, G. Methodology for calibration soil test. Soil Crop Sci. Soc Florida Proc. 1993, 52, 70–73. [Google Scholar]
- Lambot, C.; Herrera, J.C.; Bertrand, B.; Sadeghian, S.; Benavides, P.; Gaitan, A. Cultivating coffee-quality- Terrior and Agro-Ecosystem. In The craft and science of Coffee; Folmer, B., Ed.; Academic Press/Elsevier, 2017; pp. 17–45. [Google Scholar]
- Buffo, A.R.; Cardelli, F.C. Coffee flavour: An overview. Flavour Fragr. J. 2004, 19, 99–104. [Google Scholar] [CrossRef]
- Campiño, L.I.; Villegas, H.A.M.; Arana, V.; Posada, H. Characterization of Chlorogenic acids (CGA) and nine isomers in a F2 population derived from Coffea arabica L. Agronomia Colombiana. 2020, 31, 19–28. [Google Scholar] [CrossRef]
- Dessalegn, Y.; Labuschagne, T.M.; Osthoff, G.; Herselman, L. Genetic diviersity and correlation of bean caffeine content with cup quality and green bean physical characteristics in coffee (Coffea arabica L.). J Sci Food Agric. 2008, 88, 1726–1730. [Google Scholar] [CrossRef]
- Chen, B.; Furtado, A.; Smyth, E.H.; Henry, J.R. Influence of genotype and environment on coffee quality. Trends in Food Sci. Tech. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Figueiredo, P.L.; Borém, M.F.; Ribeiro, C.F.; Giomao, S.G.; Taveira, S.J.H.; Malta, R.M. Fatty acids profiles and parameters of quality of specialty coffees produced in different Brazilian regions. Afr. J Agr. Res. 2015, 10, 3484–3493. [Google Scholar] [CrossRef]
- Toci, T.A.; Neto, J.M.F.; Torres, G.A.; Farah, A. Changes in triacylglycerols and free fatty acids composition during storage of roasted coffee. Food Sci. Tech. 2013, 50, 581–590. [Google Scholar] [CrossRef]
- Bellaloui, N. Effect of water stress and foliar boron application on seed protein, oil, fatty acids, and nitrogen metabolism in soybean. Amer. J. Plant Sci. 2011, 2, 692–701. [Google Scholar] [CrossRef]
- He, M.; Ding, N.Z. Plant unsaturated fatty acids: Multiple roles in stress response. Front. Plant. Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Upchurch, G.R. Fatty acids unsaturation, mobilization, and regulation in the response of coffee plants to stress. Biotechnol Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Wu, W.; Muhammad, R.; Yan, L.; Zhang, Z.; Jian, C. How the cells were injured and the secondary metabolites in the shikimate pathway were changed by boron deficiency in trifoliate orange root. Plant. Physiol Biochem. 2020, 151, 630–639. [Google Scholar] [CrossRef]
- Chen, L.; Xia, F.; Wang, M.; Mao, P. Metabolomic analyses of alfalfa (Medicago sativa L. cv. Aohan) reproductive organs under boron deficit and surplus conditions. Ecotoxicology and Environmental Safety. 2020, 202, 111011. [Google Scholar] [CrossRef]
- Quiles, P.C.; Navarro, G.M.T.; Herrera, R.M.B.; Camacho, C.J.J.; Gonzales, F.A.; Rexach, J. Boron deficits increase cytosolic Ca+2 levels mainly via Ca+2 influx from the apoplast in Arabidopsis thaliana roots. Int. J. Mol. Sci. 2019, 20, 2297. [Google Scholar] [CrossRef] [PubMed]
| Year | T. min (°C) |
T. max (°C) |
T. med (°C) |
R.H (%) |
Rainfall (mm) |
Sunshine (hours) |
|---|---|---|---|---|---|---|
| 2014 | 15.7 | 23.6 | 19.1 | 74.6 | 1741.3 | 1233.1 |
| 2015 | 15.8 | 24.2 | 19.5 | 72.3 | 1319.6 | 1243.1 |
| 2016 | 16.1 | 24.1 | 19.6 | 73.4 | 1625.3 | 1241.4 |
| 2017 | 15.7 | 23.6 | 19.1 | 70.5 | 1976.3 | 1211.2 |
| 2018 | 15.7 | 23.5 | 19.0 | 75.3 | 1761.9 | |
| 2019 | 1482.1 | |||||
| Mean | 15.8 | 23.8 | 19.3 | 73.2 | 1651.1 | 1231.2 |
| Season | N | P2O5 | K2O | MgO | CaO | S |
|---|---|---|---|---|---|---|
| kg.ha-1 | ||||||
| 2014-2016± | 251 | 84 | 186 | 38 | 109 | 35 |
| 2016-2017 | 150 | 77 | 119 | 26 | 59 | 23 |
| 2017-2018 | 250 | 90 | 240 | 49 | 52 | 56 |
| 2018-2019 | 250 | 118 | 290 | 37 | 86 | 48 |
| Average | 225 | 92 | 209 | 38 | 77 | 41 |
| B Treatments/Season | 2014-2016± | 2016-2017 | 2017-2018 | 2018-2019 | Average |
|---|---|---|---|---|---|
| kg. B ha-1 | |||||
| High B rate | 0.8 | 1.1 | 1.05 | 1.39 | 1.1 |
| Reduced B rate (50% less) | 0.6 | 0.76 | 0.76 | 0.40 | 0,6 |
| Water Level (WL) |
B Level (BL) |
Nutrient Content | Nutrient Uptake by Plant | ||||
|---|---|---|---|---|---|---|---|
| Young Leaves | Mature Leaves | N | B | ||||
| N (%) |
B (mg.kg-1) |
N (%) |
B (mg.kg-1) |
mg. plant-1 | |||
| Without Water Stress | +B | 3.12ab | 63.72b | 2.41a | 265.07d | 1873.4d | 11.63d |
| -B | 3.70d | 12.73a | 3.72bc | 9.56a | 1553.6b | 6.32b | |
| With Water Stress | +B | 3.37bc | 249.14c | 3.86c | 80.54b | 1413.3a | 0.38a |
| -B | 4.69e | 11.44a | 4.27d | 11.93a | 1402.8a | 0.37a | |
| WL | *** | *** | *** | *** | *** | *** | |
| BL | *** | *** | *** | *** | *** | *** | |
| WL x BL | ** | *** | *** | *** | ** | *** | |
| Period | Harvest Year | Net Rainfall | Crop Evapotranspiration (ETc) |
|---|---|---|---|
| mm | |||
| 2015-2016 | 2016 | 529.0 | 565.0 |
| 2016-2017 | 2017 | 1096.0 | 980.0 |
| 2017-2018 | 2018 | 925.0 | 901.0 |
| 2018-2019 | 2019 | 837.0 | 833.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





